Effect of Soil Drought Stress on Selected Biochemical Parameters and Yield of Oat × Maize Addition (OMA) Lines
Abstract
1. Introduction
2. Results
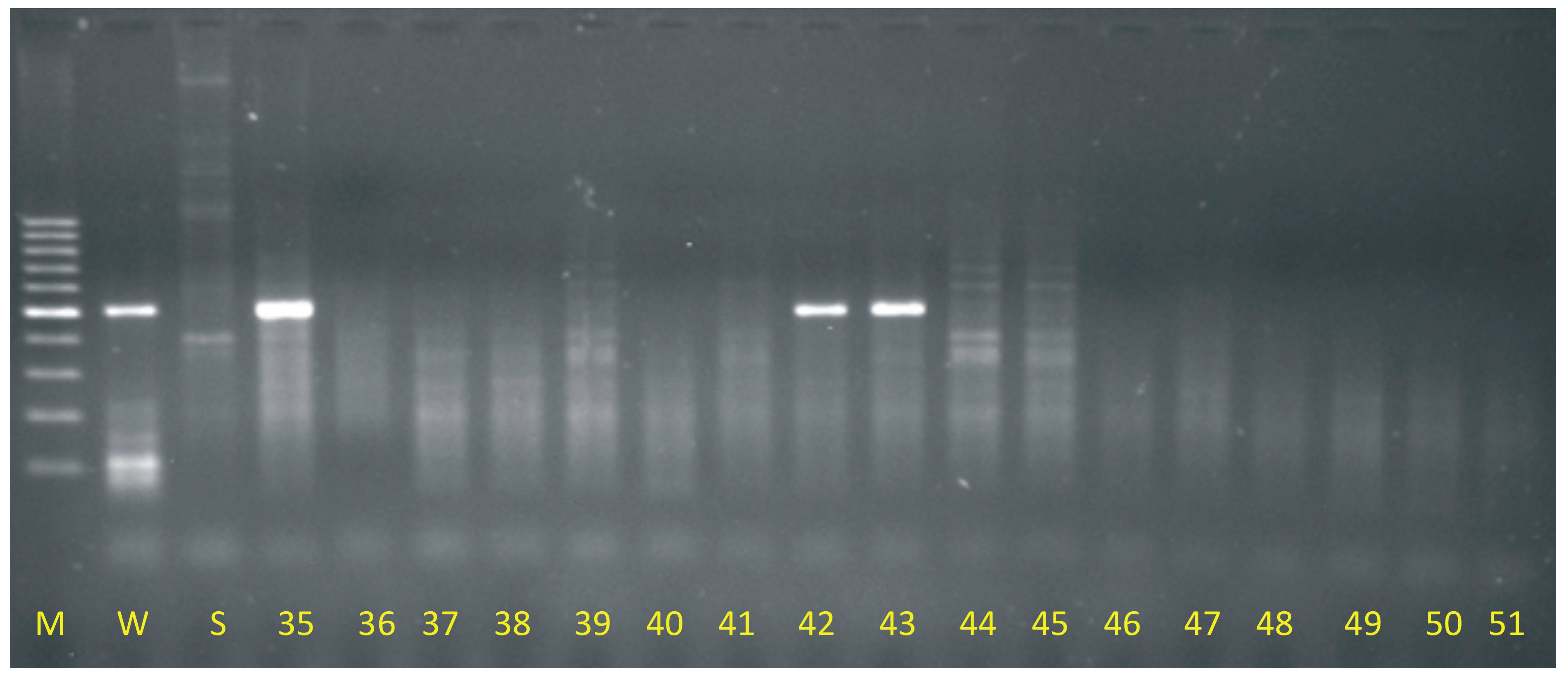
2.1. Molecular Identification of OMA Lines
2.2. Excised Leaf Water Loss Test and Biochemical Analysis
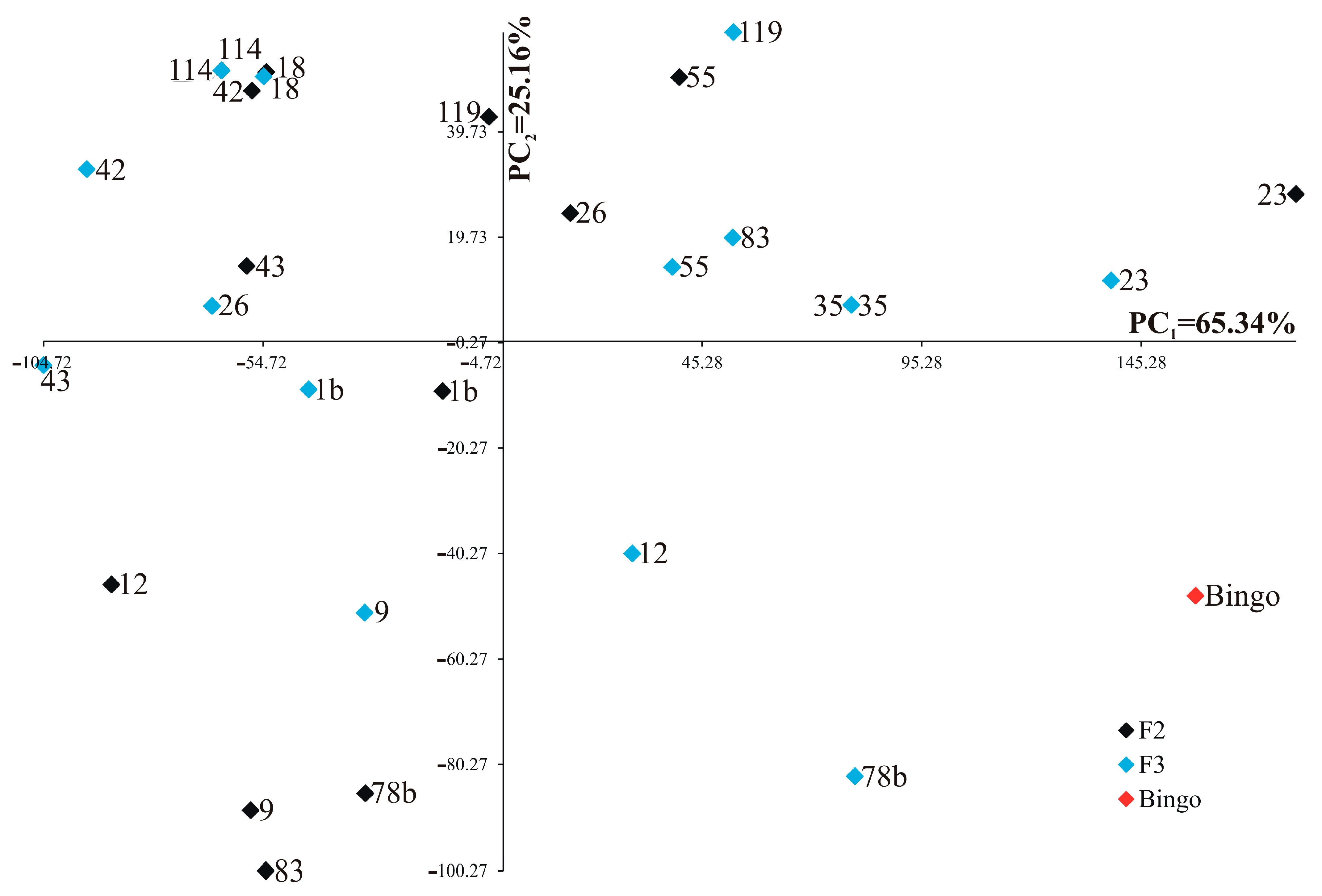
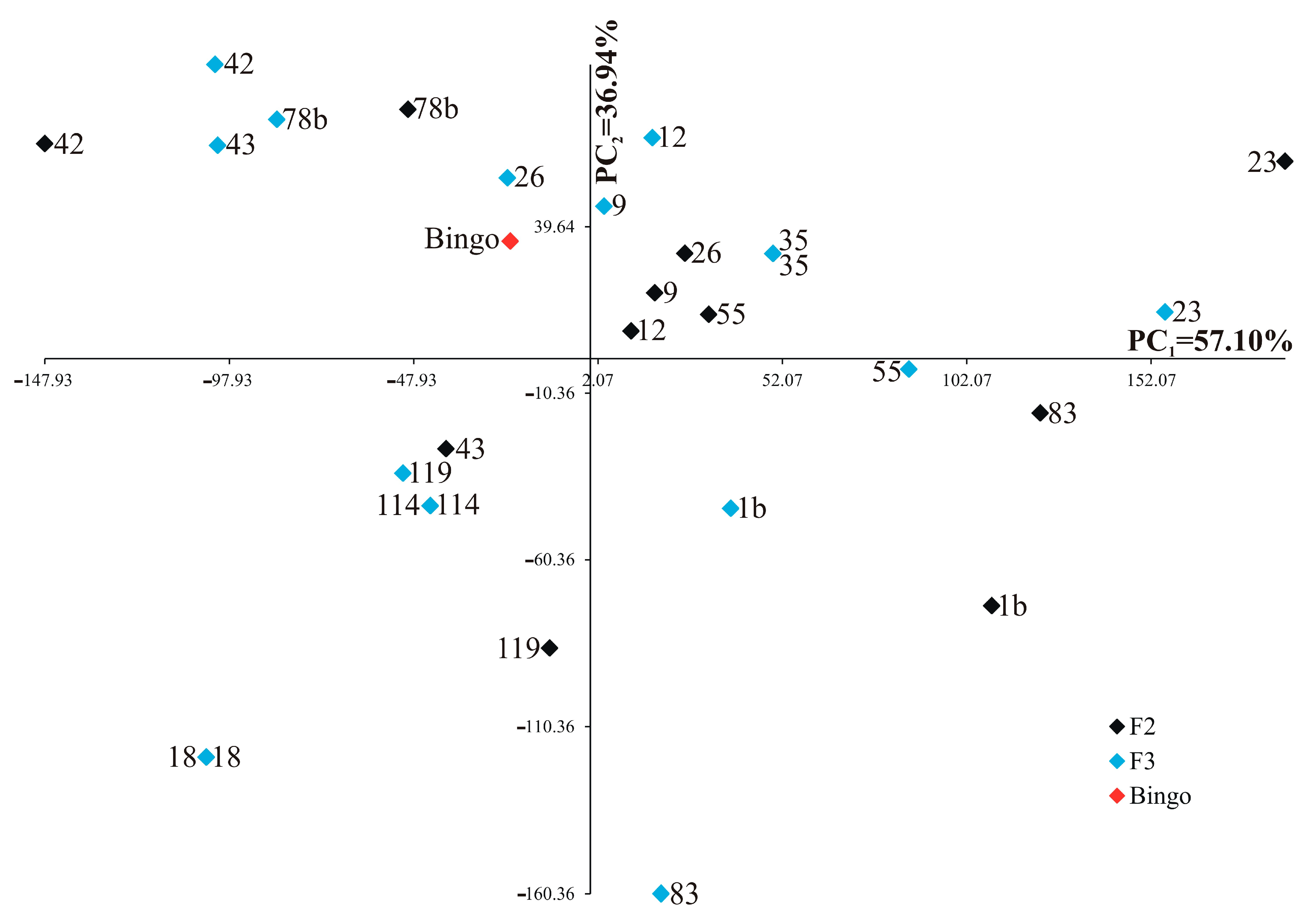
2.3. Analysis of Primary and Secondary Shoots Biomass and Selected Yield Elements
