The Dual Role of Autophagy in Postischemic Brain Neurodegeneration of Alzheimer’s Disease Proteinopathy
Abstract
1. Introduction
1.1. Epidemiology of Brain Ischemia
1.2. Medical, Financial and Social Burdens of Brain Ischemia
1.3. Postischemic Neurodegeneration
1.4. Autophagy as Hope after an Ischemic Episode
2. Search of the Literature
3. Autophagy versus Postischemic Brain Cells
4. Dysregulation of Autophagy Genes in Postischemic Brain
5. Autophagy and Neuronal Death in Ischemic Brain Injury
6. Crosstalk between Autophagy, Necroptosis and Apoptosis after Brain Ischemia
7. Autophagy versus Postischemic Brain Injury
7.1. The Protective Role of Autophagy in Postischemic Brain
7.2. The Deleterious Role of Autophagy in Postischemic Brain
8. Potential Therapeutic Strategies for Autophagy Modulation Postischemia
9. Conclusions
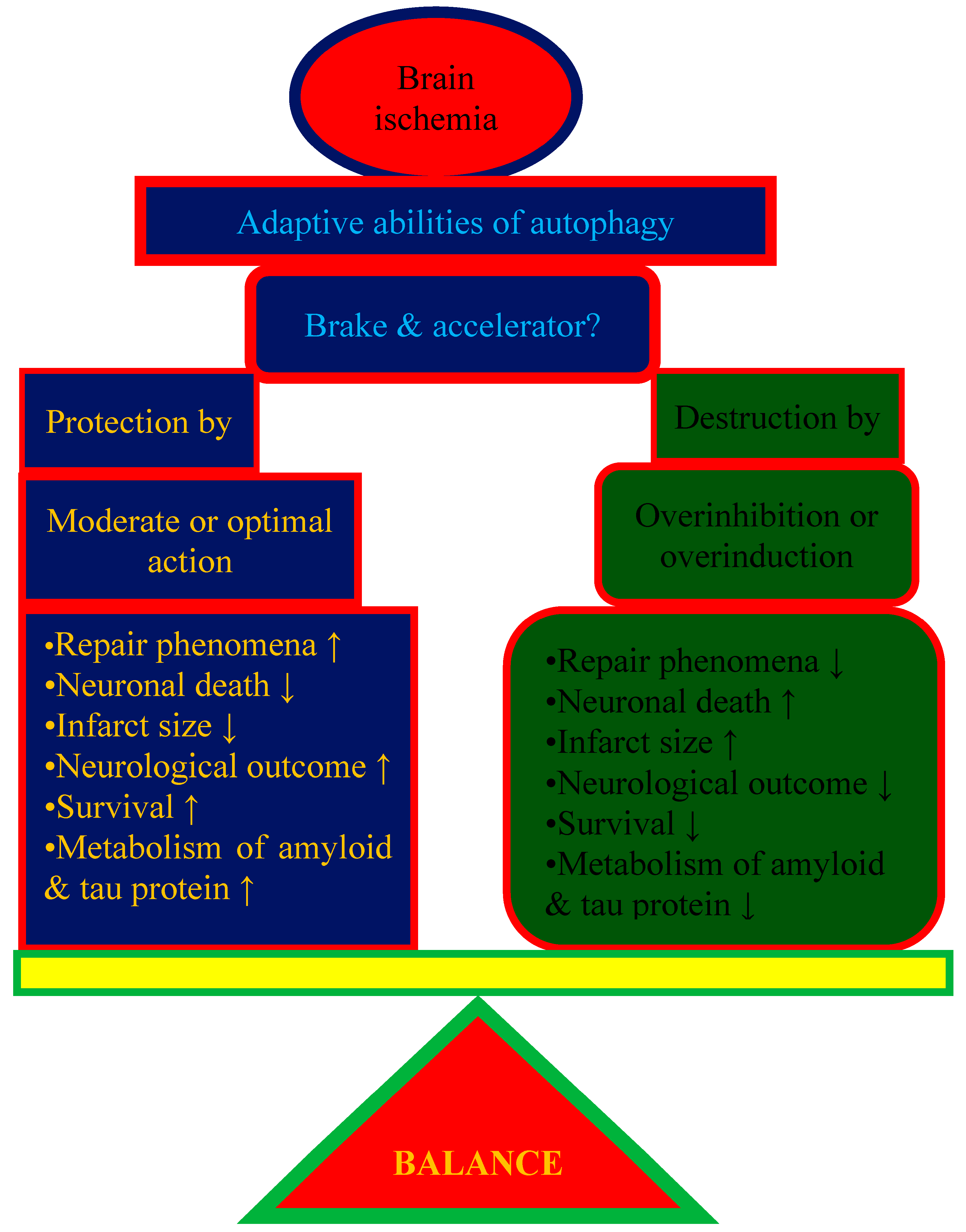
10. Good or Bad Autophagy: A Matter of Balance?
11. Challenges
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farina, M.; Vieira, L.E.; Buttari, B.; Profumo, E.; Saso, L. The Nrf2 pathway in ischemic stroke: A review. Molecules 2021, 26, 5001. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Parrella, E.; Gussago, C.; Porrini, V.; Benarese, M.; Pizzi, M. From Preclinical Stroke Models to Humans: Polyphenols in the Prevention and Treatment of Stroke. Nutrients 2021, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B.; Lynch, J.K. Stroke in newborn infants. Lancet Neurol. 2004, 3, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Faustino-Mendes, T.; Machado-Pereira, M.; Castelo-Branco, M.; Ferreira, R. The Ischemic Immature Brain: Views on Current Experimental Models. Front. Cell. Neurosci. 2018, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the immune response in ischemic stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Hernández, I.H.; Villa-González, M.; Martín, G.; Soto, M.; Pérez-Álvarez, M.J. Glial Cells as therapeutic approaches in brain ischemia-reperfusion injury. Cells 2021, 10, 1639. [Google Scholar] [CrossRef]
- Patabendige, A.; Singh, A.; Jenkins, S.; Sen, J.; Chen, R. Astrocyte activation in neurovascular damage and repair following ischaemic stroke. Int. J. Mol. Sci. 2021, 22, 4280. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Neuroinflammation in post-ischemic neurodegeneration of the brain: Friend, foe, or both? Int. J. Mol. Sci. 2021, 22, 4405. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2021 update: A report from the American heart association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Kamarova, M.; Baig, S.; Patel, H.; Monks, K.; Wasay, M.; Ali, A.; Redgrave, J.; Majid, A.; Bell, S.M. Antiplatelet use in ischemic stroke. Ann. Pharmacother. 2022, 56, 1159–1173. [Google Scholar] [CrossRef]
- Wang, Y.; Leak, R.K.; Cao, G. Microglia-mediated neuroinflammation and neuroplasticity after stroke. Front. Cell. Neurosci. 2022, 16, 980722. [Google Scholar] [CrossRef]
- Dang, H.; Mao, W.; Wang, S.; Sha, J.; Lu, M.; Cong, L.; Meng, X.; Li, H. Systemic inflammation response index as a prognostic predictor in patients with acute ischemic stroke: A propensity score matching analysis. Front. Neurol. 2023, 13, 1049241. [Google Scholar] [CrossRef] [PubMed]
- Venketasubramanian, N.; Yoon, B.W.; Pandian, J.; Navarro, J.C. Stroke epidemiology in south, east, and south-east Asia: A review. J. Stroke 2017, 19, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.J.; Wang, L.D. Special Writing Group of China Stroke Surveillance Report. China stroke surveillance report 2021. Mil. Med. Res. 2023, 10, 33. [Google Scholar]
- Tu, W.J. Is the world of stroke research entering the Chinese era? Front. Neurol. 2023, 14, 1189760. [Google Scholar] [CrossRef]
- Varkey, B.P.; Joseph, J.; Varghese, A.; Sharma, S.K.; Mathews, E.; Dhandapani, M.; Narasimha, V.L.; Kuttan, R.; Shah, S.; Dabla, S.; et al. The Distribution of Lifestyle Risk Factors Among Patients with Stroke in the Indian Setting: Systematic Review and Meta-Analysis. Ann. Neurosci. 2023, 30, 40–53. [Google Scholar] [CrossRef]
- Dhandapani, M.; Joseph, J.; Sharma, S.; Dabla, S.; Varkey, B.P.; Narasimha, V.L.; Varghese, A.; Dhandapani, S. The Quality of Life of Stroke Survivors in the Indian Setting: A Systematic Review and Meta-Analysis. Ann. Indian Acad. Neurol. 2022, 25, 376–382. [Google Scholar]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Bulygin, K.V.; Beeraka, N.M.; Saitgareeva, A.R.; Nikolenko, V.N.; Gareev, I.; Beylerli, O.; Akhmadeeva, L.R.; Mikhaleva, L.M.; Torres Solis, L.F.; Solís Herrera, A.; et al. Can miRNAs be considered as diagnostic and therapeutic molecules in ischemic stroke pathogenesis? Current Status. Int. J. Mol. Sci. 2020, 21, 6728. [Google Scholar] [CrossRef]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: A global response is needed. Bull. World Health Organ. 2016, 94, 634. [Google Scholar] [CrossRef]
- Owolabi, M.O.; Akarolo-Anthony, S.; Akinyemi, R.; Arnett, D.; Gebregziabher, M.; Jenkins, C.; Tiwari, H.; Arulogun, O.; Akpalu, A.; Sarfo, F.S.; et al. Members of the H3 Africa Consortium. Members of the H3 Africa Consortium. The burden of stroke in Africa: A glance at the present and a glimpse into the future. Cardiovasc. J. Afr. 2015, 26 (Suppl. S1), S27–S38. [Google Scholar] [CrossRef]
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar] [PubMed]
- Appelros, P.; Nydevik, I.; Viitanen, M. Poor outcome after first-ever stroke: Predictors for death, dependency, and recurrent stroke within the first year. Stroke 2002, 34, 122. [Google Scholar] [CrossRef]
- Feigin, V.L.; Lawes, C.M.M.; Bennett, D.A.; Anderson, C.S. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003, 2, P43–P53. [Google Scholar] [CrossRef]
