The Effect of Orally Administered Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Obesity Parameters in Mice
Abstract
:1. Introduction
2. Results
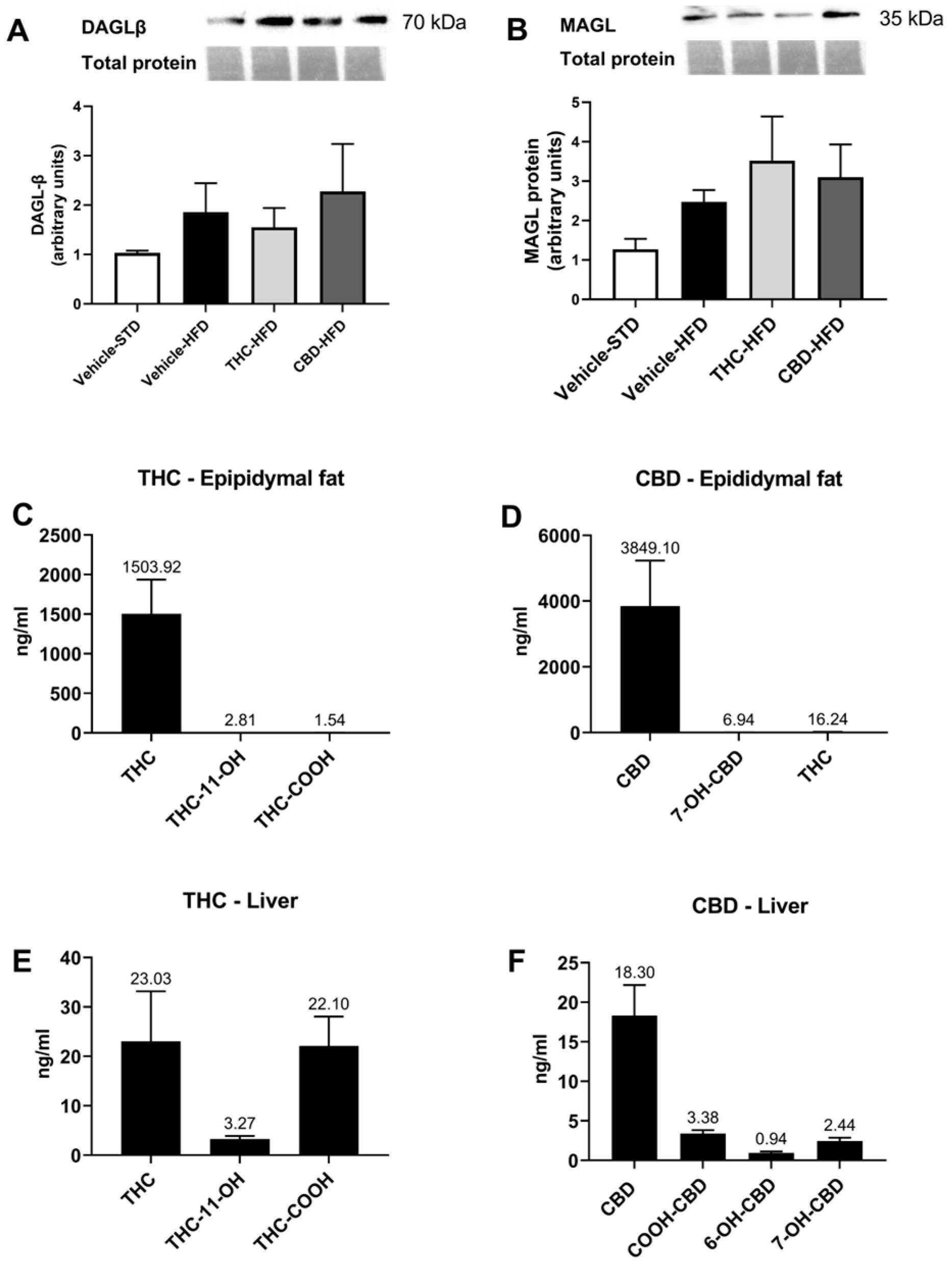
2.1. Pharmacokinetic Profile of Orally Administered THC and CBD
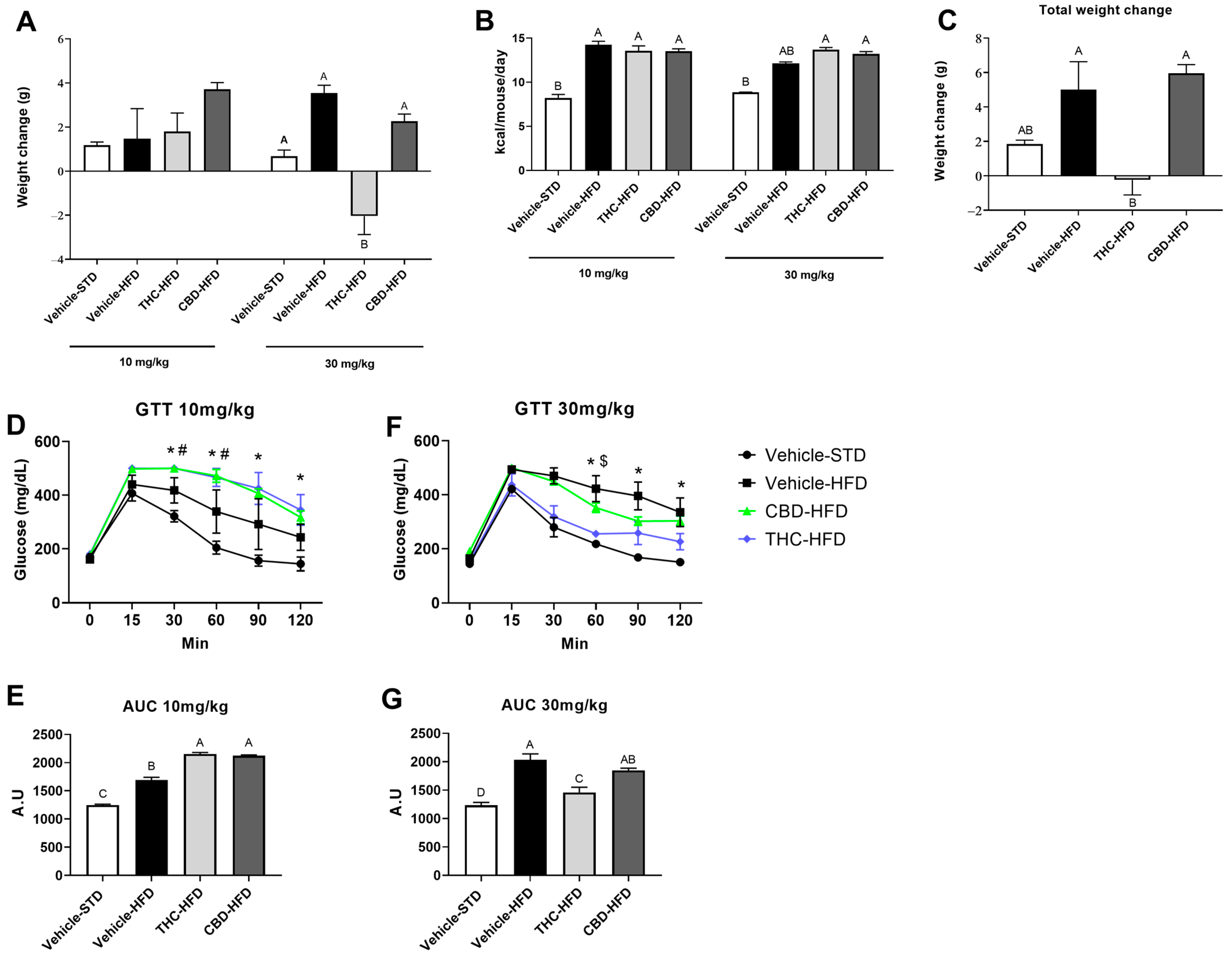
2.2. The Effect of THC and CBD on Weight Gain, Caloric Intake, and Glucose Tolerance
2.3. The Effect of THC and CBD on the ECS in Liver and Adipose Tissues
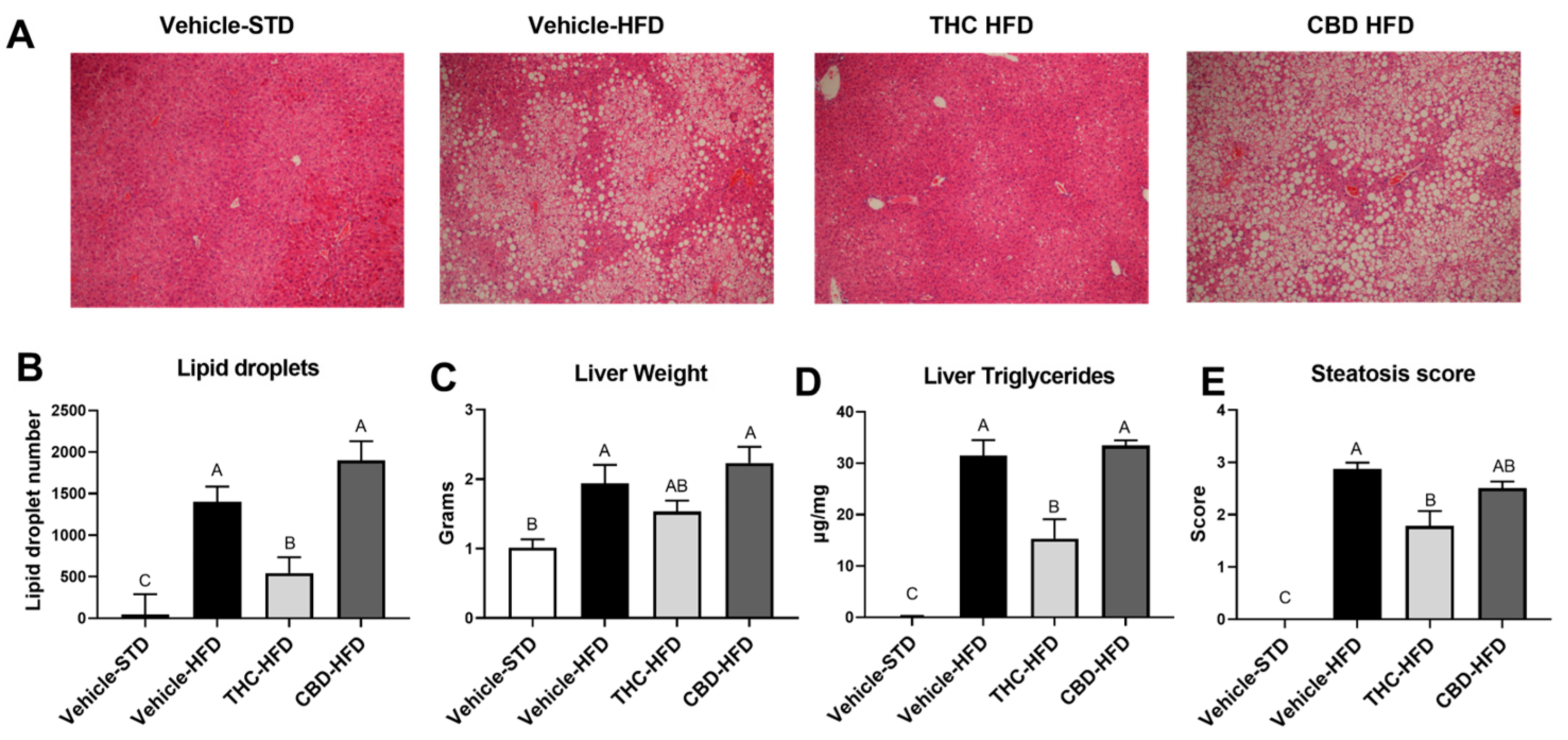
2.4. The Effect of THC and CBD on Diet-Induced Steatosis
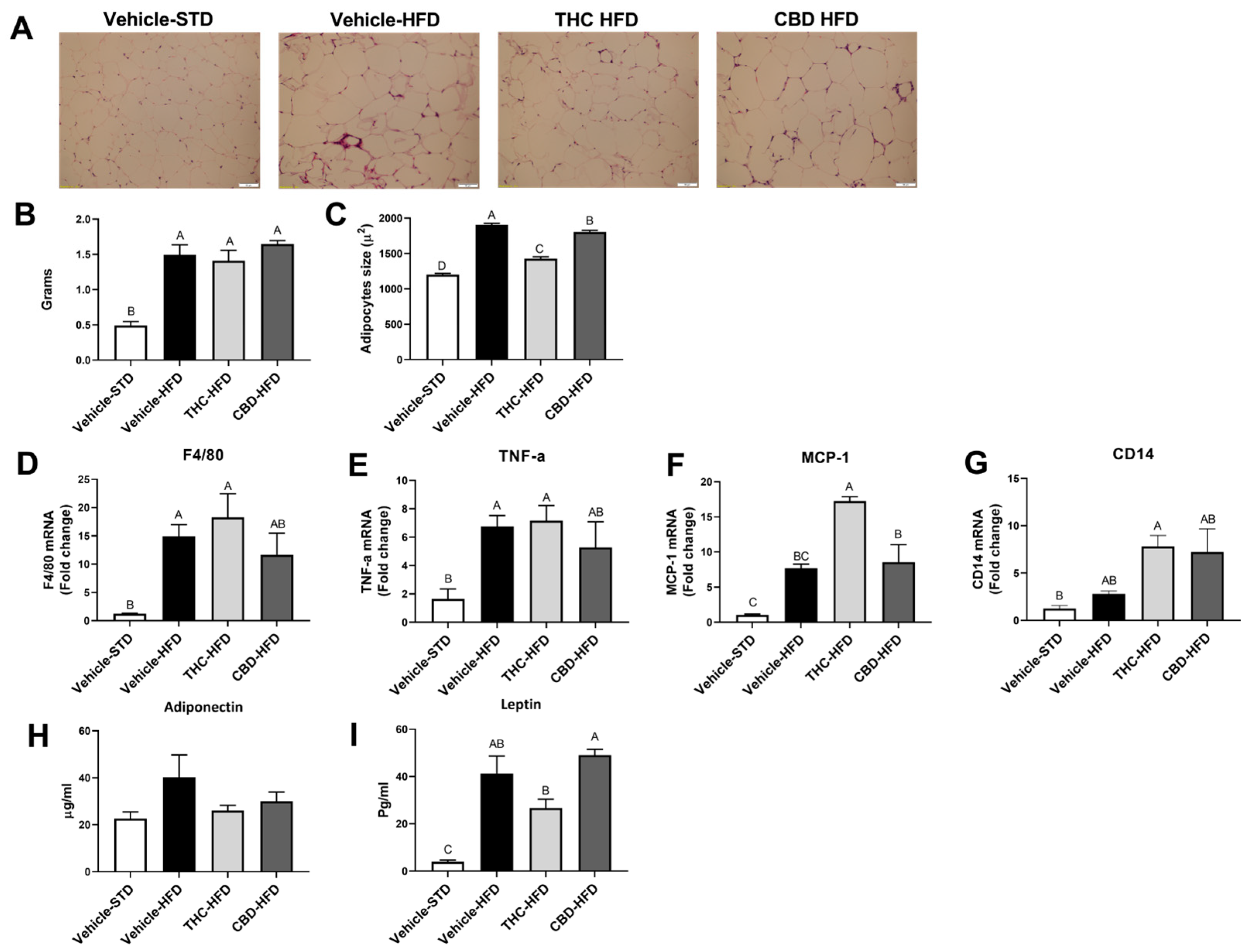
2.5. The Effect of THC and CBD on Adiposity and Inflammation
2.6. The Effect of THC and CBD on Lipid Metabolism Gene Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Treatments
4.3. Pharmacokinetics (PK)
4.4. Metabolic Cages
4.5. Glucose Tolerance Test
4.6. Liver Triglyceride Quantification
4.7. Histology and Microscopy
4.8. Liquid Chromatography–High-Resolution Mass Spectrometry (LC-HRMS) Chemical Analysis
4.9. Western Blot
4.10. RNA Extraction and Real-Time Quantitative PCR
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef]
- Di Marzo, V. New Approaches and Challenges to Targeting the Endocannabinoid System. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Mazier, W.; Saucisse, N.; Gatta-Cherifi, B.; Cota, D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol. Metab. 2015, 26, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Sandoval, D.A.; Olivieri, M.; Prodi, E.; D’Alessio, D.A.; Woods, S.C.; Seeley, R.J.; Obici, S. Food Intake-Independent Effects of CB1 Antagonism on Glucose and Lipid Metabolism. Obesity 2009, 17, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 Cannabinoid Receptor Alters Gut Microbiota and Attenuates Inflammation and Diet-Induced Obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Guzman, M. Cannabis in Cancer Care. Clin. Pharmacol. Ther. 2015, 97, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Alshaarawy, O.; Anthony, J.C. Are Cannabis Users Less Likely to Gain Weight? Results from a National 3-Year Prospective Study. Int. J. Epidemiol. 2019, 48, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Rajavashisth, T.B.; Shaheen, M.; Norris, K.C.; Pan, D.; Sinha, S.K.; Ortega, J.; Friedman, T.C. Decreased Prevalence of Diabetes in Marijuana Users: Cross-Sectional Data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012, 2, e000494. [Google Scholar] [CrossRef]
- Alshaarawy, O.; Anthony, J.C. Brief Report: Cannabis Smoking and Diabetes Mellitus: Results from Meta-Analysis with Eight Independent Replication Samples. Epidemiology 2015, 26, 597. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, A.C.; Alliu, S.; Ajayi, T.O.; Adejumo, K.L.; Adegbala, O.M.; Onyeakusi, N.E.; Akinjero, A.M.; Durojaiye, M.; Bukong, T.N. Cannabis Use Is Associated with Reduced Prevalence of Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. PLoS ONE 2017, 12, e0176416. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Keenan, C.M.; Reimer, R.A.; Le Foll, B.; Sharkey, K.A. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Δ9-Tetrahydrocannabinol. PLoS ONE 2015, 10, e0144270. [Google Scholar] [CrossRef] [PubMed]
