Potential Inflammatory Biomarkers for Major Depressive Disorder Related to Suicidal Behaviors: A Systematic Review
Abstract
:1. Introduction
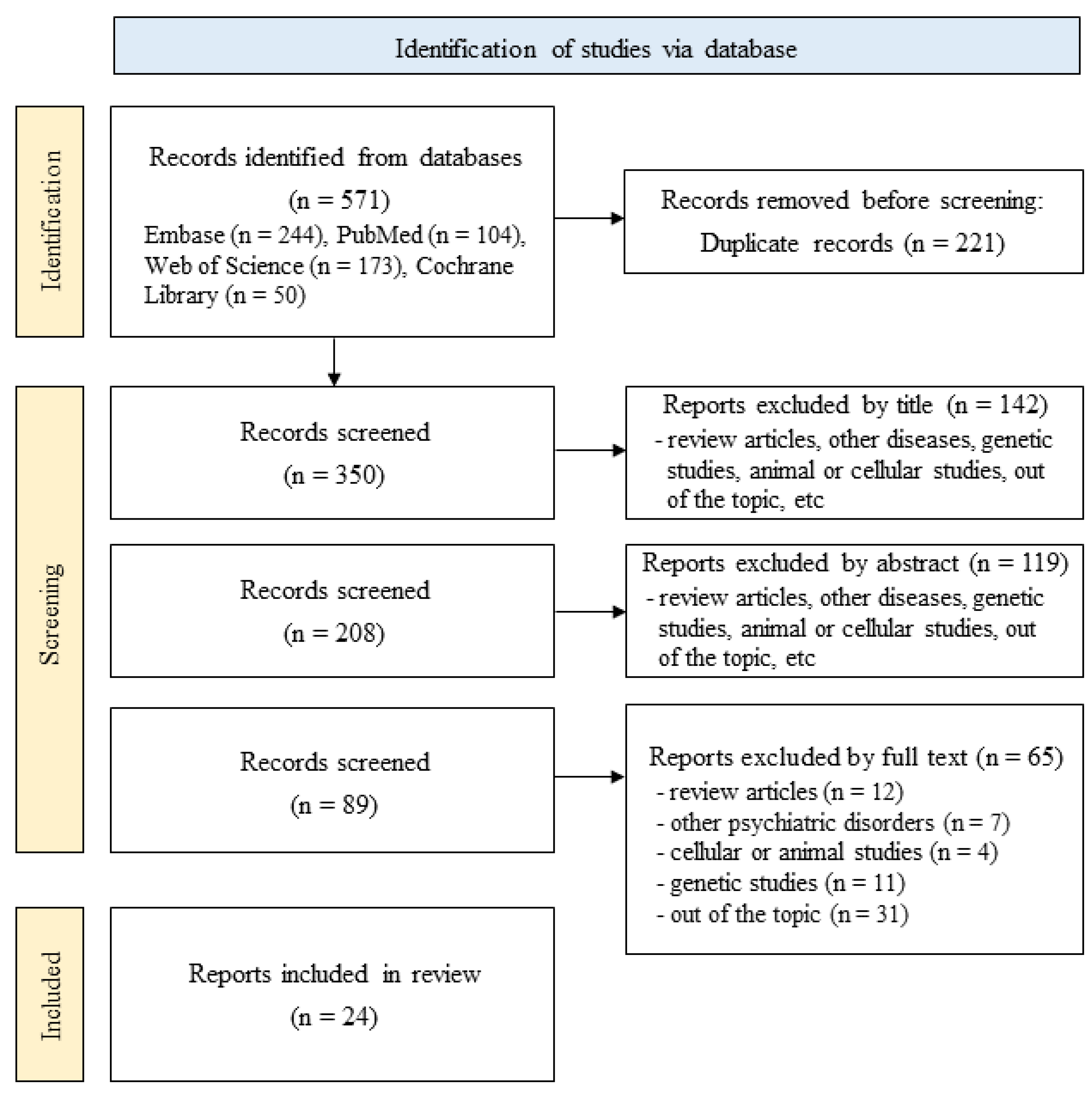
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Data Extraction and Analysis
2.4. Statistical Analysis and Quality Assessment
3. Results
3.1. Characteristics of the Included Studies
3.2. Potential Inflammatory Biomarkers in Patients with MDD with Suicide-Related Behaviors
3.3. Analyzed Potential Inflammatory Biomarkers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Suicide. Available online: https://www.who.int/news-room/fact-sheets/detail/suicide (accessed on 18 November 2022).
