Studying the Relationship between the Antiviral Activity and the Structure of ἰ-Carrageenan Using Ultrasonication
Abstract
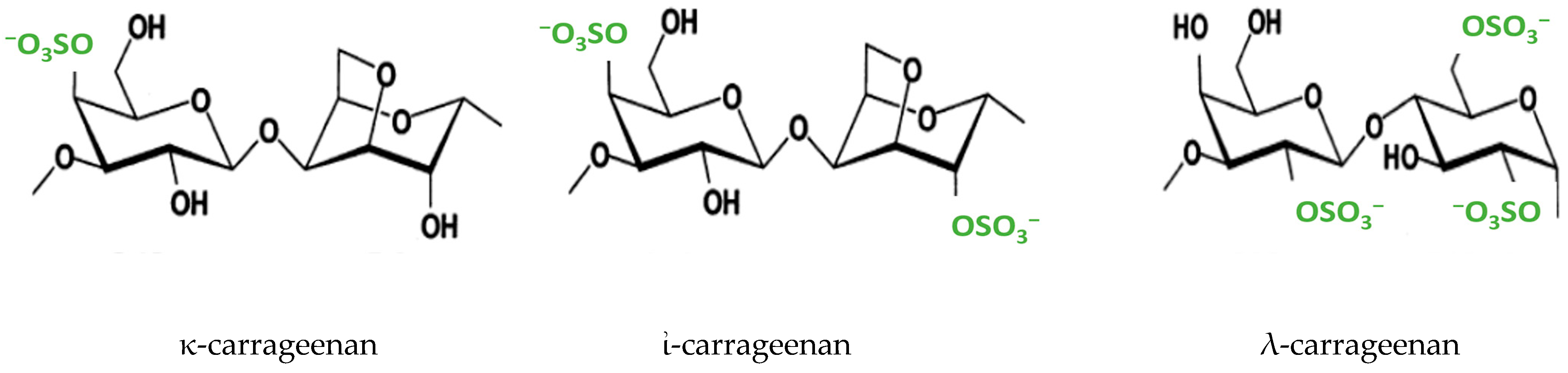
:1. Introduction
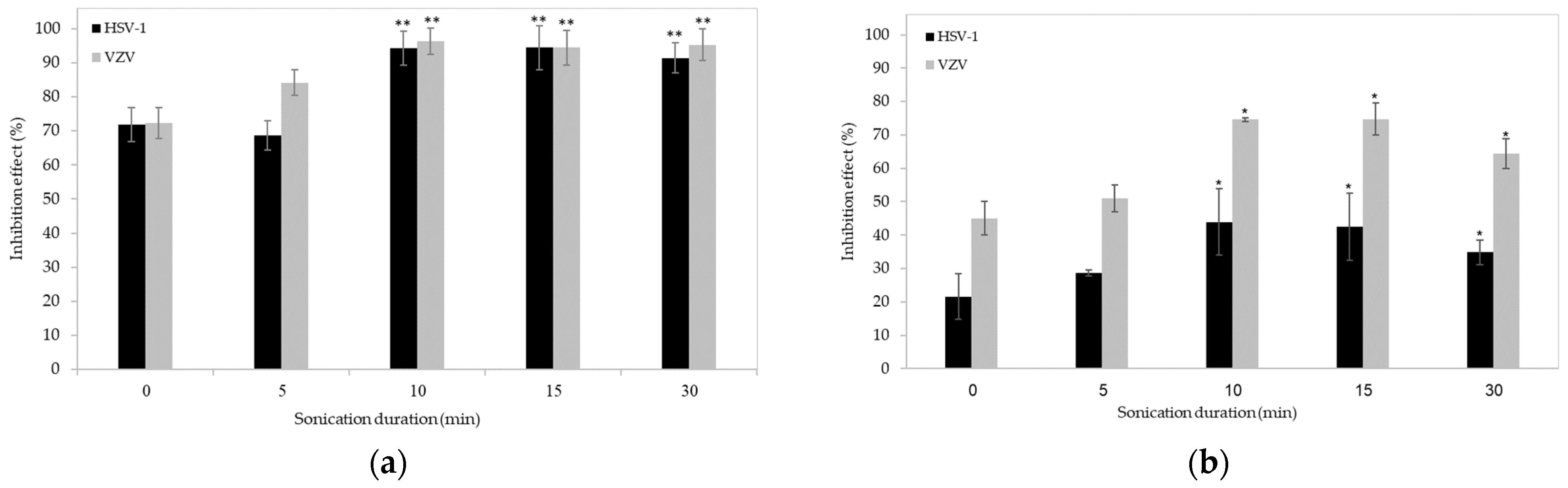
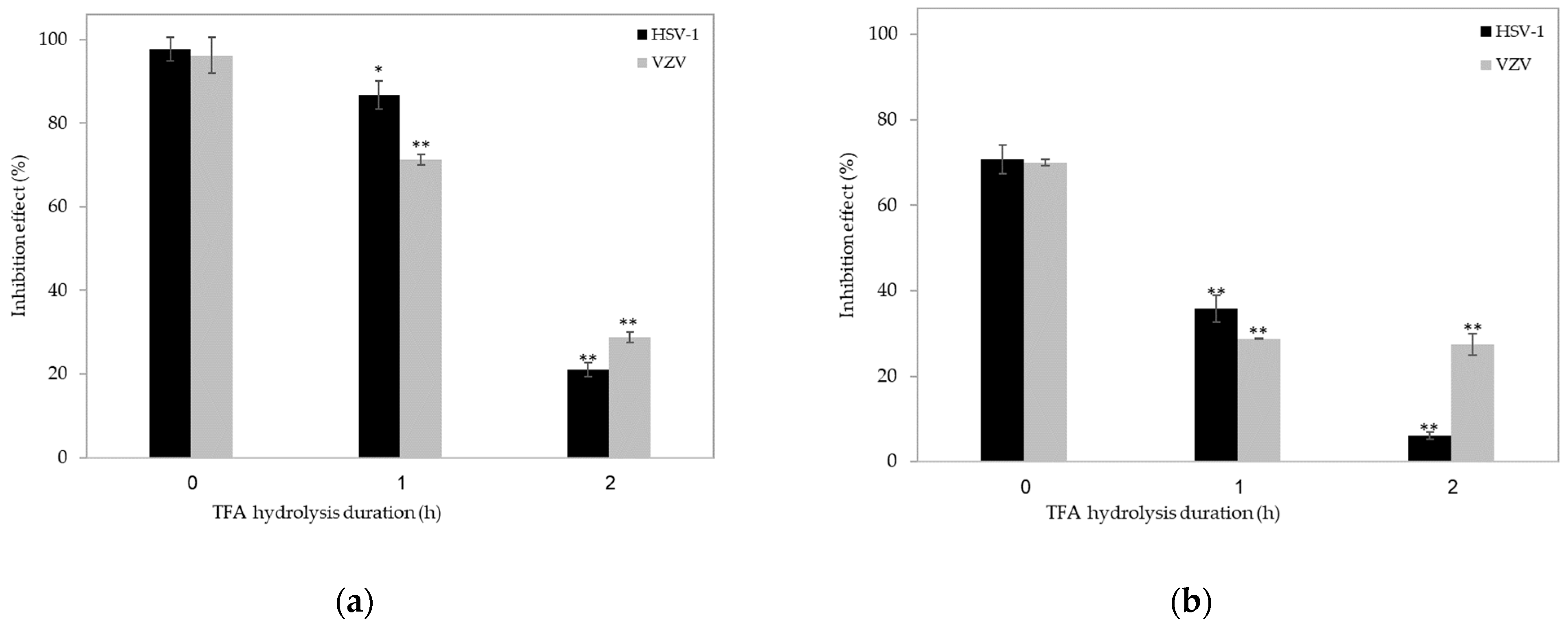
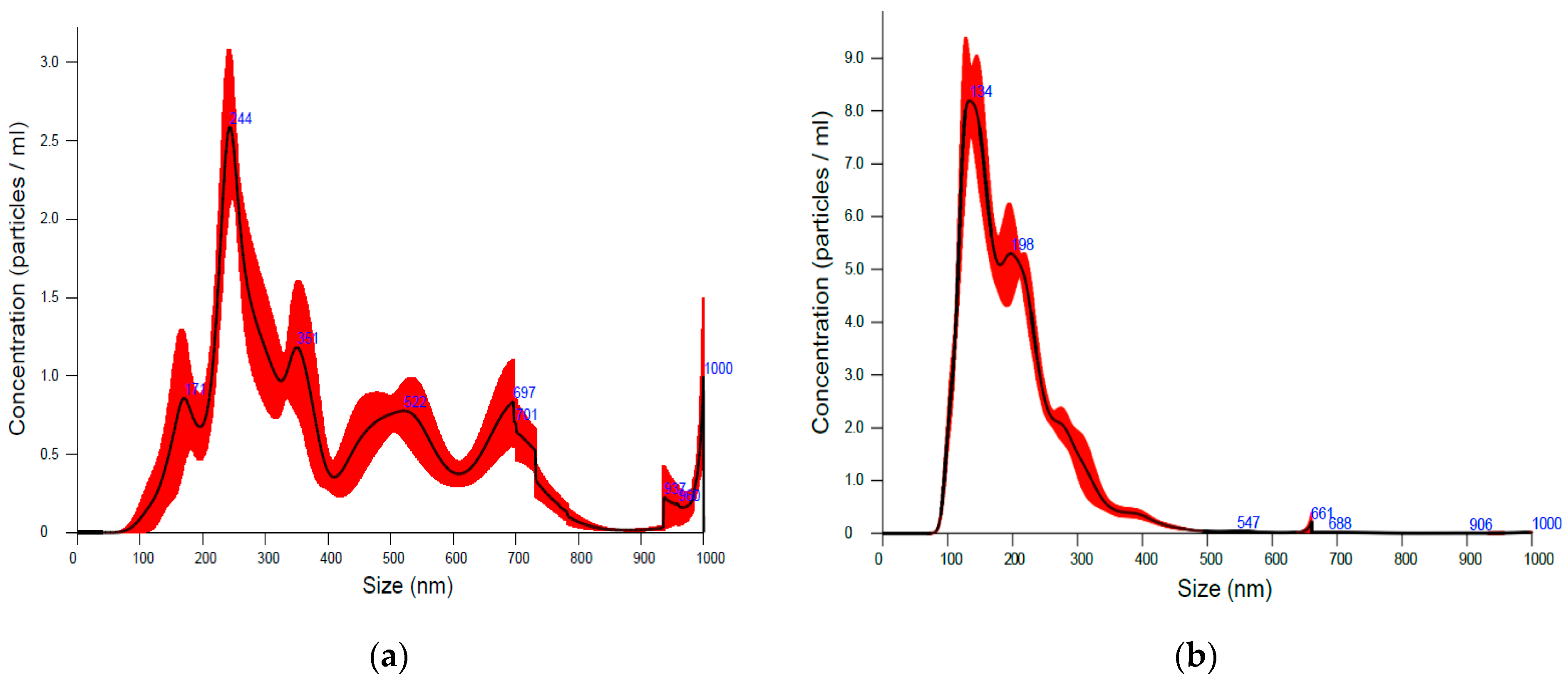
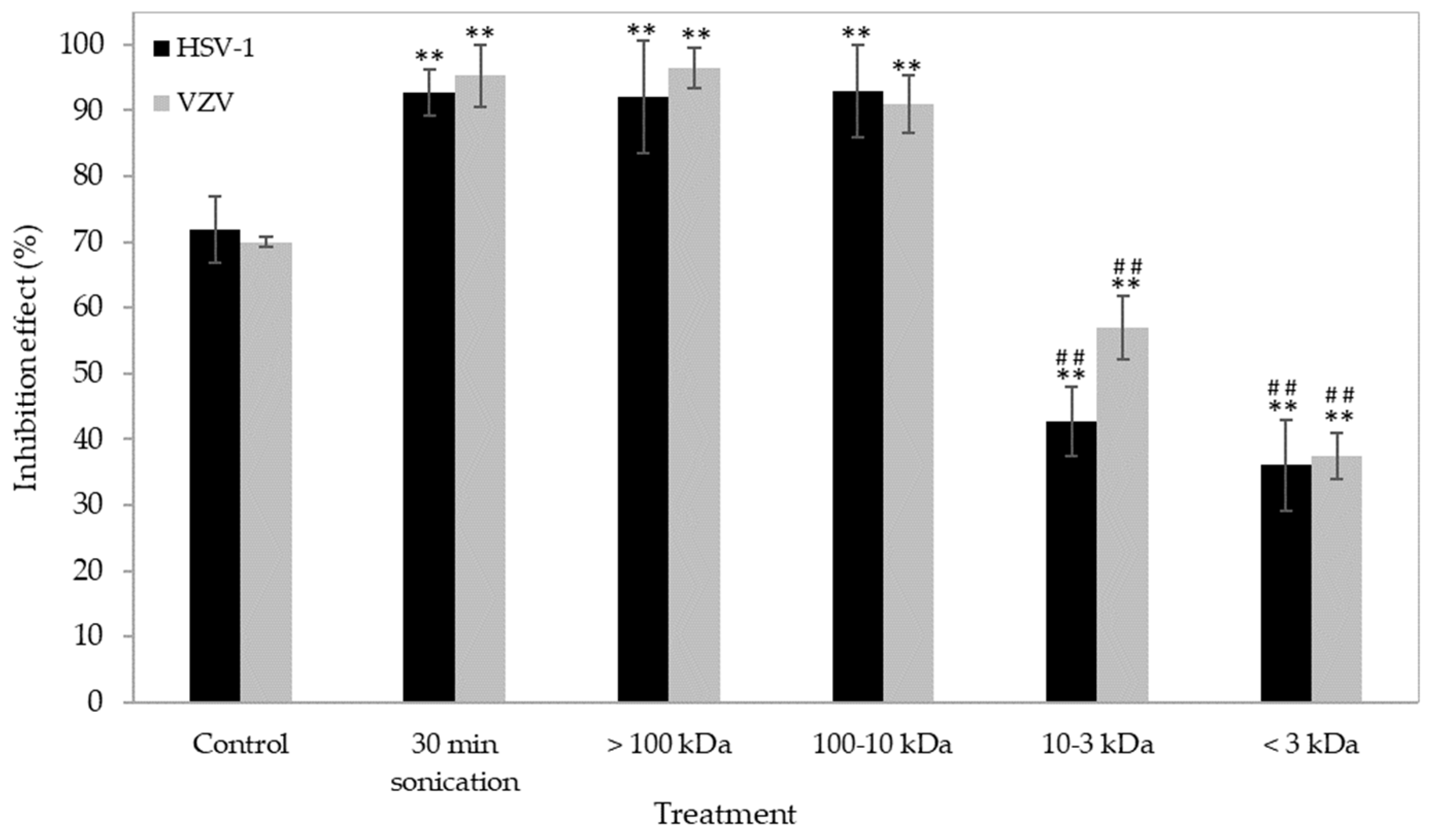
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of ἰ-Carrageenan and Its Ultrasonicated Forms
3.2. Acid Hydrolysis
3.3. Fractionation
3.4. Sugar Content Determination
3.5. In Vitro Cytotoxicity Measurements in Vero Cells
3.6. In Vitro Antiviral Activity Efficacy
3.7. Statistical Analyses
3.8. Fourier-Transform Infrared (FTIR) Analyses
3.9. Rheological Measurements
3.10. Nanoparticle Tracking Analysis (NTA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barros, F.C.; da Silva, D.C.; Sombra, V.G.; Maciel, J.S.; Feitosa, J.P.; Freitas, A.L.; de Paula, R.C. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh). Carbohydr. Polym. 2013, 92, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan from Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, structure, production, and application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. κ-CG: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Saxena, A. Bacterial carrageenases: An overview of production and biotechnological applications. Biotech 2016, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries-A review. J. Food Sci. Technol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohyd. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Lee, C. Carrageenans as broad-spectrum microbicides: Current status and challenges. Mar. Drugs 2020, 18, 435. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral Activity of Carrageenans and Processing Implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef]
- Eccles, R.; Meier, C.; Jawad, M.; Weinmüllner, R.; Grassauer, A.; Prieschl-Grassauer, E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: A randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir. Res. 2010, 11, 108. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure–activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Abu-Galiyun, E.; Huleihel, M.; Levy-Ontman, O. Antiviral bioactivity of renewable polysaccharides against Varicella Zoster. Cell Cycle 2019, 18, 3540–3549. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.-H.; Elahi, R.; Zhang, M.; Sun, H.-N. Ultrasonic modification of selected polysaccharides-review. J. Food Sci. Technol. 2015, 6, 446. [Google Scholar] [CrossRef]
- Ducatti, D.; Colodi, F.; Gonçalves, A.; Duarte, M.; Noseda, M. Production of agaro-and carra-oligosaccharides by partial acid hydrolysis of galactans. Rev. Bras. Farmacogn. 2011, 21, 296–304. [Google Scholar] [CrossRef]
- Tarman, K.; Sadi, U.; Santoso, J.; Hardjito, L. Carrageenan and its enzymatic extraction. Encycl. Mar. Biotechnol. 2020, 1, 147–159. [Google Scholar]
