Protonated Organic Diamines as Templates for Layered and Microporous Structures: Synthesis, Crystal Chemistry, and Structural Trends among the Compounds Formed in Aqueous Systems Transition Metal Halide or Nitrate–Diamine–Selenious Acid
Abstract
1. Introduction
2. Results and Discussion
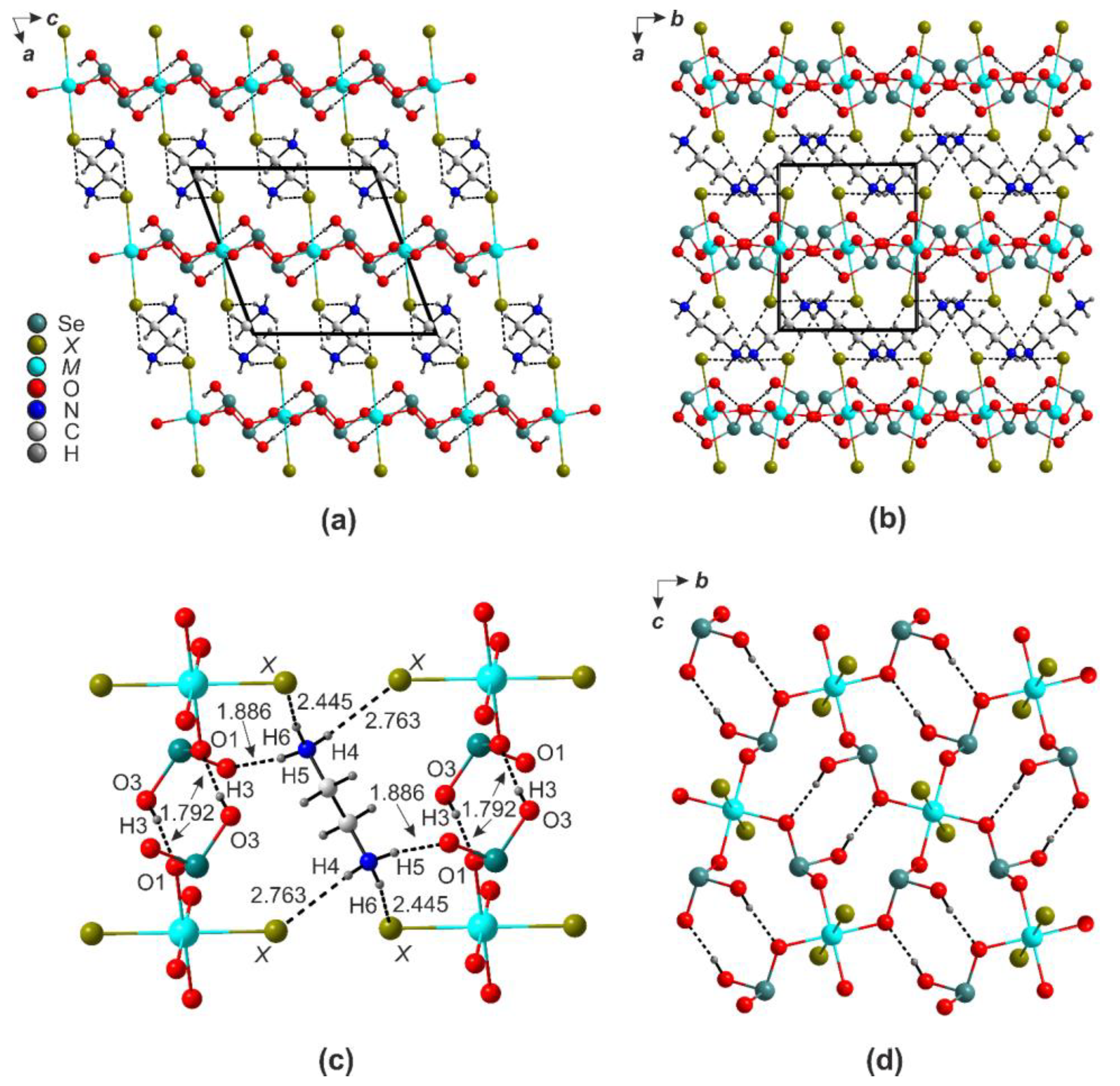
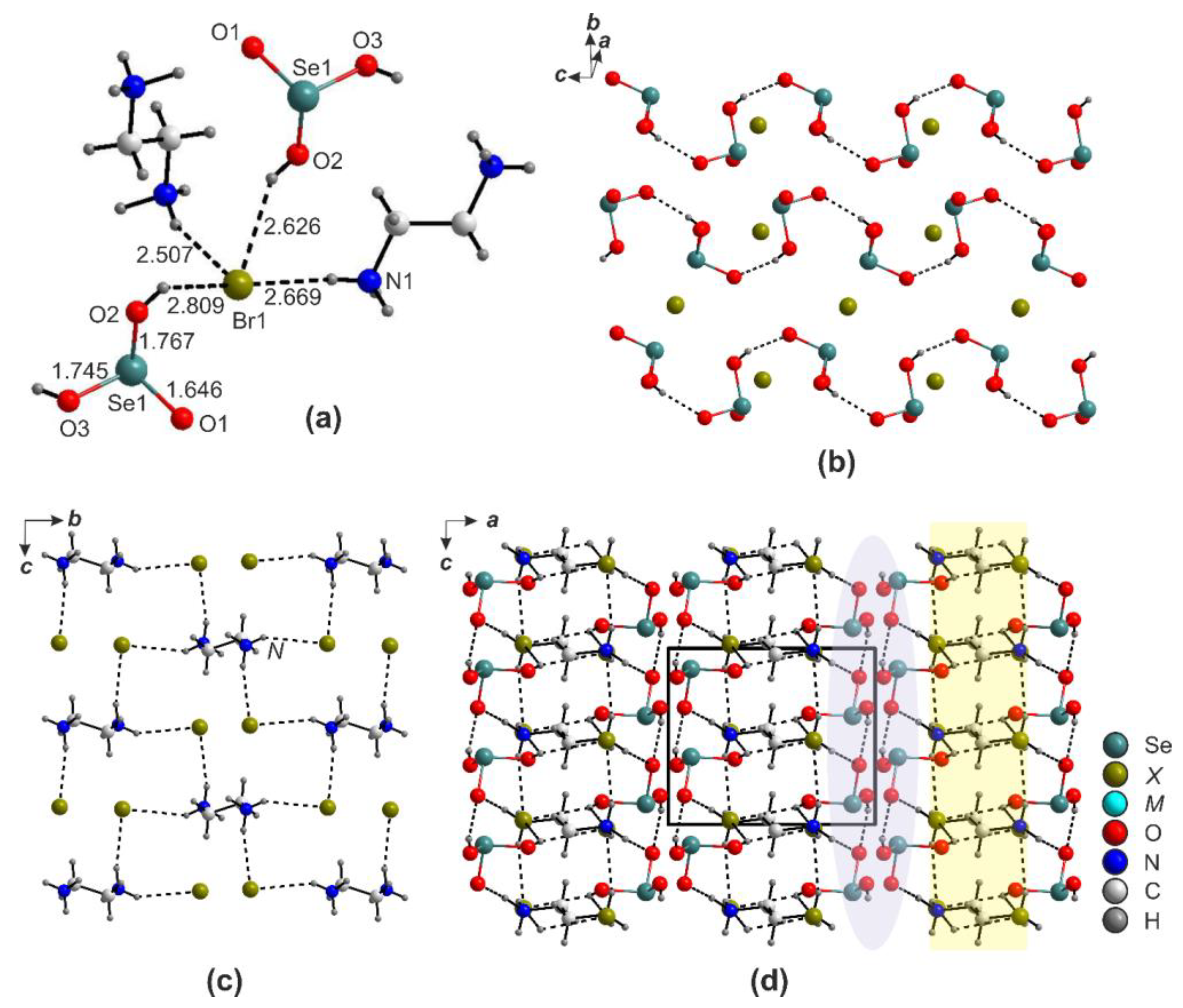
2.1. (enH2)[M(HSeO3)2X2] (X = Cl and Br)
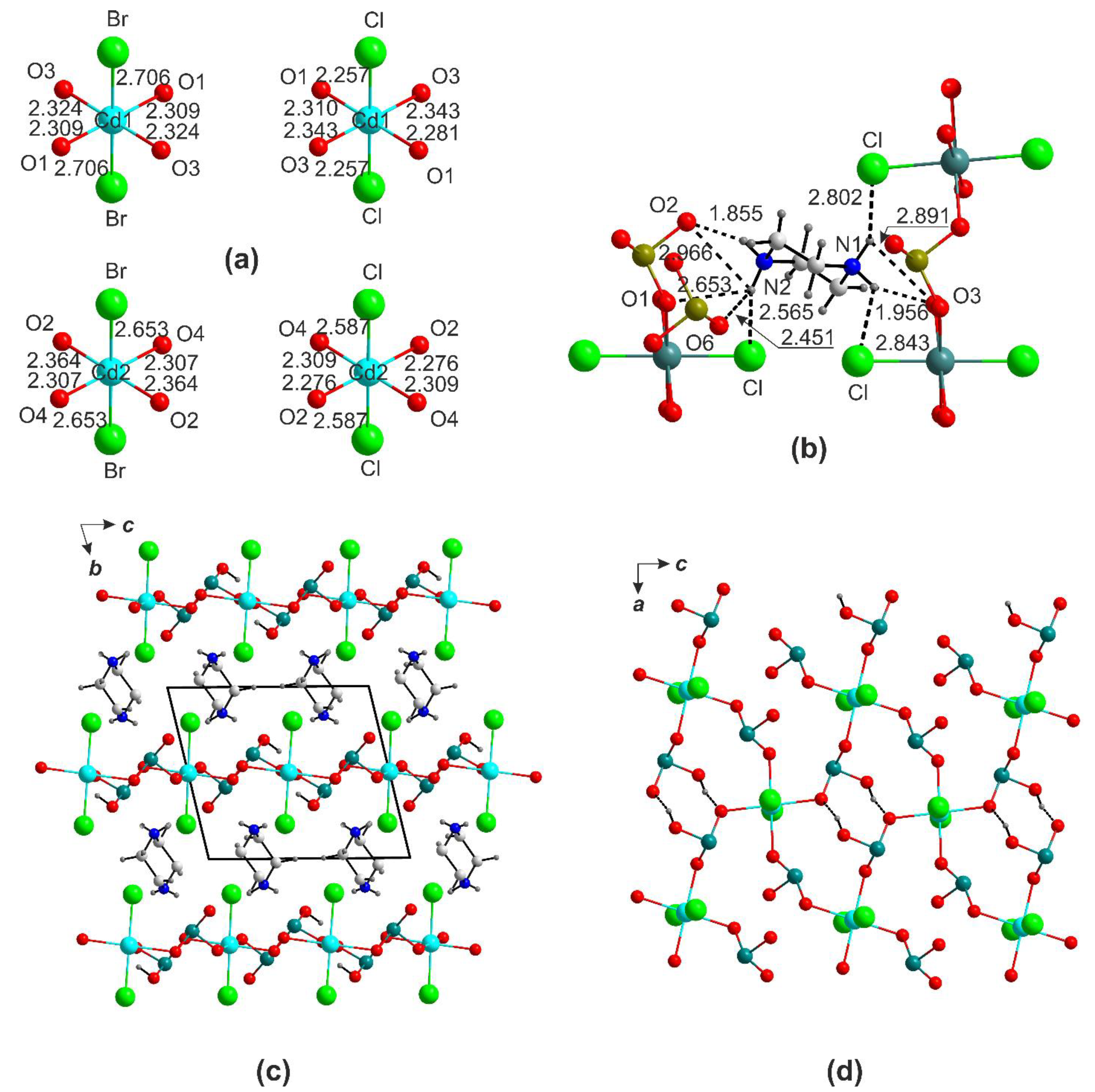
2.2. The (pipH2)[Cd(HSeO3)2X2] (X = Cl, Br) Series
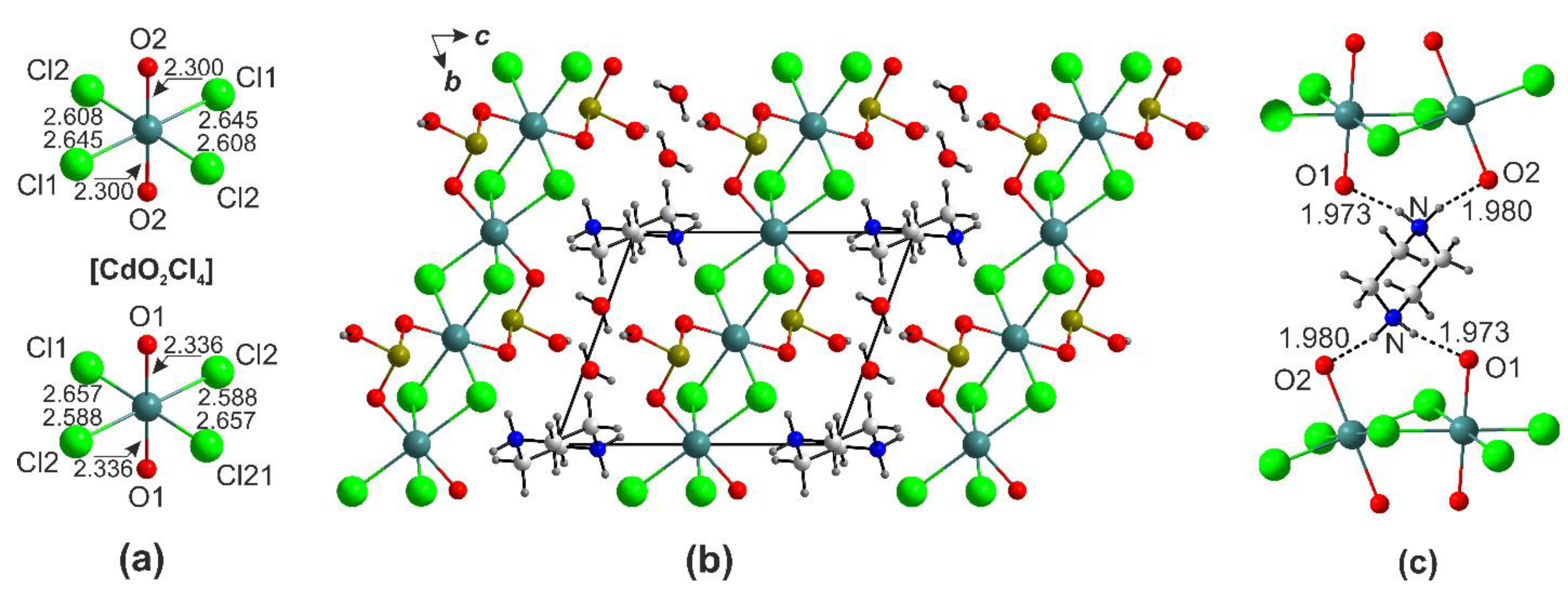
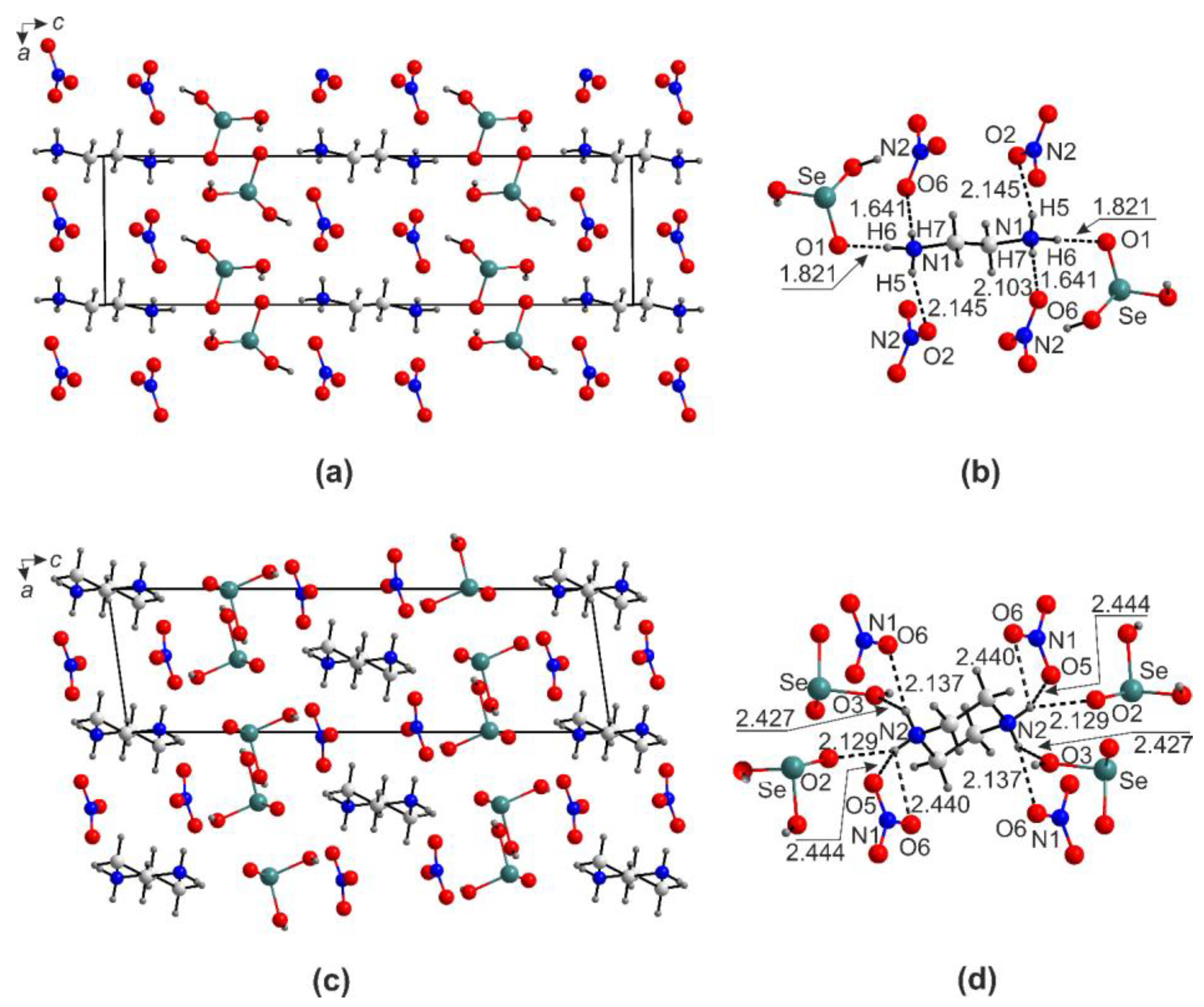
2.3. Halide-Free Framework Structures
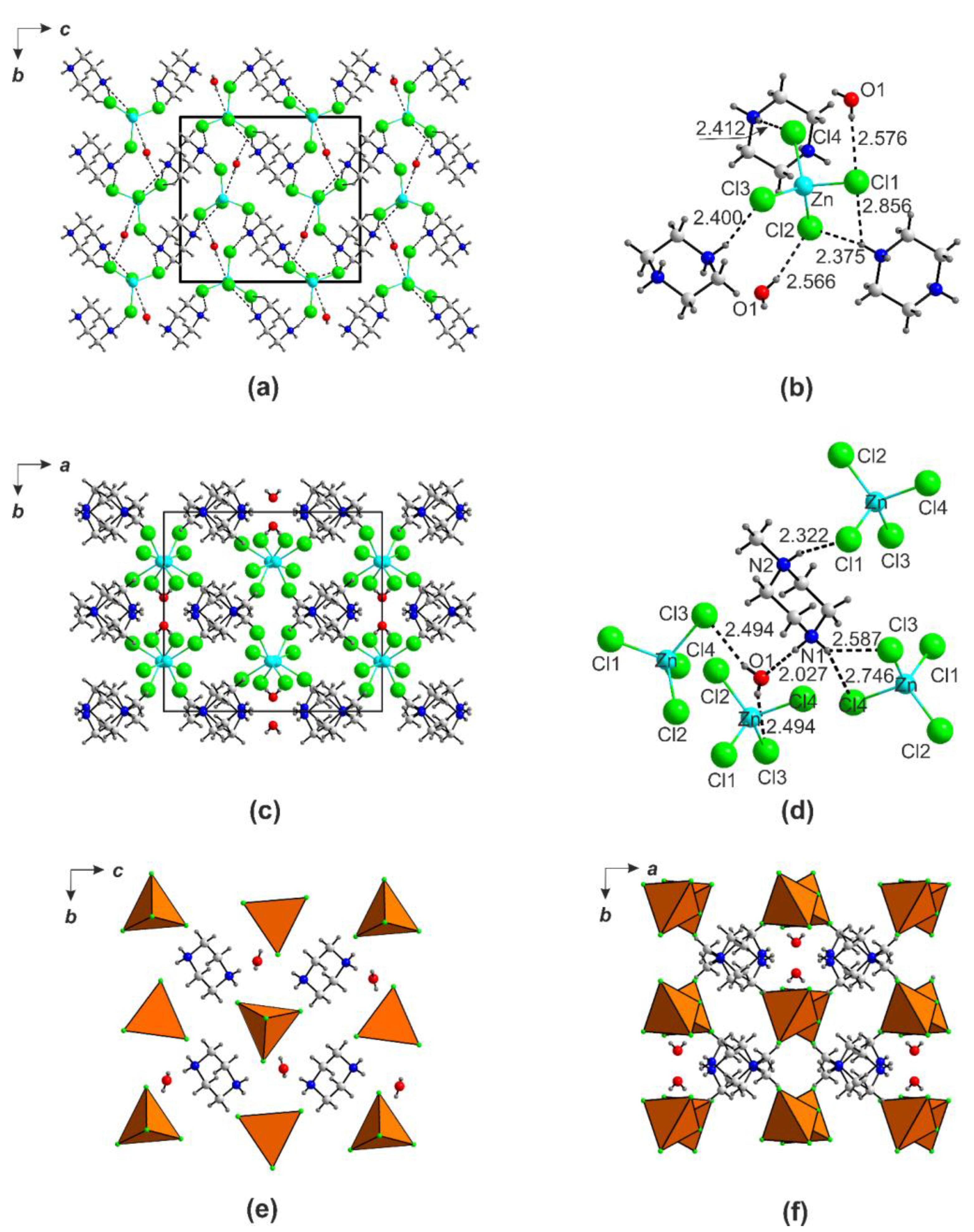
2.4. Ion-Molecular Crystals and Halometallates
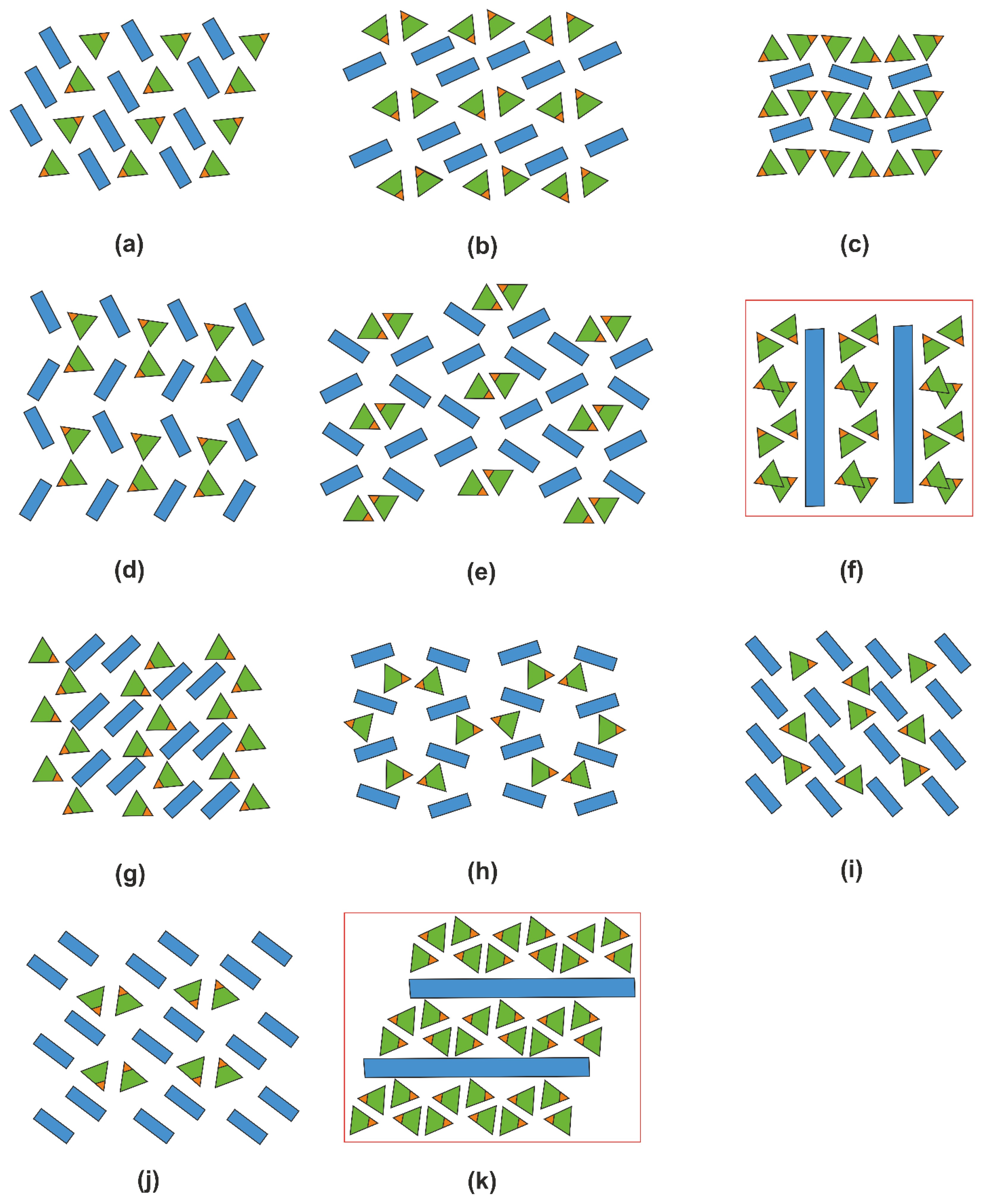
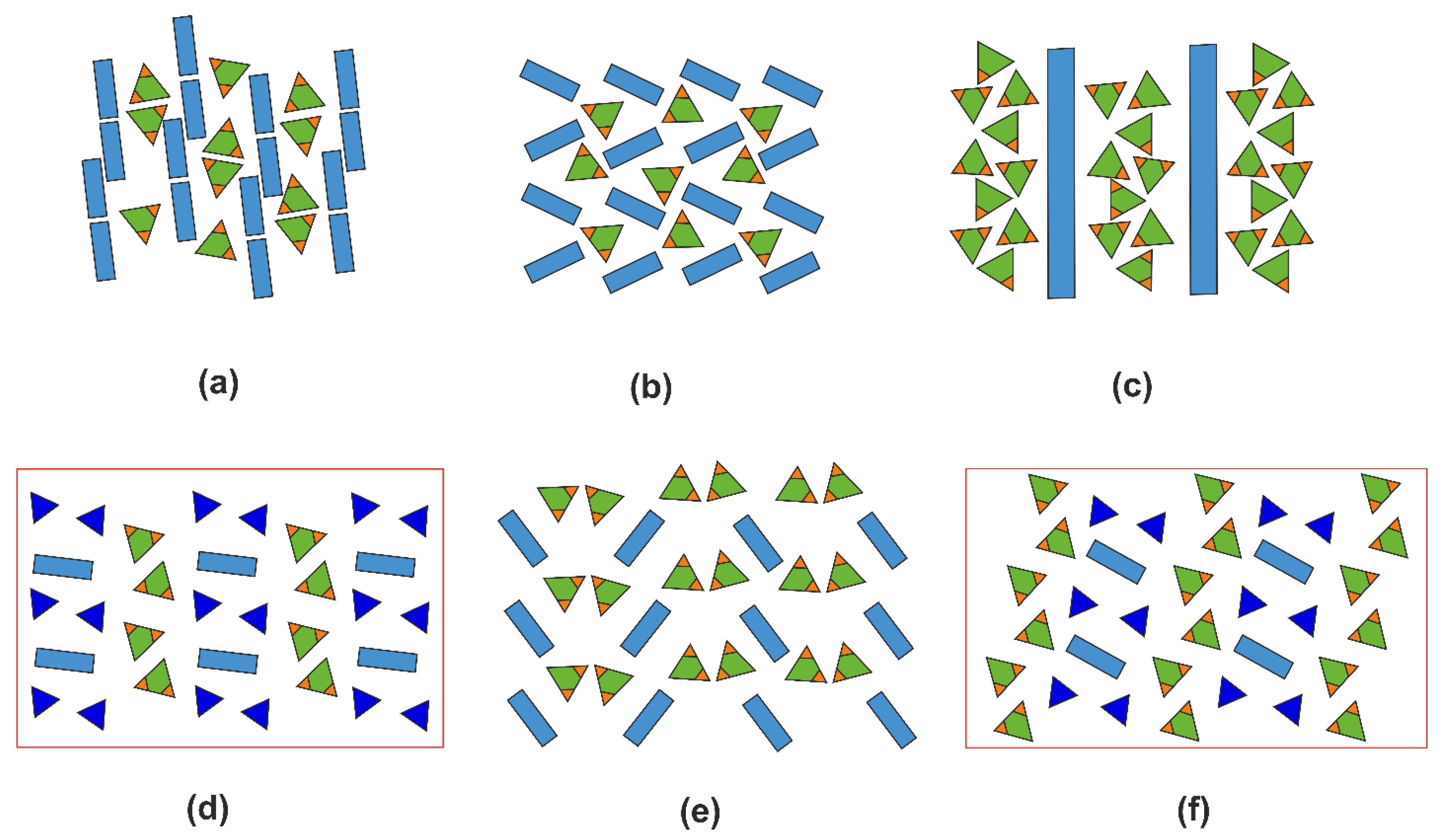
2.5. Structural Trends in Organic Hydrogen Selenites
2.6. Overall Remarks
3. Materials and Methods
3.1. Synthesis
3.2. Single Crystal X-ray Study