- Yoo, J.W.; Hong, B.Y.; Jo, L.; Kim, J.S.; Park, J.G.; Shin, B.K.; Lim, S.H. Effects of Age on Long-Term Functional Recovery in Patients with Stroke. Medicina 2020, 56, 451. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Miziak, B.; Czuczwar, S.J. Apitherapy in Post-Ischemic Brain Neurodegeneration of Alzheimer’s Disease Proteinopathy: Focus on Honey and Its Flavonoids and Phenolic Acids. Molecules 2023, 28, 5624. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Miziak, B.; Czuczwar, S.J. Post-Ischemic Permeability of the Blood-Brain Barrier to Amyloid and Platelets as a Factor in the Maturation of Alzheimer’s Disease-Type Brain Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 10739. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; Goff, D.C. Population shifts and the future of stroke: Forecasts of the future burden of stroke. Ann. N. Y. Acad. Sci. 2012, 1268, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Simats, A.; Liesz, A. Systemic inflammation after stroke: Implications for post-stroke comorbidities. EMBO Mol. Med. 2022, 14, e16269. [Google Scholar] [CrossRef] [PubMed]
- Neurology, T.L. Editorial. A unified European action plan on stroke. Lancet Neurol. 2020, 19, 963. [Google Scholar] [CrossRef]
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [CrossRef]
- Wang, Q.H.; Wang, X.; Bu, X.L.; Lian, Y.; Xiang, Y.; Luo, H.B.; Zou, H.Q.; Pu, J.; Zhou, Z.H.; Cui, X.P.; et al. Comorbidity burden of dementia: A hospital-based retrospective study from 2003 to 2012 in seven cities in China. Neurosci. Bull. 2017, 33, 703–710. [Google Scholar] [CrossRef]
- Mandzia, J.; Cipriano, L.E.; Kapral, M.K.; Fang, J.; Hachinski, V.; Sposato, L.A. Intravenous thrombolysis after first-ever ischemic stroke and reduced incident dementia rate. Stroke 2022, 53, 1170–1177. [Google Scholar]
- Pluta, R.; Ułamek, M.; Jabłoński, M. Alzheimer’s mechanisms in ischemic brain degeneration. Anat. Rec. 2009, 292, 1863–1881. [Google Scholar] [CrossRef]
- Sekeljic, V.; Bataveljic, D.; Stamenkovic, S.; Ułamek, M.; Jabłoński, M.; Radenovic, L.; Pluta, R.; Andjus, P.R. Cellular markers of neuroinflammation and neurogenesis after ischemic brain injury in the long-term survival rat model. Brain Struct. Funct. 2012, 217, 411–420. [Google Scholar] [CrossRef]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Radenovic, L.; Nenadic, M.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J.; Andjus, P.R.; Pluta, R. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging 2020, 12, 12251–12267. [Google Scholar] [CrossRef]
- Goulay, R.; Mena Romo, L.; Hol, E.M.; Dijkhuizen, R.M. From stroke to dementia: A Comprehensive review exposing tight interactions between stroke and amyloid-β formation. Transl. Stroke Res. 2020, 11, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.Y.; Kim, B.; Lee, S.R.; Cha, S.H.; Lee, D.S.; Lee, H.J. Prospects of therapeutic target and directions for ischemic stroke. Pharmaceuticals 2021, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, M. Risk factors and their correlation with severity of cerebral microbleed in acute large artery atherosclerotic cerebral infarction patients. Clin. Neurol. Neurosurg. 2022, 221, 107380. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-stroke cognitive impairment and dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, D.A.; Greiner, L.H.; Mortimer, J.A.; Riley, K.P.; Greiner, P.A.; Markesbery, W.R. Brain infarction and the clinical expression of Alzheimer disease: The Nun Study. JAMA 1997, 277, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Van Groen, T.; Puurunen, K.; Mäki, H.M.; Sivenius, J.; Jolkkonen, J. Transformation of diffuse beta-amyloid precursor protein and beta-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke 2005, 36, 1551–1556. [Google Scholar] [CrossRef]
- Qi, J.; Wu, H.; Yang, Y.; Wand, D.; Chen, Y.; Gu, Y.; Liu, T. Cerebral ischemia and Alzheimer’s disease: The expression of amyloid-β and apolipoprotein E in human hippocampus. J. Alzheimers Dis. 2007, 12, 335–341. [Google Scholar] [CrossRef]
- Hatsuta, H.; Takao, M.; Nogami, A.; Uchino, A.; Sumikura, H.; Takata, T.; Morimoto, S.; Kanemaru, K.; Adachi, T.; Arai, T.; et al. Tau and TDP-43 accumulation of the basal nucleus of Meynert in individuals with cerebral lobar infarcts or hemorrhage. Acta Neuropathol. Commun. 2019, 7, 49. [Google Scholar] [CrossRef]
- Ihle-Hansen, H.; Thommessen, B.; Wyller, T.B.; Engedal, K.; Oksengard, A.R.; Stenset, V.; Loken, K.; Aaberg, M.; Fure, B. Incidence and subtypes of MCI and dementia 1 year after first-ever stroke in patients without pre-existing cognitive impairment. Dement. Geriatr. Cogn. Disord. 2011, 32, 401–407. [Google Scholar] [CrossRef]
- Douiri, A.; Rudd, A.G.; Wolfe, C.D. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke 2013, 44, 138–145. [Google Scholar] [CrossRef]
- Jacquin, A.; Binquet, C.; Rouaud, O.; Graule-Petot, A.; Daubail, B.; Osseby, G.V.; Bonithon-Kopp, C.; Giroud, M.; Bejot, Y. Post-stroke cognitive impairment: High prevalence and determining factors in a cohort of mild stroke. J. Alzheimers Dis. 2014, 40, 1029–1038. [Google Scholar] [CrossRef]
- Lo, J.W.; Crawford, J.D.; Desmond, D.W.; Godefroy, O.; Jokinen, H.; Mahinrad, S.; Bae, H.J.; Lim, J.S.; Kohler, S.; Douven, E.; et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology 2019, 93, e2257–e2271. [Google Scholar] [CrossRef]
- Hashim, S.; Ahmad, S.; Al Hatamleh, M.A.I.; Mustafa, M.Z.; Mohamed, M.; Mohamud, R.; Kadir, R.; Kub, T.N.T. Trigona honey as a potential supplementary therapy to halt the progression of post-stroke vascular cognitive impairment. Int. Med. J. 2021, 28, 335–338. [Google Scholar]