- Ansari, S.A.; Matricardi, P.; Cencetti, C.; DiMeo, C.; Carafa, M.; Mazzuca, C.; Palleschi, A.; Capitani, D.; Alhaique, F.; Coviello, T. Sonication-based improvement of the physicochemical properties of Guar Gum as a potential substrate for modified drug delivery systems. BioMed Res. Int. 2013, 2013, 985259. [Google Scholar] [CrossRef]
- Rastogi, N.K. Opportunities and challenges in application of ultrasound in food processing. Crit. Rev. Food Sci. Nut. 2011, 51, 705–722. [Google Scholar] [CrossRef]
- Reich, G. Ultrasound-induced degradation of PLA and PLGA during microspere processing: Influence of formulation variables. Eur. J. Pharm. Biopharm. 1998, 45, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, X.; Zhan, Q.; Zhong, L.; Hu, Q.; Zhao, L. Effects of ultrasound on the degradation kinetics, physicochemical properties and prebiotic activity of Flammulina velutipes polysaccharide. Ultrason. Sonochem. 2022, 82, 105901. [Google Scholar] [CrossRef] [PubMed]
- Basedow, A.M.; Ebert, K.H. Ultrasonic Degradation of Polymers in Solution. In Physical Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; pp. 83–148. [Google Scholar]
- De Ruiter, G.; Rudolph, B. Carrageenan biotechnology. Trends Food Sci. Technol. 1997, 8, 389–395. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Rokita, B.; Lotfy, S.; Ulanski, P.; Rosiak, J.M. Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr. Polym. 2005, 60, 175–184. [Google Scholar] [CrossRef]
