A Review of the Molecular Landscape of Adenoid Cystic Carcinoma of the Lacrimal Gland
Abstract
1. Introduction
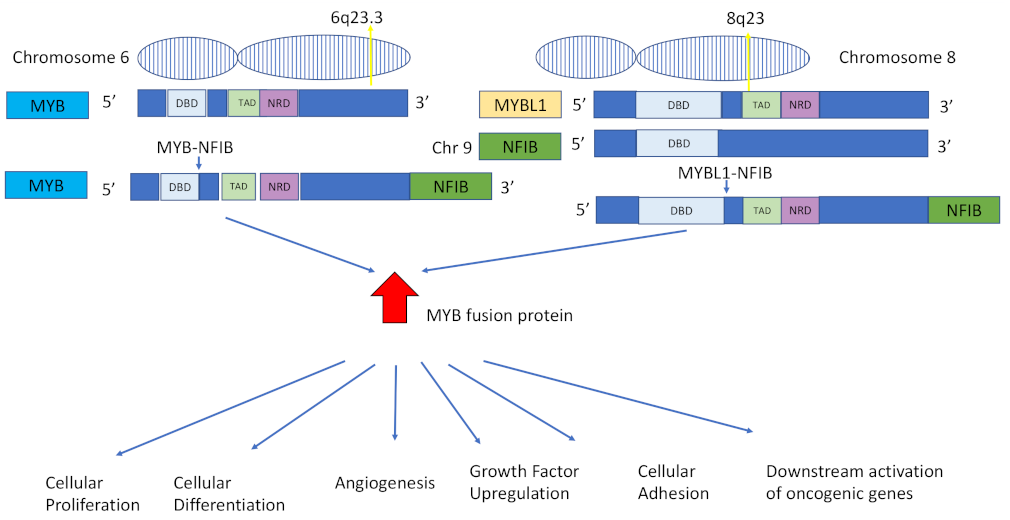
2. MYB/NFIB Translocation
2.1. MYB–NFIB Fusion in Salivary Gland ACC
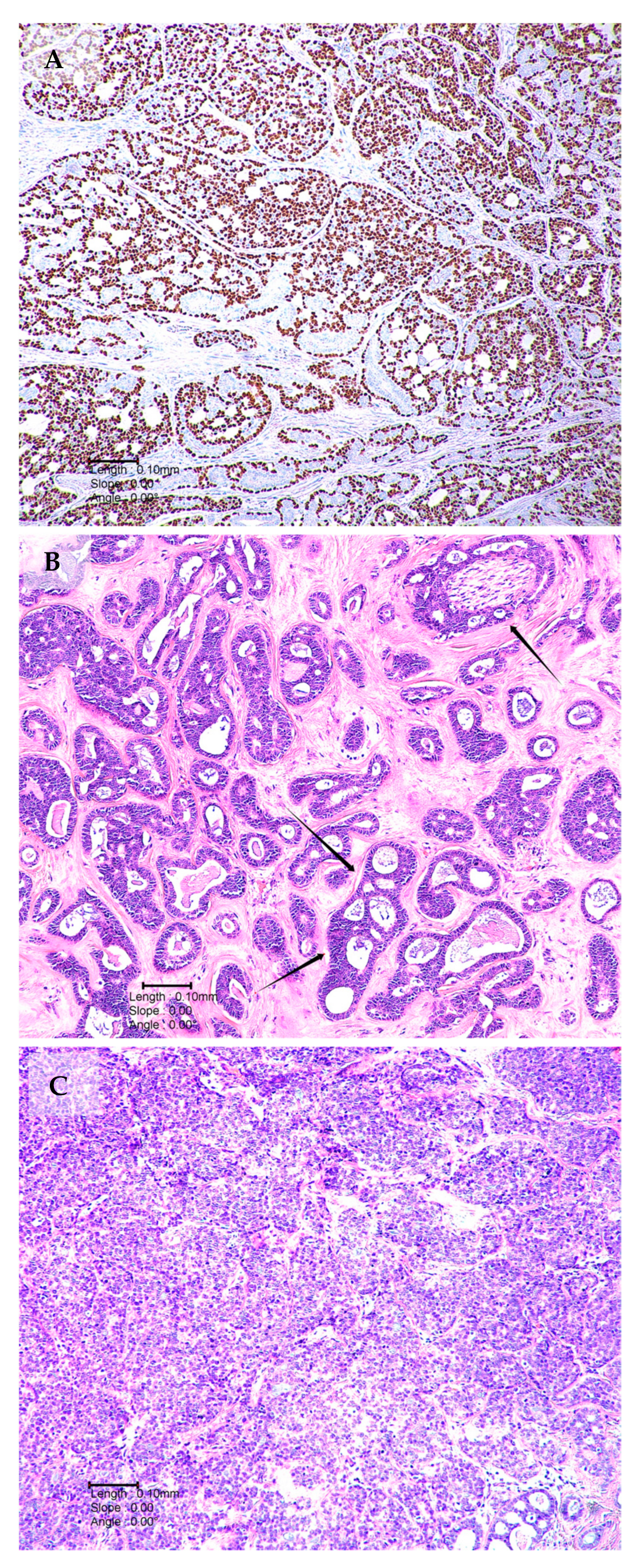
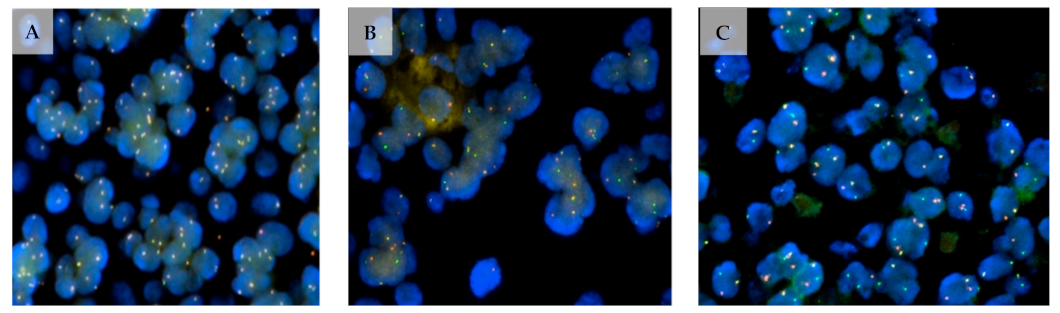
2.2. MYB–NFIB Fusion in Lacrimal Gland ACC
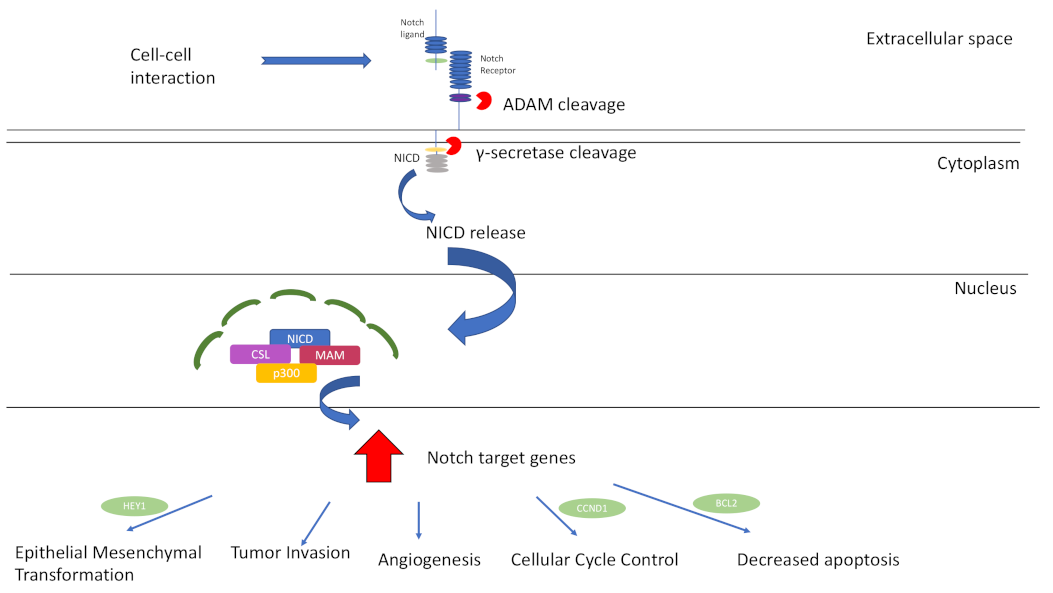
3. Notch-Signalling Pathway
Notch Signalling and Lacrimal Gland ACC
4. DNA Damage Repair Gene (DDRG) Mutations
5. Epigenetic Modifications in ACC Pathogenesis
6. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coca-Pelaz, A.; Rodrigo, J.P.; Bradley, P.J.; Poorten, V.V.; Triantafyllou, A.; Hunt, J.L.; Strojan, P.; Rinaldo, A.; Haigentz, M.; Takes, R.P.; et al. Adenoid cystic carcinoma of the head and neck–An update. Oral Oncol. 2015, 51, 652–661. [Google Scholar] [CrossRef]
- Thierauf, J.; Ramamurthy, N.; Jo, V.Y.; Robinson, H.; Frazier, R.P.; Gonzalez, J.; Pacula, M.; Meneses, E.D.; Nose, V.; Nardi, V.; et al. Clinically Integrated Molecular Diagnostics in Adenoid Cystic Carcinoma. Oncologist 2019, 24, 1356–1367. [Google Scholar] [CrossRef]
- Chummun, S.; McLean, N.; Kelly, C.; Dawes, P.; Fellows, S.; Meikle, D.; Soames, J. Adenoid cystic carcinoma of the head and neck. Br. J. Plast. Surg. 2001, 54, 476–480. [Google Scholar] [CrossRef]
- Belulescu, I.C.; Margaritescu, C.; Dumitrescu, C.I.; DĂguci, L.; Munteanu, C.; Margaritescu, O.C. Adenoid Cystic Carcinoma of Salivary Gland: A Ten-Year Single Institute Experience. Curr. Health Sci. J. 2020, 46, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, L.; Zhao, H.; El-Naggar, A.K.; Sturgis, E.M. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer 2012, 118, 3945–3953. [Google Scholar] [CrossRef]
- von Holstein, S.L.; Therkildsen, M.H.; Prause, J.U.; Stenman, G.; Siersma, V.D.; Heegaard, S. Lacrimal gland lesions in Denmark between 1974 and 2007. Acta Ophthalmol. 2013, 91, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Eberhart, C.G.; Kivelä, T.T. WHO Classification of Tumours of the Eye; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Bernardini, F.P.; Devoto, M.H.; Croxatto, J.O. Epithelial tumors of the lacrimal gland: An update. Curr. Opin. Ophthalmol. 2008, 19, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Sen, S.; Pushker, N.; Bajaj, M.S.; Kashyap, S.; Bakhshi, S.; Chosdol, K.; Meel, R.; Sharma, M.C. Prognostic impact of Notch1 receptor and clinicopathological High-Risk Predictors in lacrimal gland adenoid cystic carcinoma. Acta Ophthalmol. 2021, 99, e1467–e1473. [Google Scholar] [CrossRef]