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ok, K.M. Toward the rational design of novel noncentrosymmetric materials: Factors influencing the framework structures. Acc. Chem. Res. 2016, 49, 2774–2785. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.Y.; Li, M.Y.; Ma, Z.; Wu, L.M.; Wu, X.T.; Zhu, Q.L. Centric-to-acentric structure transformation induced by a stereochemically active lone pair: A new insight for design of IR nonlinear optical materials. J. Mater. Chem. C 2019, 7, 4638–4643. [Google Scholar] [CrossRef]
- Handy, J.V.; Zaheer, W.; Rothfuss, A.R.M.; McGranahan, C.R.; Agbeworfi, G.; Andrews, J.L.; García-Pedraza, K.E.; Ponis, J.D.; Ayala, J.R.; Ding, Y.; et al. Lone but not alone: Precise positioning of lone pairs for the design of photocatalytic architectures. Chem. Mater. 2022, 34, 1439–1458. [Google Scholar] [CrossRef]
- Hu, C.L.; Mao, J.G. Recent advances on second-order NLO materials based on metal iodates. Coord. Chem. Rev. 2015, 288, 1–17. [Google Scholar] [CrossRef]
- Yan, M.; Xue, H.G.; Guo, S.P. Recent achievements in lone-pair cation-based infrared second-order nonlinear optical materials. Cryst. Growth Des. 2021, 21, 698–720. [Google Scholar] [CrossRef]