- Wang, Q. Editorial: A global perspective on suicidal behaviour and ideation: Demographics, biomarkers and treatment. Front. Psychiatry 2023, 14, 1218831. [Google Scholar] [CrossRef] [PubMed]
- Kouter, K.; Salamon Arcan, I.; Videtic Paska, A. Epigenetics in psychiatry: Beyond DNA methylation. World J. Psychiatry 2023, 13, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Q.; Tay, G.W.N.; Ho, C.S.H. Clinical Utility of Functional Near-Infrared Spectroscopy for Assessment and Prediction of Suicidality: A Systematic Review. Front. Psychiatry 2021, 12, 716276. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, P.; Ji, S.; Ghosh, S.; Ghosh, S.; Raghunath, M.; Kim, H.; Bhaskar, R.; Sinha, J.K.; Han, S.S. Plausible Role of Stem Cell Types for Treating and Understanding the Pathophysiology of Depression. Pharmaceutics 2023, 15, 814. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Galvao, L.P.; Paiva, H.S.; Perico, C.D.; Ventriglio, A.; Torales, J.; Castaldelli-Maia, J.M.; Martins-da-Silva, A.S. Major depressive disorder as a risk factor for suicidal ideation for attendees of educational institutions: A meta-analysis and meta-regression. Rev. Paul. Pediatr. 2023, 41, e2021344. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Li, C.T.; Juan, C.H. A review of critical brain oscillations in depression and the efficacy of transcranial magnetic stimulation treatment. Front. Psychiatry 2023, 14, 1073984. [Google Scholar] [CrossRef]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Tura, A.; Goya-Maldonado, R. Brain connectivity in major depressive disorder: A precision component of treatment modalities? Transl. Psychiatry 2023, 13, 196. [Google Scholar] [CrossRef]
- Karabatsiakis, A.; De Punder, K.; Salinas-Manrique, J.; Todt, M.; Dietrich, D.E. Hair cortisol level might be indicative for a 3PM approach towards suicide risk assessment in depression: Comparative analysis of mentally stable and depressed individuals versus individuals after completing suicide. Epma J. 2022, 13, 383–395. [Google Scholar] [CrossRef]
- Angst, J.; Angst, F.; Stassen, H.H. Suicide risk in patients with major depressive disorder. J. Clin. Psychiatry 1999, 60 Suppl. 2, 57–62; discussion 75–56, 113–116. [Google Scholar]
- Li, Y.; Gui, Y.; Zhao, M.; Chen, X.; Li, H.; Tian, C.; Zhao, H.; Jiang, C.; Xu, P.; Zhang, S.; et al. The roles of extracellular vesicles in major depressive disorder. Front. Psychiatry 2023, 14, 1138110. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Mun, S.; Joo, E.J.; Lee, K.Y.; Kang, H.G.; Lee, J. Discovery of Screening Biomarkers for Major Depressive Disorder in Remission by Proteomic Approach. Diagnostics 2021, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.X.; Xia, J.J.; Deng, F.L.; Liang, W.W.; Wu, J.; Yin, B.M.; Dong, M.X.; Chen, J.J.; Ye, F.; Wang, H.Y.; et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: A targeted metabolomics study. Transl. Psychiatry 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, R.; Arnone, D.; Danese, A.; Papadopoulos, A.; Herane Vives, A.; Cleare, A.J. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 2015, 25, 1532–1543. [Google Scholar] [CrossRef]
- Dadkhah, M.; Jafarzadehgharehziaaddin, M.; Molaei, S.; Akbari, M.; Gholizadeh, N.; Fathi, F. Major depressive disorder: Biomarkers and biosensors. Clin. Chim. Acta 2023, 547, 117437. [Google Scholar] [CrossRef]
- Orsolini, L.; Pompili, S.; Tempia Valenta, S.; Salvi, V.; Volpe, U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int. J. Mol. Sci. 2022, 23, 1616. [Google Scholar] [CrossRef]
- Neupane, S.P.; Daray, F.M.; Ballard, E.D.; Galfalvy, H.; Itzhaky, L.; Segev, A.; Shelef, A.; Tene, O.; Rizk, M.M.; Mann, J.J.; et al. Immune-related biomarkers and suicidal behaviors: A meta-analysis. Eur. Neuropsychopharmacol. 2023, 75, 15–30. [Google Scholar] [CrossRef]
- Ko, G.R.; Lee, J.S. Engineering of Immune Microenvironment for Enhanced Tissue Remodeling. Tissue Eng. Regen. Med. 2022, 19, 221–236. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Panam. Salud Publ. 2022, 46, e112. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP (Case-Control-Study or Cohort-Study) Checklist. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 20 June 2023).
- Amitai, M.; Taler, M.; Ben-Baruch, R.; Lebow, M.; Rotkopf, R.; Apter, A.; Fennig, S.; Weizman, A.; Chen, A. Increased circulatory IL-6 during 8-week fluoxetine treatment is a risk factor for suicidal behaviors in youth. Brain Behav. Immun. 2020, 87, 301–308. [Google Scholar] [CrossRef]