- Lesueur, P.; Rapeaud, E.; De Marzi, L.; Goudjil, F.; Levy, C.; Galatoire, O.; Jacomet, P.V.; Dendale, R.; Calugaru, V. Adenoid Cystic Carcinoma of the Lacrimal Gland: High Dose Adjuvant Proton Therapy to Improve Patients Outcomes. Front. Oncol. 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Drill, E.; Ho, A.; Ho, A.; Dunn, L.; Prieto-Granada, C.N.; Chan, T.; Ganly, I.; Ghossein, R.; Katabi, N. Predictors of Outcome in Adenoid Cystic Carcinoma of Salivary Glands: A Clinicopathologic Study with Correlation between MYB Fusion and Protein Expression. Am. J. Surg. Pathol. 2017, 41, 1422–1432. [Google Scholar] [CrossRef]
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.T.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.A.; Eng, S.; Huse, J.T.; et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013, 45, 791–798. [Google Scholar] [CrossRef]
- Williams, M.D.; Al-Zubidi, N.; Debnam, J.M.; Shinder, R.; DeMonte, F.; Esmaeli, B. Bone Invasion by Adenoid Cystic Carcinoma of the Lacrimal Gland: Preoperative Imaging Assessment and Surgical Considerations. Ophthalmic Plast. Reconstr. Surg. 2010, 26, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Gamel, J.W.; Font, R.L. Adenoid cystic carcinoma of the lacrimal gland: The clinical significance of a basaloid histologic pattern. Hum. Pathol. 1982, 13, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Font, R.L.; Smith, S.L.; Bryan, R.G. Malignant Epithelial Tumors of the Lacrimal Gland: A Clinicopathologic Study of 21 Cases. Arch. Ophthalmol. 1998, 116, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Emerick, C.; Mariano, F.V.; Vargas, P.A.; Nör, J.E.; Squarize, C.H.; Castilho, R.M. Adenoid Cystic Carcinoma from the salivary and lacrimal glands and the breast: Different clinical outcomes to the same tumor. Crit. Rev. Oncol. Hematol. 2022, 179, 103792. [Google Scholar] [CrossRef]
- Esmaeli, B.; Ahmadi, M.A.; Youssef, A.; Diba, R.; Amato, M.; Myers, J.; Kies, M.; El-Naggar, A. Outcomes in Patients with Adenoid Cystic Carcinoma of the Lacrimal Gland. Ophthalmic Plast. Reconstr. Surg. 2004, 20, 156. [Google Scholar] [CrossRef]
- Andersson, M.K.; Mangiapane, G.; Nevado, P.T.; Tsakaneli, A.; Carlsson, T.; Corda, G.; Nieddu, V.; Abrahamian, C.; Chayka, O.; Rai, L.; et al. ATR is a MYB regulated gene and potential therapeutic target in adenoid cystic carcinoma. Oncogenesis 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.W.; Speight, P.M. Perineural invasion in adenoid cystic carcinoma of the salivary glands: A valid prognostic indicator? Oral Oncol. 2009, 45, 936–940. [Google Scholar] [CrossRef]