- Millet, P.; Johnson, M.; Pashchenko, V.; Ksari, Y.; Stepanov, A.; Mila, F. New copper(II)–lone electron pair elements–oxyhalides compounds: Syntheses, crystal structures, and magnetic properties. Solid State Ion. 2001, 141–142, 559–565. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, J.; Sefat, A.S.; Mandrus, D.G.; Halasyamani, P.S. Stereo-active lone-pair control on the ferromagnetic behavior in VO(SeO2OH)2: A new acentric ferromagnetic material. Chem. Mater. 2010, 22, 6665–6672. [Google Scholar] [CrossRef]
- Charkin, D.O.; Grishaev, V.Y.; Omelchenko, T.A.; Nazarchuk, E.V.; Stefanovich, S.Y.; Siidra, O.I. KNO3 3H2SeO3 and NaHSeO3 3H2SeO3: Two non-centrosymmetric co-crystals. Solid State Sci. 2023, 137, 107116. [Google Scholar] [CrossRef]
- Lafront, A.M.; Trombe, J.C. ‘Layered hydrogenselenite’ I. Synthesis, structure redetermination of Cu(HSeO3)2(H2O)2 and determination of Cu(HSeO3)2(NO3)22−⋅2NH4+⋅NH4NO3. Structural relationships of these complexes with Cu(HSeO3)2. Inorg. Chim. Acta 1995, 234, 19–25. [Google Scholar] [CrossRef]
