Move Your Body toward Healthy Aging: Potential Neuroprotective Mechanisms of Irisin in Alzheimer’s Disease
Abstract
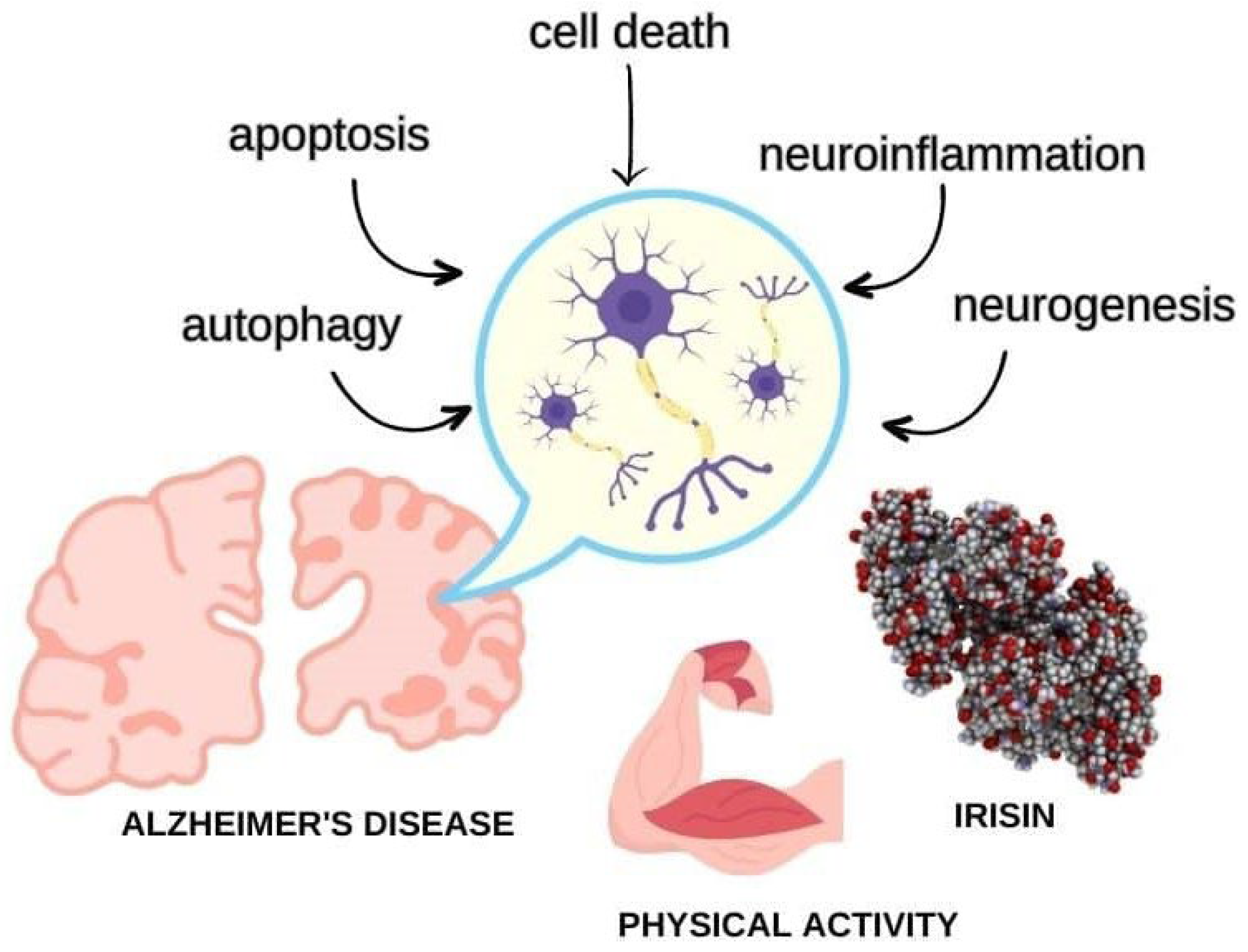
1. Introduction
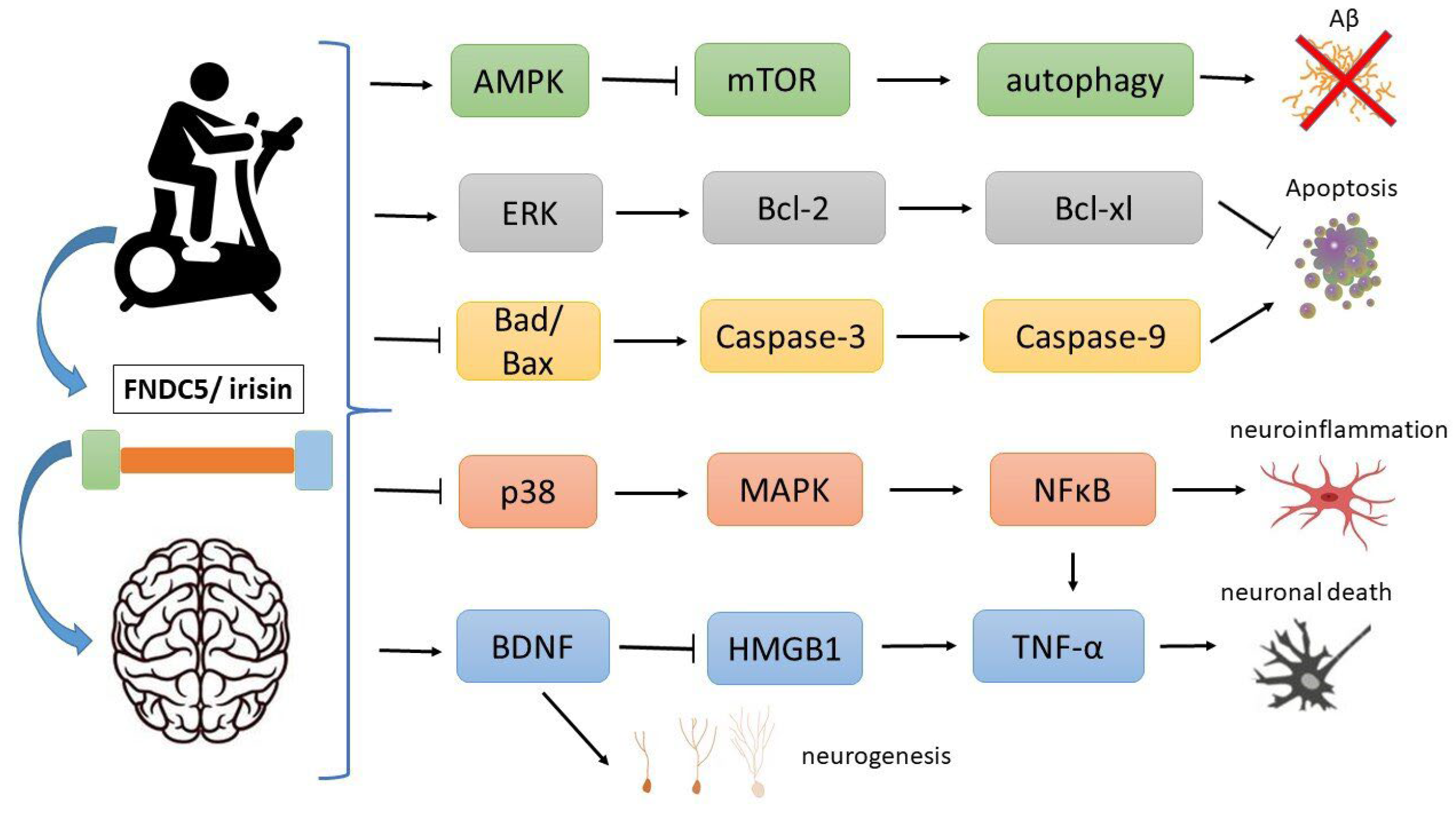
2. Irisin
3. Irisin and AD
4. Autophagy
5. Cell Death
6. Apoptosis
7. Oxidative Stress
8. Neuroinflammation
9. Conclusions
10. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Feldman, H.; Fillit, H.; Sano, M.; Schmitt, F.; Aisen, P.; Leibman, C.; Mucha, L.; Ryan, J.M.; Sullivan, S.D.; et al. Dependence as a Unifying Construct in Defining Alzheimer’s Disease Severity. Alzheimers Dement. 2010, 6, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimers Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Physical Activity and Risk of Neurodegenerative Disease: A Systematic Review of Prospective Evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- So, W.Y.; Leung, P.S. Irisin Ameliorates Hepatic Glucose/lipid Metabolism and Enhances Cell Survival in Insulin-Resistant Human HepG2 Cells through Adenosine Monophosphate-Activated Protein Kinase Signaling. Int. J. Biochem. Cell Biol. 2016, 78, 237–247. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The Structure of Irisin Reveals a Novel Intersubunit β-Sheet Fibronectin Type III (FNIII) Dimer: Implications for Receptor Activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. mRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Dun, S.L.; Lyu, R.-M.; Chen, Y.-H.; Chang, J.-K.; Luo, J.J.; Dun, N.J. Irisin-Immunoreactivity in Neural and Non-Neural Cells of the Rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-Linked FNDC5/irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Kim, O.Y.; Song, J. The Role of Irisin in Alzheimer’s Disease. J. Clin. Med. Res. 2018, 7, 407. [Google Scholar] [CrossRef]
- Wong, T.-S.; Li, G.; Li, S.; Gao, W.; Chen, G.; Gan, S.; Zhang, M.; Li, H.; Wu, S.; Du, Y. G Protein-Coupled Receptors in Neurodegenerative Diseases and Psychiatric Disorders. Signal Transduct. Target. Ther. 2023, 8, 177. [Google Scholar]
- Alzoughool, F.; Al-Zghoul, M.B.; Al-Nassan, S.; Alanagreh, L.; Mufleh, D.; Atoum, M. The Optimal Therapeutic Irisin Dose Intervention in Animal Model: A Systematic Review. Vet. World 2020, 13, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Berthold, H.K.; Huh, J.Y.; Berman, R.; Spenrath, N.; Krone, W.; Mantzoros, C.S. Effects of Lipid-Lowering Drugs on Irisin in Human Subjects In Vivo and in Human Skeletal Muscle Cells Ex Vivo. PLoS ONE 2013, 8, e72858. [Google Scholar] [CrossRef]