- Spiro, R.H. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am. J. Surg. 1997, 174, 495–498. [Google Scholar] [CrossRef]
- Chen, T.Y.; Keeney, M.G.; Chintakuntlawar, A.V.; Knutson, D.L.; Kloft-Nelson, S.; Greipp, P.T.; Garrity, J.A.; Salomao, D.R.; Garcia, J.J. Adenoid cystic carcinoma of the lacrimal gland is frequently characterized by MYB rearrangement. Eye 2017, 31, 720–725. [Google Scholar] [CrossRef]
- Singh, F.M.; Mak, S.Y.; Bonington, S.C. Patterns of spread of head and neck adenoid cystic carcinoma. Clin. Radiol. 2015, 70, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.A.; Ho, A.L.; Fury, M.G.; Sherman, E.; Pfister, D.G. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: A systematic review. Lancet Oncol. 2011, 12, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-russo, C.A.; Junn, J.C.; Yom, S.S.; Bakst, R.L. Radiation Therapy for Adenoid Cystic Carcinoma of the Head and Neck. Cancers 2021, 13, 6335. [Google Scholar] [CrossRef] [PubMed]
- Maniar, A.; Saqi, A.; Troob, S.H.; Belinsky, I.; Charles, N.C.; Gobin, Y.P.; Marr, B.P. Targeted Neoadjuvant Intra-arterial Chemotherapy in Lacrimal Gland Adenoid Cystic Carcinoma: A Histological Correlation Using Apoptotic Tumor Markers. Ophthalmic Plast. Reconstr. Surg. 2022, 38, e28–e33. [Google Scholar] [CrossRef]
- Du, F.; Zhou, C.-X.; Gao, Y. Myoepithelial differentiation in cribriform, tubular and solid pattern of adenoid cystic carcinoma: A potential involvement in histological grading and prognosis. Ann. Diagn. Pathol. 2016, 22, 1092–9134. [Google Scholar] [CrossRef]
- Szanto, P.A.; Luna, M.A.; Tortoledo, M.E.; White, R.A. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 1984, 54, 1062–1069. [Google Scholar] [CrossRef]
- Batsakis, J.G.; Luna, M.A.; El-Naggar, A. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. Ann. Otol. Rhinol. Laryngol. 1990, 99, 1007–1009. [Google Scholar] [CrossRef]
- Perzin, K.H.; Gullane, P.; Clairmont, A.C. Adenoid cystic carcinomas arising in salivary glands. A correlation of histologic features and clinical course. Cancer 1978, 42, 265–282. [Google Scholar] [CrossRef]
- Osborn, D.A. Morphology and the natural history of cribriform adenocarcinoma (adenoid cystic carcinoma). J. Clin. Pathol. 1977, 30, 195–205. [Google Scholar] [CrossRef]