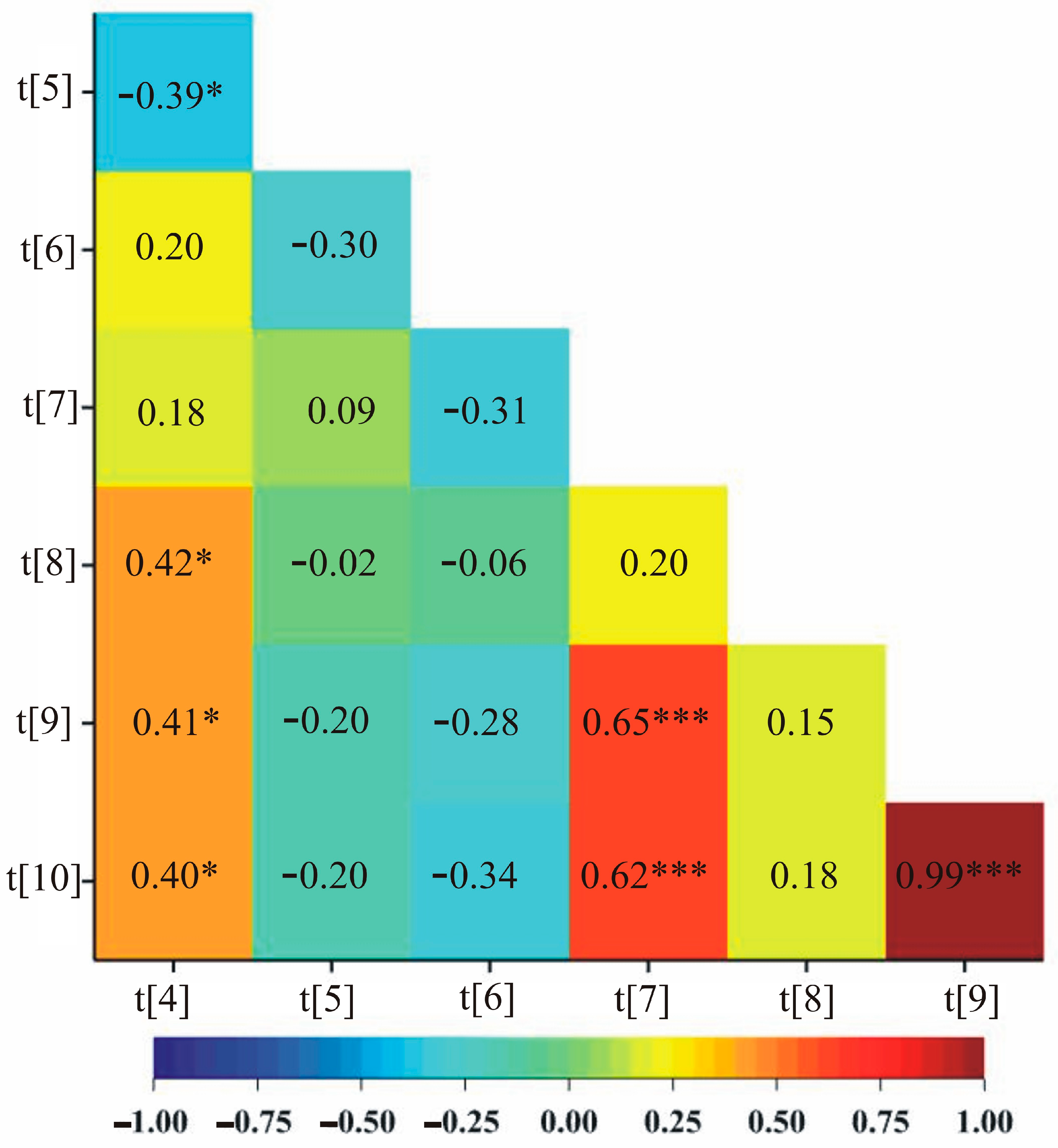
2.4. Correlations among Biochemical Parameters, Biomass and Yield Elements
3. Discussion
4. Materials and Methods
4.1. Molecular Identification of OMA Lines
4.2. Grande I Amplification
4.3. Greenhouse Experiment
- Performing ELWL (Excised Leave Water Loss)
- Collection of leaves for soluble sugars and phenolic compound content on the first day of reaching 20% humidity simultaneously with a control combination (70% soil humidity).
