Heterologous codA Gene Expression Leads to Mitigation of Salt Stress Effects and Modulates Developmental Processes
Abstract
:1. Introduction
2. Results
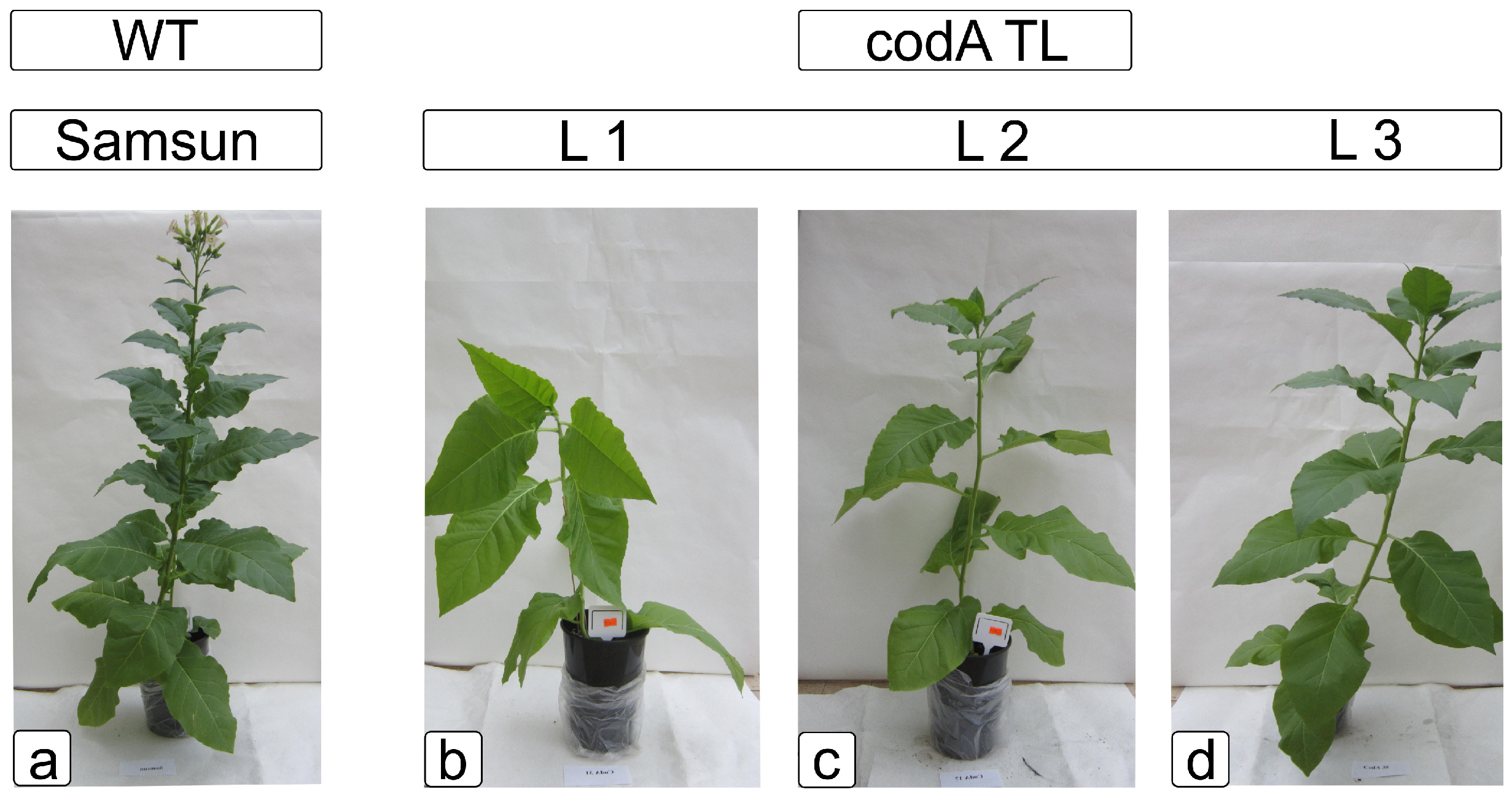
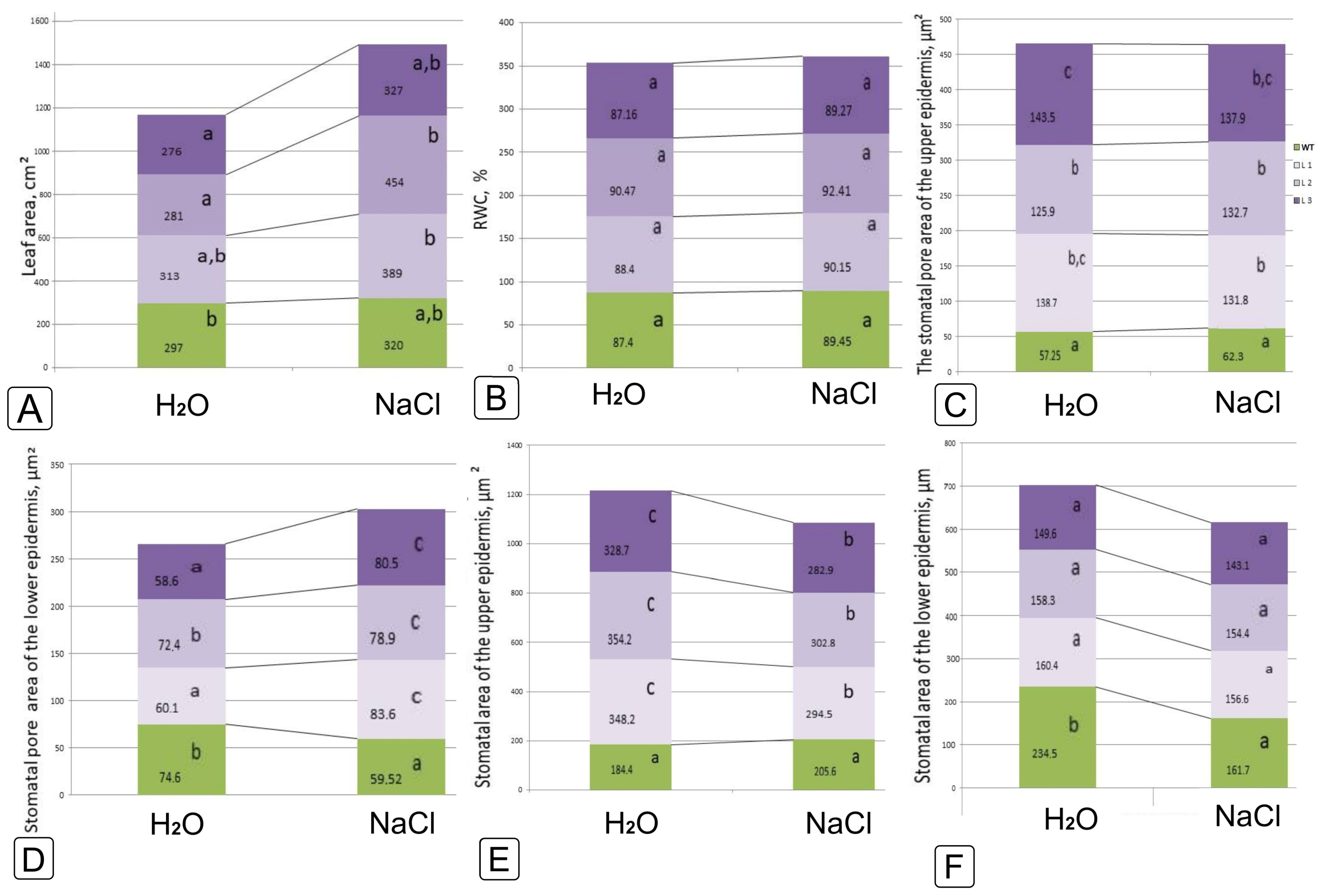
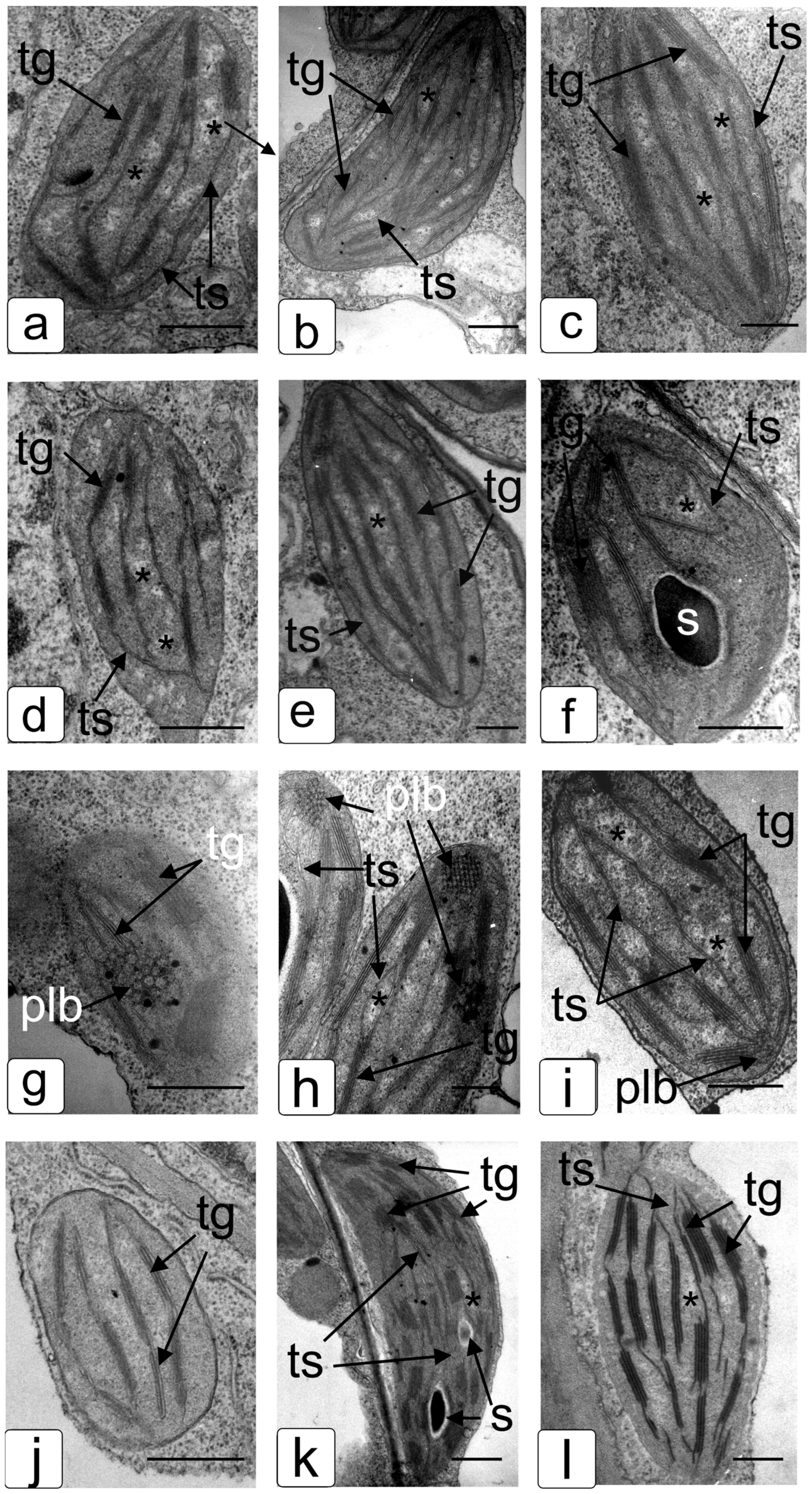