- Levendal, R.A.; Schumann, D.; Donath, M.; Frost, C.L. Cannabis Exposure Associated with Weight Reduction and β-Cell Protection in an Obese Rat Model. Phytomedicine 2012, 19, 575–582. [Google Scholar] [CrossRef]
- Ignatowska-Jankowska, B.; Jankowski, M.M.; Swiergiel, A.H. Cannabidiol Decreases Body Weight Gain in Rats: Involvement of CB2 Receptors. Neurosci. Lett. 2011, 490, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Hložek, T.; Uttl, L.; Kadeřábek, L.; Balíková, M.; Lhotková, E.; Horsley, R.R.; Nováková, P.; Šíchová, K.; Štefková, K.; Tylš, F.; et al. Pharmacokinetic and Behavioural Profile of THC, CBD, and THC+CBD Combination after Pulmonary, Oral, and Subcutaneous Administration in Rats and Confirmation of Conversion in Vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Baglot, S.L.; Hume, C.; Petrie, G.N.; Aukema, R.J.; Lightfoot, S.H.M.; Grace, L.M.; Zhou, R.; Parker, L.; Rho, J.M.; Borgland, S.L.; et al. Pharmacokinetics and Central Accumulation of Delta-9-Tetrahydrocannabinol (THC) and Its Bioactive Metabolites Are Influenced by Route of Administration and Sex in Rats. Sci. Rep. 2021, 11, 23990. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Casari, I.; Falasca, M. Modulatory Role of the Endocannabinoidome in the Pathophysiology of the Gastrointestinal Tract. Pharmacol. Res. 2022, 175, 106025. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Bhattacharyya, S. Cannabis Use and the Development of Tolerance: A Systematic Review of Human Evidence. Neurosci. Biobehav. Rev. 2018, 93, 1–25. [Google Scholar] [CrossRef]