- Collection of leaves for soluble sugars and phenolic compound content on the fourteenth day of drought (maintaining 20% humidity) simultaneously with a control combination (70% soil humidity).
- Harvesting mature shoots, weighing the biomass of aboveground parts and total mass of grains from all shoots.
4.4. Water Loss Test
- ELWL0–3h = (FW0 − FW3)/(FW0 − DW),
- ELWL4–6h = (FW3 − FW6)/(FW3 − DW),
- ELWL0–6h = (FW0 − FW6)/(FW0 − DW),
4.5. Analysis of Biochemical Parameters
4.6. Analysis of Primary and Secondary Shoots Biomass and Selected Yield Elements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.D.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef]
- Okagaki, R.J.; Kynast, R.G.; Livingston, S.M.; Russell, C.D.; Rines, H.W.; Phillips, R.L. Mapping maize sequences to chromosomes using oat-maize chromosome addition materials. Plant Physiol. 2001, 125, 1228–1235. [Google Scholar] [CrossRef]
- Thomas, H. Drought resistance in plants. In Mechanisms of Environmental Stress Resistance in Plants; Basra, A.S., Basra, R.K., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1997; pp. 1–42. [Google Scholar]
- Hoekstra, F.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 8, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Hurry, V. A plant for all seasons: Alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr. Opin. Plant Biol. 2002, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Paul, M.; Pellny, T. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003, 54, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.F. Fluxes and turnover of sucrose and fructans in healthy and diseased plants. J. Plant Physiol. 1989, 134, 137–140. [Google Scholar] [CrossRef]
- Yoshida, M.; Abe, J.; Moriyama, M.; Shimokawa, S.; Nakamura, Y. Seasonal changes in the physical state od crown water associated with freezing tolerance in winter wheat. Physiol. Plant. 1997, 99, 363–370. [Google Scholar] [CrossRef]
- Woloshuk, C.P.; Meulenhof, J.S.; Sela-Buurlage, M.; van den Elzen, P.J.M.; Cornelissen, B.J.C. Pathogen-induced proteins with inhibitor activity toward Phytophtora infestans. Plant Cell 1991, 3, 619–628. [Google Scholar]
- Tejeda-Sartorius, O.; Soto-Hernández, R.M.; San Miguel-Chávez, R.; Trejo-Téllez, L.I.; Caamal-Velázquez, H. Endogenous Hormone Profile and Sugars Display Differential Distribution in Leaves and Pseudobulbs of Laelia anceps Plants Induced and Non-Induced to Flowering by Exogenous Gibberellic Acid. Plants 2022, 11, 845. [Google Scholar] [CrossRef]
- Basharat, S.; Huang, Z.; Gong, M.; Lv, X.; Ahmed, A.; Hussain, I.; Li, J.; Du, G.; Liu, L. A review on current conventional and biotechnical approaches to enhance biosynthesis od steviol glycoside in Stevia rebaudiana. Chin. J. Chem. Eng. 2021, 30, 92–104. [Google Scholar] [CrossRef]
- Rines, H.W.; Phillips, R.L.; Kynast, R.G.; Okagaki, R.J.; Galatowitsch, M.W.; Huettl, P.A.; Stec, A.O.; Jacobs, M.S.; Suresh, J.; Porter, H.L.; et al. Addition of individual chromosomes of maize inbreds B73 and Mo17 to oat cultivars Starter and Sun II: Maize chromosome retention, transmission, and plant phenotype. Theor. Appl. Genet. 2009, 119, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Ananiev, E.V.; Phillips, R.L.; Rines, H.W. Chromosome-specific molecular organization of maize (Zea mays L.) centromeric regions. Proc. Natl. Acad. Sci. USA 1998, 95, 13073–13078. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, G.J.; Riera-Lizarazu, O.; Kynast, R.G.; Martin, D.; Phillips, R.L.; Rines, H.W. A maize chromosome 3 addition line of oat exhibits expression of the maize homeobox gene liguleless3 and alteration of cell fates. Genome 2000, 43, 1055–1064. [Google Scholar] [CrossRef]
- Kowles, R.V.; Walch, M.D.; Minnerath, J.M.; Bernacchi, C.J.; Stec, A.O.; Rines, H.W.; Phillips, R.L. Expression of C4 photosynthetic enzymes in oat-maize chromosome addition lines. Maydica 2008, 53, 69–78. [Google Scholar]
- Walch, M.D. Expression of Maize Pathogenesis-Related and Photosynthetic Genes in Oat × Maize Addition Lines; University of Minnesota: Saint Paul, MN, USA, 2007. [Google Scholar]
- Warzecha, T.; Bathelt, R.; Skrzypek, E.; Warchoł, M.; Bocianowski, J.; Sutkowska, A. Studies of Oat-Maize Hybrids Tolerance to Soil Drought Stress. Agriculture 2023, 13, 243. [Google Scholar] [CrossRef]
- Kynast, R.G.; Riera-Lizarazu, O.; Vales, M.I.; Okagaki, R.J.; Maquieira, S.B.; Chen, G.; Ananiev, E.V.; Odland, W.E.; Russell, C.D.; Stec, A.O.; et al. A complete set of maize individual chromosome additions to the oat genome. Plant Physiol. 2001, 125, 1216–1227. [Google Scholar] [CrossRef][Green Version]
- Dong, Z.; Yu, J.; Li, H.; Huang, W.; Xu, L.; Zhao, Y.; Zhang, T.; Xu, W.; Jiang, J.; Su, Z.; et al. Transcriptional and epigenetic adaptation of maize chromosomes in Oat-Maize addition lines. Nucleic Acids Res. 2018, 46, 5012–5028. [Google Scholar] [CrossRef]
- Sosnowska, K.; Majka, M.; Majka, J.; Bocianowski, J.; Kasprowicz, M.; Książczyk, T.; Szała, L.; Cegielska-Taras, T. Chromosome instabilities in resynthesized Brassica napus revealed by FISH. J. Appl. Genet. 2020, 61, 323–335. [Google Scholar] [CrossRef]
- Amarillo, F.I.E.; Bass, H.W. A transgenomic cytogenetic sorghum (Sorghum propinquum) bacterial artificial chromosome fluorescence in situ hybridization map of maize (Zea mays L.) pachytene chromosome 9, evidence for regions of genome hyperexpansion. Genetics 2007, 177, 1509–1526. [Google Scholar] [CrossRef]
- Li, L.J.; Arumuganathan, K.; Rines, H.W.; Phillips, R.L.; Riera-Lizarazu, O.; Sandhu, D.; Zhou, Y.; Gill, K.S. Flow cytometric sorting of maize chromosome 9 from an oat-maize chromosome addition line. Theor. Appl. Genet. 2001, 102, 658–663. [Google Scholar] [CrossRef]
- Kynast, R.; Okagaki, R.; Rines, H.; Phillips, R. Maize individualized chromosome and derived radiation hybrid lines and their use in functional genomics. Funct. Integr. Genom. 2002, 2, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ohm, H.; Shaner, G. Breeding oat for resistance to diseases. In Oat Science and Technology; Marshall, H., Sorrells, M., Eds.; American Society of Agronomy: Madison, WI, USA, 1992; pp. 657–698. [Google Scholar]
- Rines, H.W.; Phillips, R.L.; Kynast, R.G.; Okagaki, R.; Odland, W.E.; Stec, A.O.; Jacobs, M.S.; Granath, S.R. Maize chromosome additions and radiation hybrids in oat and their use in dissecting the maize genome. In Proceedings of the International Congress “In the Wake of the Double Helix: From the Green Revolution to the Gene Revolution”, Bologna, Italy, 27–31 May 2003; pp. 427–441. [Google Scholar]
- Sadras, V.O.; Calderini, D.F. (Eds.) Crop Physiology Case Histories for Major Crops 2021; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAOSTAT). 2022. Available online: https://www.fao.org/faostat/en/%3F%23data/ (accessed on 22 March 2022).