- Rachid, T.L.; Penna-de-Carvalho, A.; Bringhenti, I.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Fenofibrate (PPARalpha Agonist) Induces Beige Cell Formation in Subcutaneous White Adipose Tissue from Diet-Induced Male Obese Mice. Mol. Cell. Endocrinol. 2015, 402, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-J.; Huang, F.; Lu, W.-J.; Jiang, G.-J.; Deng, Y.-P.; Shen, F.-M. Metformin Promotes Irisin Release from Murine Skeletal Muscle Independently of AMP-Activated Protein Kinase Activation. Acta Physiol. 2015, 213, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, L.; Zhang, Y.; Zhang, L.; An, Y.; Liu, J.; Wang, G. Effect of Sitagliptin on Serum Irisin Levels in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Diabetes Ther. 2021, 12, 1029–1039. [Google Scholar] [CrossRef]
- Amengual, J.; García-Carrizo, F.J.; Arreguín, A.; Mušinović, H.; Granados, N.; Palou, A.; Bonet, M.L.; Ribot, J. Retinoic Acid Increases Fatty Acid Oxidation and Irisin Expression in Skeletal Muscle Cells and Impacts Irisin In Vivo. Cell. Physiol. Biochem. 2018, 46, 187–202. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Liu, J.; Xie, W.; Xu, W.; Liang, F.; Huang, K.; He, X. Intraperitoneal Administration of Follistatin Promotes Adipocyte Browning in High-Fat Diet-Induced Obese Mice. PLoS ONE 2019, 14, e0220310. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Lourenco, M.V.; Oliveira, M.M.; De Felice, F.G. Soluble Amyloid-β Oligomers as Synaptotoxins Leading to Cognitive Impairment in Alzheimer’s Disease. Front. Cell. Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Lepeta, K.; Lourenco, M.V.; Schweitzer, B.C.; Martino Adami, P.V.; Banerjee, P.; Catuara-Solarz, S.; de La Fuente Revenga, M.; Guillem, A.M.; Haidar, M.; Ijomone, O.M.; et al. Synaptopathies: Synaptic Dysfunction in Neurological Disorders—A Review from Students to Students. J. Neurochem. 2016, 138, 785–805. [Google Scholar] [PubMed]
- de Freitas, G.B.; Lourenco, M.V.; De Felice, F.G. Protective Actions of Exercise-Related FNDC5/Irisin in Memory and Alzheimer’s Disease. J. Neurochem. 2020, 155, 602–611. [Google Scholar] [CrossRef]
- Flori, L.; Testai, L.; Calderone, V. The “Irisin System”: From Biological Roles to Pharmacological and Nutraceutical Perspectives. Life Sci. 2021, 267, 118954. [Google Scholar] [CrossRef]