- El Husseini, N.; Katzan, I.L.; Rost, N.S.; Blake, M.L.; Byun, E.; Pendlebury, S.T.; Aparicio, H.J.; Marquine, M.J.; Gottesman, R.F.; Smith, E.E.; et al. cognitive impairment after ischemic and hemorrhagic stroke: A scientific statement from the American heart association/American stroke association. Stroke 2023, 54, e272–e291. [Google Scholar] [CrossRef] [PubMed]
- Rasquin, S.M.; Lodder, J.; Verhey, F.R. Predictors of reversible mild cognitive impairment after stroke: A 2-year follow-up study. J. Neurol. Sci. 2005, 229–230, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xie, W.; He, A.D.; Da, X.W.; Liang, M.L.; Yao, G.Q.; Xiang, J.Z.; Gao, C.J.; Ming, Z.Y. Antiplatelet activity of chrysin via inhibiting platelet αIIbβ3-mediated signaling pathway. Mol. Nutr. Food Res. 2016, 60, 1984–1993. [Google Scholar] [CrossRef]
- Dichgans, M.; Leys, D. Vascular cognitive impairment. Circ. Res. 2017, 120, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Wadling, S.; Silver, L.E.; Mehta, Z.; Rothwell, P.M. Transient cognitive impairment in TIA and minor stroke. Stroke 2011, 42, 3116–3121. [Google Scholar] [CrossRef]
- Elman-Shina, K.; Efrati, S. Ischemia as a common trigger for Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1012779. [Google Scholar] [CrossRef]
- Carloni, S.; Girelli, S.; Scopa, C.; Buonocore, G.; Longini, M.; Balduini, W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 2010, 6, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Ułamek-Kozioł, M.; Furmaga-Jabłońska, W.; Januszewski, S.; Brzozowska, J.; Ściślewska, M.; Jabłoński, M.; Pluta, R. Neuronal autophagy: Self-eating or self-cannibalism in Alzheimer’s disease. Neurochem. Res. 2013, 38, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Ułamek-Kozioł, M.; Kocki, J.; Bogucka-Kocka, A.; Petniak, A.; Gil-Kulik, P.; Januszewski, S.; Bogucki, J.; Jabłoński, M.; Furmaga-Jabłońska, W.; Brzozowska, J.; et al. Dysregulation of autophagy, mitophagy and apoptotic genes in the medial temporal lobe cortex in an ischemic model of Alzheimer’s disease. J. Alzheimers Dis. 2016, 54, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ułamek-Kozioł, M.; Pluta, R.; Januszewski, S.; Kocki, J.; Bogucka-Kocka, A.; Czuczwar, S.J. Expression of Alzheimer’s disease risk genes in ischemic brain degeneration. Pharmacol. Rep. 2016, 68, 1345–1349. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Dysregulation of Alzheimer’s disease-related genes and proteins following cardiac arrest. Folia Neuropathol. 2017, 55, 283–288. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Kocki, J.; Bogucka-Kocka, A.; Januszewski, S.; Bogucki, J.; Czuczwar, S.J.; Pluta, R. Autophagy, mitophagy and apoptotic gene changes in the hippocampal CA1 area in a rat ischemic model of Alzheimer’s disease. Pharmacol. Rep. 2017, 69, 1289–1294. [Google Scholar] [CrossRef]
- Wang, P.; Shao, B.Z.; Deng, Z.; Chen, S.; Yue, Z.; Miao, C.Y. Autophagy in ischemic stroke. Prog. Neurobiol. 2018, 163, 98–117. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Kocki, J.; Januszewski, S.; Bogucki, J.; Bogucka-Kocka, A.; Pluta, R. Dysregulation of Autophagy, Mitophagy, and Apoptosis Genes in the CA3 Region of the Hippocampus in the Ischemic Model of Alzheimer’s Disease in the Rat. J. Alzheimers Dis. 2019, 72, 1279–1286. [Google Scholar] [CrossRef]
- Das, T.K.; Ganesh, B.P.; Fatima-Shad, K. Common Signaling Pathways Involved in Alzheimer’s Disease and Stroke: Two Faces of the Same Coin. J. Alzheimers Dis. Rep. 2023, 7, 381–398. [Google Scholar] [CrossRef]
- Zhang, H.; Bezprozvanny, I. “Dirty Dancing” of Calcium and Autophagy in Alzheimer’s Disease. Life 2023, 13, 1187. [Google Scholar] [CrossRef]
- Wang, P.; Guan, Y.F.; Du, H.; Zhai, Q.W.; Su, D.F.; Miao, C.Y. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyl-transferase in cerebral ischemia. Autophagy 2012, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Hadley, G.; Xilouri, M.; Hoyte, L.C.; Nagel, S.; McMenamin, M.M.; Tsaknakis, G.; Watt, S.M.; Drakesmith, C.W.; Chen, R.; et al. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat. Med. 2013, 19, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zou, X.; Zhu, W.; Mao, Y.; Chen, L.; Zhao, F. Minocycline is effective in intracerebral hemorrhage by inhibition of apoptosis and autophagy. J. Neurol. Sci. 2016, 371, 88–95. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Han, S.; Liu, S.; Zhang, N.; Zhao, L.; Li, S.; Li, J. cPKCγ-Modulated Autophagy in Neurons Alleviates Ischemic Injury in Brain of Mice with Ischemic Stroke through Akt-mTOR Pathway. Transl. Stroke Res. 2016, 7, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Nitatori, T.; Sato, N.; Waguri, S.; Karasawa, Y.; Araki, H.; Shibanai, K.; Kominami, E.; Uchiyama, Y. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J. Neurosci. 1995, 15, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Adhami, F.; Liao, G.; Morozov, Y.M.; Schloemer, A.; Schmithorst, V.J.; Lorenz, J.N.; Dunn, R.S.; Vorhees, C.V.; Wills-Karp, M.; Degen, J.L.; et al. Cerebral ischemia hypoxia induces intravascular coagulation and autophagy. Am. J. Pathol. 2006, 169, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, T.Y.; Wei, K.; Guan, Y.F.; Wang, X.; Xu, H.; Su, D.F.; Pei, G.; Miao, C.Y. ARRB1/β-arrestin-1 mediates neuroprotection through coordination of BECN1-dependent autophagy in cerebral ischemia. Autophagy 2014, 10, 1535–1548. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, X.; Li, Q.; Wang, J.; Jia, W.; Sun, X. Exacerbation of ischemia induced amyloid-beta generation by diabetes is associated with autophagy activation in mice brain. Neurosci. Lett. 2010, 479, 215–220. [Google Scholar] [CrossRef]