- van Weert, S.; van der Waal, I.; Witte, B.I.; René Leemans, C.; Bloemena, E. Histopathological grading of adenoid cystic carcinoma of the head and neck: Analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 2015, 51, 71–76. [Google Scholar] [CrossRef]
- Ouyang, D.; Liang, L.; Zheng, G.; Ke, Z.; Weng, D.; Yang, W.; Su, Y.; Liao, G. Risk factors and prognosis for salivary gland adenoid cystic carcinoma in southern China: A 25-year retrospective study. Medicine 2017, 96, e5964. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.K.; Afshari, M.K.; Andrén, Y.; Wick, M.J.; Stenman, G. Targeting the Oncogenic Transcriptional Regulator MYB in Adenoid Cystic Carcinoma by Inhibition of IGF1R/AKT Signaling. JNCI J. Natl. Cancer Inst. 2017, 109, djx017. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; Van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole exome sequencing of adenoid cystic carcinoma. J. Clin. Investig. 2013, 123, 2965–2968. [Google Scholar] [CrossRef]
- Nambiar, M.; Kari, V.; Raghavan, S.C. Chromosomal translocations in cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2008, 1786, 139–152. [Google Scholar] [CrossRef]
- Wagner, V.P.; Bingle, C.D.; Bingle, L. MYB-NFIB fusion transcript in adenoid cystic carcinoma: Current state of knowledge and future directions. Crit. Rev. Oncol. Hematol. 2022, 176, 103745. [Google Scholar] [CrossRef]
- Nowell, P.C. A minute chromosome in human chronic granulocytic leukemia. Science 1960, 132, 1497. [Google Scholar]
- Drier, Y.; Cotton, M.J.; Williamson, K.E.; Gillespie, S.M.; Ryan, R.J.H.; Kluk, M.J.; Carey, C.D.; Rodig, S.J.; Sholl, L.M.; Afrogheh, A.H.; et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat. Genet. 2016, 48, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.; Cohen, C.; Siddiqui, M.T. MYB expression: Potential role in separating adenoid cystic carcinoma (ACC) from pleomorphic adenoma (PA). Diagn. Cytopathol. 2016, 44, 799–804. [Google Scholar] [CrossRef]
- Brill, L.B., II; Kanner, W.A.; Fehr, A.; Andrén, Y.; Moskaluk, C.A.; Löning, T.; Stenman, G.; Frierson, H.F. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod. Pathol. 2011, 24, 1169–1176. [Google Scholar] [CrossRef]
- Claringbould, A.; Zaugg, J.B. Enhancers in disease: Molecular basis and emerging treatment strategies. Trends Mol. Med. 2021, 27, 1060–1073. [Google Scholar] [CrossRef]