- Bai, S.; Fang, L.; Xie, J.; Bai, H.; Wang, W.; Chen, J.J. Potential Biomarkers for Diagnosing Major Depressive Disorder Patients with Suicidal Ideation. J. Inflamm. Res. 2021, 14, 495–503. [Google Scholar] [CrossRef]
- Bergmans, R.S.; Kelly, K.M.; Mezuk, B. Inflammation as a unique marker of suicide ideation distinct from depression syndrome among U.S. adults. J. Affect. Disord. 2019, 245, 1052–1060. [Google Scholar] [CrossRef]
- Brundin, L.; Sellgren, C.M.; Lim, C.K.; Grit, J.; Palsson, E.; Landen, M.; Samuelsson, M.; Lundgren, K.; Brundin, P.; Fuchs, D.; et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl. Psychiatry 2016, 6, e865. [Google Scholar] [CrossRef]
- Choi, K.W.; Jang, E.H.; Kim, A.Y.; Kim, H.; Park, M.J.; Byun, S.; Fava, M.; Mischoulon, D.; Papakostas, G.I.; Yu, H.Y.; et al. Predictive inflammatory biomarkers for change in suicidal ideation in major depressive disorder and panic disorder: A 12-week follow-up study. J. Psychiatr. Res. 2021, 133, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Coryell, W.; Wilcox, H.; Evans, S.J.; Pandey, G.N.; Jones-Brando, L.; Dickerson, F.; Yolken, R. Aggression, impulsivity and inflammatory markers as risk factors for suicidal behavior. J. Psychiatr. Res. 2018, 106, 38–42. [Google Scholar] [CrossRef]
- Falcone, T.; Fazio, V.; Lee, C.; Simon, B.; Franco, K.; Marchi, N.; Janigro, D. Serum S100B: A Potential Biomarker for Suicidality in Adolescents? PLoS ONE 2010, 5, e11089. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sevillano, J.; González-Ortega, I.; MacDowell, K.; Zorrilla, I.; López, M.P.; Courtet, P.; Gabilondo, A.; Martínez-Cengotitabengoa, M.; Leza, J.C.; Sáiz, P.; et al. Inflammation biomarkers in suicide attempts and their relation to abuse, global functioning and cognition. World J. Biol. Psychiatry 2022, 23, 307–317. [Google Scholar] [CrossRef]
- Ganança, L.; Galfalvy, H.C.; Cisneros-Trujillo, S.; Basseda, Z.; Cooper, T.B.; Ren, X.; Figueira, M.L.; Oquendo, M.A.; Mann, J.J.; Sublette, M.E. Relationships between inflammatory markers and suicide risk status in major depression. J. Psychiatr. Res. 2021, 134, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Grassi-Oliveira, R.; Brieztke, E.; Teixeira, A.; Pezzi, J.C.; Zanini, M.; Lopes, R.P.; Bauer, M.E. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Braz. J. Psychiatry 2012, 34, 71–75. [Google Scholar] [CrossRef]
- Grudet, C.; Malm, J.; Westrin, A.; Brundin, L. Suicidal patients are deficient in vitamin D, associated with a pro-inflammatory status in the blood. Psychoneuroendocrinology 2014, 50, 210–219. [Google Scholar] [CrossRef]
- Lin, L.Y.; Fu, X.Y.; Zhou, X.F.; Liu, D.; Bobrovskaya, L.; Zhou, L. Analysis of blood mature BDNF and proBDNF in mood disorders with specific ELISA assays. J. Psychiatr. Res. 2021, 133, 166–173. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Li, H.; Mou, T.; Zhou, L.; Huang, B.; Huang, M.; Xu, Y. Changes in plasma NPY, IL-1β and hypocretin in people who died by suicide. Neuropsychiatr. Dis. Treat. 2019, 15, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Nowak, W.; Grendas, L.N.; Sanmarco, L.M.; Estecho, I.G.; Arena, R.; Eberhardt, N.; Rodante, D.E.; Aoki, M.P.; Daray, F.M.; Carrera Silva, E.A.; et al. Pro-inflammatory monocyte profile in patients with major depressive disorder and suicide behaviour and how ketamine induces anti-inflammatory M2 macrophages by NMDAR and mTOR. EBioMedicine 2019, 50, 290–305. [Google Scholar] [CrossRef]
- Ohlsson, L.; Gustafsson, A.; Lavant, E.; Suneson, K.; Brundin, L.; Westrin, Å.; Ljunggren, L.; Lindqvist, D. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 2019, 139, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.M.H.; Eidan, A.J.; Al-Dujaili, A.H.; Abada, L.H.; Al-Charrakh, A.H. Different cytokines and lipid profile in suicidal and non suicidal adults with major depression. Ann. Trop. Med. Public Health 2019, 22, 255–260. [Google Scholar] [CrossRef]
- Rui, P.; Wen, D.; Yan, L. High serum levels of tenascin-C are associated with suicide attempts in depressed patients. Psychiatry Res. 2018, 268, 60–64. [Google Scholar] [CrossRef]
- Su, Y.A.; Lin, J.Y.; Liu, Q.; Lv, X.Z.; Wang, G.; Wei, J.; Zhu, G.; Chen, Q.L.; Tian, H.J.; Zhang, K.R.; et al. Associations among serum markers of inflammation, life stress and suicide risk in patients with major depressive disorder. J. Psychiatr. Res. 2020, 129, 53–60. [Google Scholar] [CrossRef]