- Novelle, M.G.; Contreras, C.; Romero-Picó, A.; López, M.; Diéguez, C. Irisin, Two Years Later. Int. J. Endocrinol. 2013, 2013, 746281. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined Adult Neurogenesis and BDNF Mimic Exercise Effects on Cognition in an Alzheimer’s Mouse Model. Science 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Bretland, K.A.; Lin, L.; Bretland, K.M.; Smith, M.A.; Fleming, S.M.; Dengler-Crish, C.M. Irisin Treatment Lowers Levels of Phosphorylated Tau in the Hippocampus of Pre-Symptomatic Female but Not Male Htau Mice. Neuropathol. Appl. Neurobiol. 2021, 47, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.S.; Anderson, B.G.; Conner, J.D.; Wolden-Hanson, T. Circulating Irisin Levels and Muscle FNDC5 mRNA Expression Are Independent of IL-15 Levels in Mice. Endocrine 2015, 50, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Atci, İ.B.; Kalayci, M.; Yilmaz, M.; Kuloglu, T.; Aydin, S.; Kom, M.; Ayden, O.; Aydin, S. Effect of Carnosine, Methylprednisolone and Their Combined Application on Irisin Levels in the Plasma and Brain of Rats with Acute Spinal Cord Injury. Neuropeptides 2015, 52, 47–54. [Google Scholar] [CrossRef]
- Piya, M.K.; Harte, A.L.; Sivakumar, K.; Tripathi, G.; Voyias, P.D.; James, S.; Sabico, S.; Al-Daghri, N.M.; Saravanan, P.; Barber, T.M.; et al. The Identification of Irisin in Human Cerebrospinal Fluid: Influence of Adiposity, Metabolic Markers, and Gestational Diabetes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E512–E518. [Google Scholar] [CrossRef]
- Ferrer-Martínez, A.; Ruiz-Lozano, P.; Chien, K.R. Mouse PeP: A Novel Peroxisomal Protein Linked to Myoblast Differentiation and Development. Dev. Dyn. 2002, 224, 154–167. [Google Scholar] [CrossRef]
- Jodeiri Farshbaf, M.; Alviña, K. Multiple Roles in Neuroprotection for the Exercise Derived Myokine Irisin. Front. Aging Neurosci. 2021, 13, 649929. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Korsmeyer, S.J. Irisin and the Therapeutic Benefits of Exercise. BMC Proc. 2012, 6, O23. [Google Scholar] [CrossRef]
- Briken, S.; Rosenkranz, S.C.; Keminer, O.; Patra, S.; Ketels, G.; Heesen, C.; Hellweg, R.; Pless, O.; Schulz, K.-H.; Gold, S.M. Effects of Exercise on Irisin, BDNF and IL-6 Serum Levels in Patients with Progressive Multiple Sclerosis. J. Neuroimmunol. 2016, 299, 53–58. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Flouris, A.D.; Dinas, P.C.; Valente, A.; Andrade, C.M.B.; Kawashita, N.H.; Sakellariou, P. Exercise-Induced Effects on UCP1 Expression in Classical Brown Adipose Tissue: A Systematic Review. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.H.; Weaver, A.N.; Coker, M.S.; Murphy, C.J.; Gunga, H.