- Pisulewska, E.; Tobiasz-Salach, R.; Witkowicz, R.; Cieślik, E.; Bobrecka-Jamro, D. Effect of habitat conditions on content and quality of lipids in selected oat forms. Żywność. Nauka. Technol. Jakość 2011, 3, 66–77. [Google Scholar] [CrossRef]
- Oleksiak, T.; Spyroglou, I.; Pacoń, D.; Matysik, P.; Pernisová, M.; Rybka, K. Effect of drought on wheat production in Poland between 1961 and 2019. Crop Sci. 2021, 62, 728–743. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Shakhatreh, Y.; Kafawin, O.; Ceccarelli, S.; Saoub, H. Selection of barley lines for drought tolerance in low rainfall areas. J. Agron. Sci. 2001, 186, 119–127. [Google Scholar] [CrossRef]
- Samarah, N.; Alqudah, A.; Amayreh, J.; McAndrews, G. The Effect of Late-terminal Drought Stress on Yield Components of Four Barley Cultivars. J. Agron. Crop Sci. 2009, 195, 427–441. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P. Global Synthesis of Drought Effects on Maize and Wheat Production. Public Libr. Sci. ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Ishii, T.; Tanaka, H.; Eltayeb, A.; Tsujimoto, H. Wide hybridization between oat and pearl millet belonging to different subfamilies of Poaceae. Plant Reprod. 2013, 26, 25–32. [Google Scholar] [CrossRef]
- Skrzypek, E.; Warzecha, T.; Noga, A.; Warchol, M.; Czyczylo-Mysza, I.; Dziurka, K.; Marcinska, I.; Kaploniak, K.; Sutkowska, A.; Nita, Z.; et al. Complex characterization of oat (Avena sativa L.) lines obtained by wide crossing with maize (Zea mays L.). PeerJ 2018, 6, e5107. [Google Scholar] [CrossRef]
- Juzoń, K.; Idziak-Helmcke, D.; Rojek-Jelonek, M.; Warzecha, T.; Warchoł, M.; Czyczyło-Mysza, I.; Dziurka, K.; Skrzypek, E. Functioning of the Photosynthetic Apparatus in Response to Drought Stress in Oat × Maize Addition Lines. Int. J. Mol. Sci. 2020, 21, 6958. [Google Scholar] [CrossRef] [PubMed]
- Munjal, R.; Dhanda, S. Physiological evaluation of wheat (Triticum aestivum L.) genotypes for drough tresistance. Indian J. Genet. Plant Breed. 2005, 65, 307–308. [Google Scholar]
- Sallam, A.; Alqudah, A.; Dawood, M.; Baenziger, S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, T.; Skrzypek, E.; Sutkowska, A. Effect of Fusarium culmorum infection on the selected physiological and biochemical parameters of barley (Hordeum vulgare L.) DH lines. Physiol. Mol. Plant Pathol. 2015, 89, 62–69. [Google Scholar] [CrossRef]
- Warzecha, T.; Skrzypek, E.; Adamski, T.; Surma, M.; Kaczmarek, Z.; Sutkowska, A. Chlorophyll a fluorescence parameters of hulled and hull-less barley (Hordeum vulgare L.) DH lines inoculated with Fusarium culmorum. Plant Pathol. J. 2019, 35, 112–124. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Redillas, M.; Park, S.H.; Lee, J.; Kim, Y.; Jeong, J.; Jung, H.; Bang, S.; Hahn, T.R.; Kim, J.K. Accumulation of trehalose increases soluble sugar contents in rice plants conferring tolerance to drought and salt stress. Plant Biotechnol. Rep. 2011, 6, 89–96. [Google Scholar] [CrossRef]
- Sinay, H.; Karuwal, R. Proline and total soluble sugar content at the vegetative phase of six corn cultivars from Kisar Island Maluku, grown under drought stress conditions. Int. J. Adv. Agric. Res. 2014, 2, 77–82. [Google Scholar]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Differential responses of phenolic compounds of Brassica napus under drought stress. Iran. J. Plant Physiol. 2018, 8, 2417–2425. [Google Scholar]
- Cyplik, A.; Czyczyło-Mysza, I.M.; Jankowicz-Cieslak, J.; Bocianowski, J. QTL × QTL × QTL Interaction Effects for Total Phenolic Content of Wheat Mapping Population of CSDH Lines under Drought Stress by Weighted Multiple Linear Regression. Agriculture 2023, 13, 850. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, S. Leaf dehydration induces different content of phenolics and ferulic acid in drought-resistant and -sensitive genotypes of spring triticale. Verl. Z. Naturforschung 2014, 64, 85–95. [Google Scholar] [CrossRef]
- André, C.; Schafleitner, R.; Guignard, C.; Oufir, M.; Aliaga, C.; Nomberto, G.; Hoffmann, L.; Hausman, J.F.; Evers, D.; Larondelle, Y. Modification of the health-promoting value of potato tubers field grown under drought stress: Emphasis on dietary antioxidant and glycoalkaloid contents in five native Andean cultivars (Solanum tuberosum L.). J. Agric. Food Chem. 2009, 57, 599–609. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Ahmadi, J.; Mehrabi, A.; Etminan, A.; Moghaddam, M.; Siddique, K. Physiological responses to drought stress in wild relatives of wheat: Implications for wheat improvement. Acta Physiol. Plant. 2017, 39, 106. [Google Scholar] [CrossRef]