- Botten, G.A.; Zhang, Y.; Dudnyk, K.; Liu, X.; Sanders, J.T.; Imanci, A.; Droin, N.; Cao, H.; Kaphle, P.; Dickerson, K.E.; et al. Structural Variation Cooperates with Permissive Chromatin to Control Enhancer Hijacking-Mediated Oncogenic Transcription. Blood 2022, 140 (Suppl. 1), 1007–1008. [Google Scholar] [CrossRef]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Mayor, C.V.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Sniegowski, M.C.; Wani, K.; Prieto, V.; Esmaeli, B. Mutational landscape of lacrimal gland carcinomas and implications for treatment. Head Neck 2016, 38, E724–E729. [Google Scholar] [CrossRef]
- Fujii, K.; Murase, T.; Beppu, S.; Saida, K.; Takino, H.; Masaki, A.; Ijichi, K.; Kusafuka, K.; Iida, Y.; Onitsuka, T.; et al. MYB, MYBL1, MYBL2 and NFIB gene alterations and MYC overexpression in salivary gland adenoid cystic carcinoma. Histopathology 2017, 71, 823–834. [Google Scholar] [CrossRef]
- Mitani, Y.; Li, J.; Rao, P.H.; Zhao, Y.-J.; Bell, D.; Lippman, S.M.; Weber, R.S.; Caulin, C.; El-Naggar, A.K. Comprehensive Analysis of the MYB-NFIB Gene Fusion in Salivary Adenoid Cystic Carcinoma: Incidence, Variability, and Clinicopathologic Significance. Clin. Cancer Res. 2010, 16, 4722–4731. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Andrén, Y.; Mark, J.; Horlings, H.M.; Persson, F.; Stenman, G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc. Natl. Acad. Sci. USA 2009, 106, 18740–18744. [Google Scholar] [CrossRef]
- Lin, Q.-Q.; Sun, J.-L.; Wang, F.; Zhang, H.-Z.; Zhou, G.; Xi, Q. Current understanding of adenoid cystic carcinoma in the gene expression and targeted therapy. Holist. Integr. Oncol. 2023, 2, 7. [Google Scholar] [CrossRef]
- Brayer, K.J.; Frerich, C.A.; Kang, H.; Ness, S.A. Recurrent fusions in MYB and MYBL1 define a common, transcription factor–driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 2016, 6, 176–187. [Google Scholar] [CrossRef]
- Andreasen, S.; Tan, Q.; Agander, T.K.; Steiner, P.; Bjørndal, K.; Høgdall, E.; Larsen, S.R.; Erentaite, D.; Olsen, C.H.; Ulhøi, B.P.; et al. Adenoid cystic carcinomas of the salivary gland, lacrimal gland, and breast are morphologically and genetically similar but have distinct microRNA expression profiles. Mod. Pathol. 2018, 31, 1211–1225. [Google Scholar] [CrossRef]
- Shakoori, A.R. Fluorescence in situ hybridization (FISH) and its applications. In Chromosome Structure and Aberrations; Bhat, T.A., Wani, A.A., Eds.; Springer: New Delhi, India, 2017; pp. 343–367. [Google Scholar] [CrossRef]
- Heyer, E.E.; Deveson, I.W.; Wooi, D.; Selinger, C.I.; Lyons, R.J.; Hayes, V.M.; O’toole, S.A.; Ballinger, M.L.; Gill, D.; Thomas, D.M.; et al. Diagnosis of fusion genes using targeted RNA sequencing. Nat. Commun. 2019, 10, 1388. [Google Scholar] [CrossRef]