- Lafront, A.M.; Trombe, J.C.; Bonvoisin, J. ‘Layered hydrogenselenites’ II. Synthesis, structure studies and magnetic properties of a novel series of bimetallic hydrogenselenites: Cu(HSeO3)2⋅MCl2(H2O)4, M(II) = Mn, Co, Ni, Cu, Zn. Inorg. Chim. Acta 1995, 238, 15–22. [Google Scholar] [CrossRef]
- Trombe, J.C.; Lafront, A.M.; Bonvoisin, J. Synthesis, structure and magnetic measurement of a new layered copper hydrogenselenite: Cu(HSeO3)2⋅NH4Cl. Inorg. Chim. Acta 1997, 262, 47–51. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Krivovichev, S.V.; Mentré, O.; Colmont, M. [NaCl][Cu(HSeO3)2], NaCl-intercalated Cu(HSeO3)2: Synthesis, crystal structure and comparison with related compounds. Z. Krist.–Cryst. Mat. 2015, 230, 573–577. [Google Scholar] [CrossRef]
- Charkin, D.O.; Grishaev, V.Y.; Markovski, M.R.; Nekrasova, D.O.; Siidra, O.I. Influence of the alkali cation size on the Cu2+ coordination environments in (AX)[Cu(HSeO3)2] (A = Na, K, NH4, Rb, Cs; X = Cl, Br) layered copper hydrogen selenite halides. Z. Krist.–Cryst. Mat. 2019, 234, 739–747. [Google Scholar] [CrossRef]