- Matias, I.; Petrosino, S.; Racioppi, A.; Capasso, R.; Izzo, A.A.; di Marzo, V. Dysregulation of Peripheral Endocannabinoid Levels in Hyperglycemia and Obesity: Effect of High Fat Diets. Mol. Cell. Endocrinol. 2008, 286, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Mittal, B. Subcutaneous Adipose Tissue & Visceral Adipose Tissue. Indian J. Med. Res. 2019, 149, 571. [Google Scholar] [PubMed]
- Pertwee, R.G. The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: Δ9-Tetrahydrocannabinol, Cannabidiol and Δ9-Tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- McKinney, D.L.; Cassidy, M.P.; Collier, L.M.; Martin, B.R.; Wiley, J.L.; Selley, D.E.; Sim-Selley, L.J. Dose-Related Differences in the Regional Pattern of Cannabinoid Receptor Adaptation and In Vivo Tolerance Development to Δ9-Tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 2008, 324, 664–673. [Google Scholar] [CrossRef]
- Sim-Selley, L.J. Regulation of Cannabinoid CB1 Receptors in the Central Nervous System by Chronic Cannabinoids. Crit. Rev. Neurobiol. 2003, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Geurts, L.; Matamoros, S.; Plovier, H.; Duparc, T. Glucose Metabolism: Focus on Gut Microbiota, the Endocannabinoid System and Beyond. Diabetes Metab. 2014, 40, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Mukhopadhyay, P.; Haskó, G.; Pacher, P. The Endocannabinoid System and Plant-Derived Cannabinoids in Diabetes and Diabetic Complications. Am. J. Pathol. 2012, 180, 432–442. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.M.; Cristino, L.; Matias, I.; Capasso, R.; Racioppi, A.; Izzo, A.A.; Di Marzo, V. Endocannabinoid Dysregulation in the Pancreas and Adipose Tissue of Mice Fed with a High-Fat Diet. Obesity 2008, 16, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Alvheim, A.R.; Torstensen, B.E.; Lin, Y.H.; Lillefosse, H.H.; Lock, E.