- Samarah, N. Effects of drought stress on growth and yield of barley. Agron. Sustain. Dev. 2005, 25, 145–149. [Google Scholar] [CrossRef]
- Marcińska, I.; Nowakowska, A.; Skrzypek, E.; Czyczyło-Mysza, I. Production of double haploids in oat (Avena sativa L.) by pollination with maize (Zea mays L.). Cent. Eur. J. Biol. 2013, 8, 306–313. [Google Scholar] [CrossRef]
- Czyczyło-Mysza, I.; Marcińska, I.; Skrzypek, E.; Bocianowski, J.; Dziurka, K.; Rančić, D.; Radošević, R.; Pekić-Quarrie, S.; Dodig, D.; Quarrie, S. Genetic analysis of water loss of excised leaves associated with drought tolerance in wheat. PeerJ 2018, 6, e5063. [Google Scholar] [CrossRef]
- Clarke, J.M.; McCaig, T.N. Evaluation of techniques for screening for drought resistance in wheat. Crop Sci. 1982, 22, 503–506. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Roberts, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kozak, M.; Bocianowski, J.; Rybiński, W. Selection of promising genotypes based on path and cluster analyses. J. Agric. Sci. 2008, 146, 85–92. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Skomra, U.; Bocianowski, J.; Agacka, M. Agro-morphological differentiation between European hop (Humulus lupulus L.) cultivars in relation to their origin. J. Food Agric. Environ. 2013, 11, 1123–1128. [Google Scholar]
- VSN International. Genstat for Windows, 23rd ed.; VSN International: Hemel Hempstead, UK, 2023. [Google Scholar]


| Trait | Excised-Leaf Water Loss (ELWL) | ||||||
|---|---|---|---|---|---|---|---|
| After 0–3 h | After 4–6 h | After 0–6 h | |||||
| Genotype | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| Bingo | 19.7 c–g | 3.68 | 14.0 c–e | 3.32 | 30.8 b–e | 5.67 | |
| F2 | 114 | 22.8 bc | 5.23 | 13. 0 c–f | 4.27 | 32.6 b–d | 7.85 |
| 119 | 17.4 e–h | 1.49 | 12.9 c–f | 3.04 | 28.0 b–f | 3.55 | |
| 12 | 16.6 gh | 2.03 | 13.7 c–e | 11.02 | 28.0 b–f | 9.05 | |
| 18 | 28.3 a | 5.77 | 19.3 b | 2.40 | 42.1 a | 6.26 | |
| 1b | 22.3 b–d | 7.37 | 14.1 c–e | 6.47 | 32.9 b–d | 11.08 | |
| 23 | 15.3 gh | 3.66 | 10.3 e–h | 4.05 | 23.8 e–g | 6.56 | |
| 26 | 15.4 gh | 1.83 | 10.0 e–h | 1.21 | 23.9 e–g | 2.62 | |
| 35 | 21.3 b–f | 3.19 | 16.4 b–d | 2.72 | 34. 1 bc | 4.47 | |
| 42 | 16.3 gh | 2.91 | 10.8 e–h | 3.34 | 25.3 e–g | 5.30 | |
| 43 | 18.9 c–h | 6.30 | 11.1 e–h | 6.23 | 27.6 c–g | 10.28 | |
| 55 | 21.7 b–e | 3.93 | 16.9 bc | 4.98 | 34.8 b | 7.02 | |
| 78b | 15.7 gh | 0.82 | 10.1 e–h | 0.75 | 24.2 e–g | 0.11 | |
| 83 | 18.2 d–h | 3.52 | 11.4 e–h | 4.06 | 27.5 c–g | 5.89 | |
| 9 | 15.6 gh | 2.29 | 7.6 gh | 1.30 | 22.0 fg | 3.20 | |
| F3 | 114 | 22.8 bc | 5.23 | 13.0 c–f | 4.27 | 32.6 b–d | 7.85 |
| 119 | 16.9 f–h | 3.88 | 11.7 d–h | 1.96 | 26.7 d–g | 3.32 | |
| 12 | 17.1 f–h | 3.99 | 9.5 e–h | 2.46 | 24.8 e–g | 5.66 | |
| 18 | 28.3 a | 5.77 | 19.3 b | 2.40 | 42.1 a | 6.26 | |
| 1b | 17.2 e–h | 2.19 | 8.5 f–h | 3.02 | 24.3 e–g | 4.33 | |
| 23 | 15.3 gh | 1.64 | 8.4 f–h | 0.91 | 22.4 fg | 2.12 | |
| 26 | 16.9 f–h | 1.48 | 12.3 c–g | 2.54 | 27.1 d–g | 3.35 | |
| 35 | 21.3 b–f | 3.19 | 16.4 b–d | 2.72 | 34.2 bc | 4.47 | |
| 42 | 16.3 gh | 4.26 | 10.1 e–h | 3.37 | 24.7 e–g | 6.52 | |
| 43 | 16.0 gh | 2.69 | 11.3 e–h | 3.18 | 25.4 eg | 5.05 | |
| 55 | 16.7 gh | 1.09 | 12.8 c–f | 1.81 | 27.4 c–g | 1.87 | |
| 78b | 25.6 ab | 4.52 | 26.8 a | 6.27 | 45.3 a | 7.68 | |
| 83 | 18.2 d–h | 0.50 | 11.6 e–h | 0.75 | 27.7 c–g | 0.17 | |
| 9 | 14.8 h | 1.00 | 7.1 h | 1.68 | 20.8 g | 2.28 | |
| LSD0.05 | 4.5 | 4. 8 | 7.1 | ||||
| Trait | First Day of Drought (20% of Soil Field Capacity) | After Two Weeks of Drought (Maintaining 20% of Soil Field Capacity) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | S | Average | C | S | Average | |||||||
| Genotype | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| Bingo | 374.9 | 19.98 | 340.1 | 42.86 | 357.5 bc | 36.96 | 263.9 | 77.13 | 162.1 | 8.13 | 213.0 b–g | 74.64 | |
| F2 | 114 | 204.6 | 28.81 | 295.8 | 26.54 | 250.2 g–l | 55.09 | 139.6 | 12.26 | 218.6 | 10.10 | 179.1 f–k | 43.47 |