- Markovski, M.R.; Charkin, D.O.; Nekrasova, D.O.; Siidra, O.I. Novel layered copper hydrogen selenite nitrates: Synthesis, structure, and spectroscopic properties. Z. Krist.–Cryst. Mat. 2019, 234, 749–756. [Google Scholar] [CrossRef]
- Grishaev, V.Y.; Siidra, O.I.; Markovski, M.R.; Charkin, D.O.; Omelchenko, T.A.; Nazarchuk, E.V. Synthesis and crystal structure of two novel polymorphs of (NaCl)[Cu(HSeO3)2]: A further contribution to the family of layered copper hydrogen selenites. Z. Krist.–Cryst. Mater. 2023, 238, 177–185. [Google Scholar] [CrossRef]
- Spirovski, F.; Wagener, M.; Stefov, V.; Engelen, B. Crystal structures of rubidium zinc bis(hydrogenselenate(IV)) chloride RbZn(HSeO3)2Cl, and rubidium zinc bis(hydrogenselenate(IV)) bromide RbZn(HSeO3)2Br. Z. Krist.-New Cryst. Struct. 2007, 222, 91–92. [Google Scholar] [CrossRef]
- Wagener, M. Synthese, Charakterisierung Und Struktur-Chemische Aspekte Von Kupfer- Und Silberchalkogenohalogeniden Sowie Von Halogeno- Und Oxochalkogenaten(IV). Ph.D. Thesis, Uinversität Siegen, Siegen, Germany, 2005. Available online: https://d-nb.info/97663936X/34 (accessed on 15 May 2023).