- Lii, C.; Chen, C.; Yeh, A.; Lai, V. Preliminary study on the degradation kinetics of agarose and carrageenans by ultrasound. Food Hydrocoll. 1999, 13, 477–481. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Sanchez, J.A.; Motohiro, T.; Takaomi, K. Ultrasound effect used as external stimulus for viscosity change of aqueous carrageenans. Ultrason. Sonochem. 2013, 20, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Tecson, M.G.; Abad, L.V.; Ebajo, V.D., Jr.; Camacho, D.H. Ultrasound-assisted depolymerization of kappa-carrageenan and characterization of degradation product. Ultrason. Sonochem. 2021, 73, 105540. [Google Scholar] [CrossRef] [PubMed]
- Azizi, R.; Farahnaky, A. Ultrasound assisted-viscosifying of kappa carrageenan without heating. Food Hydrocoll. 2016, 61, 85–91. [Google Scholar] [CrossRef]
- Verzosa, A.L.; McGeever, L.A.; Bhark, S.J.; Delgado, T.; Salazar, N.; Sanchez, E.L. Herpes Simplex Virus 1 Infection of Neuronal and Non-Neuronal Cells Elicits Specific Innate Immune Responses and Immune Evasion Mechanisms. Front Immunol. 2021, 31, 644664. [Google Scholar] [CrossRef]
- Sasivimolphan, P.; Lipipun, V.; Likhitwitayawuid, K.; Takemoto, M.; Pramyothin, P.; Hattori, M.; Shiraki, K. Inhibitory activity of oxyresveratrol on wild-type and drug-resistant varicella-zoster virus replication in vitro. Antivir. Res. 2009, 84, 95–97. [Google Scholar] [CrossRef]
- Galetta, K.M.; Gilden, D. Zeroing in on zoster: A tale of many disorders produced by one virus. J. Neurol. Sci. 2015, 358, 38–45. [Google Scholar] [CrossRef]
- Stephanie, B.; Eric, D.; Sophie, F.M.; Christian, B.; Yu, G. Carrageenan from Solieria chordalis (Gigartinales): Structural analysis and immunological activities of the low molecular weight fractions. Carbohydr. Polym. 2010, 81, 448–460. [Google Scholar] [CrossRef]