2.1. Comparative Characteristics of codA Transgenic and WT Plants
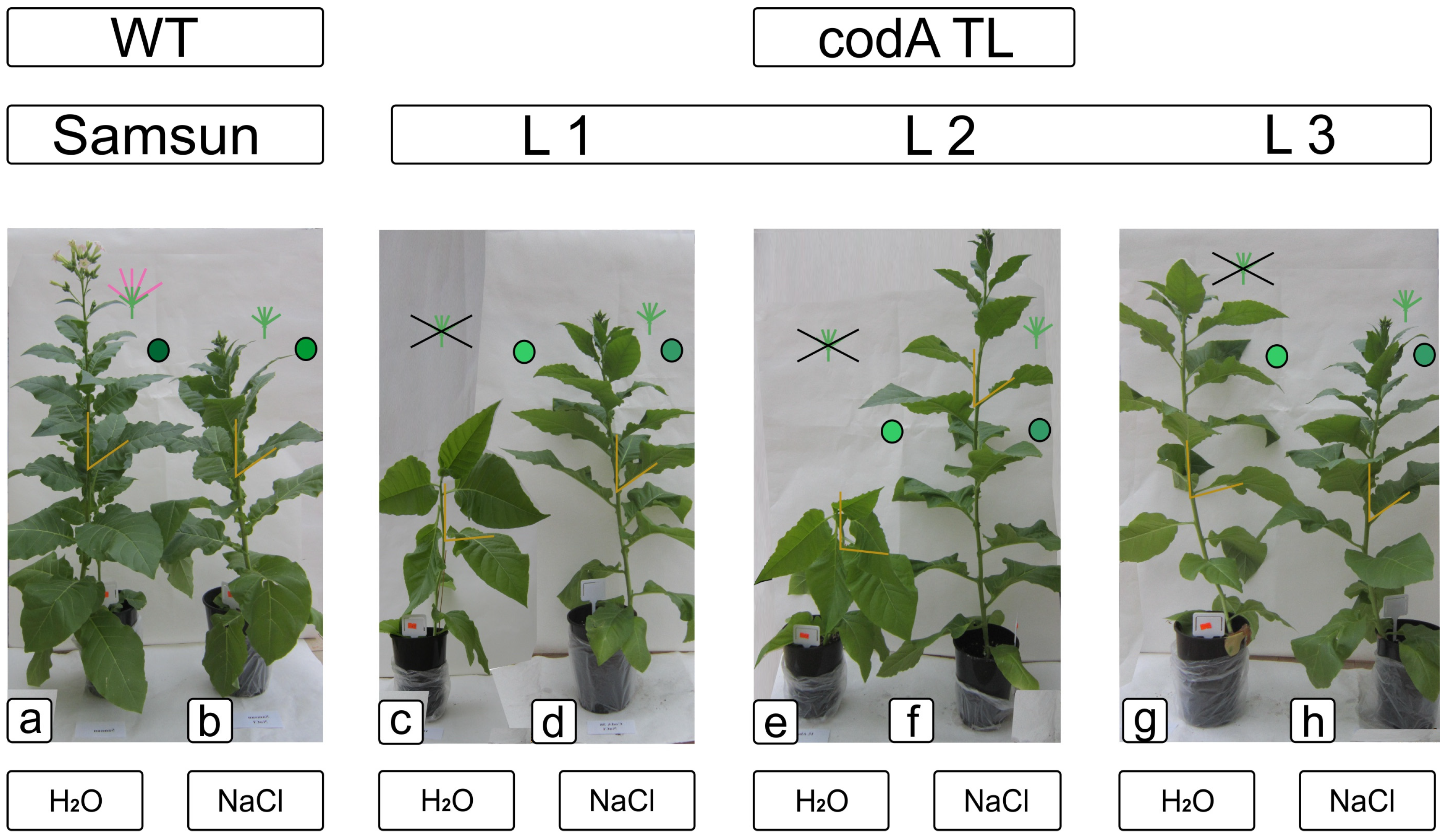
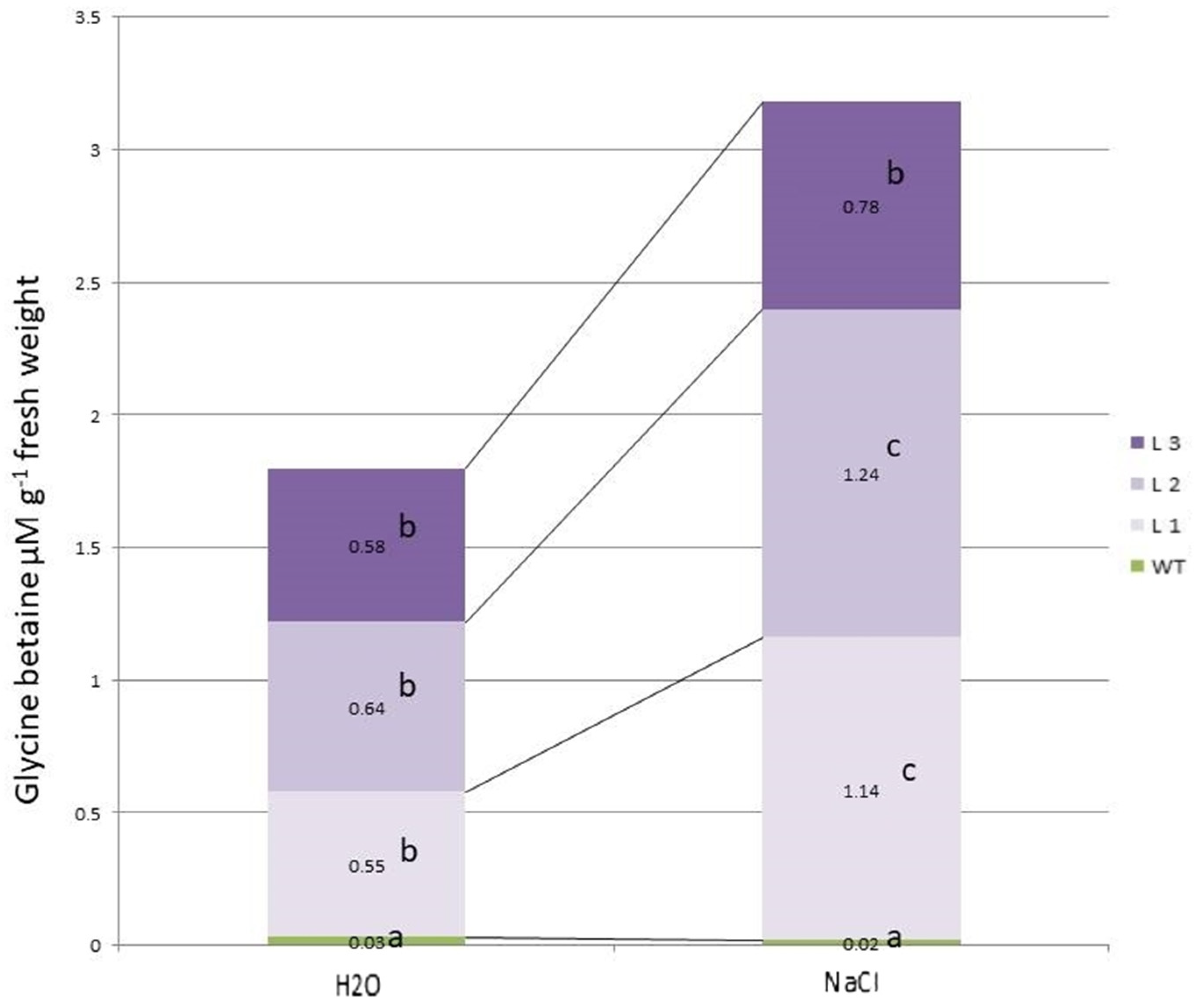
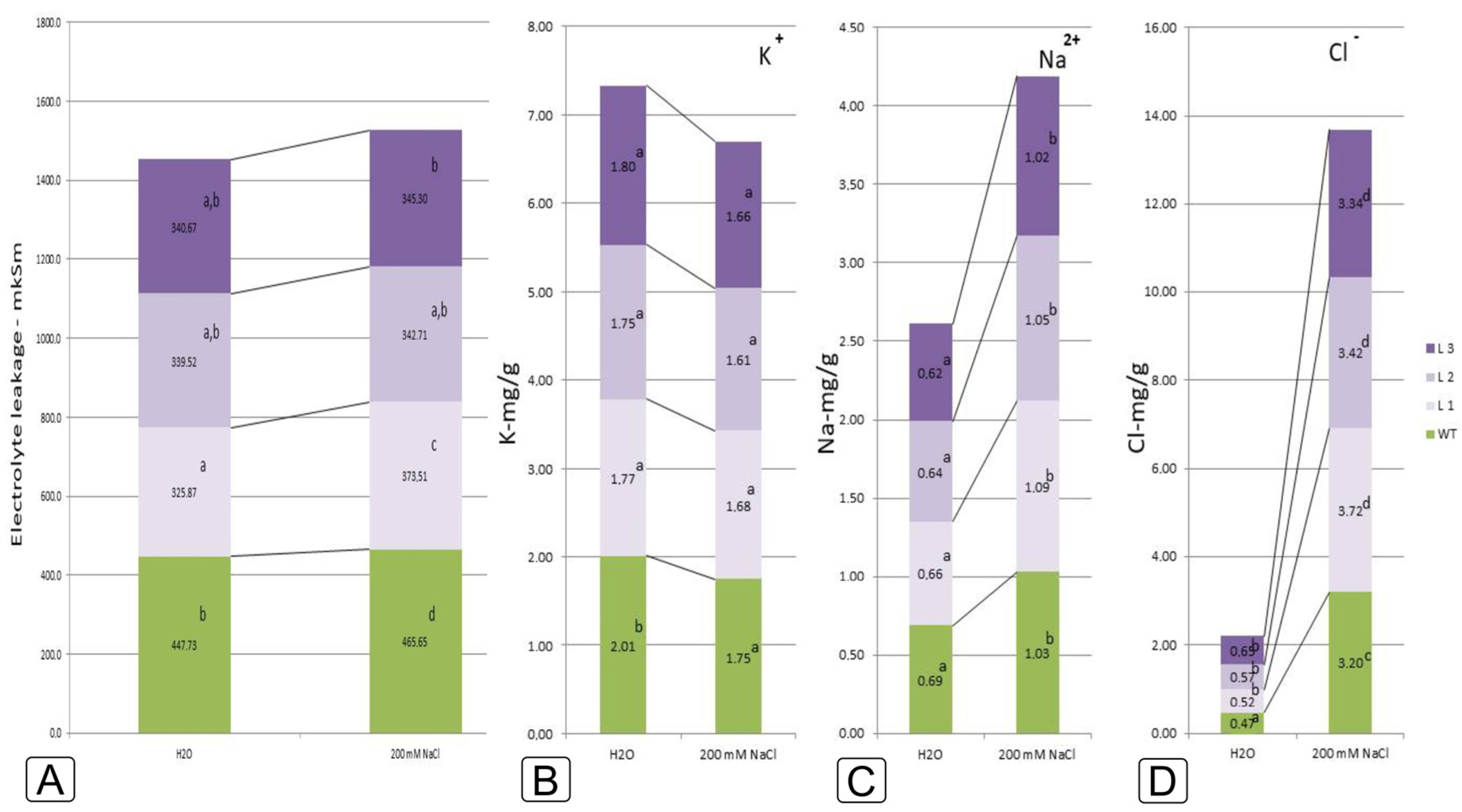
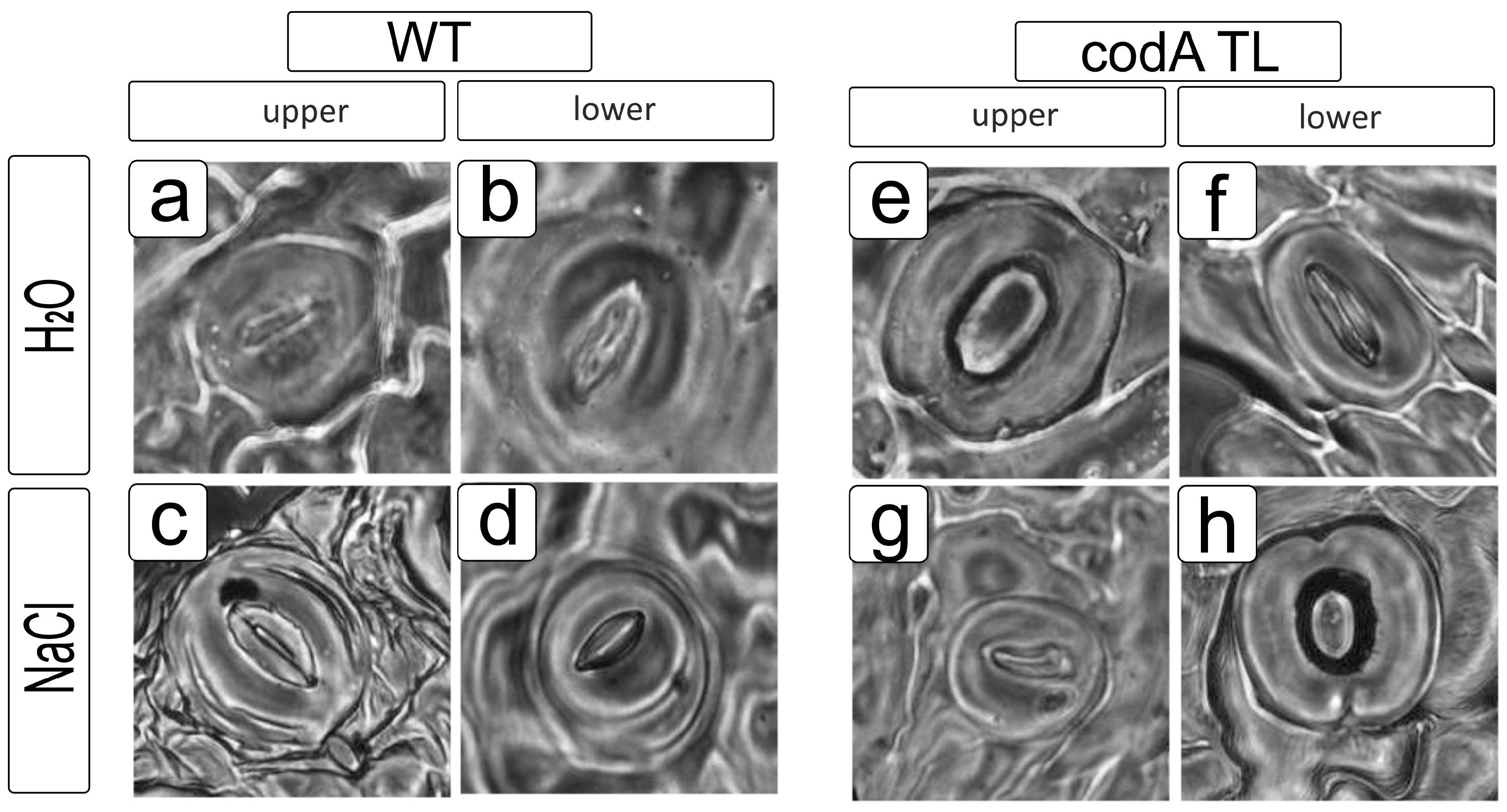
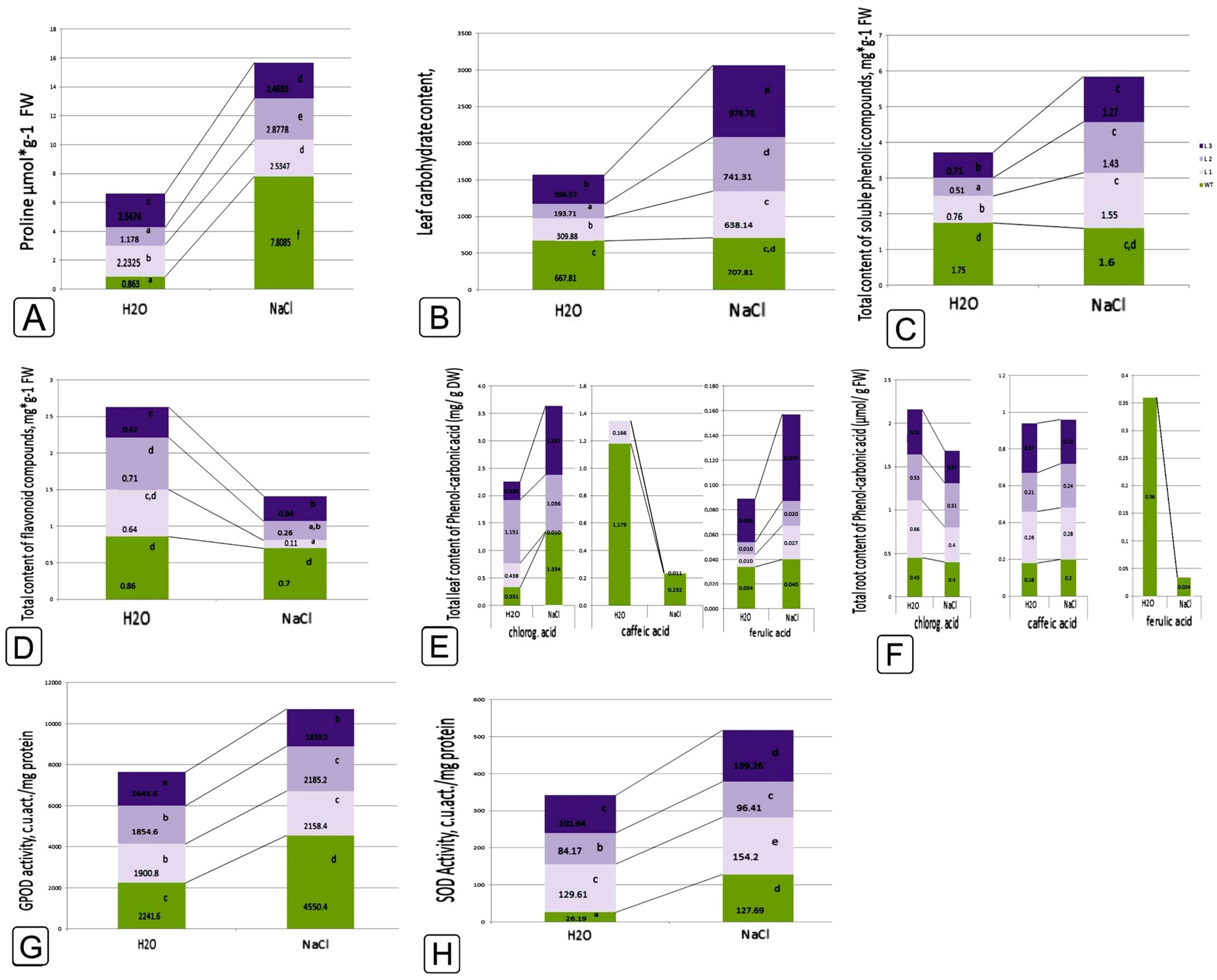