-J.; Madsen, L.; Frøyland, L.; Hibbeln, J.R.; Malde, M.K. Dietary Linoleic Acid Elevates the Endocannabinoids 2-AG and Anandamide and Promotes Weight Gain in Mice Fed a Low Fat Diet. Lipids 2014, 49, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bolsoni-Lopes, A.; Alonso-Vale, M.I.C. Lipolysis and Lipases in White Adipose Tissue—An Update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Bátkai, S.; Harvey-White, J.; Mackie, K.; Offertáler, L.; Wang, L.; et al. Endocannabinoid Activation at Hepatic CB1 Receptors Stimulates Fatty Acid Synthesis and Contributes to Diet-Induced Obesity. J. Clin. Investig. 2005, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and Related N-Acylethanolamines: Biological Activities and Metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Osei-Hyiaman, D.; Park, O.; Liu, J.; Bátkai, S.; Mukhopadhyay, P.; Horiguchi, N.; Harvey-White, J.; Marsicano, G.; Lutz, B.; et al. Paracrine Activation of Hepatic CB1 Receptors by Stellate Cell-Derived Endocannabinoids Mediates Alcoholic Fatty Liver. Cell Metab. 2008, 7, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Westerbacka, J.; Kotronen, A.; Fielding, B.A.; Wahren, J.; Hodson, L.; Perttilä, J.; Seppänen–Laakso, T.; Suortti, T.; Arola, J.; Hultcrantz, R.; et al. Splanchnic Balance of Free Fatty Acids, Endocannabinoids, and Lipids in Subjects with Nonalcoholic Fatty Liver Disease. Gastroenterology 2010, 139, 1961–1971.e1. [Google Scholar] [CrossRef] [PubMed]