- Myslabodski, D.E.; Stancioff, D.; Heckert, R.A. Effect of acid hydrolysis on the molecular weight of kappa carrageenan by GPC-LS. Carbohydr. Polym. 1996, 31, 83–92. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G.; Zhao, X.; Jiao, G.; Ren, S.; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J. 2009, 276, 2125–2137. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Barabanova, A.B.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Kelco, C.P. Genu® Carrageenan Book; CP Kelco ApS: Lille Skensved, Denmark, 2001. [Google Scholar]
- Volery, P.; Besson, R.; Schaffer-Lequart, C. Characterization of commercial carrageenans by Fourier transform infrared spectroscopy using single-reflection attenuated total reflection. J. Agric. Food Chem. 2004, 52, 7457–7463. [Google Scholar] [CrossRef]
- Jumaah, F.N.; Mobarak, N.N.; Ahmad, A.; Ghani, M.A.; Rahman, M.Y.A. Derivative of iota-carrageenan as solid polymer electrolyte. Ionics 2015, 21, 1311–1320. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- McKim, J.M.; Willoughby, J.A., Sr.; Blakemore, W.R.; Weiner, M.L. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: A review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Cri. Rev. Food Sci. Nutr. 2019, 59, 3054–3073. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Shi, Y.; Kornovski, B.S.; Savani, R.; Turley, E.A. A rapid, multiwell colorimetric assay for chemotaxis. J. Immunol. Methods 1993, 164, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]