- Uchitomi, Y.; Kugaya, A.; Akechi, T.; Nakano, T.; Inagaki, M.; Matsuoka, Y.; Kagaya, A.; Yamawaki, S. Lack of association between suicidal ideation and enhanced platelet 5-HT2A receptor-mediated calcium mobilization in cancer patients with depression. Biol. Psychiatry 2002, 52, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.O.; Nunes, S.O.; de Castro, M.R.; Vargas, M.M.; Barbosa, D.S.; Bortolasci, C.C.; Venugopal, K.; Dodd, S.; Berk, M. Oxidative stress and inflammatory markers are associated with depression and nicotine dependence. Neurosci. Lett. 2013, 544, 136–140. [Google Scholar] [CrossRef]
- Ventorp, F.; Gustafsson, A.; Traskman-Bendz, L.; Westrin, A.; Ljunggren, L. Increased Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) Levels in Plasma of Suicide Attempters. PLoS ONE 2015, 10, e0140052. [Google Scholar] [CrossRef]
- Wiener, C.D.; Moreira, F.P.; Portela, L.V.; Strogulski, N.R.; Lara, D.R.; da Silva, R.A.; Souza, L.D.D.M.; Jansen, K.; Oses, J.P. Interleukin-6 and Interleukin-10 in mood disorders: A population-based study. Psychiatry Res. 2019, 273, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Liang, J.; Gao, W.F.; Sun, Y.H.; Zhang, Y.Y.; Shan, F.; Ge, J.F.; Xia, Q.R. Peripheral blood cytokines as potential diagnostic biomarkers of suicidal ideation in patients with first-episode drug-naive major depressive disorder. Front. Public Health 2022, 10, 1021309. [Google Scholar] [CrossRef]
- Yang, Y.T.; Chen, J.; Liu, C.Y.; Fang, L.; Liu, Z.; Guo, J.; Cheng, K.; Zhou, C.J.; Zhan, Y.; Melgiri, N.D.; et al. The Extrinsic Coagulation Pathway: A Biomarker for Suicidal Behavior in Major Depressive Disorder. Sci. Rep. 2016, 6, 32882. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Lorkiewicz, P.; Waszkiewicz, N. Biomarkers of Post-COVID Depression. J. Clin. Med. 2021, 10, 4142. [Google Scholar] [CrossRef]
- Guan, X.; Fu, Y.; Liu, Y.; Cui, M.; Zhang, C.; Zhang, Q.; Li, C.; Zhao, J.; Wang, C.; Song, J.; et al. The role of inflammatory biomarkers in the development and progression of pre-eclampsia: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1156039. [Google Scholar] [CrossRef]
- Zhou, F.; Sun, Y.; Xie, X.; Zhao, Y. Blood and CSF chemokines in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Alzheimers Res. Ther. 2023, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Tirandi, A.; Sgura, C.; Carbone, F.; Montecucco, F.; Liberale, L. Inflammatory biomarkers of ischemic stroke. Intern. Emerg. Med. 2023, 18, 723–732. [Google Scholar] [CrossRef]
- Dinca, A.L.; Melit, L.E.; Marginean, C.O. Old and New Aspects of H. pylori-Associated Inflammation and Gastric Cancer. Children 2022, 9, 1083. [Google Scholar] [CrossRef]
- Negishi, Y.; Shima, Y.; Kato, M.; Ichikawa, T.; Ino, H.; Horii, Y.; Suzuki, S.; Morita, R. Inflammation in preterm birth: Novel mechanism of preterm birth associated with innate and acquired immunity. J. Reprod. Immunol. 2022, 154, 103748. [Google Scholar] [CrossRef]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2022, 24, 578. [Google Scholar] [CrossRef] [PubMed]
- Grunberg, D.; Martin, J.A.; Cryan, J.F.; O’Halloran, K.D.; Kelleher, E.; Dinan, T.G.; Clarke, G. The effect of exercise interventions on inflammatory markers in major depressive disorder: Protocol for a systematic review and meta-analysis. HRB Open Res. 2021, 4, 42. [Google Scholar] [CrossRef]
- Roohi, E.; Jaafari, N.; Hashemian, F. On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J. Neuroinflamm. 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, J.; Li, R.; McIntyre, R.S.; Teopiz, K.M.; Cao, B.; Yang, F. The Impact of Cognitive Behavioral Therapy on Peripheral Interleukin-6 Levels in Depression: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 844176. [Google Scholar] [CrossRef]
- Tamimou, R.; Lumbroso, S.; Mouzat, K.; Lopez-Castroman, J. Genetic variations related to inflammation in suicidal ideation and behavior: A systematic review. Front. Psychiatry 2022, 13, 1003034. [Google Scholar] [CrossRef]
- Kern, S.; Skoog, I.; Borjesson-Hanson, A.; Blennow, K.; Zetterberg, H.; Ostling, S.; Kern, J.; Gudmundsson, P.; Marlow, T.; Rosengren, L.; et al. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav. Immun. 2014, 41, 55–58. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef]
- Jha, M.K.; Trivedi, M.H. Personalized Antidepressant Selection and Pathway to Novel Treatments: Clinical Utility of Targeting Inflammation. Int. J. Mol. Sci. 2018, 19, 233. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Sabo, C.M.; Ismaiel, M.; Ismaiel, A.; Leucuta, D.C.; Popa, S.L.; Grad, S.; Dumitrascu, D.L. Do Colonic Mucosal Tumor Necrosis Factor Alpha Levels Play a Role in Diverticular Disease? A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 9934. [Google Scholar] [CrossRef] [PubMed]