- Chen, W.; Kalscheuer, V.; Tzschach, A.; Menzel, C.; Ullmann, R.; Schulz, M.H.; Erdogan, F.; Li, N.; Kijas, Z.; Arkesteijn, G.; et al. Mapping translocation breakpoints by next-generation sequencing. Genome Res. 2008, 18, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Razzaq, S.K.; Vo, A.D.; Gautam, M.; Li, H. Identifying fusion transcripts using next generation sequencing. WIREs RNA 2016, 7, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Abel, H.J.; Al-Kateb, H.; Cottrell, C.E.; Bredemeyer, A.J.; Pritchard, C.C.; Grossmann, A.H.; Wallander, M.L.; Pfeifer, J.D.; Lockwood, C.M.; Duncavage, E.J. Detection of gene rearrangements in targeted clinical next-generation sequencing. J. Mol. Diagn. 2014, 16, 405–417. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.B.; Ko, J.J.; Siever, J.; Chan, A.M.Y.; Simpson, R.H.W.; Hao, D.; Lau, H.Y. MYB-NFIB gene fusions identified in archival adenoid cystic carcinoma tissue employing NanoString analysis: An exploratory study. Diagn. Pathol. 2019, 14, 78. [Google Scholar] [CrossRef]
- Humtsoe, J.O.; Kim, H.-S.; Jones, L.; Cevallos, J.; Boileau, P.; Kuo, F.; Morris, L.G.T.; Ha, P. Development and Characterization of MYB-NFIB Fusion Expression in Adenoid Cystic Carcinoma. Cancers 2022, 14, 2263. [Google Scholar] [CrossRef]
- Pham, T.; Pereira, L.; Roth, S.; Galletta, L.; Link, E.; Akhurst, T.; Solomon, B.; Michael, M.; Darcy, P.; Sampurno, S.; et al. First-in-human phase I clinical trial of a combined immune modulatory approach using TetMYB vaccine and Anti-PD-1 antibody in patients with advanced solid cancer including colorectal or adenoid cystic carcinoma: The MYPHISMO study protocol (NCT03287427). Contemp. Clin. Trials Commun. 2019, 16, 100409. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; McGrail, D.J.; Li, K.; Karpinets, T.V.; Bell, D.; Frank, S.J.; Song, X.; Kupferman, M.E.; Liu, B.; et al. Proteogenomic Analysis of Salivary Adenoid Cystic Carcinomas Defines Molecular Subtypes and Identifies Therapeutic Targets. Clin. Cancer Res. 2023, 27, 852–864. [Google Scholar] [CrossRef]
- Brayer, K.J.; Kang, H.; El-Naggar, A.K.; Andreasen, S.; Homøe, P.; Kiss, K.; Mikkelsen, L.; Heegaard, S.; Pelaez, D.; Moeyersoms, A.; et al. Dominant Gene Expression Profiles Define Adenoid Cystic Carcinoma (ACC) from Different Tissues: Validation of a Gene Signature Classifier for Poor Survival in Salivary Gland ACC. Cancers 2023, 15, 1390. [Google Scholar] [CrossRef]