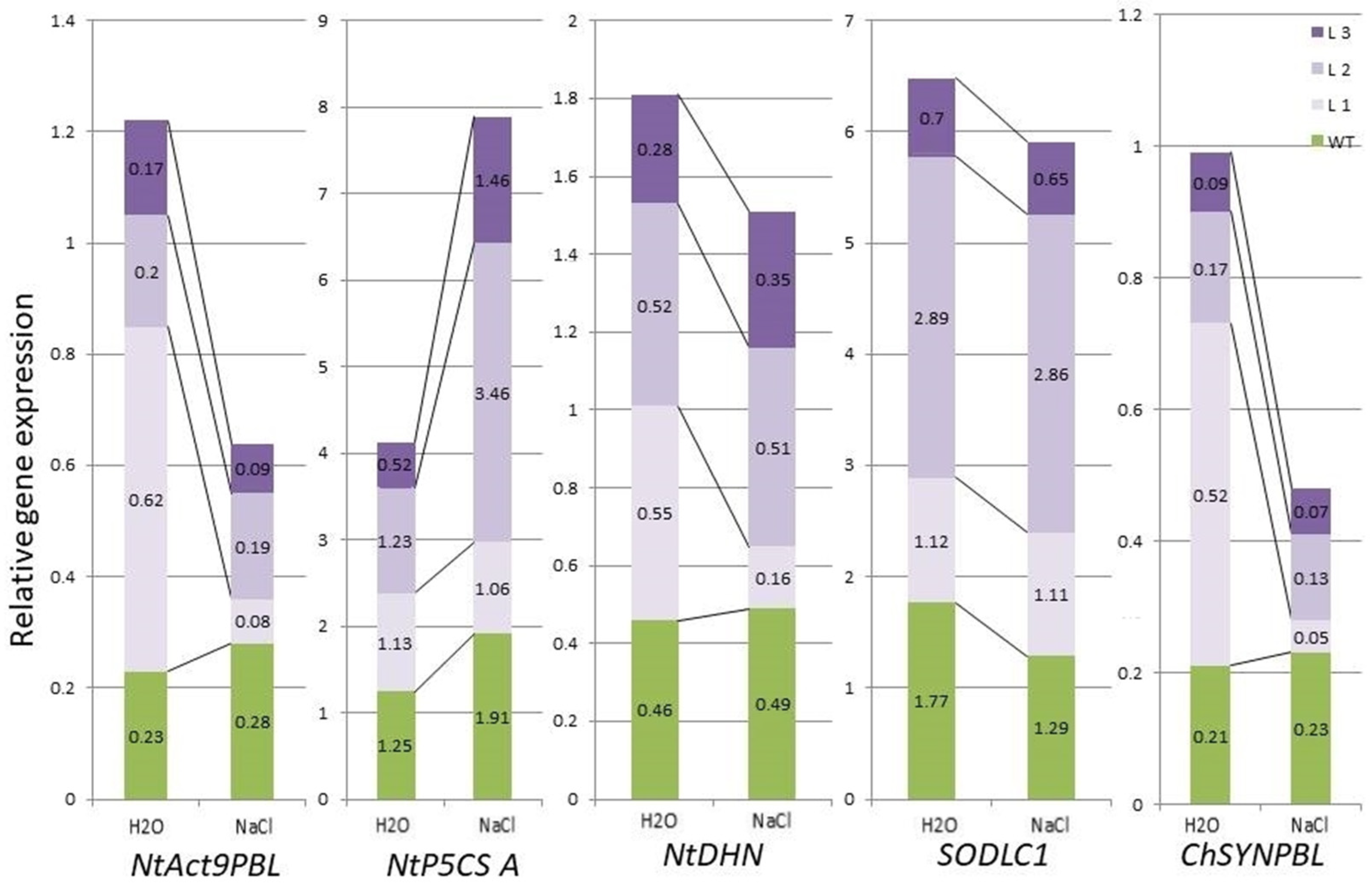
2.2. Comparative Characteristics of codA Transgenic and WT Plants under Saline Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Material and General Growth Conditions
- Wa is soil moisture;
- Mv is the weight of wet soil;
- Ms is the weight of dried soil.
4.2. Determination of Glycine Betaine Content
4.3. Ions Uptake Determination
4.4. Water Content Measurement
4.5. Measurement of Leaf Area
4.6. Study of the Stomatal Apparatus
4.7. Determination of Pigments
- chl a is chlorophyll a, chl b is chlorophyll b.
4.8. Lipid Peroxidation
- C is the concentration of MDA, µmol MDA g−1 fresh weight
- E—optical density of the solution
- n—dilution
- ε—Coefficient of molar extinction MDA (156 mM−1·cm−1)
- l—The length of the optical path of the light beam, cm.