- Harrison, W.T.A.; Johnston, M.G. Syntheses and structures of two selenite chloride hydrates: Co(HSeO3)Cl⋅3H2O and Cu(HSeO3)Cl⋅2H2O. Z. Anorg. Allg. Chem. 2000, 626, 2487–2490. [Google Scholar] [CrossRef]
- Feng, M.-L.; Prosvirin, A.V.; Mao, J.G.; Dunbar, K.R. Syntheses, structural studies, and magnetic properties of divalent Cu and Co selenites with organic constituents. Chem. Eur. J. 2006, 12, 8312–8323. [Google Scholar] [CrossRef]
- Johnston, M.G.; Harrison, W.T.A. Cobalt hydrogen selenite chloride dihydrate, Co(HSeO3)Cl 2H2O. Acta Crystallogr. E 2003, 59, i62–i64. [Google Scholar] [CrossRef]
- Pasha, I.; Choudhury, A.; Rao, C.N.R. An organically templated open-framework cadmium selenite. Solid State Sci. 2003, 5, 257–262. [Google Scholar] [CrossRef]
- Effenberger, H. Cu(SeO2OH)2: Synthesis and crystal structure. Z. Krist. 1985, 173, 267–272. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Udayakumar, D.; Rao, C.N.R. Organically templated three-dimensional open-framework metal selenites with a diamondoid network. J. Mater Chem. 2003, 13, 1635–1638. [Google Scholar] [CrossRef]
- Koskenlinna, M.; Valkonen, J. The crystal structure of PrH3(SeO3)2(Se2O5), a compound with selenite and diselenite groups. Acta Chem. Scand. 1977, 31a, 457–460. [Google Scholar] [CrossRef][Green Version]
- Koskenlinna, M.; Valkonen, J. Crystal structure of manganese(III) hydrogen selenite diselenite, MnH(SeO3)(Se2O5). Acta Chem. Scand. 1977, 31a, 638–640. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Behera, J.N.; Dan, M. Organically-templated metal sulfates, selenites and selenates. Chem. Soc. Rev. 2006, 35, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Wickleder, M.S. Sm2Se5O13: A selenite–diselenite according to Sm2(SeO3)(Se2O5)2. Z. Anorg. Allg. Chem. 2006, 632, 2377–2379. [Google Scholar] [CrossRef]
- Jiang, H.-G.; Mao, J.-G. Syntheses, crystal structures and optical properties of the first strontium selenium(IV) and tellurium(IV) oxychlorides: Sr3(SeO3)(Se2O5)Cl2 and Sr4(Te3O8)Cl4. J. Solid State Chem. 2008, 181, 345–354. [Google Scholar] [CrossRef]
- Jones, P.G.; Schwartzmann, E.; Sheldrick, G.M.; Timpe, H. Preparation and crystal structure of di-gold(III)bis(selenite) diselenite, Au2(SeO3)2(Se2O5). Z. Natuforsch. 1981, 36, 1050–1052. [Google Scholar] [CrossRef]
- Markovski, M.R.; Siidra, O.I.; Charkin, D.O.; Vladimirova, V.A.; Tsirlin, A.A.; Grishaev, V.Y. Li2(Se2O5)(H2O)1.5·CuCl2, a salt-inclusion diselenite structurally based on tetranuclear Li4 complexes. Dalton Trans. 2020, 49, 7790–7795. [Google Scholar] [CrossRef]
- Markovski, M.R.; Siidra, O.I.; Charkin, D.O.; Grishaev, V.Y. Layered calcium hydrogen selenite chlorides Ca(HSeO3)Cl and Ca(HSeO3)Cl(H2O), the first halides obtained in the CaCl2–SeO2–H2O system. Z. Krist.–Cryst. Mat. 2020, 235, 439–443. [Google Scholar] [CrossRef]
- Markovski, M.R.; Siidra, O.I.; Charkin, D.O.; Nazarchuk, E.V.; Grishaev, V.Y. Molecular inorganic polymers: Synthesis and crystal structures of KCl∙2H2SeO3 and CsCl∙H2SeO3. Z. Krist.–Cryst. Mat. 2020, 235, 553–557. [Google Scholar] [CrossRef]
- Wang, H.; Liu, L.; Hu, Z.; Wang, J.; Zhu, M.; Meng, Y.; Xu, J. RbCl·(H2SeO3)2: A salt-inclusion selenite featuring short UV cut-off edge and large birefringence. Inorg. Chem. 2023, 62, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Hatano, N.; Horiuchi, K.; Terao, H. NQR Study of piperazinium tetrahalogenometalates(II) [C4H12N2]MX4 (M = Zn, Cd, Hg; X = Br, I). Z. Naturforsch. 2002, 57, 343–347. [Google Scholar] [CrossRef]
- Kefi, R.; Ben, N.C. Crystal structure of piperazinium tetrachlorozincate monohydrate, (C4H12N2)[ZnCl4]⋅H2O. Z. Krist.–New Cryst. Struct. 2005, 220, 241–242. [Google Scholar] [CrossRef]
- Krishnan, V.G.; Dou, S.Q.; Paulus, H.; Weiss, A. Solid phases from the liquid system H2N(CH2)2NH2–CdBr2–HBr–H2O. An X-ray and 79,81Br–NQR study of (H3N(CH2)2NH3)CdBr4, (H3N(CH2)2NH3)(CdBr3)2⋅H2O, and (H3N(CH2)2NH3)2CdBr6. Ber. Bunsenges. Phys. Chem. 1991, 95, 1256–1264. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural Crystallography of Inorganic Oxysalts; Oxford University Press: Oxford, UK, 2009; 320p. [Google Scholar]
- Nemec, I.; Cisarova, I.; Micka, Z. Study of the family of glycine–selenious acid addition compounds: Crystal structure of diglycine hydrogen selenite and vibrational spectra and DSC measurement of diglycine hydrogen selenite and monoglycine–selenious acid crystals. J. Solid State Chem. 1998, 140, 71–82. [Google Scholar] [CrossRef]
- Lukevics, E.; Arsenyan, P.; Shestakova, I.; Domracheva, I.; Kanepe, I.; Belyakov, S.; Popelis, J.; Pudova, O. Cycloaddition reactions of nitrile oxides to silyl- and germyl-substituted thiophene-1,1-dioxides. Organometallics 2002, 18, 3187–3193. [Google Scholar] [CrossRef]
- Nemec, I.; Chudoba, V.; Havlicek, D.; Cisarova, I.; Micka, Z. Preparation, crystal structure, vibrational spectra, and thermal behavior of N,N′-dimethylpiperazinium(2+) hydrogen selenite. J. Solid State Chem. 2001, 161, 312–318. [Google Scholar] [CrossRef]
- Takouachet, R.; Benali-Cherif, R.; Benali-Cherif, N. Cytosinium hydrogen selenite. Acta Crystallogr. 2014, E70, o186–o187. [Google Scholar] [CrossRef]
- Gharzaryan, V.V.; Fleck, M.; Petrosyan, A.M. New salts of amino acids with dimeric cations. Proc. SPIE Int. Conf. Laser Phys. 2010, 7998, 107–115. [Google Scholar] [CrossRef]
- Paixao, J.A.; Silva, M.R.; Beja, A.M.; Eusebio, E. Crystal structure and properties of L-tryptophanium hydrogen selenite. Polyhedron 2006, 25, 2021–2025. [Google Scholar] [CrossRef][Green Version]
- Paixao, J.A.; Matos Beja, A.; Ramos Silva, M.; de Matos Gomes, E.; Martin-Gil, J.; Martin-Gil, F.J. N,N’-diphenylguanidinium hydrogenselenite monohydrate. Acta Crystallogr. 1997, C53, 1113–1115. [Google Scholar] [CrossRef]