- Sayana, P.; Colpo, G.D.; Simoes, L.R.; Giridharan, V.V.; Teixeira, A.L.; Quevedo, J.; Barichello, T. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J. Psychiatr. Res. 2017, 92, 160–182. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Neuroinflammation in Mood Disorders: Role of Regulatory Immune Cells. Neuroimmunomodulation 2021, 28, 99–107. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child. Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Gananca, L.; Oquendo, M.A.; Tyrka, A.R.; Cisneros-Trujillo, S.; Mann, J.J.; Sublette, M.E. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology 2016, 63, 296–310. [Google Scholar] [CrossRef]
- Dri, E.; Lampas, E.; Lazaros, G.; Lazarou, E.; Theofilis, P.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Endothelial Dysfunction. Life 2023, 13, 1420. [Google Scholar] [CrossRef]
- Hsuchou, H.; Kastin, A.J.; Mishra, P.K.; Pan, W. C-reactive protein increases BBB permeability: Implications for obesity and neuroinflammation. Cell Physiol. Biochem. 2012, 30, 1109–1119. [Google Scholar] [CrossRef]
- Jeon, M.T.; Kim, K.S.; Kim, E.S.; Lee, S.; Kim, J.; Hoe, H.S.; Kim, D.G. Emerging pathogenic role of peripheral blood factors following BBB disruption in neurodegenerative disease. Ageing Res. Rev. 2021, 68, 101333. [Google Scholar] [CrossRef]
- Serafini, G.; Parisi, V.M.; Aguglia, A.; Amerio, A.; Sampogna, G.; Fiorillo, A.; Pompili, M.; Amore, M. A Specific Inflammatory Profile Underlying Suicide Risk? Systematic Review of the Main Literature Findings. Int. J. Environ. Res. Public Health 2020, 17, 2393. [Google Scholar] [CrossRef]
- Laing, K.J.; Secombes, C.J. Chemokines. Dev. Comp. Immunol. 2004, 28, 443–460. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Mednova, I.A.; Boiko, A.S.; Buneva, V.N.; Ivanova, S.A. Chemokine Dysregulation and Neuroinflammation in Schizophrenia: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2215. [Google Scholar] [CrossRef]
- Yadav, A.; Saini, V.; Arora, S. MCP-1: Chemoattractant with a role beyond immunity: A review. Clin. Chim. Acta 2010, 411, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xu, W.Y.; Shen, A.; Fu, X.M.; Cen, H.Y.; Wang, S.R.; Lin, Z.X.; Zhang, L.M.; Lin, F.Y.; Zhang, X.; et al. Inhibition of YAP1 activity ameliorates acute lung injury through promotion of M2 macrophage polarization. Medcomm 2023, 4, e293. [Google Scholar] [CrossRef] [PubMed]
- Curzytek, K.; Leskiewicz, M. Targeting the CCL2-CCR2 axis in depressive disorders. Pharmacol. Rep. 2021, 73, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.F.; Xu, Y.Y.; Liang, J.; Sun, Y.H.; Zhang, Y.Y.; Shan, F.; Ge, J.F.; Xia, Q.R. Serum CC Chemokines as Potential Biomarkers for the Diagnosis of Major Depressive Disorder. Psychol. Res. Behav. Manag. 2022, 15, 2971–2978. [Google Scholar] [CrossRef]
- Kornhuber, J.; Gulbins, E. New Molecular Targets for Antidepressant Drugs. Pharmaceuticals 2021, 14, 894. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Turkheimer, F.E.; Veronese, M.; Mondelli, V.; Cash, D.; Pariante, C.M. Sickness behaviour and depression: An updated model of peripheral-central immunity interactions. Brain Behav. Immun. 2023, 111, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef]
- Ting, E.Y.; Yang, A.C.; Tsai, S.J. Role of Interleukin-6 in Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 2194. [Google Scholar] [CrossRef]
- Nikkheslat, N. Targeting inflammation in depression: Ketamine as an anti-inflammatory antidepressant in psychiatric emergency. Brain Behav. Immun. Health 2021, 18, 100383. [Google Scholar] [CrossRef]
- Chang, H.C.; Lin, K.H.; Tai, Y.T.; Chen, J.T.; Chen, R.M. Lipoteichoic acid-induced TNF-alpha and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating Toll-like receptor 2-mediated activation oF ERK1/2 and NFkappaB. Shock 2010, 33, 485–492. [Google Scholar] [CrossRef]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef]
- Gorlova, A.; Svirin, E.; Pavlov, D.; Cespuglio, R.; Proshin, A.; Schroeter, C.A.; Lesch, K.P.; Strekalova, T. Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies. Int. J. Mol. Sci. 2023, 24, 915. [Google Scholar] [CrossRef] [PubMed]
- Somoza-Moncada, M.M.; Turrubiates-Hernandez, F.J.; Munoz-Valle, J.F.; Gutierrez-Brito, J.A.; Diaz-Perez, S.A.; Aguayo-Arelis, A.; Hernandez-Bello, J. Vitamin D in Depression: A Potential Bioactive Agent to Reduce Suicide and Suicide Attempt Risk. Nutrients 2023, 15, 1765. [Google Scholar] [CrossRef]