4.9. Determination of SOD Activity
- A—SOD activity, c.u./mg of protein
- Econtr—optical density of the control sample
- Eexper—optical density of the experimental sample
- n—reaction mixture volume, mL
- Valiq—volume of enzyme aliquot, mL
- C—concentration of fresh biomass in the enzyme extract, mg.
4.10. Determination of Peroxidase Activity
- A—enzyme activity, µmol guaiacol/mg biomass × min
- ΔE—average optical density per minute (t2 − t1)/2
- n—dilution
- W—wet tissue weight, mg
- l—length of the optical path of the light beam, cm
- ε—molar extinction coefficient, 5.6 µmol−1 × cm.
4.11. Determination of Endogenous Content of Free Proline
- C—proline concentration, µmol g−1 wet weight
- E—optical density
- k—coefficient calculated from the calibration curve
- V—extract volume, mL
- W—sample weight, g.
4.12. Determination of the Content of Soluble Sugars
4.13. Determination of the Content of Phenolic Compounds and Flavonoids
4.14. Determination of Phenolic Acids
4.15. Determination of the Content of Abscisic Acid
4.16. Plant RNA Extraction
4.17. Gene Expression Analysis
4.18. Phenotyping Analisis
4.19. Transmission Electron Microscopy
4.20. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhodes, D.; Hanson, A.D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. GB protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [PubMed]
- Kishitani, S.; Watanabe, K.; Yasuda, S.; Arakawa, K.; Takabe, T.J. Accumulation of glycinebetaine during cold acclimation and freezing tolerance in leaves of winter and spring barley plants. Plant Cell Environ. 1994, 17, 89–95. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Banerjee, A. Endogenous glycine betaine accumulation mediates abiotic stress tolerance in plants. Trop. Plant Res. 2016, 3, 105–111. [Google Scholar]
- Papageorgiou, G.C.; Murata, N. The unusually strong stabilizing effects of GB on the structure and function of the oxygen-evolving photosystem-II complex. Photosynth. Res. 1995, 44, 243–252. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar]
- Hayashi, H.; Mustardy, L.; Deshnium, P.; Ida, M.; Murata, N. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 1997, 12, 133–142. [Google Scholar] [CrossRef]
- Prasad, K.V.S.K.; Sharmila, P.; Saradhi, P.P. Enhanced tolerance of transgenic Brassica juncea to choline confirms successful expression of the bacterial codA gene. Plant Sci. 2000, 159, 233–242. [Google Scholar] [CrossRef]
- Huang, J.; Hirji, R.; Adam, L.; Rozwadowski, K.L.; Hammerlindl, J.K.; Keller, W.A.; Selvaraj, G. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: Metabolic limitations. Plant Physiol. 2000, 122, 747–756. [Google Scholar] [CrossRef]
- Holmstrom, K.O.; Somersalo, S.; Mandal, A.; Palva, T.E.; Welin, B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 2000, 51, 177–185. [Google Scholar] [CrossRef]
- Sulpice, R.; Tsukaya, H.; Nonaka, H.; Mustardy, L.; Chen, T.H.; Murata, N. Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J. 2003, 36, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, H.; Giri, J.; Nataraja, K.N.; Murata, N.; Udayakumar, M.; Tyagi, A.K. Glycinebetaine-induced water-stress tolerance in codA-expressing transgenic indica rice is associated with up-regulation of several stress responsive genes. Plant Biotechnol. J. 2009, 7, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Murata, N.; Bansal, K.C. Transformation of tomato with a bacterial codA gene enhances tolerance to salt and water stresses. J. Plant Physiol. 2011, 168, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Wang, Z.; Ji, C.Y.; Jeong, J.C.; Lee, H.S.; Li, H.; Xu, B.; Deng, X.; Kwak, S.S. Transgenic poplar expressing codA exhibits enhanced growth and abiotic stress tolerance. Plant Physiol. Biochem. 2016, 100, 75–84. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef]
- Park, E.J.; Jeknic, Z.; Pino, M.T.; Murata, N.; Chen, T.H.H. Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007, 30, 994–1005. [Google Scholar] [CrossRef]
- Gulevich, A.A.; Kurenina, L.V.; Baranova, E.N. Application of a system for targeting Fe-dependent superoxide dismutase and choline oxidase enzymes to chloroplast as a strategy for effective plant resistance to abiotic stresses. Russ. Agric. Sci. 2018, 44, 118–123. [Google Scholar] [CrossRef]
- Raldugina, G.N.; Evsukov, S.V.; Bogoutdinova, L.R.; Gulevich, A.A.; Baranova, E.N. Morpho-physiological testing of NaCl sensitivity of tobacco plants overexpressing choline oxidase gene. Plants 2021, 10, 1102. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl– ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Fakhrfeshani, H.; Shahriari-Ahmadi, F.; Niazi, A.; Moshtagni, N.; Zare-Mehrjerdi, M. The effect of salinity stress on Na+, K+ concentration, Na+/K+ ratio, electrolyte leakage and HKT expression profile in roots of Aeluropus littoralis. J. Plant Mol. Breed. 2015, 3, 1–10. [Google Scholar]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef]
- Khan, M.S.; Yu, X.; Kikuchi, A.; Asahina, M.; Watanabe, K.N. Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotechnol. 2009, 26, 125–134. [Google Scholar] [CrossRef]
- Weretilnyk, E.A.; Bednarek, S.; McCue, K.F.; Rhodes, D.; Hanson, A.D. Comparative biochemical and immunological studies of the glycine betaine synthesis pathway in diverse families of dicotyledons. Planta 1989, 178, 342–352. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. GB: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Hernandez-Leon, S.G.; Valenzuela-Soto, E.M. Glycine betaine is a phytohormone-like plant growth and development regulator under stress conditions. J. Plant Growth Regul. 2022, 42, 5029–5040. [Google Scholar] [CrossRef]
- Valenzuela-Soto, E.M.; Figueroa-Soto, C.G. Biosynthesis and degradation of glycine betaine and its potential to control plant growth and development. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 123–140. [Google Scholar]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Delfani, K.; Asadi, M.; Golein, B.; Babakhani, B.; Razeghi Jadid, R. Foliar application of glycine betaine affects morpho-physiological, biochemical and fruit quality traits of thomson navel orange under deficit irrigation. J. Plant Growth Regul. 2022, 42, 2867–2883. [Google Scholar] [CrossRef]