-C.; Steinach, M. Metabolic Responses to the Yukon Arctic Ultra: Longest and Coldest in the World. Med. Sci. Sports Exercise 2017, 49, 357. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miah, M.; Culbreth, M.; Aschner, M. Autophagy in Neurodegenerative Diseases and Metal Neurotoxicity. Neurochem. Res. 2016, 41, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Pourbagher-Shahri, A.M.; Farkhondeh, T.; Samarghandian, S. Molecular and Cellular Pathways Contributing to Brain Aging. Behav. Brain Funct. 2021, 17, 6. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Chung, K.M.; Hernández, N.; Sproul, A.A.; Yu, W.H. Alzheimer’s Disease and the Autophagic-Lysosomal System. Neurosci. Lett. 2019, 697, 49–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Song, Y.-Q.; Tu, J. Autophagy in Alzheimer’s Disease Pathogenesis: Therapeutic Potential and Future Perspectives. Ageing Res. Rev. 2021, 72, 101464. [Google Scholar] [CrossRef]
- Liu, J.; Li, L. Targeting Autophagy for the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Front. Mol. Neurosci. 2019, 12, 203. [Google Scholar] [CrossRef]
- Bujak, A.L.; Crane, J.D.; Lally, J.S.; Ford, R.J.; Kang, S.J.; Rebalka, I.A.; Green, A.E.; Kemp, B.E.; Hawke, T.J.; Schertzer, J.D.; et al. AMPK Activation of Muscle Autophagy Prevents Fasting-Induced Hypoglycemia and Myopathy during Aging. Cell Metab. 2015, 21, 883–890. [Google Scholar] [CrossRef]
- Sánchez, B.; Muñoz-Pinto, M.F.; Cano, M. Irisin Enhances Longevity by Boosting SIRT1, AMPK, Autophagy and Telomerase. Expert. Rev. Mol. Med. 2022, 25, e4. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Stachowiak, A.; Mamun, A.A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front. Aging Neurosci. 2018, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated Tau Interactome in the Human Alzheimer’s Disease Brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and Cellular Mechanisms Underlying the Pathogenesis of Alzheimer’s Disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef]
- Silva, M.V.F.; de Loures, C.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; das Carvalho, G.M. Alzheimer’s Disease: Risk Factors and Potentially Protective Measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A Crucial Pathogenic Mediator of Human Disease. JCI Insight 2019, 4, e128834. [Google Scholar] [CrossRef]
- Mangalmurti, A.; Lukens, J.R. How Neurons Die in Alzheimer’s Disease: Implications for Neuroinflammation. Curr. Opin. Neurobiol. 2022, 75, 102575. [Google Scholar] [CrossRef]