- Qin, A.P.; Liu, C.F.; Qin, Y.Y.; Hong, L.Z.; Xu, M.; Yang, L.; Liu, J.; Qin, Z.H.; Zhang, H.L. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy 2010, 6, 738–753. [Google Scholar] [CrossRef]
- Tanabe, F.; Yone, K.; Kawabata, N.; Sakakima, H.; Matsuda, F.; Ishidou, Y.; Maeda, S.; Abematsu, M.; Komiya, S.; Setoguchi, T. Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: Functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy 2011, 7, 1462–1471. [Google Scholar] [CrossRef]
- Kasprowska, D.; Machnik, G.; Kost, A.; Gabryel, B. Time-dependent changes in apoptosis upon autophagy inhibition in astrocytes exposed to oxygen and glucose deprivation. Cell. Mol. Neurobiol. 2017, 37, 223–234. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, N.; Shen, H.; Lin, C.; Lin, L.; Yuan, B. Microglial activation with reduction in autophagy limits white matter lesions and improves cognitive defects during cerebral hypoperfusion. Curr. Neurovasc. Res. 2014, 11, 223–229. [Google Scholar] [CrossRef]
- Yang, Z.; Zhong, L.; Zhong, S.; Xian, R.; Yuan, B. Hypoxia induces microglia autophagy and neural inflammation injury in focal cerebral ischemia model. Exp. Mol. Pathol. 2015, 98, 219–224. [Google Scholar] [CrossRef]
- Ni, J.; Wu, Z.; Peterts, C.; Yamamoto, K.; Qing, H.; Nakanishi, H. The critical role of proteolytic relay through cathepsins B and E in the phenotypic change of microglia/macrophage. J. Neurosci. 2015, 35, 12488–12501. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.Y.; Zhang, S.; Chu, S.F.; Wang, Z.Z.; Song, X.Y.; Zuo, W.; Gao, Y.; Yang, P.F.; Chen, N.H. Autophagic flux regulates microglial phenotype according to the time of oxygen-glucose deprivation/reperfusion. Int. Immunopharmacol. 2016, 39, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Yu, S.P.; Chen, D.; Yu, S.S.; Jiang, Y.J.; Genetta, T.; Wei, L. The regulatory role of NF-kappaB in autophagy-like cell death after focal cerebral ischemia in mice. Neuroscience 2013, 244, 16–30. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Zhu, X.C.; Wang, H.F.; Tan, M.S.; Cao, L.; Zhang, Q.Q.; Gao, L.; Shi, J.Q.; Zhang, Y.D.; et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br. J. Pharmacol. 2014, 171, 3146–3157. [Google Scholar] [CrossRef]
- Fang, L.; Li, X.; Zhong, Y.; Yu, J.; Yu, L.; Dai, H.; Yan, M. Autophagy protects human brain microvascular endothelial cells against methylglyoxal-induced injuries, reproducible in a cerebral ischemic model in diabetic rats. J. Neurochem. 2015, 135, 431–440. [Google Scholar] [CrossRef]
- Frugier, T.; Taylor, J.M.; McLean, C.; Bye, N.; Beart, P.M.; Devenish, R.J.; Crack, P.J. Evidence for the recruitment of autophagic vesicles in human brain after stroke. Neurochem. Int. 2016, 96, 62–68. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Cross-talk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Liu, R.; Dong, X.; Zhong, Q. Beclin orthologs: Integrative hubs of cell signaling membrane trafficking and physiology. Trends. Cell Biol. 2015, 25, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Goodall, M.L.; Fitzwalter, B.E.; Zahedi, S.; Wu, M.; Rodriguez, D.; Mulcahy-Levy, J.M.; Green, D.R.; Morgan, M.; Cramer, S.D.; Thorburn, A. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev. Cell 2016, 37, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, X.; Xu, F.; Bahr, B.A.; Shibata, M.; Uchiyama, Y.; Hagberg, H.; Blomgren, K. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005, 12, 162–176. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, F.; Wang, X.; Shibata, M.; Uchiyama, Y.; Blomgren, K.; Hagberg, H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J. Neurochem. 2006, 96, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Ginet, V.; Puyal, J.; Clarke, P.G.; Truttmann, A.C. Enhancement of autophagic flux after neonatal cerebral hypoxia-ischemia and its region-specific relationship to apoptotic mechanisms. Am. J. Pathol. 2009, 175, 1962–1974. [Google Scholar] [CrossRef]
- Wnuk, A.; Kajta, M. Steroid and xenobiotic receptor signalling in apoptosis and autophagy of the nervous system. Int. J. Mol. Sci. 2017, 18, 2394. [Google Scholar] [CrossRef]
- Pluta, R. The role of apolipoprotein E in the deposition of beta-amyloid peptide during ischemia-reperfusion brain injury. A model of early Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000, 903, 324–334. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, H.; Yuan, Y.; Gao, J.; Shen, Z.; Cheng, Y.; Shen, Y.; Wang, R.R.; Wang, X.; Hu, W.W.; et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 2013, 9, 1321–1333. [Google Scholar] [CrossRef]
- Buckley, K.M.; Hess, D.L.; Sazonova, I.Y.; Periyasamy-Thandavan, S.; Barrett, J.R.; Kirks, R.; Grace, H.; Kondrikova, G.; Johnson, M.H.; Hess, D.C.; et al. Rapamycin up-regulation of autophagy reduces infarct size and improves outcomes in both permanent MCAL, and embolic MCAO, murine models of stroke. Exp. Transl. Stroke Med. 2014, 6, 8. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Y.; Jiang, L.; Zhang, J.; Gao, J.; Shen, Z.; Zheng, Y.; Deng, T.; Yan, H.; Li, W.; et al. Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy 2014, 10, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.H.; Chen, T.; Li, X.; Yue, K.Y.; Luo, P.; Yang, L.K.; Zhu, J.; Wang, Y.H.; Fei, Z.; Jiang, X.F. Sirt3 confers protection against neuronal ischemia by inducing autophagy: Involvement of the AMPK-mTOR pathway. Free Radic. Biol. Med. 2017, 108, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Li, F.; Han, J.; Fang, J.; Xu, L.; Sun, C.; Hua, T.; Zhang, Z.; Feng, Z.; Jiang, X. Hif-1alpha overexpression improves transplanted bone mesenchymal stem cells survival in rat MCAO stroke model. Front. Mol. Neurosci. 2017, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Choi, J.; Park, S. Ghrelin protects adult rat hippocampal neural stem cells from excessive autophagy during oxygen-glucose deprivation. Endocr. J. 2018, 65, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.; Jiang, J.; Stavrovskaya, I.G.; Li, M.; Li, W.; Wu, Q.; Zhang, X.; Luo, C.; Zhou, S.; et al. N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J. Neurosci. 2014, 34, 2967–2978. [Google Scholar] [CrossRef]