- Graziani, G.; Cirillo, A.; Giannini, P.; Conti, S.; El-Nakhel, C.; Rouphael, Y.; Ritieni, A.; Di Vaio, C. Biostimulants improve plant growth and bioactive compounds of young olive trees under abiotic stress conditions. Agriculture 2022, 12, 227. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Li, S.; Chen, J.; Amin, A.S.; Wang, G.; Xiaojun, S.; Zain, M.; Gao, Y. Linking exogenous foliar application of glycine betaine and stomatal characteristics with salinity stress tolerance in cotton (Gossypium hirsutum L.) seedlings. BMC Plant Biol. 2021, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Pandey, B.; Singh, P.; Singh, R.; Singh, S.P.; Sonkar, S.; Singh, A.K. Plant performance and defensive role of glycine betaine under environmental stress. In Plant Performance Under Environmental Stress: Hormones, Biostimulants and Sustainable Plant Growth Management; Husen, A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 225–248. [Google Scholar]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Xu, W.; Zhang, Y.; Ni, W. Improving albino tea quality by foliar application of glycinebetaine as a green regulator under lower temperature conditions. J. Agric. Food Chem. 2021, 69, 1242–1250. [Google Scholar] [CrossRef]
- Shirokikh, I.G.; Ogorodnikova, S.Y.; Nazarova, Y.I.; Shupletsova, O.N. Effect of salt stress on plants of wild-type Nicotiana tabacum L. and transformants with a choline oxidase (codA) gene. Trudy po Prikladnoj Botanike Genetike i Selekcii 2022, 183, 86–94. [Google Scholar] [CrossRef]
- Sofy, A.R.; Dawoud, R.A.; Sofy, M.R.; Mohamed, H.I.; Hmed, A.A.; El-Dougdoug, N.K. Improving regulation of enzymatic and non-enzymatic antioxidants and stress-related gene stimulation in Cucumber mosaic cucumovirus-infected cucumber plants treated with glycine betaine, chitosan and combination. Molecules 2020, 25, 2341. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A.H. Influence of glycine betaine (natural and synthetic) on growth, metabolism and yield production of drought-stressed maize (Zea mays L.) plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef]
- Dustgeer, Z.; Seleiman, M.F.; Khan, I.; Chattha, M.U.; Ali, E.F.; Alhammad, B.A.; Jalal, R.S.; Refay, Y.; Hassan, M.U. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. 2021, 49, 12248. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Li, D.; Li, C.; Wei, D.; Li, S.; Liu, Y.; Chen, T.H.H.; Yang, X. Comparative effects of glycinebetaine on the thermotolerance in codA-and BADH-transgenic tomato plants under high temperature stress. Plant Cell Rep. 2020, 39, 1525–1538. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Sadat-Noori, S.A.; Tohidfar, M.; Mortazavian, S.M.M.; Sabbatini, P. Betaine aldehyde dehydrogenase (BADH) vs. flavodoxin (Fld): Two important genes for enhancing plants stress tolerance and productivity. Front. Plant Sci. 2021, 12, 650215. [Google Scholar] [CrossRef] [PubMed]
- Serapicos, M.; Afonso, S.; Gonçalves, B.; Silva, A.P. Exogenous application of glycine betaine on sweet cherry tree (Prunus avium L.): Effects on tree physiology and leaf properties. Plants 2022, 11, 3470. [Google Scholar] [CrossRef] [PubMed]
- Zuzunaga-Rosas, J.; González-Orenga, S.; Tofei, A.M.; Boscaiu, M.; Moreno-Ramón, H.; Ibáñez-Asensio, S.; Vicente, O. Effect of a biostimulant based on polyphenols and glycine betaine on tomato plants’ responses to salt stress. Agronomy 2022, 12, 2142. [Google Scholar] [CrossRef]
- Ntanos, E.; Tsafouros, A.; Denaxa, N.-K.; Kosta, A.; Bouchagier, P.; Roussos, P.A. Mitigation of high solar irradiance and heat stress in kiwifruit during summer via the use of alleviating products with different modes of action—Part 1 Effects on leaf physiology and biochemistry. Agriculture 2022, 12, 2121. [Google Scholar] [CrossRef]
- Nawaz, M.; Wang, Z. Abscisic acid and glycine betaine mediated tolerance mechanisms under drought stress and recovery in Axonopus compressus: A new insight. Sci. Rep. 2020, 10, 6942. [Google Scholar] [CrossRef]
- Ghorbani, A.; Ghasemi-Omran, V.O.; Chen, M. The effect of glycine betaine on nitrogen and polyamine metabolisms, expression of glycoside-related biosynthetic enzymes, and K/Na balance of stevia under salt stress. Plants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Eisakhani, M.R.; Ghooshchi, F.; Moghaddam, H.T.; Kasraie, P.; Oveysi, M. Mitigation the adverse effects of salinity on red bean plants via exogenous application of glycine betaine, zinc, and manganese: Physiological and morphological approach. Russ. J. Plant Physiol. 2023, 70, 51. [Google Scholar] [CrossRef]
- Hernandez, J.O.; An, J.Y.; Combalicer, M.S.; Chun, J.-P.; Oh, S.-K.; Park, B.B. Morpho-anatomical traits and soluble sugar concentration largely explain the responses of three deciduous tree species to progressive water stress. Front. Plant Sci. 2021, 12, 738301. [Google Scholar] [CrossRef]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef]
- Li, S.; Liu, F. Vapour pressure deficit and endogenous ABA level modulate stomatal responses of tomato plants to soil water deficit. Environ. Exp. Bot. 2022, 199, 104889. [Google Scholar] [CrossRef]
- Guo, Z.F. A review: Molecular regulation of stomatal development related to environmental factors and hormones in plants. Appl. Ecol. Environ. Res. 2019, 17, 12091–12109. [Google Scholar] [CrossRef]
- Wang, Y.; Nil, N. Changes in chlorophyll, ribulose biphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J. Hortic. Sci. Biotechnol. 2000, 75, 623–627. [Google Scholar] [CrossRef]
- Noreen, Z.; Ashraf, M. Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J. Plant Physiol. 2009, 166, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.V.; Karadge, B.A.; Samant, J.S. Salinity induced changes in photosynthetic pigments and polyphenols of Cymbopogon nardus (L.) Rendle. J. Chem. Pharm. Res. 2010, 2, 338–347. [Google Scholar]
- Mansour, M.M.F.; Salama, K.H. Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 2004, 52, 113–122. [Google Scholar] [CrossRef]
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Marcek, T.; Tkalec, M.; Vidakovic-Cifrek, Ž.; Ježic, M.; Curkovic-Perica, M. Effect of NaCl stress on dihaploid tobacco lines tolerant to Potato virus Y. Acta Physiol. Plant 2014, 36, 1739–1747. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, M.I.R.; Anjum, N.A.; Masood, A.; Hussain, S.J.; Khan, N.A. Jasmonates in plants under abiotic stresses: Crosstalk with other phytohormones matters. Environ. Exp. Bot. 2018, 145, 104–120. [Google Scholar] [CrossRef]
- Boriboonkaset, T.; Theerawitaya, C.; Pichakum, A.; Cha-Um, S.; Takabe, T.; Kirdmanee, C. Expression levels of some starch metabolism related genes in flag leaf of two contrasting rice genotypes exposed to salt stress. Aust. J. Crop Sci. 2012, 6, 1579–1586. [Google Scholar]
- Mišić, D.; Dragićević, M.; Šiler, B.; Živković, J.N.; Maksimović, V.; Momčilović, I.; Nikolic, M. Sugars and acid invertase mediate the physiological response of Schenkia spicata root cultures to salt stress. J. Plant Physiol. 2012, 169, 1281–1289. [Google Scholar] [CrossRef]
- Poor, P.; Gemes, K.; Horvath, F.; Szepesi, A.; Simon, M.L.; Tari, I. Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biol. 2011, 13, 105–114. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Chowdhary, V.A.; Tank, J.G. Biomolecules regulating defense mechanism in plants. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 93, 17–25. [Google Scholar] [CrossRef]