| 119 | 244.0 | 48.65 | 305.7 | 10.88 | 274.8 e–j | 46.38 | 202.2 | 32.22 | 272.6 | 14.41 | 237.4 a–c | 44.14 | |
| 12 | 160.1 | 19.47 | 352.3 | 114.43 | 256.2 g–l | 127.8 | 142.5 | 22.68 | 206.6 | 3.88 | 174.5 g–j | 37.45 | |
| 18 | 221.2 | 38.25 | 206.7 | 70.09 | 214.0 kl | 52.84 | 122.0 | 16.74 | 264.8 | 37.10 | 193.4 d–j | 80.89 | |
| 1b | 250.3 | 24.60 | 415.6 | 26.73 | 332.9 b–e | 91.51 | 130.7 | 18.47 | 314.2 | 64.12 | 222.5 b–e | 107.39 | |
| 23 | 419.7 | 29.45 | 548.0 | 45.13 | 483.8 a | 77.09 | 243.4 | 20.49 | 219.9 | 34.43 | 231.6 b–d | 29.09 | |
| 26 | 279.1 | 75.43 | 383.6 | 62.29 | 331.3 b–e | 84.98 | 150.4 | 32.41 | 183 | 38.38 | 166.7 i–m | 37.22 | |
| 35 | 330.2 | 36.01 | 402.3 | 15.70 | 366.3 b | 46.36 | 186.7 | 25.69 | 195.6 | 22.16 | 191.1 e–j | 22.72 | |
| 42 | 225.1 | 41.08 | 241.1 | 31.87 | 233.1 j–l | 35.09 | 101.9 | 22.33 | 79.9 | 4.82 | 90.9 p | 19.02 | |
| 43 | 190.8 | 26.63 | 303.5 | 20.56 | 247.1 h–l | 64.17 | 177.4 | 40.47 | 207.7 | 35.99 | 192.6 d–j | 38.97 | |
| 55 | 310.6 | 30.05 | 379.5 | 39.92 | 345.1 b–d | 49.27 | 147.3 | 4.55 | 204.7 | 21.07 | 176.0 f–k | 33.73 | |
| 78b | 195.8 | 33.04 | 324.3 | 26.41 | 260 g–l | 74.04 | 193.8 | 54.08 | 120.8 | 30.89 | 157.3 j–m | 56.46 | |
| 83 | 167.4 | 44.50 | 437.7 | 48.72 | 302.5 c–h | 150.79 | 199.1 | 33.07 | 279.7 | 38.16 | 239.4 ab | 54.31 | |
| 9 | 186.5 | 42.29 | 356.6 | 57.23 | 271.5 f–k | 102.13 | 142.2 | 35.16 | 204.7 | 13.83 | 173.4 g–k | 41.58 | |
| F3 | 114 | 321.7 | 27.73 | 253.5 | 21.01 | 287.6 d–j | 50.27 | 123.4 | 17.29 | 128.7 | 15.45 | 126.1 n–p | 39.55 |
| 119 | 309.3 | 19.76 | 291.3 | 34.64 | 300.3 c–h | 27.82 | 190.5 | 26.76 | 208.1 | 46.86 | 199.3 c–i | 36.56 | |
| 12 | 269.3 | 66.51 | 383.4 | 29.55 | 326.4 b–f | 77.43 | 188.5 | 42.90 | 153.8 | 33.26 | 171.2 h–l | 40.08 | |
| 18 | 192.9 | 37.5 | 214.0 | 66.17 | 203.5 l | 47.67 | 132.9 | 12.89 | 128.0 | 25.67 | 130.5 m–p | 54.04 | |
| 1b | 210.6 | 29.86 | 360.2 | 38.35 | 285.4 e–j | 86.03 | 151.8 | 30.76 | 261.4 | 61.75 | 206.6 b–h | 73.99 | |
| 23 | 389.3 | 26.23 | 500.1 | 30.32 | 444.7 a | 64.76 | 197.1 | 51.67 | 247.7 | 15.50 | 222.4 b–e | 44.48 | |
| 26 | 201.2 | 25.52 | 349.7 | 45.73 | 275.5 e–j | 86.46 | 120.7 | 6.20 | 141.9 | 23.68 | 131.3 lm–o | 19.64 | |
| 35 | 303.1 | 29.47 | 284.9 | 12.58 | 294.0 d–i | 40.94 | 190.2 | 19.04 | 238.4 | 17.80 | 214.3 b–f | 19.08 | |
| 42 | 178.9 | 47.00 | 294.0 | 1.54 | 236.5 i–l | 68.81 | 113.1 | 16.64 | 75.9 | 2.94 | 94.5 op | 22.75 | |
| 43 | 147.8 | 12.36 | 279.5 | 33.99 | 213.6 kl | 74.25 | 152.7 | 32.65 | 103.3 | 0.00 | 128.0 m–p | 33.97 | |
| 55 | 292.6 | 31.49 | 421.2 | 138.04 | 356.9 bc | 115.38 | 175.9 | 8.81 | 243.0 | 7.91 | 209.4 b–h | 36.70 | |
| 78b | 327.1 | 108.05 | 289.1 | 28.59 | 308.1 b–g | 75.94 | 154.7 | 18.25 | 110.7 | 16.95 | 132.7 l–o | 28.62 | |
| 83 | 301.9 | 113.68 | 300.4 | 75.66 | 301.1 c–h | 89.40 | 193.3 | 43.89 | 354.7 | 0.00 | 274.0 a | 90.95 | |
| 9 | 231.9 | 1.51 | 358.0 | 28.28 | 294.9 d–h | 69.88 | 109.1 | 10.60 | 172.3 | 70.26 | 140.7 k–n | 57.50 | |
| LSD0.05 | 58.341 | 39.71 | |||||||||||
| Trait | First Day of Drought (20% of Soil Field Capacity) | After Two Weeks of Drought (Maintaining 20% of Soil Field Capacity) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | S | Average | C | S | Average | |||||||
| Genotype | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| Bingo | 19.4 | 3.57 | 23.2 | 1.86 | 21.3 o | 3.38 | 36.6 | 2.22 | 41.8 | 2.87 | 39.2 d–g | 3.64 | |
| F2 | 114 | 32.8 | 2.41 | 37.9 | 7.6 | 35.4 cd | 5.9 | 41.4 | 6.32 | 32.3 | 1.91 | 36.8 e–j | 6.49 |
| 119 | 27.0 | 3.46 | 32.6 | 3.35 | 29.8 f–j | 4.38 | 35.9 | 2.42 | 30.1 | 0.64 | 33.0 i–k | 3.50 | |
| 12 | 27.7 | 3.06 | 34.3 | 7.75 | 30.9 e–h | 6.51 | 27.7 | 0.86 | 38.1 | 5.55 | 32.9 i–k | 6.69 | |
| 18 | 30.1 | 2.42 | 24.7 | 4.50 | 27.4 g–m | 4.42 | 27.5 | 3.82 | 25.3 | 3.17 | 26.4 l | 3.45 | |
| 1b | 33.4 | 0.41 | 20.9 | 1.88 | 27.1 g–m | 6.82 | 38.3 | 2.27 | 38.3 | 4.65 | 38.3 d–h | 3.39 | |
| 23 | 24.79 | 1.78 | 25.7 | 2.02 | 25.2 k–o | 1.82 | 31.2 | 2.35 | 33.5 | 5.41 | 32.4 jk | 4.04 | |
| 26 | 22.9 | 0.77 | 25.5 | 3.64 | 24.2 m–o | 2.83 | 31.2 | 3.63 | 34.1 | 1.87 | 32.6 jk | 3.10 | |
| 35 | 26.7 | 3.36 | 24.6 | 1.85 | 25.7 j–n | 2.76 | 31.6 | 2.15 | 31.4 | 4.10 | 31.5 k | 3.56 | |