- Wang, J.-J.; Tessier, C.; Holm, R.H. Analogue reaction systems of selenate reductase. Inorg. Chem. 2006, 45, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.K.; Harrison, W.T.A. 1-Carbamoylguanidinium hydrogenselenite. Acta Crystallogr. 2003, E59, o1296–o1298. [Google Scholar] [CrossRef]
- Ondracek, J.; Walzelova, M.; Micka, Z.; Novotny, J. Structure of glycine-selenious acid (1/1). Acta Crystallogr. 1992, C48, 391–392. [Google Scholar] [CrossRef]
- Paixao, J.A.; Matos Beja, A.; Ramos Silva, M.; Alte da Veiga, L.; Martin-Gil, J.; Martin-Gil, F.; de Matos Gomes, E. Crystal structure of betaine dihydrogen selenite, C5H13NO5Se. Z. Krist. New Cryst. Struct. 1997, 212, 51–52. [Google Scholar] [CrossRef]
- De Matos Gomes, E.; Matos Beja, A.; Paixao, J.A.; de Veiga, L.A.; Ramos Silva, M.; Martin-Gil, J.; Martin-Gil, F.J. Synthesis, structure and thermal behavior of benzyltrimethylammonium trihydrogen selenite. Z. Krist. Cryst. Mater. 1995, 210, 929–933. [Google Scholar] [CrossRef]
- Kamali, N.; OMalley, C.; Mahon, M.F.; Erxleben, A.; McArdle, P. The use of sublimation catalysis and polycrystalline powder templates for polymorph control of gas phase crystallization. Cryst. Growth Des. 2018, 18, 3510–3516. [Google Scholar] [CrossRef]
- Takouachet, R.; Benali-Cherif, R.; Bendeif, E.-E.; Benali-Cherif, N.; Pillet, S.; Schaniel, D. Structural analysis and IR-spectroscopy of a new anilinium hydrogenselenite hybrid compound: A subtle structural phase transition. Inorg. Chim. Acta 2016, 446, 6–12. [Google Scholar] [CrossRef]
- De Matos Gomes, E.; Nogueira, E.; Fernandes, I.; Belsley, M.; Paixao, J.A.; Matos Beja, A.; Ramos, S.M.; Martin-Gil, J.; Martin-Gil, F.; Mano, J.F. Synthesis, structure, thermal and non-linear optical properties of L-argininium hydrogen selenite. Acta Crystallogr. 2001, B57, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Canossa, S.; Graiff, C. Role of bis(triphenylphosphine)iminium cation [PNP]+ on the crystal packing of [PNP]+[HSeO3]− solvate salt. Crystals 2018, 8, 151. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Duhlev, R.; Macicek, J. Structure of magnesium zinc tetrachloride hexahydrate MgZnCl4⋅6H2O. Acta Crystallogr. 1991, C47, 1573–1575. [Google Scholar] [CrossRef]
- Waizumi, K.; Masuda, H.; Fukushima, N. Structural and energetic studies on double salts of M(II)Mg2Cl6⋅12H2O (M=Ca, Mn, Cd) by X-ray diffraction and density functional methods. Inorganica Chim. Acta 1995, 238, 121–127. [Google Scholar] [CrossRef]
- Waizumi, K.; Masuda, H.; Ohtaki, H.; Scripkin, M.Y.; Burkov, K.A. Crystallographic investigations of [Mg(H2O)6)XCl3 double salts (X+ = K+, Rb+, Cs+, NH4+): Crystal structure of [Mg(H2O)6]CsCl3. Am. Miner. 1991, 76, 1884–1888. [Google Scholar]
- Leclaire, A.; Borel, M.M. Structure de l’hexachlorure de dicadmium et de nickel dodecahydrate. Acta Crystallogr. 1980, B36, 3088–3090. [Google Scholar] [CrossRef]
- Olshansky, J.H.; Tran, T.T.; Hernandez, K.J.; Zeller, M.; Halasyamani, P.S.; Schrier, J.; Norquist, A.J. Role of hydrogen-bonding in the formation of polar achiral and nonpolar chiral vanadium selenite frameworks. Inorg. Chem. 2012, 51, 11040–11048. [Google Scholar] [CrossRef]
- Tlilli, H.; Walha, S.; Elleuch, S.; Ali, B.F.; Naïli, H. Structural, vibrational, DFT and optical studies of a new non-centrosymmetric hybrid material (C4H12N2)[CoBr4]. J. Mol. Struct. 2018, 1152, 303–330. [Google Scholar] [CrossRef]
- Ishihara, H.; Horiuchi, K.; Gesing, T.M.; Dou, S.Q.; Buhl, J.C.; Erk, P. Crystal structures of piperazinium tetrabromocadmate(II)-monohydrate [C4H12N2]CdBr4⋅H2O, piperazinium tetraiodocadmate(II) [C4H12N2]CdI4, and Bis(trimethylsulphonium) Tetrabromocadmate(II) [(CH3)3S]2CdBr4. Z. Naturforsch. 2002, 57b, 503–508. [Google Scholar] [CrossRef]
- Battaglia, L.P.; Corradi, A.B.; Bruni, S.; Cariati, F.; Koman, M. Piperazinium and N-methyl-piperazinium tetrahalocadmates(II) containing discrete [CdX4]2− units. Inorg. Chim. Acta. 1991, 187, 141–147. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Chen, H.; Gao, W.; Zhu, M.; Gao, H.; Xue, J.; Li, Y. A highly selective OFF–ON fluorescent sensor for zinc in aqueous solution and living cells. Chem. Commun. 2010, 46, 8389–8391. [Google Scholar] [CrossRef] [PubMed]
- Krumbe, W.; Haussuhl, S. Structure and physical properties of orthorhombic guanidinium phthalate, [CN3H6]2C8H4O4, and guanidinium hydrogen L-aspartate, [CN3H6]C4H6NO4. Z. Kristallogr. Cryst. Mater. 1987, 179, 267–279. [Google Scholar] [CrossRef]
- Lukevics, E.; Arsenyan, P.; Shestakova, I.; Domracheva, I.; Kanepe, I.; Belyakov, S.; Popelis, J.; Pudova, O. Synthesis, structure and cytotoxicity of organoammonium selenites. Appl. Organomet. Chem. 2002, 16, 228–234. [Google Scholar] [CrossRef]




| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiation | MoKα, 0.71073 | ||||||||||
| Crystal system | monoclinic | ||||||||||
| Space group | P21/c | ||||||||||
| a (Å) | 8.8222(4) | 8.2957(5) | 8.9395(6) | 8.6750(4) | 9.1833(4) | 8.9953(3) | 8.9465(4) | 8.2958(3) | 9.1862(4) | 8.6699(4) | 8.6420(8) |
| b (Å) | 7.6140(3) | 7.8094(3) | 7.3147(4) | 7.3277(3) | 7.1548(3) | 7.1677(2) | 7.4460(3) | 7.6712(2) | 7.1516(2) | 7.3448(2) | 7.3081(4) |
| c (Å) | 10.2481(5) | 9.9433(5) | 9.7558(8) | 9.6812(4) | 9.4845(5) | 9.3819(4) | 10.0240(5) | 9.5977(4) | 9.4849(4) | 9.6996(4) | 9.6000(9) |
| β (°) | 114.196(6) | 112.430(6) | 111.904(9) | 113.093(6) | 110.608(5) | 111.215(4) | 112.720(6) | 111.293(4) | 110.624(4) | 113.228(5) | 112.999(11) |
| Volume (Å3) | 627.91(6) | 595.44(6) | 591.88(8) | 566.10(4) | 583.30(5) | 563.91(3) | 615.94(5) | 569.09(3) | 583.19(4) | 567.59(4) | 558.11(8) |
| Dcalc (g/cm3) | 3.122 | 2.726 | 3.012 | 2.628 | 3.083 | 2.665 | 2.829 | 2.590 | 3.094 | 2.658 | 2.664 |
| θ range (°) | 3.45–33.64 | 3.41–33.26 | 3.58–38.06 | 3.60–35.53 | 3.66–33.49 | 3.68–35.65 | 3.51–33.72 | 3.50–35.49 | 3.66–35.66 | 3.59–35.42 | 3.68–27.99 |
| h, k, l ranges | 12→−12 11→−9 15→−14 | 12→−12 8→−12 14→−13 | 15→−15 11→−12 15→−16 | −9→13 −9→11 −15→14 | −13→12 −10→10 −12→12 | −14→13 −11→11 −15→15 | −13→12 10→−8 14→−13 | −13→11 −10→12 −13→15 | −14→14 −11→11 −14→15 | −11→14 −11→7 −15→14 | 11→−11 9→−9 12→−12 |