- Borgognone, D.; Cardarelli, M.; Rea, E.; Lucini, L.; Colla, G. Salinity source-induced changes in yield, mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon grown in floating system. J. Sci. Food Agric. 2014, 94, 1231–1237. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 2008, 165, 813–824. [Google Scholar] [CrossRef]
- Sehmer, L.; Alaoui-Sosse, B.; Dizengremel, P. Effect of salt stress on growth and on the detoxifying pathway of pedunculate oak seedlings (Quercus robur L.). J. Plant Physiol. 1995, 147, 144–151. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Grimm, B.; Wobus, U.; Weschke, W. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plant. 2000, 109, 435–442. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Lee, M.H.; Cho, E.J.; Wi, S.G.; Bae, H.; Kim, J.E.; Cho, J.Y.; Lee, S.; Kim, J.H.; Chung, B.Y. Divergences in morphological changes and antioxidant responses in salt-tolerant and salt-sensitive rice seedlings after salt stress. Plant Physiol. Biochem. 2013, 70, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci. Total Environ. 2022, 804, 150059. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, X.; Zhang, L. Structural and functional dynamics of dehydrins: A plant protector protein under abiotic stress. Int. J. Mol. Sci. 2018, 19, 3420. [Google Scholar] [CrossRef]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2011, 10, 397–412. [Google Scholar]
- Joseph, J.T.; Poolakkalody, N.J.; Shah, J.M. Plant reference genes for development and stress response studies. J. Biosci. 2018, 43, 173–187. [Google Scholar] [PubMed]
- Wang, M.; Wang, Q.; Zhang, B. Evaluation and selection of reliable reference genes for gene expression under abiotic stress in cotton (Gossypium hirsutum L.). Gene 2013, 530, 44–50. [Google Scholar] [PubMed]
- Grimm, B.; Dehesh, K.; Zhang, L.; Leister, D. Intracellular communication. Mol. Plant 2014, 7, 1071–1074. [Google Scholar] [PubMed]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez-Juez, E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2022, 233, 2000–2016. [Google Scholar]
- Sakamoto, W.; Uno, Y.; Zhang, Q.; Miura, E.; Kato, Y.; Sodmergen. Arrested differentiation of proplastids into chloroplasts in variegated leaves characterized by plastid ultrastructure and nucleoid morphology. Plant Cell Physiol. 2009, 50, 2069–2083. [Google Scholar]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar]
- Zhang, F.; Yang, Y.L.; He, W.L.; Zhao, X.; Zhang, L.X. Effects of salinity on growth and compatible solutes of callus induced from Populus euphratica. In Vitro Cell. Devel. Biol. Plant 2004, 40, 491–494. [Google Scholar]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Gangaiah, C.; Ahmad, A.A.; Radovich, T.J. A comparison of the accuracy and efficiency of two ionic strength adjustment buffers in measuring potassium using an ion-selective electrode. Commun. Soil Sci. Plant Anal. 2019, 50, 586–593. [Google Scholar]
- Beerling, D.J.; Fry, J.C. A comparison of the accuracy, variability and speed of five different methods for estimating leaf area. Ann. Bot. 1990, 65, 483–488. [Google Scholar]
- Shlyk, A.A. Definition of a Chlorophyll and Carotenoids in Extracts of Green Leaves. Biochemical Methods in Physiology of Plants; Nauka: Moscow, Russia, 1971. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation on isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [PubMed]
- Lukatkin, A.S. Contribution of oxidative stress to the development of cold-induced damage to leaves of chilling-sensitive plants: 1. Reactive oxygen species formation during plant chilling. Russ. J. Plant Physiol. 2002, 49, 622–627. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [PubMed]
- Ridge, I.; Osborne, D.J. Role of peroxidase when hydroxyproline-rich protein in plant cell walls is increased by ethylene. Nat. New Biol. 1971, 229, 205–208. [Google Scholar] [PubMed]
- Bates, L.S.; Waldren, R.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar]
- Olenichenko, N.A.; Zagoskina, N.V.; Astakhova, N.V.; Trunova, T.I.; Kuznetsov, Y.V. Primary and secondary metabolism of winter wheat under cold hardening and treatment with antioxidants. Appl. Biochem. Microbiol. 2008, 44, 535–540. [Google Scholar]
- Turkina, M.V.; Sokolova, S.V. Methods for determination of monosaccharides and oligosaccharides. In Biokhimicheskie Metody v Fiziologii Rastenii (Biochemical Methods in Plant Physiology); Nauka: Moscow, Russia, 1971. [Google Scholar]
- Olenichenko, N.A.; Zagoskina, N.V. Response of winter wheat to cold: Production of phenolic compounds and L-phenylalanine ammonia lyase activity. Appl. Biochem. Microbiol. 2005, 41, 600–603. [Google Scholar]
- Nikolaeva, T.N.; Lapshin, P.V.; Zagoskina, N.V. Method for determining the total content of phenolic compounds in plant extracts with Folin–Denis reagent and Folin–Ciocalteu reagent: Modification and comparison. Russ. J. Bioorg. Chem. 2022, 48, 1519–1525. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raldugina, G.N.; Bogoutdinova, L.R.; Shelepova, O.V.; Kondrateva, V.V.; Platonova, E.V.; Nechaeva, T.L.; Kazantseva, V.V.; Lapshin, P.V.; Rostovtseva, H.I.; Aniskina, T.S.; et al. Heterologous codA Gene Expression Leads to Mitigation of Salt Stress Effects and Modulates Developmental Processes. Int. J. Mol. Sci. 2023, 24, 13998. https://doi.org/10.3390/ijms241813998
Raldugina GN, Bogoutdinova LR, Shelepova OV, Kondrateva VV, Platonova EV, Nechaeva TL, Kazantseva VV, Lapshin PV, Rostovtseva HI, Aniskina TS, et al. Heterologous codA Gene Expression Leads to Mitigation of Salt Stress Effects and Modulates Developmental Processes. International Journal of Molecular Sciences. 2023; 24(18):13998. https://doi.org/10.3390/ijms241813998
Chicago/Turabian StyleRaldugina, Galina N., Lilia R. Bogoutdinova, Olga V. Shelepova, Vera V. Kondrateva, Ekaterina V. Platonova, Tatiana L. Nechaeva, Varvara V. Kazantseva, Pyotr V. Lapshin, Helen I. Rostovtseva, Tatiana S. Aniskina, and et al. 2023. "Heterologous codA Gene Expression Leads to Mitigation of Salt Stress Effects and Modulates Developmental Processes" International Journal of Molecular Sciences 24, no. 18: 13998. https://doi.org/10.3390/ijms241813998
APA StyleRaldugina, G. N., Bogoutdinova, L. R., Shelepova, O. V., Kondrateva, V. V., Platonova, E. V., Nechaeva, T. L., Kazantseva, V. V., Lapshin, P. V., Rostovtseva, H. I., Aniskina, T. S., Kharchenko, P. N., Zagoskina, N. V., Gulevich, A. A., & Baranova, E. N. (2023). Heterologous codA Gene Expression Leads to Mitigation of Salt Stress Effects and Modulates Developmental Processes. International Journal of Molecular Sciences, 24(18), 13998. https://doi.org/10.3390/ijms241813998