- Frerich, C.A.; Brayer, K.J.; Painter, B.M.; Kang, H.; Mitani, Y.; El-Naggar, A.K.; Ness, S.A. Transcriptomes define distinct subgroups of salivary gland adenoid cystic carcinoma with different driver mutations and outcomes. Oncotarget 2017, 9, 7341–7358. [Google Scholar] [CrossRef]
- von Holstein, S.L.; Fehr, A.; Persson, M.; Therkildsen, M.H.; Prause, J.U.; Heegaard, S.; Stenman, G. Adenoid Cystic Carcinoma of the Lacrimal Gland: MYB Gene Activation, Genomic Imbalances, and Clinical Characteristics. Ophthalmology 2013, 120, 2130–2138. [Google Scholar] [CrossRef]
- Kim, J.; Geyer, F.C.; Martelotto, L.G.; Ng, C.K.Y.; Lim, R.S.; Selenica, P.; Li, A.; Pareja, F.; Fusco, N.; Edelweiss, M.; et al. MYBL1 rearrangements and MYB amplification in breast adenoid cystic carcinomas lacking the MYB–NFIB fusion gene. J. Pathol. 2018, 244, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Weaver, K.L.; Capobianco, A.J. Notch signalling in solid tumours: A little bit of everything but not all the time. Nat. Rev. Cancer. 2011, 11, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, M.; Wu, H.; Xu, H.; Han, N.; Chu, Q.; Yu, S.; Chen, Y.; Wu, K. Expression of Notch1 Correlates with Breast Cancer Progression and Prognosis. PLoS ONE 2015, 10, e0131689. [Google Scholar] [CrossRef]
- Misiorek, J.O.; Przybyszewska-Podstawka, A.; Kałafut, J.; Paziewska, B.; Rolle, K.; Rivero-Müller, A.; Nees, M. Context Matters: NOTCH Signatures and Pathway in Cancer Progression and Metastasis. Cells 2021, 10, 94. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhang, J.; Yoo, S.Y.; Bengtsson, L.; Moorthy, S.; Neskey, D.M.; Zhao, M.; Alves, M.V.O.; Chang, K.; Drummond, J.; et al. Integrative Genomic Characterization of Oral Squamous Cell Carcinoma Identifies Frequent Somatic Drivers. Cancer Discov. 2013, 3, 770–781. [Google Scholar] [CrossRef]
- Gallenstein, N.; Tichy, L.; Weigand, M.A.; Schenz, J. Notch Signaling in Acute Inflammation and Sepsis. Int. J. Mol. Sci. 2023, 24, 3458. [Google Scholar] [CrossRef]
- O’Brien, K.A.; Murray, A.J.; Simonson, T.S. Notch Signaling and Cross-Talk in Hypoxia: A Candidate Pathway for High-Altitude Adaptation. Life 2022, 12, 437. [Google Scholar] [CrossRef]
- Takebe, N.; Nguyen, D.; Yang, S.X. Targeting Notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol. Ther. 2014, 141, 140–149. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Rand, J.V.; Sheehan, C.E.; Jennings, T.A.; Al-Rohil, R.N.; Otto, G.A.; Curran, J.C.; Palmer, G.; Downing, S.R.; et al. Comprehensive Genomic Profiling of Relapsed and Metastatic Adenoid Cystic Carcinomas by Next-generation Sequencing Reveals Potential New Routes to Targeted Therapies. Am. J. Surg. Pathol. 2014, 38, 235–238. [Google Scholar] [CrossRef]