- Yang, T.; Li, D.; Liu, F.; Qi, L.; Yan, G.; Wang, M. Regulation on Beclin-1 expression by mTOR in CoCl2-induced HT22 cell ischemia-reperfusion injury. Brain. Res. 2015, 1614, 60–66. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, K.; Hu, Z.; Li, W.; Davies, H.; Ling, S.; Rudd, J.A.; Fang, M. Autophagy upregulation and apoptosis downregulation in DAHP and triptolide treated cerebral ischemia. Mediat. Inflamm. 2015, 2015, 120198. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Luo, Y.; Zhu, Y.M.; Liu, Z.H.; Kent, T.A.; Rong, J.G.; Li, W.; Qiao, S.G.; Li, M.; Ni, Y.; et al. Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes. Cell Death Dis. 2017, 8, e2618. [Google Scholar] [CrossRef]
- Vieira, M.; Fernandes, J.; Carreto, L.; Anuncibay-Soto, B.; Santos, M.; Han, J.; Fernandez-Lopez, A.; Duarte, C.B.; Carvalho, A.L.; Santos, A.E. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol. Dis. 2014, 68, 26–36. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Gertner, M.; Pontarelli, F.; Court-Vazquez, B.; Bennett, M.V.; Ofengeim, D.; Zukin, R.S. Global ischemia induces lysosomal-mediated degradation of mTOR and activation of autophagy in hippocampal neurons destined to die. Cell Death Differ. 2017, 24, 317–329. [Google Scholar] [CrossRef]
- Sun, X.; Wang, D.; Zhang, T.; Lu, X.; Duan, F.; Ju, L.; Zhuang, X.; Jiang, X. Eugenol Attenuates Cerebral Ischemia-Reperfusion Injury by Enhancing Autophagy via AMPK-mTOR-P70S6K Pathway. Front. Pharmacol. 2020, 11, 84. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Zhu, X.C.; Zhang, Q.Q.; Tan, M.S.; Cao, L.; Wang, H.F.; Shi, J.Q.; Gao, L.; Qin, H.; et al. Ischemic preconditioning provides neuroprotection by induction of AMP-activated protein kinase-dependent autophagy in a rat model of ischemic stroke. Mol. Neurobiol. 2015, 51, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Yue, J.; Hu, L.N.; Tian, Z.; Zhang, K.; Yang, L.; Zhang, H.N.; Guo, Y.Y.; Feng, B.; Liu, H.Y.; et al. Activation of G protein-coupled receptor 30 protects neurons by regulating autophagy in astrocytes. Glia 2020, 68, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sheng, H.; Liu, S.; Zhao, S.; Glembotski, C.C.; Warner, D.S.; Paschen, W.; Yang, W. Activation of the ATF6 branch of the unfolded protein response in neurons improves stroke outcome. J. Cereb. Blood Flow Metab. 2017, 37, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, C.; Dong, H.; Xia, F.; Zhou, H.; Jiang, X.; Pei, C.; Ren, H.; Li, H.; Li, R.; et al. Immune-related GTPase M (IRGM1) regulates neuronal autophagy in a mouse model of stroke. Autophagy 2012, 8, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Li, N.; Zhang, X.; Li, Y.; Ma, K.; Wang, R.; Qin, X.; Yin, J.; Wang, S. Isoflurane Enhances Autophagy by Activating AMPK/ULK1, Inhibits NLRP3, and Reduces Cognitive Impairment after Cerebral Ischemia-Reperfusion Injury in Rats. J. Mol. Neurosci. 2023, 30. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, M.; Chen, Z. Schaftoside improves cerebral ischemia-reperfusion injury by enhancing autophagy and reducing apoptosis and inflammation through the AMPK/mTOR pathway. Adv. Clin. Exp. Med. 2022, 31, 1343–1354. [Google Scholar] [CrossRef]
- Feng, D.; Wang, B.; Wang, L.; Abraham, N.; Tao, K.; Huang, L.; Shi, W.; Dong, Y.; Qu, Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J. Pineal Res. 2017, 62, e12395. [Google Scholar] [CrossRef]
- Baek, S.H.; Noh, A.R.; Kim, K.A.; Akram, M.; Shin, Y.J.; Kim, E.S.; Yu, S.W.; Majid, A.; Bae, O.N. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 2014, 45, 2438–2443. [Google Scholar] [CrossRef]
- Wang, M.; Liang, X.; Cheng, M.; Yang, L.; Liu, H.; Wang, X.; Sai, N.; Zhang, X. Homocysteine enhances neural stem cell autophagy in in vivo and in vitro model of ischemic stroke. Cell Death Dis. 2019, 10, 561. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.; Tao, L.; Wang, Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 2010, 6, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Zhang, Y.; Li, J.; Zhang, J.; Li, Y.; Dang, C.; Li, C.; Fan, Y.; Yu, J.; Pei, Z.; et al. Beclin 1 knockdown inhibits autophagic activation and prevents the secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy 2012, 8, 63–76. [Google Scholar] [CrossRef]
- Wen, Y.D.; Sheng, R.; Zhang, L.S.; Han, R.; Zhang, X.; Zhang, X.D.; Han, F.; Fukunaga, K.; Qin, Z.H. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 2008, 4, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, S.; Dai, Q.; Zheng, J.; Liu, C.; Li, S.; Li, J. IL-17 Amediated excessive autophagy aggravated neuronal ischemic injuries via Src-PP2B-mTOR pathway. Front. Immunol. 2019, 10, 2952. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.J.; Ding, Y.; Zhang, H.N.; Sun, T.; Zhang, K.; Yang, L.; Guo, Y.Y.; Liu, S.B.; Zhao, M.G.; et al. Silibinin prevents autophagic cell death upon oxidative stress in cortical neurons and cerebral ischemia-reperfusion injury. Mol. Neurobiol. 2016, 53, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, T.; Zhang, Y.; Li, F.; Yu, B.; Kou, J. Schizandrin protects against OGD/R-induced neuronal injury by suppressing autophagy: Involvement of the AMPK/mTOR pathway. Molecules 2019, 24, 3624. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.H.; Qin, C.; Fan, W.H.; Tian, D.S.; Liu, J.L. Fingolimod suppresses neuronal autophagy through the mTOR/p70S6K pathway and alleviates ischemic brain damage in mice. PLoS ONE 2017, 12, e0188748. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.; Zhang, Y.; Bai, Y.; Chen, X.; Huang, R.; Wu, F.; Leng, S.; Chao, J.; Zhang, J.H.; et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: Implications for cerebral ischemic stroke. Autophagy 2018, 14, 1164–1184. [Google Scholar] [CrossRef]