- Bhatt, S.; Devadoss, T.; Jha, N.K.; Baidya, M.; Gupta, G.; Chellappan, D.K.; Singh, S.K.; Dua, K. Targeting inflammation: A potential approach for the treatment of depression. Metab. Brain Dis. 2023, 38, 45–59. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef]
- Marini, S.; Vellante, F.; Matarazzo, I.; De Berardis, D.; Serroni, N.; Gianfelice, D.; Olivieri, L.; Di Renzo, F.; Di Marco, A.; Fornaro, M.; et al. Inflammatory markers and suicidal attempts in depressed patients: A review. Int. J. Immunopathol. Pharmacol. 2016, 29, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Warriach, Z.I.; Patel, S.; Khan, F.; Ferrer, G.F. Association of Depression With Cardiovascular Diseases. Cureus 2022, 14, e26296. [Google Scholar] [CrossRef] [PubMed]
- Mosiolek, A.; Pieta, A.; Jakima, S.; Zborowska, N.; Mosiolek, J.; Szulc, A. Effects of Antidepressant Treatment on Peripheral Biomarkers in Patients with Major Depressive Disorder (MDD). J. Clin. Med. 2021, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Scharpe, S.; Meltzer, H.Y.; Bosmans, E.; Suy, E.; Calabrese, J.; Cosyns, P. Relationships between Interleukin-6 Activity, Acute-Phase Proteins, and Function of the Hypothalamic-Pituitary-Adrenal Axis in Severe Depression. Psychiatry Res. 1993, 49, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Langenecker, S.A.; Phan, K.L.; Keating, S.M.; Neigh, G.N.; Weber, K.M.; Maki, P.M. Remitted depression and cognition in HIV: The role of cortisol and inflammation. Psychoneuroendocrinology 2020, 114, 104609. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Pariante, C.M.; Miller, A.H. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA Axis as Target for Depression Outdated, or Is There a New Hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef]
| Questions | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score out of 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitai et al. [23] | Y | Y | Y | Y | ? | ? | significant (p < 0.05) | can’t tell | Y | ? | Y | 7 |
| Bai et al. [24] | Y | Y | Y | Y | Y | ? | significant (p < 0.01) | Precise (95% CI used) | Y | ? | Y | 9 |
| Bergmans et al. [25] | Y | Y | Y | Y | ? | ? | significant (p < 0.001) | Precise (95% CI used) | Y | ? | Y | 8 |
| Brundin et al. [26] | Y | Y | Y | Y | ? | ? | significant (p < 0.001) | can’t tell | Y | ? | Y | 7 |
| Choi et al. [27] | Y | Y | Y | Y | Y | ? | significant (p < 0.05) | can’t tell | Y | ? | Y | 8 |
| Coryell et al. [28] | Y | Y | Y | Y | Y | ? | significant (p < 0.05) | can’t tell | Y | ? | Y | 8 |
| Falcone et al. [29] | Y | Y | Y | Y | Y | Y | significant (p < 0.01) | can’t tell | Y | ? | Y | 9 |
| Fernández-Sevillano et al. [30] | Y | Y | Y | Y | ? | ? | significant (p < 0.05) | can’t tell | Y | ? | Y | 7 |
| Ganança et al. [31] | Y | Y | Y | Y | ? | ? | significant (p < 0.001) | can’t tell | Y | ? | Y | 7 |
| Grassi-Oliveira et al. [32] | Y | Y | Y | Y | ? | Y | significant (p < 0.001) | can’t tell | Y | ? | Y | 8 |
| Grudet et al. [33] | Y | Y | Y | Y | ? | ? | significant (p < 0.05) | can’t tell | Y | ? | Y | 7 |
| Lin et al. [34] | Y | Y | Y | Y | ? | Y | significant (p < 0.001) | can’t tell | Y | ? | Y | 8 |
| Lu et al. [35] | Y | Y | Y | Y | ? | Y | significant (p < 0.05) | can’t tell | Y | ? | Y | 8 |
| Nowak et al. [36] | Y | Y | Y | Y | ? | Y | significant (p < 0.05) | can’t tell | Y | ? | Y | 8 |
| Ohlsson et al. [37] | Y | Y | Y | Y | Y | ? | significant (p < 0.001) | can’t tell | Y | ? | Y | 8 |
| Rasheed et al. [38] | Y | Y | Y | Y | Y | ? | significant (p < 0.05) | can’t tell | Y | ? | Y | 8 |
| Rui et al. [39] | Y | Y | Y | Y | Y | ? | significant (p < 0.001) | can’t tell | Y | ? | Y | 8 |
| Su et al. [40] | Y | Y | Y | Y | Y | ? | significant (p < 0.05) | can’t tell | Y | ? | Y | 8 |
| Uchitomi et al. [41] | Y | Y | Y | Y | Y | ? | not significant | can’t tell | Y | ? | Y | 7 |
| Vargas et al. [42] | Y | Y | Y | Y | ? | ? | significant (p < 0.05) | Precise (95% CI used) | Y | ? | Y | 8 |
| Ventorp et al. [43] | Y | Y | Y | Y | ? | ? | significant (p < 0.001) | can’t tell | Y | ? | Y | 7 |
| Wiener et al. [44] | Y | Y | Y | Y | ? | ? | significant (p < 0.001) | can’t tell | Y | ? | Y | 7 |
| Xu et al. [45] | Y | Y | Y | Y | Y | ? | significant (p < 0.05) | Precise (95% CI used) | Y | ? | Y | 9 |
| Yang et al. [46] | Y | Y | Y | Y | ? | ? | significant (p < 0.05) | can’t tell | Y | ? | Y | 7 |
| Author, Year | Country | Group Characteristic (Group = n) | Gender (F/M or Male %) | Age (Mean ± SD or (Median)) | Diagnosis Tool | Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | MDD | MDD + SI | MDD + SA | HC | MDD | MDD + SI | MDD + SA | MDD | Suicide | ||||