- Chen, K.; Wang, K.; Wang, T. Protective Effect of Irisin against Alzheimer’s Disease. Front. Psychiatry 2022, 13, 967683. [Google Scholar] [CrossRef]
- Butt, Z.D.; Hackett, J.D.; Volkoff, H. Irisin in Goldfish (Carassius auratus): Effects of Irisin Injections on Feeding Behavior and Expression of Appetite Regulators, Uncoupling Proteins and Lipoprotein Lipase, and Fasting-Induced Changes in FNDC5 Expression. Peptides 2017, 90, 27–36. [Google Scholar] [CrossRef]
- Ibrahim, R.R.; Shafik, N.M.; El-Esawy, R.O.; El-Sakaa, M.H.; Arakeeb, H.M.; El-Sharaby, R.M.; Ali, D.A.; El-deeb, O.S.; El-Khalik, S.R.A. The Emerging Role of Irisin in Experimentally Induced Arthritis: A Recent Update Involving HMGB1/MCP1/Chitotriosidase I-mediated Necroptosis. Redox Rep. 2022, 27, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yu, R.; Liu, S.; Huwatibieke, B.; Li, Z.; Zhang, W. Irisin Inhibits Hepatic Cholesterol Synthesis via AMPK-SREBP2 Signaling. EBioMedicine 2016, 6, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Du, F.; Li, X.; Wang, M.; Duan, R.; Zhang, J.; Wu, Y.; Zhang, Q. Effects and Underlying Mechanisms of Irisin on the Proliferation and Apoptosis of Pancreatic β Cells. PLoS ONE 2017, 12, e0175498. [Google Scholar] [CrossRef]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin Protects against Endothelial Injury and Ameliorates Atherosclerosis in Apolipoprotein E-Null Diabetic Mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wu, F.; Zhang, Y.; Zhang, Y.; Wang, F.; Jiang, M.; Wang, Z.; Zhang, M.; Li, S.; Yang, L.; et al. Irisin Promotes Human Umbilical Vein Endothelial Cell Proliferation through the ERK Signaling Pathway and Partly Suppresses High Glucose-Induced Apoptosis. PLoS ONE 2014, 9, e110273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, M.; Tan, J.; Pei, X.; Lu, C.; Xin, Y.; Deng, S.; Zhao, F.; Gao, Y.; Gong, Y. Irisin Ameliorates Neuroinflammation and Neuronal Apoptosis through Integrin αVβ5/AMPK Signaling Pathway after Intracerebral Hemorrhage in Mice. J. Neuroinflamm. 2022, 19, 82. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative Stress and Neurodegeneration: Where Are We Now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants and Human Diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Liang, J.; Kirberger, M.; Chen, N. Irisin, an Exercise-Induced Bioactive Peptide Beneficial for Health Promotion during Aging Process. Ageing Res. Rev. 2022, 80, 101680. [Google Scholar] [CrossRef] [PubMed]