- Ruby, M.A.; Nomura, D.K.; Hudak, C.S.S.; Mangravite, L.M.; Chiu, S.; Casida, J.E.; Krauss, R.M. Overactive Endocannabinoid Signaling Impairs Apolipoprotein E-Mediated Clearance of Triglyceride-Rich Lipoproteins. Proc. Natl. Acad. Sci. USA 2008, 105, 14561–14566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, Y.; Schultz, R.D.; Li, N.; He, Z.; Zhang, Z.; Caron, A.; Zhu, Q.; Sun, K.; Xiong, W.; et al. Partial Leptin Reduction as an Insulin Sensitization and Weight Loss Strategy. Cell Metab. 2019, 30, 706–719.e6. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Uña, M.; López-Mancheño, Y.; Diéguez, C.; Fernández-Rojo, M.A.; Novelle, M.G. Unraveling the Role of Leptin in Liver Function and Its Relationship with Liver Diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef]
- Scott, J.C.; Rosen, A.F.G.; Moore, T.M.; Roalf, D.R.; Satterthwaite, T.D.; Calkins, M.E.; Ruparel, K.; Gur, R.E.; Gur, R.C. Cannabis Use in Youth Is Associated with Limited Alterations in Brain Structure. Neuropsychopharmacology 2019, 44, 1362–1369. [Google Scholar] [CrossRef]
- Mina, A.I.; LeClair, R.A.; LeClair, K.B.; Cohen, D.E.; Lantier, L.; Banks, A.S. CalR: A Web-Based Analysis Tool for Indirect Calorimetry Experiments. Cell Metab. 2018, 28, 656–666.e1. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Sulimani, L.; Gelfand, A.; Amsalem, K.; Lewitus, G.M.; Meiri, D. Cannabinoidomics—An Analytical Approach to Understand the Effect of Medical Cannabis Treatment on the Endocannabinoid Metabolome. Talanta 2020, 219, 121336. [Google Scholar] [CrossRef] [PubMed]


| Cmax (ng/mL) | AUC (ng·min/mL) | Tmax (h) | ||
|---|---|---|---|---|
| THC | Δ9-THC | 119 ± 55.3 | 295.2 ± 75.4 | 1 |
| 11-OH-THC | 7.4 ± 1.5 | 31.66 ± 8.4 | 1 | |
| 11-COOH-THC | 7.25 ± 1.34 | 80.3 ± 23.3 | 1 | |