| Ultrasonication Duration (min) | n | R2 |
|---|---|---|
| 0 | 0.154 | 0.997 |
| 5 | 0.119 | 0.999 |
| 10 | 0.324 | 0.999 |
| 15 | 0.490 | 0.985 |
| 30 | 0.980 | 0.946 |
| Fraction Size | % Sugar Content (Fraction/Total × 100) | |
|---|---|---|
| Native | Sonicated | |
| >100 kDa | 99.27 | 90.20 |
| 10–100 kDa | 0.60 | 8.80 |
| 3–10 kDa | 0.08 | 0.49 |
| <3 kDa | 0.05 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy-Ontman, O.; Abu-Galiyun, E.; Huleihel, M. Studying the Relationship between the Antiviral Activity and the Structure of ἰ-Carrageenan Using Ultrasonication. Int. J. Mol. Sci. 2023, 24, 14200. https://doi.org/10.3390/ijms241814200
Levy-Ontman O, Abu-Galiyun E, Huleihel M. Studying the Relationship between the Antiviral Activity and the Structure of ἰ-Carrageenan Using Ultrasonication. International Journal of Molecular Sciences. 2023; 24(18):14200. https://doi.org/10.3390/ijms241814200
Chicago/Turabian StyleLevy-Ontman, Oshrat, Eiman Abu-Galiyun, and Mahmoud Huleihel. 2023. "Studying the Relationship between the Antiviral Activity and the Structure of ἰ-Carrageenan Using Ultrasonication" International Journal of Molecular Sciences 24, no. 18: 14200. https://doi.org/10.3390/ijms241814200
APA StyleLevy-Ontman, O., Abu-Galiyun, E., & Huleihel, M. (2023). Studying the Relationship between the Antiviral Activity and the Structure of ἰ-Carrageenan Using Ultrasonication. International Journal of Molecular Sciences, 24(18), 14200. https://doi.org/10.3390/ijms241814200