- Gella, A.; Durany, N. Oxidative Stress in Alzheimer Disease. Cell Adh. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Agrawal, I.; Jha, S. Mitochondrial Dysfunction and Alzheimer’s Disease: Role of Microglia. Front. Aging Neurosci. 2020, 12, 252. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.; Tremblay, M.-È. Chronic Stress as a Risk Factor for Alzheimer’s Disease: Roles of Microglia-Mediated Synaptic Remodeling, Inflammation, and Oxidative Stress. Neurobiol. Stress. 2018, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Tamagno, E.; Robino, G.; Obbili, A.; Bardini, P.; Aragno, M.; Parola, M.; Danni, O. H2O2 and 4-Hydroxynonenal Mediate Amyloid Beta-Induced Neuronal Apoptosis by Activating JNKs and p38MAPK. Exp. Neurol. 2003, 180, 144–155. [Google Scholar] [CrossRef]
- Prasad, K.N. Oxidative Stress and pro-Inflammatory Cytokines May Act as One of the Signals for Regulating microRNAs Expression in Alzheimer’s Disease. Mech. Ageing Dev. 2017, 162, 63–71. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Valaris, S.; Wrann, C.D. A Role for FNDC5/Irisin in the Beneficial Effects of Exercise on the Brain and in Neurodegenerative Diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise Benefits on Alzheimer’s Disease: State-of-the-Science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A Glance at the Therapeutic Potential of Irisin against Diseases Involving Inflammation, Oxidative Stress, and Apoptosis: An Introductory Review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.; Stevens, B. Microglia: The Brain’s First Responders. Cerebrum 2017, 2017, cer-14-17. [Google Scholar]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia Emerge as Central Players in Brain Disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Zecca, C.; Dell’Abate, M.T.; Buccoliero, C.; Logroscino, G.; Colucci, S.; Grano, M. FNDC5/Irisin System in Neuroinflammation and Neurodegenerative Diseases: Update and Novel Perspective. Int. J. Mol. Sci. 2021, 22, 1605. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased Clearance of CNS Beta-Amyloid in Alzheimer’s Disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Fernandez, F.; Nikolic, W.V.; Obregon, D.; Rrapo, E.; Town, T.; Tan, J. Inflammaging as a Prodrome to Alzheimer’s Disease. J. Neuroinflam. 2008, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Madhu, L.N.; Somayaji, Y.; Shetty, A.K. Promise of Irisin to Attenuate Cognitive Dysfunction in Aging and Alzheimer’s Disease. Ageing Res. Rev. 2022, 78, 101637. [Google Scholar] [CrossRef]