- Clarkson, B.D.; Ling, C.; Shi, Y.; Harris, M.G.; Rayasam, A.; Sun, D.; Salamat, M.S.; Kuchroo, V.; Lambris, J.D.; Sandor, M.; et al. T cell-derived interleukin (IL)-21 promotes brain injury following stroke in mice. J. Exp. Med. 2014, 211, 595–604. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Roughton, K.; Wang, X.; Kroemer, G.; Blomgren, K.; Zhu, C. Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death Dis. 2010, 1, e56. [Google Scholar] [CrossRef]
- McCrary, M.R.; Jiang, M.Q.; Giddens, M.M.; Zhang, J.Y.; Owino, S.; Wei, Z.Z.; Zhong, W.; Gu, X.; Xin, H.; Hall, R.A.; et al. Protective effects of GPR37 via regulation of inflammation and multiple cell death pathways after ischemic stroke in mice. FASEB J. 2019, 33, 10680–10691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Zhang, T.; Wang, M.M.; Wang, X.X.; Qin, Y.Y.; Wu, J.; Han, R.; Sheng, R.; Wang, Y.; Chen, Z.; et al. TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic. Biol. Med. 2019, 137, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of non apoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Gu, W.W.; Liu, Z.H.; Zhu, Y.M.; Rong, J.G.; Kent, T.A.; Li, M.; Qiao, S.G.; An, J.Z.; Zhang, H.L. RIP1K contributes to neuronal and astrocytic cell death in ischemic stroke via activating autophagic-lysosomal pathway. Neuroscience 2018, 371, 60–74. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Mitochondrial and nuclear cross talk in cell death: Parthanatos. Ann. N. Y. Acad. Sci. 2008, 1147, 233–241. [Google Scholar] [CrossRef]
- Zille, M.; Karuppagounder, S.S.; Chen, Y.; Gough, P.J.; Bertin, J.; Finger, J.; Milner, T.A.; Jonas, E.A.; Ratan, R.R. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 2017, 48, 1033–1043. [Google Scholar] [CrossRef]
- Huang, C.T.; Huang, D.Y.; Hu, C.J.; Wu, D.; Lin, W.W. Energy adaptive response during parthanatos is enhanced by PD98059 and involves mitochondrial function but not autophagy induction. Biochim. Biophys. Acta 2014, 1843, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Oliván, S.; Calvo, A.C.; Manzano, R.; Zaragoza, P.; Osta, R. Sex differences in constitutive autophagy. BioMed. Res. Int. 2014, 2014, 652817. [Google Scholar] [CrossRef]
- Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocrinol. 2019, 55, 100787. [Google Scholar] [CrossRef]
- Shang, D.; Wang, L.; Klionsky, D.J.; Cheng, H.; Zhou, R. Sex differences in autophagy-mediated diseases: Toward precision medicine. Autophagy 2021, 17, 1065–1076. [Google Scholar] [CrossRef]
- Mo, Y.; Sun, Y.Y.; Liu, K.Y. Autophagy and inflammation in ischemic stroke. Neural. Regen. Res. 2020, 15, 1388–1396. [Google Scholar]
- Wang, W.; Kang, J.; Li, H.; Su, J.; Wu, J.; Xu, Y.; Yu, H.; Xiang, X.; Yi, H.; Lu, Y.; et al. Regulation of endoplasmic reticulum stress in rat cortex by p62/ZIP through the Keap1-Nrf2-ARE signaling pathway after transient focal cerebral ischaemia. Brain. Inj. 2013, 27, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Chu, K.; Jung, K.H.; Lee, S.T.; Bahn, J.J.; Kim, M.; Lee, S.K.; Roh, J.K. Autophagy is involved in the ischemic preconditioning. Neurosci. Lett. 2009, 451, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Song, D.D.; Zhang, T.T.; Chen, J.L.; Xia, Y.F.; Qin, Z.H.; Waeber, C.; Sheng, R. Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain. Cell Death Dis. 2017, 8, e2912. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 2007, 8, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Barré, B.; Perkins, N.D. The Skp2 promoter integrates signaling through the NF-kappaB, p53, and Akt/GSK3beta pathways to regulate autophagy and apoptosis. Mol. Cell 2010, 38, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Duronio, V. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 2008, 415, 333–344. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Menzies, F.M.; Rubinsztein, D.C. Broadening the therapeutic scope for rapamycin treatment. Autophagy 2010, 6, 286–287. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Chen, J.; Sun, S.; Zhao, J.; Dong, X.; Wang, J. Effects of Estradiol on Autophagy and Nrf-2/ARE Signals after Cerebral Ischemia. Cell Physiol. Biochem. 2017, 41, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, X.; Wang, J.; Mang, J.; Xu, Z. Betulinic acid ameliorates cerebral injury in middle cerebral artery occlusion rats through regulating autophagy. ACS Chem. Neurosci. 2021, 12, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Zheng, D.; Cheng, X.; Sun, Y. Lnc RNA-MIAT promotes neural cell autophagy and apoptosis in ischemic stroke by up-regulating REDD1. Brain Res. 2021, 1763, 147436. [Google Scholar] [CrossRef]