| 42 | 33.4 | 3.57 | 31.0 | 0.55 | 32.2 d–f | 2.68 | 46.3 | 0.87 | 42.6 | 1.84 | 44.5 a–c | 2.40 | |
| 43 | 38.3 | 0.83 | 34.6 | 9.86 | 36.4 c | 6.76 | 40.6 | 1.87 | 39.6 | 8.26 | 40.1 c–e | 5.57 | |
| 55 | 23.3 | 1.68 | 24.8 | 2.32 | 24.0 m–o | 2.05 | 36.0 | 0.28 | 35.3 | 5.00 | 35.6 f–k | 3.31 | |
| 78b | 31.8 | 4.03 | 28.1 | 3.14 | 29.9 f–i | 3.89 | 33.4 | 2.43 | 32.2 | 8.70 | 32.8 jk | 5.94 | |
| 83 | 28.6 | 3.09 | 25.3 | 1.19 | 26.9 h–n | 2.78 | 32.5 | 2.46 | 40.9 | 2.63 | 36.7 e–j | 5.05 | |
| 9 | 28.7 | 0.90 | 29.4 | 4.22 | 29.0 f–l | 2.85 | 34.8 | 1.97 | 48.4 | 2.96 | 41.6 b–d | 7.63 | |
| F3 | 114 | 43.7 | 1.07 | 50.2 | 6.66 | 47.0 a | 5.07 | 39.9 | 4.01 | 54.2 | 2.37 | 47.1 a | 6.17 |
| 119 | 22.1 | 2.45 | 30.9 | 2.23 | 26.5 i–n | 5.20 | 35.3 | 3.00 | 36.4 | 0.49 | 35.9 e–k | 2.04 | |
| 12 | 27.8 | 4.93 | 29.4 | 2.53 | 28.6 f–l | 3.72 | 34.7 | 1.46 | 39.9 | 3.81 | 37.3 d–i | 3.85 | |
| 18 | 36.3 | 3.08 | 45.6 | 5.07 | 41.0 b | 5.57 | 43.8 | 2.99 | 51.27 | 4.07 | 47.5 a | 4.02 | |
| 1b | 32.0 | 4.87 | 26.5 | 5.51 | 29.3 f–k | 5.64 | 32.9 | 1.20 | 40.0 | 1.27 | 36.5 e–j | 4.00 | |
| 23 | 19.8 | 2.06 | 25.7 | 3.10 | 22.74 no | 3.98 | 32.6 | 2.51 | 32.9 | 0.90 | 32.8 jk | 1.80 | |
| 26 | 27.2 | 1.26 | 24.0 | 3.43 | 25.6 k–n | 2.94 | 28.6 | 2.81 | 35.5 | 2.74 | 32.0 k | 4.46 | |
| 35 | 42.3 | 3.17 | 45.9 | 3.09 | 44.1 ab | 3.04 | 44.9 | 2.78 | 47.8 | 4.57 | 46.4 a | 4.91 | |
| 42 | 34.2 | 2.53 | 28.3 | 1.41 | 31.2 e–g | 3.68 | 42.7 | 4.23 | 36.7 | 3.24 | 39.7 d–f | 4.74 | |
| 43 | 37.1 | 1.39 | 31.6 | 1.71 | 34.3 c–e | 3.29 | 38.2 | 5.29 | 31.9 | 0.00 | 35.0 g–k | 4.84 | |
| 55 | 26.9 | 0.45 | 21.9 | 2.42 | 24.4 m–o | 3.14 | 30.1 | 1.92 | 38.9 | 3.63 | 34.7 h–k | 5.24 | |
| 78b | 24.0 | 2.91 | 30.1 | 1.90 | 27.3 g–m | 3.74 | 31.9 | 2.96 | 44.8 | 3.598 | 38.4 d–h | 7.54 | |
| 83 | 29.3 | 0.23 | 25.13 | 1.89 | 27.2 g–m | 2.53 | 33.7 | 4.32 | 29.7 | 7.93 | 31.7 k | 6.29 | |
| 9 | 20.5 | 3.05 | 29.5 | 4.48 | 25.0 l–o | 5.99 | 32.8 | 0.83 | 57.9 | 8.00 | 45.4 ab | 14.4 | |
| LSD0.05 | 4.18 | 4.45 | |||||||||||
| Trait | The Mass of Stems/Plant (g) | The Number of Grains | The Mass of Grains/Plant (g) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | D | Average | C | D | Average | C | D | Average | ||||||||||
| Genotype | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| Bingo | 17.2 | 3.16 | 4.3 | 3.09 | 10.7 c–f | 7.30 | 124.3 | 17.34 | 47.8 | 31.13 | 86.0 bc | 46.40 | 6.2 | 0.88 | 1.8 | 1.57 | 4.0 b–d | 2.59 | |
| F2 | 114 | 9.0 | 4.20 | 6.2 | 1.50 | 7.6 f | 3.28 | 1.0 | 1.15 | 2.7 | 1.70 | 1.8 i | 1.61 | 0.1 | 0.05 | 0.1 | 0.08 | 0.1 jk | 0.07 |
| 119 | 14.9 | 2.86 | 10.6 | 1.72 | 12.7 a–d | 3.17 | 8.5 | 3.00 | 7.8 | 2.87 | 8.1 i | 2.8 | 0.4 | 0.07 | 0.3 | 0.11 | 0.4 jk | 0.11 | |
| 12 | 16.6 | 3.52 | 9.13 | 1.21 | 12.9 a–d | 4.66 | 90.8 | 39.51 | 76.3 | 21.39 | 83.5 b–d | 30.42 | 3.6 | 1.45 | 3.0 | 0.80 | 3.3 c–f | 1.13 | |
| 18 | 13.6 | 2.22 | 5.72 | 1.50 | 9.7 d–f | 4.55 | 8.3 | 2.50 | 0.3 | 0.50 | 4.3 j | 4.59 | 0.3 | 0.09 | 0.01 | 0.01 | 0.2 jk | 0.17 | |
| 1b | 17.3 | 2.15 | 13.0 | 2.87 | 15.1 a | 3.29 | 76.8 | 12.69 | 35.5 | 21.11 | 56.1 d–f | 27.32 | 3.5 | 0.43 | 1.5 | 0.86 | 2.5 e–h | 1.23 | |
| 23 | 15.0 | 2.45 | 11.3 | 0.77 | 13.2 a–c | 2.56 | 58.8 | 17.21 | 37.0 | 4.69 | 47.9 e–g | 16.48 | 2.4 | 0.75 | 1.5 | 0.24 | 1.9 g–i | 0.73 | |
| 26 | 13.7 | 3.18 | 9.2 | 1.20 | 11.5 c–e | 3.28 | 44.5 | 17.18 | 43.3 | 10.63 | 43.9 e–h | 13.24 | 2.0 | 0.69 | 2.0 | 0.45 | 1.9 g–i | 0.54 | |
| 35 | 14.8 | 1.18 | 7.8 | 1.51 | 11.3 c–e | 3.94 | 69.3 | 23.91 | 58.3 | 10.40 | 63.7 c–e | 18.05 | 3.0 | 0.80 | 2.6 | 0.39 | 2.8 d–g | 0.62 | |
| 42 | 14.1 | 1.96 | 7.9 | 2.90 | 11.0 c–e | 4.01 | 16.0 | 14.21 | 25.8 | 15.13 | 20.9 h–j | 14.55 | 0.9 | 0.80 | 1.3 | 0.87 | 1.1 i–k | 0.83 | |
| 43 | 12.9 | 1.66 | 9.4 | 1.99 | 11.1 c–e | 2.55 | 29.8 | 14.45 | 17.8 | 21.6 | 23.8 g–j | 18.19 | 1.4 | 0.65 | 0.9 | 1.19 | 1.1 i–k | 0.92 | |
| 55 | 14.2 | 1.97 | 9.7 | 1.07 | 11.9 a–e | 2.82 | 26.3 | 7.41 | 51.5 | 17.62 | 38.9 e–i | 18.40 | 1.2 | 0.29 | 2.2 | 0.76 | 1.7 g–i | 0.75 | |
| 78b | 16.8 | 3.28 | 8.9 | 1.05 | 12.8 a–d | 4.79 | 132.0 | 73.14 | 82.0 | 9.27 | 107.0 ab | 55.17 | 5.1 | 2.40 | 3.3 | 0.39 | 4.2 bc | 1.84 | |
| 83 | 12.7 | 1.02 | 6.9 | 1.25 | 9.8 d–e | 3.28 | 139.5 | 36.57 | 104.0 | 22.38 | 121.8 a | 33.88 | 5.4 | 1.07 | 4.0 | 0.76 | 4.7 ab | 1.15 | |