| CBD | CBD | 96.9 ± 37.1 | 500 ± 115 | 1 |
| 6-OH-CBD | 3.88 ± 1.76 | 8.7 ± 4 | 1 | |
| 7-OH-CBD | 18 ± 7.03 | 48.9 ± 16.44 | 1 |
| Lipid Family | ECs (ng/g Tissue) | Vehicle-STD | Vehicle-HFD | THC-HFD | CBD-HFD | |
|---|---|---|---|---|---|---|
| Fat | NAEs | AEA | 6.7 ± 0.2 | 8.5 ± 0.7 | 6.9 ± 0.2 | 8.1 ± 0.4 |
| α-LEA | 0.9 ± 0.1 a | 0.2 ± 0.01 b | 0.2 ± 0.01 b | 0.2 ± 0.02 b | ||
| DHEA | 2.6 ± 0.1 a | 1.8 ± 0.1 b | 1.6 ± 0.1 b | 1.7 ± 0.09 b | ||
| LEA | 43.7 ± 1.5 a | 31.1 ± 3 b | 24.2 ± 1.8 b | 33.6± 3 ab | ||
| OEA | 43.3 ± 4 | 54 ± 5.4 | 39.6 ± 3.5 | 55.7± 4.1 | ||
| PEA | 54.4 ± 3.7 a | 36.8 ± 1.7 b | 30.4 ± 4.6 b | 41 ± 1.9 ab | ||
| SEA | 112 ± 9.4 | 130 ± 11.5 | 115 ± 5.9 | 127 ± 2.1 | ||
| DEA | 1.4 ± 0.7 | 1.2 ± 0.07 | 1.3 ± 0.04 | 1.5 ± 0.09 | ||
| 2-MAGs | 2-AG | 144 ± 108 | 147 ± 32 | 104 ± 14 | 164 ± 45 | |
| 2-LG | 352 ± 86 b | 2534 ± 601 a | 1128 ± 250 ab | 2773 ± 739 a | ||
| NAAAs | NL-Gly | 2.8 ± 0.2 | 1.5 ± 0.3 | 1.3 ± 0.2 | 1.5 | |
| NP-Gly | 0.6 ± 0.1 | 1 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.09 | ||
| Liver | NAEs | AEA | 28.6 ± 3.6 | 23.8 ± 4.8 | 20.6 ± 4.1 | 12.9 ± 0.5 |
| DHEA | 8.8 ± 1 a | 6 ± 1.5 ab | 4.6 ± 0.6 ab | 2.9 ± 0.1 b | ||
| LEA | 69.4 ± 10.7 a | 36.8 ± 7.8 b | 30.3 ± 4.5 b | 20.8 ± 1.5 b | ||
| OEA | 40.5 ± 7.9 | 33.6 ± 2.4 | 24.5 ± 6.6 | 25.5 ± 1.8 | ||
| PEA | 326 ± 27 a | 157 ± 16 b | 107 ± 4.7 b | 100 ± 8.6 b | ||
| SEA | 288 ± 39 a | 134 ± 27 b | 111 ± 4.7 b | 75.2 ± 3.6 b | ||
| 2-MAGs | 2-AG | 1975 ± 171 a | 1763 ± 572 a | 767 ± 102 ab | 390 ± 23 b | |
| 2-LG | 12,682 ± 2214 a | 5748 ± 2132 b | 4112 ± 1208 b | 1951 ± 341 b | ||