| Amitai et al. [23] | Israel | No FLX-associated suicidality = 57 (MDD = 45), FLX-associated suicidality = 35 (MDD = 29) | 23 (34%) | 12 (40%) | 13.9 ± 2.4 | 13.9 ± 2.5 | BDI | Plasma | |||||
| Bai et al. [24] | China | C = 86, MDD = 20, MDD + SI = 53 | 52/34 | 12/8 | 33/20 | 37.4 (13.9) | 32.3 (14.4) | 37.3 (13.2) | HDRS | BSI-CV | Serum | ||
| Bergmans et al. [25] | USA | Not depressed = 12,516, Depressed = 867 Not depressed + SI = 199, Depressed + SI = 330 | 49.7% | 33% | 44.2% | 20 to 65< | 20 to 65< | 20 to 65< | PHQ-9 | Serum | |||
| Brundin et al. [26] | Sweden | C = 35, SA = 73(included MDD = 15) | 16/19 | 42/31 | 40 (19–66) | 43 (20–67) | DSM-III | Plasma | |||||
| Choi et al. [27] | USA | C = 59, MDD = 41, PD = 52 | 22/37 | 11/30 | 38.5 ± 14.6 | 41.0 ± 16.5 | HAM-D | SSI | Serum | ||||
| Coryell et al. [28] | USA | MDD = 123, MDD + SA = 79 | 87/36 | 54/25 | 38.5 (15.8) | 30.7 (12.9) | PHQ-8 | CSSRS | Plasma | ||||
| Falcone et al. [29] | USA | C = 20, Acute psychosis = 40, Mood disorders = 24 | 50–60% | Low risk, 50.0%; High risk, 66.7% | Low risk, 14.5 ± 0.5; High risk, 14.1 ± 0.5 | DSM-IV | BPRS-C | Serum | |||||
| Fernández-Sevillano et al. [30] | Spain | C = 20, MDD = 23, MDD + SI = 33, MDD + SA = 20 | 14/6 | 18/5 | 26/7 | 13/7 | 44.6 (9.2) | 50.6 (9.9) | 44.5 (12.8) | 44.7 (8.8) | HDRS | Plasma | |
| Ganança et al. [31] | USA | C = 24, MDD = 38, MDD + SI = 22, MDD + SA = 20 | 15 (62.5) | 6 (16) | 12 (55) | 10 (50) | 33.7 (9.8) | 35.1 (9.7) | 37.7 (12.0) | 32.7 (13.4) | 17-HDRS | Plasma/Serum | |
| Grassi-Oliveira et al. [32] | Brazil | C = 16, MDD = 12, MDD + SI = 18 | 30/0 | 38.1 (3.9) | 37.8 (9.5) | 40.2 (8.3) | BDI | Serum | |||||
| Grudet et al. [33] | USA | C = 14, MDD = 17, MDD + SA = 59 | 7/7 | 8/9 | 34/25 | 33 (23–55) | 35 (22–54) | 38 (18–73) | DSM-IV | Serum | |||
| Lin et al. [34] | China | C = 96, MDD = 90, MDD + SA = 14 | 73/23 | 56/34 | 14/0 | 37.5 ± 9.6 | 38.1 ± 11.4 | 44.2 ± 12.2 | HRSD-24 | Serum | |||
| Lu et al. [35] | China | C = 22, People who died by suicide (including MDD) = 22 | 15/7 | Suicide (including MDD) 11/11 | 38.7 ± 16.4 | Suicide (including MDD) 38.9 ± 14.0 | Plasma | ||||||
| Nowak et al. [36] | Argentina | HC = 20, MDD + SI = 8, MDD + SA = 25 | MDD + SI/SA 25/8 | MDD + SI/SA 36.4 ± 12.8 | DSM-IV | Plasma | |||||||
| Ohlsson et al. [37] | USA | HC = 17, MDD = 13, recent SA = 54 | 8/9 | 7/6 | 30/24 | 34.4 ± 11.4 | 34.5 ± 11.5 | 38.5 ± 14.5 | MADRS | SUAS | Plasma | ||
| Rasheed et al. [38] | Iraq | C = 30, MDD = 38, MDD + SA = 22 | 17/13 | 14/24 | 6/16 | 31.1 ± 15.4 | 30.8 ± 14.1 | 36.9 ± 10.3 | DSM-IV | CDC | Plasma | ||
| Rui et al. [39] | China | C = 109, MDD = 86, MDD + SA = 43 | 56/53 | 47/39 | 27/16 | 32.6 ± 12.8 | 37.4 ± 14.6 | 34.8 ± 12.5 | HDRS | SSI-Beck | Serum | ||
| Su et al. [40] | China | MDD = 118, MDD + suicide risk = 50 | 95/23 | MDD + suicide risk 35/15 | 39 ± 10.7 | MDD + suicide risk 35.9 ± 11.3 | HAMD-17 | suicidal module of MINI | Serum | ||||
| Uchitomi et al. [41] | Japan | Cancer patients (MDD = 16, MDD + SI = 8) | 7/9 | 5/3 | 61.1 ± 11.3 | 57.6 ± 9.1 | DSM-IV/HDRS | DSM-IV | Blood | ||||
| Vargas et al. [42] | Brazil | Non-smoker: Non-depressed = 123, Depressed = 68 Smoker: Non-depressed = 78, Depressed = 72 | Non-smoker 76/47 Smoker 43/35 (including 2 SA) | Non-smoker 54/14 (including 2 SA) Smoker 52/20 (including 22 SA) | 18–60 | DSM-IV/HDRS | Plasma/serum | ||||||
| Ventorp et al. [43] | Sweden | C = 19, MDD = 19, MDD + SA = 54 | 10/9 | 10/9 | 30/24 | 34.7 ± 10.8 | 34.0 ± 10.3 | 38.5 ± 14.5 | DSM-IV | Plasma | |||
| Wiener et al. [44] | Brazil | HC = 743, MDD = 149 (including suicide risk), BD = 142 (including suicide risk) | 354/389 | MDD (including suicide risk) 113/36 BD (including suicide risk) 84/58 | 18–35 | DSM-IV | Serum | ||||||
| Xu et al. [45] | China | MDD = 26, MDD + SI = 29 | 19/7 | 16/13 | 37.0 ± 2.9 | 35.5 ± 2.7 | DSM-V/HAMD-24 | Serum | |||||
| Yang et al. [46] | China | HC = 12, MDD = 12, MDD + SA = 12 | 33/16 | 34/15 | 31/18 | 33.1 ± 5.8 | 36.3 ± 11.2 | 33.7 ± 12.2 | HAM-D | Plasma | |||
| Author, Year | Screened Biomarkers | Candidate Biomarkers |
|---|---|---|
| Amitai et al. [23] | Interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α | IL-6 |
| Bai et al. [24] | Alpha 1-antitrypsin (AAT), apolipoprotein A1 (APOA1), C-reactive protein (CRP), high-density lipoprotein cholesterol (HDLC), homocysteine (HCY), transferring (TRSF) | AAT, TRSF |