| 9 | 12.9 | 2.53 | 9.2 | 5.21 | 11.0 c–e | 4.27 | 141.8 | 21.61 | 104.0 | 70.15 | 122.9 a | 52.12 | 7.0 | 2.73 | 4.0 | 2.71 | 5.5 a | 3.00 | |
| F3 | 114 | 11.3 | 5.01 | 9.8 | 2.45 | 10.6 c–f | 5.08 | 2.5 | 1.07 | 0.5 | 0.09 | 1.5 j | 0.55 | 0.1 | 0.01 | 0.03 | 0.02 | 0.07 k | 0.04 |
| 119 | 18.9 | 6.16 | 11.1 | 1.25 | 15.0 a | 5.84 | 9.3 | 3.20 | 9.8 | 1.50 | 9.5 ij | 2.33 | 0.4 | 0.09 | 0.4 | 0.04 | 0.4 jk | 0.07 | |
| 12 | 13.1 | 2.38 | 9.1 | 0.34 | 11.1 c–e | 2.68 | 103.3 | 25.2 | 74.8 | 15.22 | 89.0 bc | 24.58 | 4.0 | 1.09 | 3.0 | 0.53 | 3.5 b–e | 0.97 | |
| 18 | 7.8 | 3.07 | 7.6 | 2.99 | 7.7 f | 4.92 | 9.3 | 2.94 | 3.3 | 0.74 | 6.3 j | 2.73 | 0.07 | 0.02 | 0.02 | 0.01 | 0.05 k | 0.05 | |
| 1b | 17.3 | 4.16 | 12.4 | 0.82 | 14.8 ab | 3.80 | 62.8 | 31.6 | 47.5 | 13.4 | 55.1 d–f | 23.9 | 2.7 | 1.31 | 2.0 | 0.63 | 2.4 e–h | 1.02 | |
| 23 | 18.0 | 1.88 | 9.6 | 1.60 | 13.8 a–c | 4.80 | 76.3 | 18.1 | 27.5 | 8.35 | 51.9 e–g | 29.1 | 3.0 | 0.64 | 1.1 | 0.32 | 2.1 f–i | 1.16 | |
| 26 | 13.4 | 1.55 | 9.4 | 0.91 | 11.4 c–e | 2.45 | 49.3 | 14.5 | 39.88 | 5.56 | 44.5 e–h | 11.4 | 2.4 | 0.75 | 1.8 | 0.29 | 2.1 f–i | 0.62 | |
| 35 | 11.3 | 4.07 | 7.2 | 2.91 | 9.3 ef | 4.58 | 97.0 | 20.7 | 78.7 | 27.27 | 87.9 bc | 22.61 | 2.9 | 0.17 | 2.2 | 0.27 | 2.6 e–g | 0.97 | |
| 42 | 11.8 | 1.97 | 8.0 | 6.70 | 9.9 d–f | 5.00 | 18.5 | 13.58 | 27.8 | 32.95 | 23.1 g–j | 23.85 | 0.7 | 0.52 | 1.3 | 1.69 | 1.0 i–k | 1.20 | |
| 43 | 15.1 | 2.14 | 8.4 | 0.238 | 11.7 b–e | 3.82 | 43.0 | 42.58 | 48.0 | 16.17 | 45.5 e–h | 29.93 | 1.9 | 1.83 | 2.3 | 0.48 | 2.1 f–i | 1.26 | |
| 55 | 13.9 | 2.33 | 7.5 | 0.970 | 10.7 c–f | 3.79 | 54.3 | 24.30 | 57.0 | 11.34 | 55.6 d–f | 17.61 | 2.5 | 1.01 | 2.3 | 0.65 | 2.4 e–h | 0.80 | |
| 78b | 19.8 | 2.44 | 7.4 | 0.69 | 13.6 a–c | 6.85 | 167.5 | 56.97 | 84.8 | 18.39 | 126.1 a | 59.10 | 6.2 | 1.17 | 3.4 | 0.62 | 4.7 ab | 1.76 | |
| 83 | 6.3 | 6.31 | 2.1 | 2.93 | 4.2 g | 5.06 | 48.0 | 55.43 | 11.5 | 22.34 | 29.8 f–j | 43.72 | 2.2 | 2.48 | 0.4 | 0.81 | 1.3 h–j | 1.94 | |
| 9 | 13.6 | 1.70 | 8.4 | 1.91 | 11.0 c–e | 3.27 | 119.0 | 26.14 | 91.3 | 23.41 | 105.1 ab | 27.35 | 4.7 | 0.88 | 3.4 | 1.10 | 4.1 bc | 1.15 | |
| LSD0.05 | 3.2 | 29.80 | 1.2 | ||||||||||||||||
| Independent Variable | Dependent Variable | Model | Percentage Variance Accounted | Standard Error of Observations |
|---|---|---|---|---|
| ELWL after 0–3 h | The mass of stems plant−1 | y = 21.53 *** − 0.314 * x | 3.0 | 4.87 |
| The number of grains | y = 20.184 *** − 0.028 * x | 3.2 | 4.86 | |
| The mass of grains plant−1 | y = 20.109 *** − 0.637 * x | 2.6 | 4.88 | |
| ELWL after 4–6 h | The mass of stems plant−1 | y = 15.14 *** − 0.285 x | 2.0 | 5.23 |
| The number of grains | y = 13.051 *** − 0.0057 x | - | 5.3 | |
| The mass of grains plant−1 | y = 12.935 *** − 0.074 x | - | 5.3 | |
| ELWL after 0–6 h | The mass of stems plant−1 | y = 33.23 *** − 0.500 * x | 3.0 | 7.77 |
| The number of grains | y = 30.39 *** − 0.0286 x | 0.8 | 7.86 | |
| The mass of grains plant−1 | y = 30.23 *** − 0.611 x | 0.4 | 7.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warzecha, T.; Bocianowski, J.; Warchoł, M.; Bathelt, R.; Sutkowska, A.; Skrzypek, E. Effect of Soil Drought Stress on Selected Biochemical Parameters and Yield of Oat × Maize Addition (OMA) Lines. Int. J. Mol. Sci. 2023, 24, 13905. https://doi.org/10.3390/ijms241813905
Warzecha T, Bocianowski J, Warchoł M, Bathelt R, Sutkowska A, Skrzypek E. Effect of Soil Drought Stress on Selected Biochemical Parameters and Yield of Oat × Maize Addition (OMA) Lines. International Journal of Molecular Sciences. 2023; 24(18):13905. https://doi.org/10.3390/ijms241813905
Chicago/Turabian StyleWarzecha, Tomasz, Jan Bocianowski, Marzena Warchoł, Roman Bathelt, Agnieszka Sutkowska, and Edyta Skrzypek. 2023. "Effect of Soil Drought Stress on Selected Biochemical Parameters and Yield of Oat × Maize Addition (OMA) Lines" International Journal of Molecular Sciences 24, no. 18: 13905. https://doi.org/10.3390/ijms241813905
APA StyleWarzecha, T., Bocianowski, J., Warchoł, M., Bathelt, R., Sutkowska, A., & Skrzypek, E. (2023). Effect of Soil Drought Stress on Selected Biochemical Parameters and Yield of Oat × Maize Addition (OMA) Lines. International Journal of Molecular Sciences, 24(18), 13905. https://doi.org/10.3390/ijms241813905