| NAAAs | NL-Gly | 359 ± 34 a | 49 ± 41 b | 95 ± 14 b | 50.3 ± 5.9 b | |
| NP-Gly | 695 ± 49 a | 366 ± 70 b | 284 ± 33 b | 177 ± 18 b | ||
| NA-Gly | 228 ± 14 a | 117 ± 44 ab | 118 ± 28 ab | 61.9 ± 10.8 b | ||
| NDH-Gly | 244 ± 15.9 a | 106 ± 33.3 b | 69.1 ± 13.3 | 45.3 ± 6.1 | ||
| NA-Ser | 83.3 ± 4.5 a | 46.3 ± 19.9 ab | 34.5 ± 8 ab | 15 ± 1.9 b | ||
| NA-GABA | 6.1 ± 1.4 | 6 ± 2.5 | 4.6 ± 1 | 1.5 ± 0.2 | ||
| NA-Ala | 126 ± 12.1 a | 91.8 ± 32.7 b | 96.7 ± 21.3 b | 41.2 ± 5.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eitan, A.; Gover, O.; Sulimani, L.; Meiri, D.; Schwartz, B. The Effect of Orally Administered Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Obesity Parameters in Mice. Int. J. Mol. Sci. 2023, 24, 13797. https://doi.org/10.3390/ijms241813797
Eitan A, Gover O, Sulimani L, Meiri D, Schwartz B. The Effect of Orally Administered Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Obesity Parameters in Mice. International Journal of Molecular Sciences. 2023; 24(18):13797. https://doi.org/10.3390/ijms241813797
Chicago/Turabian StyleEitan, Adi, Ofer Gover, Liron Sulimani, David Meiri, and Betty Schwartz. 2023. "The Effect of Orally Administered Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Obesity Parameters in Mice" International Journal of Molecular Sciences 24, no. 18: 13797. https://doi.org/10.3390/ijms241813797
APA StyleEitan, A., Gover, O., Sulimani, L., Meiri, D., & Schwartz, B. (2023). The Effect of Orally Administered Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Obesity Parameters in Mice. International Journal of Molecular Sciences, 24(18), 13797. https://doi.org/10.3390/ijms241813797