- Liu, N.; Peng, A.; Sun, H.; Zhuang, Y.; Yu, M.; Wang, Q.; Wang, J. Lnc RNA AC136007.2 alleviates cerebral ischemic-reperfusion injury by suppressing autophagy. Aging 2021, 13, 19587–19597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, Y.; Chen, X.; Xiao, X.; Tan, B.; Chen, G.; Hu, J.; Qi, D.; Li, X.; Xie, R. p53 inhibition protects against neuronal ischemia/reperfusion injury by the p 53/PRAS40/mTOR pathway. Oxid. Med. Cell. Longev. 2021, 2021, 4729465. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, X.; Gao, Z.; Chu, L. mi R-155-5p in extracellular vesicles derived from choroid plexus epithelial cells promotes autophagy and inflammation to aggravate ischemic brain injury in mice. Oxid. Med. Cell. Longev. 2022, 2022, 8603427. [Google Scholar] [PubMed]
- Hadley, G.; Beard, D.J.; Couch, Y.; Neuhaus, A.A.; Adriaanse, B.A.; DeLuca, G.C.; Sutherland, B.A.; Buchan, A.M. Rapamycin in ischemic stroke: Old drug, new tricks? JCBFM 2019, 39, 20–35. [Google Scholar] [CrossRef]
- Wang, J.; Lin, X.; Mu, Z.; Shen, F.; Zhang, L.; Xie, Q.; Tang, Y.; Wang, Y.; Zhang, Z.; Yang, G.Y. Rapamycin increases collateral circulation in rodent brain after focal ischemia as detected by multiple modality dynamic imaging. Theranostics 2019, 9, 4923–4934. [Google Scholar] [CrossRef]
- Beard, D.J.; Hadley, G.; Thurley, N.; Howells, D.W.; Sutherland, B.A.; Buchan, A.M. The effect of rapamycin treatment on cerebral ischemia: A systematic review and metaanalysis of animal model studies. Int. J. Stroke 2019, 14, 137–145. [Google Scholar] [CrossRef]
- Chi, O.Z.; Mellender, S.J.; Barsoum, S.; Liu, X.; Damito, S.; Weiss, H.R. Effects of rapamycin pretreatment on blood-brain barrier disruption in cerebral ischemia-reperfusion. Neurosci. Lett. 2016, 620, 132–136. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Kai, J.; Zhu, F.; Dong, J.; Xu, Z.; Wong, M.; Zeng, L.H. Rapamycin prevents cerebral stroke by modulating apoptosis and autophagy in penumbra in rats. Ann. Clin. Transl. Neurol. 2018, 5, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, J. Rapamycin pretreatment alleviates cerebral ischemia/reperfusion injury in dose-response manner through inhibition of the autophagy and NFκB pathways in rats. Dose Response 2020, 18, 1559325820946194. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, Q.; Zhao, Z.; Sui, H.; Xie, X. Resveratrol improves delayed r-tPA treatment outcome by reducing MMPs. Acta Neurol. Scand. 2016, 134, 54–60. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, Z.; Wang, Y.; Hou, Y.; Li, L.; Zhao, J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt 1-dependent autophagy induction. Int. Immunopharmacol. 2017, 50, 208–215. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Jia, G.; Li, L.; Chen, H.; Bi, J.; Wang, C. The synergistic neuroprotective effects of combined rosuvastatin and resveratrol pretreatment against cerebral ischemia/reperfusion injury. J. Stroke Cerebrovasc. Dis. 2018, 27, 1697–1704. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.; Liu, J.; Wen, Q.; Yu, J.; Yu, L.; Xie, K. Role of JNK signaling pathway in dexmedetomidine post-conditioning-induced reduction of the inflammatory response and autophagy effect of focal cerebral ischemia reperfusion injury in rats. Inflammation 2019, 42, 2181–2191. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, H.; Zhang, W.; Ma, X.; Liu, Y. Dexmedetomidine attenuates neuronal injury induced by cerebral ischemia reperfusion by regulating mi R199a. Mol. Med. Rep. 2021, 24, 574. [Google Scholar] [CrossRef]
- Gelb, A.W.; Bayona, N.A.; Wilson, J.X.; Cechetto, D.F. Propofol anesthesia compared to awake reduces infarct size in rats. Anesthesiology 2002, 96, 1183–1190. [Google Scholar] [CrossRef]
- Sun, B.; Ou, H.; Ren, F.; Huan, Y.; Zhong, T.; Gao, M.; Cai, H. Propofol inhibited autophagy through Ca (2+)/CaMKKbeta/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol. Med. 2018, 24, 58. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, D.; Wei, C.; Cui, V.; Wang, H.; Zhu, Y.; Wu, A.; Yue, Y. Propofol attenuates alpha synuclein aggregation and neuronal damage in a mouse model of ischemic stroke. Neurosci. Bull. 2020, 36, 289–298. [Google Scholar] [CrossRef]
- Sun, B.; Ou, H.; Ren, F.; Guan, Y.; Huan, Y.; Cai, H. Propofol protects against cerebral ischemia/reperfusion injury by down-regulating long noncoding RNA SNHG14. ACS Chem. Neurosci. 2021, 12, 3002–3014. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Long, L.; Xu, F.; Feng, L.; Liu, Y.; Shi, J.; Gong, Q. Icariside II, a phosphodiesterase 5 inhibitor, attenuates cerebral ischaemia/reperfusion injury by inhibiting glycogen synthase kinase-3beta-mediated activation of autophagy. Br. J. Pharmacol. 2020, 177, 1434–1452. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Arriola Apelo, S.I.; Lamming, D.W. Rapamycin: An InhibiTOR of aging emerges from the soil of Easter Island. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, A.; Feng, D.; Wang, Y.; Zhang, L.; Cui, Y.; Li, B.; Wang, Z.; Chen, G. Evaluation of the protective potential of brain microvascular endothelial cell autophagy on blood-brain barrier integrity during experimental cerebral ischemia-reperfusion injury. Transl. Stroke Res. 2014, 5, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Ouyang, M.W.; Fang, Y.Y.; Li, S.J.; Zhou, Q.; Fan, J.; Qin, Z.S.; Tao, T. Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1alpha. Front. Cell. Neurosci. 2017, 11, 197. [Google Scholar] [CrossRef]
- Huuskonen, M.T.; Loppi, S.; Dhungana, H.; Keksa-Goldsteine, V.; Lemarchant, S.; Korhonen, P.; Wojciechowski, S.; Pollari, E.; Valonen, P.; Koponen, J.; et al. Bexarotene targets autophagy and is protective against thromboembolic stroke in aged mice with tauopathy. Sci. Rep. 2016, 6, 33176. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, J.; Liu, J.; Yao, M.; Li, L.; Zhang, B.; Zhu, H.; Wang, Z. Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J. Pharmacol. Sci. 2014, 124, 354–364. [Google Scholar] [CrossRef]