- Wang, K.; Song, F.; Xu, K.; Liu, Z.; Han, S.; Li, F.; Sun, Y. Irisin Attenuates Neuroinflammation and Prevents the Memory and Cognitive Deterioration in Streptozotocin-Induced Diabetic Mice. Mediat. Inflamm. 2019, 2019, 1567179. [Google Scholar] [CrossRef]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of Irisin in Physiology and Pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Ribeiro, F.C.; Sudo, F.K.; Drummond, C.; Assunção, N.; Vanderborght, B.; Tovar-Moll, F.; Mattos, P.; De Felice, F.G.; Ferreira, S.T. Cerebrospinal Fluid Irisin Correlates with Amyloid-β, BDNF, and Cognition in Alzheimer’s Disease. Alzheimers Dement. 2020, 12, e12034. [Google Scholar] [CrossRef]
- Rody, T.; De Amorim, J.A.; De Felice, F.G. The Emerging Neuroprotective Roles of Exerkines in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 965190. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellettini-Santos, T.; Batista-Silva, H.; Marcolongo-Pereira, C.; Quintela-Castro, F.C.d.A.; Barcelos, R.M.; Chiepe, K.C.M.B.; Rossoni, J.V., Jr.; Passamani-Ambrosio, R.; da Silva, B.S.; Chiarelli-Neto, O.; et al. Move Your Body toward Healthy Aging: Potential Neuroprotective Mechanisms of Irisin in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 12440. https://doi.org/10.3390/ijms241512440
Bellettini-Santos T, Batista-Silva H, Marcolongo-Pereira C, Quintela-Castro FCdA, Barcelos RM, Chiepe KCMB, Rossoni JV Jr., Passamani-Ambrosio R, da Silva BS, Chiarelli-Neto O, et al. Move Your Body toward Healthy Aging: Potential Neuroprotective Mechanisms of Irisin in Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(15):12440. https://doi.org/10.3390/ijms241512440
Chicago/Turabian StyleBellettini-Santos, Tatiani, Hemily Batista-Silva, Clairton Marcolongo-Pereira, Fernanda Cristina de Abreu Quintela-Castro, Rafael Mazioli Barcelos, Kelly Cristina Mota Braga Chiepe, Joamyr Victor Rossoni, Jr., Roberta Passamani-Ambrosio, Bruno Spalenza da Silva, Orlando Chiarelli-Neto, and et al. 2023. "Move Your Body toward Healthy Aging: Potential Neuroprotective Mechanisms of Irisin in Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 15: 12440. https://doi.org/10.3390/ijms241512440
APA StyleBellettini-Santos, T., Batista-Silva, H., Marcolongo-Pereira, C., Quintela-Castro, F. C. d. A., Barcelos, R. M., Chiepe, K. C. M. B., Rossoni, J. V., Jr., Passamani-Ambrosio, R., da Silva, B. S., Chiarelli-Neto, O., & Garcez, M. L. (2023). Move Your Body toward Healthy Aging: Potential Neuroprotective Mechanisms of Irisin in Alzheimer’s Disease. International Journal of Molecular Sciences, 24(15), 12440. https://doi.org/10.3390/ijms241512440