| Bergmans et al. [25] | Log C-reactive protein (CRP), log white blood cell count, standardized diet inflammatory index score | log CRP |
| Brundin et al. [26] | Picolinic acid (PIC), quinolinic acid (QUIN), PIC:QUIN ratio | PIC, PIC:QUIN ratio |
| Choi et al. [27] | CRP, IL-10, interferon (IFN)-γ, TNF-α | TNF-α |
| Coryell et al. [28] | CRP, IL-6, IL-1β, IL-1ra, TNF-α | log IL-1β |
| Falcone et al. [29] | S100B | S100B |
| Fernández-Sevillano et al. [30] | IL-2, IL-2R, IL-4, IL-6, TNF-α | IL-6 |
| Ganança et al. [31] | Docosahexaenoic acid (DHA; %), eicosapentaenoic acid (EPA; %), IL-6, IL-1β, plasma phospholipid levels of arachidonic acid (AA; %), TNF-α | DHA (%), IL-1β |
| Grassi-Oliveira et al. [32] | C-C motif chemokine ligand (CCL) 2, CCL5, CCL11 | CCL2, CCL5 |
| Grudet et al. [33] | IL-1β, IL-6, TNF-a, vitamin D | Vitamin D |
| Lin et al. [34] | Mature brain-derived neurotrophic factor (mBDNF) | mBDNF |
| Lu et al. [35] | Neuropeptide Y (NPY), IL-1β, hypocretin | NPY, IL-1β |
| Nowak et al. [36] | IL-12, IL-6 | IL-12, IL-6 |
| Ohlsson et al. [37] | IL-6, intestinal fatty acid binding protein (I-FABP), soluble CD14, zonulin | I-FABP, IL-6, Zonulin, |
| Rasheed et al. [38] | IL-1β, high-density lipoprotein (HDL), low-density lipoprotein (LDL), TNF-α, total cholesterol (TC), triglyceride (TG) | LDL, TC, TG, TNF-α |
| Rui et al. [39] | Tenascin-C | Tenascin-C |
| Su et al. [40] | Alpha-2-macroglobulin (α2M), CCL-2, chemokine (C-X-C motif) ligand 1 (CXCL-1), CRP, IL-1β, IL1-Rα, IL2-Rα, IL-6, IL-18, macrophage migration inhibitory factor (MIF), myeloperoxidase (MPO), TNF-α, toll like receptor 1 (TLR-1) | CCL-2, CXCL-1, IL-1β, IL2-Rα, TLR-1 |
| Uchitomi et al. [41] | Platelet Ca2+ | |
| Vargas et al. [42] | Advanced oxidation protein products (AOPP), CRP, erythrocytes sedimentation rate (ESR), fibrinogen, lipid hydroperoxides, malondialdehyde (MDA), nitric oxide metabolites (NOx), total reactive antioxidant potential (TRAP) | AOPP, CRP, ESR, Fibrinogen, NOx, TRAP |
| Ventorp et al. [43] | CRP, soluble form of the urokinase receptor-Type plasminogen activator receptor (suPAR) | suPAR |
| Wiener et al. [44] | IL-6, IL-10 | IL-6, IL-10 |
| Xu et al. [45] | IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-12p70, IL-13, IL-15, IL-16, IL-17C, IL-27, IL-31, CCL3, CCL4, CCL11, CCL17, CCL26, CXCL10, fibroblast growth factor (FGF) basic (FGF2/bFGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, intercellular cell adhesion molecular (ICAM)-1, placenta growth factor (PIGF), thymic stromal lymphopoietin (TSLP), Tie-2, TNF-α, TNF-β, vascular endothelial growth factor (VEGF), VEGF-C, VEGFR1 | CCL26, CXCL10, IL-17C, TNF-β, VEGF |
| Yang et al. [46] | Activated protein C (APC), coagulation factor (F) VII, F V, tissue factor (TF), tissue factor pathway inhibitor (TFPI), prothrombin, prothrombin fragment (F) | APC, FV, F VII, TF, TFPI |
| Characteristics of Analysis | Potential Inflammatory Biomarkers |
|---|---|
| Markers overlapping in two or more articles among all screened variables included in this study | CCL2, CCL11, CRP, IFN-γ, IL-1β, IL-1Rα, IL-2, IL-4, IL-6, IL-10, IL-12, TNF-α |
| Significant markers in two or more articles | CCL2, CRP, IL-1β, IL-6, TNF-α |
| Potential SI markers | AAT, CCL2, CCL5, CCL26, CXCL10, DHA (%), IL-1β, IL-6, IL-12, IL-17C, log CRP, TRSF, TNF-α, S100B, TNF-β, VEGF |
| Potential SA markers | APC, DHA (%), FV, FVII, I-FABP, IL-6, IL-12, LDL, log IL-1β, mBDNF, PIC, PIC:QUIN ratio, suPAR, TC, Tenascin-C, TF, TFPI, TG, TNF-α, vitamin D, zonulin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.Y.; Shin, K.Y.; Chang, K.-A. Potential Inflammatory Biomarkers for Major Depressive Disorder Related to Suicidal Behaviors: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 13907. https://doi.org/10.3390/ijms241813907
Kim KY, Shin KY, Chang K-A. Potential Inflammatory Biomarkers for Major Depressive Disorder Related to Suicidal Behaviors: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(18):13907. https://doi.org/10.3390/ijms241813907
Chicago/Turabian StyleKim, Ka Young, Ki Young Shin, and Keun-A Chang. 2023. "Potential Inflammatory Biomarkers for Major Depressive Disorder Related to Suicidal Behaviors: A Systematic Review" International Journal of Molecular Sciences 24, no. 18: 13907. https://doi.org/10.3390/ijms241813907
APA StyleKim, K. Y., Shin, K. Y., & Chang, K.-A. (2023). Potential Inflammatory Biomarkers for Major Depressive Disorder Related to Suicidal Behaviors: A Systematic Review. International Journal of Molecular Sciences, 24(18), 13907. https://doi.org/10.3390/ijms241813907