- Shi, G.S.; Qin, Q.L.; Huang, C.; Li, Z.R.; Wang, Z.H.; Wang, Y.Y.; He, X.Y.; Zhao, X.M. The Pathological Mechanism of Neuronal Autophagy-Lysosome Dysfunction after Ischemic Stroke. Cell. Mol. Neurobiol. 2023, 29. ahead of print. [Google Scholar] [CrossRef]
| Reference | Model | Animal | Autophagy Induction by | Autophagy Inhibition by | Role |
|---|---|---|---|---|---|
| [72] | 4VO | Rats | Tsc1 induction | Protective | |
| [110] | 4VO | Rats | Rapamycin induction | Protective | |
| [111] | tMCAO | Rats | Eugenol induction | Protective | |
| [87] | pMCAO | Rats | Metformin induction | Protective | |
| [112] | pMCAO | Rats | Compound C inhibition | Protective | |
| [113] | tMCAO | Mice | GPR30 knockout inhibition | Protective | |
| [77] | tMCAO | Mice | ARRB1 knockout inhibition | Protective | |
| [114] | tMCAO | Mice | ATF6 knockin induction | Protective | |
| [115] | pMCAO | Mice | Immune-related GTPase M1 knockout inhibition | Protective | |
| [116] | tMCAO | Rats | Isoflurane induction | Protective | |
| [117] | tMCAO | Rats | Schaftoside induction | Protective | |
| [118] | tMCAO | Rats | Melatonin inhibition | Harmful | |
| [108] | pMCAO | Rats | 3-methyladenine or wortmannin inhibition | Harmful | |
| [119] | tMCAO or pMCAO | Rats | Carnosine inhibition | Harmful | |
| [120] | tMCAO | Rats | Homocysteine induction | Harmful | |
| [121] | pMCAO | Rats | 3-methyladenine inhibition | Harmful | |
| [122] | tMCAO | Rats | Becn1-shRNA or 3-methyladenine inhibition | Harmful | |
| [123] | pMCAO | Rats | Bafliomycin A1 or 3-methyladenine inhibition | Harmful | |
| [124] | tMCAO | Mice | IL-17A induction | Harmful | |
| [105] | pMCAO | Mice | N-acetyl-serotonin inhibition | Harmful | |
| [125] | pMCAO | Mice | Silibinin inhibition | Harmful | |
| [126] | tMCAO | Mice | Schizandrin inhibition | Harmful | |
| [127] | tMCAO | Mice | 3-methyladenine inhibition | Harmful | |
| [128] | tMCAO | Mice | CircHECTD1 knockdown inhibition | Harmful | |
| [129] | tMCAO | Mice | IL-21 knockout inhibition | Harmful | |
| [130] | Hypoxic-ischemic | Neonatal rats | Lithium inhibition | Harmful | |
| [131] | tMCAO | Mice | GPR37 knockout induction | Harmful | |
| [132] | tMCAO | Mice | TIGAR knockout induction and TIGARtransgene inhibition | Harmful |
| References | Molecules | Effect on Autophagy | Action by | End Result on Ischemia |
|---|---|---|---|---|
| [157,158,159,160,161,162,175] | Rapamycin | Induction | mTOR-dependent activity | ↓ Infarct volume, neuronal injury, neurological deficits, endothelial cell death, BBB injury |
| [163,164,165] | Resveratrol | Induction | Sirt1-dependent autophagy activity | ↓ Infarct volume, inflammation, brain edema, apoptosis, neurological deficits. ↑ Therapeutic window |
| [166,167,176] | Dexmedetomidine | Inhibition-excessive autophagy | Upregulation of HIF-1α | ↓ Neuronal injury, neurological deficits, beclin 1, caspase 3. ↑ Learning, memory, neurological outcome |
| [168,169,170,171] | Propofol | Inhibition-excessive autophagy | Regulation of mTOR/S6K1 | ↓ Infarct volume. ↑ Neurological outcome |
| [118] | Melatonin | Inhibition-excessive autophagy | Inhibiting ER stress-dependent autophagy | ↓ Apoptosis, brain edema, neurological deficits |
| [172] | Icariside II | Inhibiting-excessive autophagy | PKG/GSK-3β signaling | ↓ Autophagic neuronal death |
| [72] | Hamartin | Initiation | Inhibition of mTORC1 | ↑ Neuroprotective effect |
| [177] | Bexarotene | Initiation | Inhibition of autophagosome degradation | ↓ Infarct size, behavioral deficits |
| [178] | Melatonin | Inhibition | Activating the PI3K/Akt pro-survival pathway and inhibiting expression of beclin-1 | ↓ Infarct size, neurological deficits |
| [73] | Minocycline | Inhibition | Suppressing beclin-1 | ↓ Cell damage, caspase 3 and 8 |
| [127] | Fingolimod | Suppression | Activation of mTOR/p70S6K pathway, decrease in autophagosome and beclin 1 | ↓ Infarct volume, neuronal apoptosis, functional deficits |
| [105] | N-acetyl-serotonin | Suppression | Reducing the activation of autophagy | ↓ Mitochondrial cell death pathway, autophagic cell death |
| [123] | 3-methyladenine | Increase in autophagos-omes | Inhibition on ischemia-induced upregulation of LC3-II | ↓ Infarct volume, brain edema, motor deficits |
| [119] | Carnosine | Attenuation | Attenuation of deleterious autophagic process | ↑ Improvement in brain mitochondrial function, mitophagy signaling |
| [87] | Metformin | Initiation | Activation of brain AMPK, | ↓ Infarct volume, neurological deficits, cell apoptosis |
| [175] | Lithium carbonate | Induction | mTOR-independent | ↓ Evans blue extravasation, brain water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluta, R. The Dual Role of Autophagy in Postischemic Brain Neurodegeneration of Alzheimer’s Disease Proteinopathy. Int. J. Mol. Sci. 2023, 24, 13793. https://doi.org/10.3390/ijms241813793
Pluta R. The Dual Role of Autophagy in Postischemic Brain Neurodegeneration of Alzheimer’s Disease Proteinopathy. International Journal of Molecular Sciences. 2023; 24(18):13793. https://doi.org/10.3390/ijms241813793
Chicago/Turabian StylePluta, Ryszard. 2023. "The Dual Role of Autophagy in Postischemic Brain Neurodegeneration of Alzheimer’s Disease Proteinopathy" International Journal of Molecular Sciences 24, no. 18: 13793. https://doi.org/10.3390/ijms241813793
APA StylePluta, R. (2023). The Dual Role of Autophagy in Postischemic Brain Neurodegeneration of Alzheimer’s Disease Proteinopathy. International Journal of Molecular Sciences, 24(18), 13793. https://doi.org/10.3390/ijms241813793