| Total reflections collected | 2119 | 2019 | 4713 | 2319 | 1992 | 2395 | 2091 | 2362 | 2414 | 2339 | 1349 |
| Unique reflections (Rint) | 1885 (0.032) | 1702 (0.031) | 3061 (0.08) | 2009 (0.043) | 1710 (0.054) | 2101 (0.059) | 1791 (0.035) | 2109 (0.031) | 2128 (0.042) | 2089 (0.041) | 1048 (0.075) |
| R1[F > 4σF], wR1[F > 4σF] | 0.023 (0.051) | 0.025 (0.053) | 0.047 (0.084) | 0.029 (0.056) | 0.035 (0.070) | 0.024 (0.057) | 0.030 (0.073) | 0.022 (0.049) | 0.028 (0.062) | 0.028 (0.058) | 0.039 (0.076) |
| Rall, wRall | 0.028 (0.052) | 0.033 (0.054) | 0.082 (0.092) | 0.036 (0.058) | 0.043 (0.073) | 0.030 (0.058) | 0.037 (0.075) | 0.026 (0.050) | 0.035 (0.063) | 0.033 (0.059) | 0.058 (0.081) |
| Goodness of fit | 1.053 | 0.984 | 0.989 | 1.056 | 1.095 | 1.026 | 1.107 | 0.981 | 1.125 | 1.047 | 1.058 |
| CCDC number | 2,271,293 | 2,271,295 | 2,271,296 | 2,271,297 | 2,271,298 | 2,271,307 | 2,271,300 | 2,271,301 | 2,271,306 | 2,271,305 | 2,271,304 |
| Polyhedron | CdO4Br2 | CdO4Cl2 | CoO4Cl2 | CuO4Br2 | CuO4Cl2 | MnO4Br2 | MnO4Cl2 | ZnO4Br2 | ZnO4Cl2 | NiO4Cl2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Bond valence for M2+ | 2.04 | 2.08 | 2.00 | 1.89 | 2.09 | 2.11 | 2.04 | 2.09 | 1.90 | 2.01 |
| Average bond length, Å | 2.4387 | 2.3950 | 2.2243 | 2.2807 | 2.2329 | 2.3587 | 2.3032 | 2.2806 | 2.2318 | 2.1973 |
| Polyhedral volume, Å3 | 19.063 | 18.177 | 14.453 | 15.083 | 14.271 | 17.124 | 16.131 | 15.081 | 14.611 | 13.9603 |
| Distortion index (bond length) | 0.0768 | 0.0546 | 0.0884 | 0.1686 | 0.1532 | 0.1086 | 0.0735 | 0.1689 | 0.0866 | 0.08296 |
| 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Radiation | MoKα, 0.71073 | |||||||||
| Crystal system | triclinic | monoclinic | triclinic | monoclinic | ||||||
| Space group | P-1 | P21/c | P21/c | P-1 | P21/n | |||||
| a (Å)/α (°) | 7.6207(3)/ 77.077(4) | 7.7373(2)/ 76.646(3) | 7.4411(3)/ 114.766(4) | 7.5632(3)/ 115.300(4) | 8.0012(5) | 6.5391(2) | 14.2117(3) | 5.9386(3) | 7.14040(10)/ 107.419(2) | 5.61000(10) |
| b (Å)/β (°) | 9.4337(4)/ 88.138(4) | 9.4999(3)/ 88.778(2) | 8.5623(4)/ 93.329(3) | 8.7844(3)/ 92.985(3) | 11.1514(7)/ 90.012(5) | 12.6400(3)/ 92.567(2) | 12.6651(3)/ 102.492(2) | 5.2221(3)/ 90.669(4) | 7.5642(2)/ 95.832(2) | 6.8184(2)/ 98.024(2) |
| c (Å)/γ (°) | 10.0207(4)/ 68.552(4) | 10.1340(3)/ 68.472(3) | 9.2460(4)/ 114.568(4) | 9.4451(3)/ 114.829(4) | 6.8168(4) | 13.8009(4) | 13.6203(3) | 21.0732(11) | 9.0469(2)/ 106.206(2) | 18.7159(5) |
| Volume (Å3)/Z | 652.57(5) | 672.53(4) | 466.85(4) | 493.96(4) | 608.23(6) | 1139.56(6) | 2393.52(9) | 653.48(6) | 438.464(18) | 708.90(3) |
| Dcalc (g/cm3) | 3.043 | 3.331 | 3.068 | 2.620 | 3.053 | 2.906 | 2.257 | 2.828 | 2.202 | |
| θ range (°) | 3.38–27.50 | 3.43–33.64 | 3.38–33.49 | 3.50–35.66 | 3.37–35.65 | 3.49–35.44 | 3.43–33.60 | 3.47–38.02 | 3.68–38.09 | |
| h, k, l ranges | 10→−10 12→−12 13→−13 | 11→−10 12→−13 13→−13 | 10→−11 13→−13 14→−13 | 12→−10 15→−17 6→−10 | 10→−10 13→−20 20→−22 | 21→−22 20→−18 22→−20 | 7→−8 7→−7 27→−32 | 12→−12 12→−12 15→−14 | 9→−8 11→−10 31→−27 | |
| Total reflections collected | 3084 | 3120 | 3331 | 2335 | 4741 | 4979 | 2234 | 4484 | 3622 | |
| Unique reflections (Rint) | 2748 (0.057) | 2624 (0.031) | 2724 (0.042) | 1887 (0.036) | 4069 (0.035) | 4379 (0.025) | 1827 (0.06) | 3997 (0.034) | 3046 (0.037) | |
| R1[F > 4σF], wR1[F > 4σF] | 0.035 (0.085) | 0.025 (0.045) | 0.038 (0.087) | 0.033 (0.076) | 0.025 (0.049) | 0.019 (0.042) | 0.053 (0.14) | 0.025 (0.56) | 0.028 (0.62) | |
| Rall, wRall | 0.040 (0.087) | 0.033 (0.046) | 0.049 (0.089) | 0.045 (0.079) | 0.033 (0.051) | 0.024 (0.043) | 0.064 (0.15) | 0.030 (0.57) | 0.038 (0.65) | |
| Goodness-of-fit | 1.024 | 1.030 | 1.150 | 1.004 | 1.055 | 1.056 | 1.081 | 1.026 | 1.029 | |
| CCDC number | * | 2,275,315 | 2,275,344 | 2,275,377 | 2,275,047 | 2,275,306 | 2,275,269 | 2,275,256 | 2,275,336 | 2,275,366 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkin, D.O.; Nazarchuk, E.V.; Dmitriev, D.N.; Grishaev, V.Y.; Omelchenko, T.A.; Spiridonova, D.V.; Siidra, O.I. Protonated Organic Diamines as Templates for Layered and Microporous Structures: Synthesis, Crystal Chemistry, and Structural Trends among the Compounds Formed in Aqueous Systems Transition Metal Halide or Nitrate–Diamine–Selenious Acid. Int. J. Mol. Sci. 2023, 24, 14202. https://doi.org/10.3390/ijms241814202
Charkin DO, Nazarchuk EV, Dmitriev DN, Grishaev VY, Omelchenko TA, Spiridonova DV, Siidra OI. Protonated Organic Diamines as Templates for Layered and Microporous Structures: Synthesis, Crystal Chemistry, and Structural Trends among the Compounds Formed in Aqueous Systems Transition Metal Halide or Nitrate–Diamine–Selenious Acid. International Journal of Molecular Sciences. 2023; 24(18):14202. https://doi.org/10.3390/ijms241814202
Chicago/Turabian StyleCharkin, Dmitri O., Evgeny V. Nazarchuk, Dmitri N. Dmitriev, Vasili Yu. Grishaev, Timofey A. Omelchenko, Darya V. Spiridonova, and Oleg I. Siidra. 2023. "Protonated Organic Diamines as Templates for Layered and Microporous Structures: Synthesis, Crystal Chemistry, and Structural Trends among the Compounds Formed in Aqueous Systems Transition Metal Halide or Nitrate–Diamine–Selenious Acid" International Journal of Molecular Sciences 24, no. 18: 14202. https://doi.org/10.3390/ijms241814202
APA StyleCharkin, D. O., Nazarchuk, E. V., Dmitriev, D. N., Grishaev, V. Y., Omelchenko, T. A., Spiridonova, D. V., & Siidra, O. I. (2023). Protonated Organic Diamines as Templates for Layered and Microporous Structures: Synthesis, Crystal Chemistry, and Structural Trends among the Compounds Formed in Aqueous Systems Transition Metal Halide or Nitrate–Diamine–Selenious Acid. International Journal of Molecular Sciences, 24(18), 14202. https://doi.org/10.3390/ijms241814202