- Miller, L.E.; Au, V.; Mokhtari, T.E.; Goss, D.; Faden, D.L.; Varvares, M.A. A Contemporary Review of Molecular Therapeutic Targets for Adenoid Cystic Carcinoma. Cancers 2022, 14, 992. [Google Scholar] [CrossRef]
- Ding, L.-C.; She, L.; Zheng, D.-L.; Huang, Q.-L.; Wang, J.-F.; Zheng, F.-F.; Lu, Y.-G. Notch-4 contributes to the metastasis of salivary adenoid cystic carcinoma. Oncol. Rep. 2010, 24, 363–368. [Google Scholar] [CrossRef]
- Su, B.H.; Qu, J.; Song, M.; Huang, X.-Y.; Hu, X.-M.; Xie, J.; Zhao, Y.; Ding, L.-C.; She, L.; Chen, J.; et al. NOTCH1 signaling contributes to cell growth, anti-apoptosis and metastasis in salivary adenoid cystic carcinoma. Oncotarget 2014, 5, 6885–6895. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; Jr, W.N.W.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef]
- Sajed, D.P.; Faquin, W.C.; Carey, C.; Severson, E.A.; Afrogheh, A.H.; Johnson, C.A.; Blacklow, S.C.; Chau, N.G.; Lin, D.T.; Krane, J.F.; et al. Diffuse Staining for Activated NOTCH1 Correlates With NOTCH1 Mutation Status and Is Associated With Worse Outcome in Adenoid Cystic Carcinoma. Am. J. Surg. Pathol. 2017, 41, 1473–1482. [Google Scholar] [CrossRef]
- Rettig, E.M.; Talbot, C.C., Jr.; Sausen, M.; Jones, S.; Bishop, J.A.; Wood, L.D.; Tokheim, C.; Niknafs, N.; Karchin, R.; Fertig, E.J.; et al. Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma. Cancer Prev. Res. 2016, 9, 265–274. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Eckhardt, G.; Patnaik, A.; LoRusso, P.; Faoro, L.; Heymach, J.; Kapoun, A.; Xu, L.; Munster, P. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann. Oncol. 2018, 29, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Lopez Miranda, E.; Stathis, A.; Hess, D.; Racca, F.; Quon, D.; Rodon, J.; Gadea, O.S.S.; Garcia, J.M.P.; Nuciforo, P.; Vivancos, A.; et al. Phase 1 study of CB-103, a novel first-in-class inhibitor of the CSL-NICD gene transcription factor complex in human cancers. J. Clin. Oncol. 2021, 39 (Suppl. 15). [Google Scholar] [CrossRef]
- Ferrarotto, R.; Metcalf, R.; Rodriguez, C.P.; Muzaffar, J.; Even, C.; Perez, C.A.; Van Herpen, C.M.L.; Oliva, M.; Xia, B.; Bowles, D.W.; et al. Results of ACCURACY: A phase 2 trial of AL101, a selective gamma secretase inhibitor, in subjects with recurrent/metastatic (R/M) adenoid cystic carcinoma (ACC) harboring Notch activating mutations (Notchmut). J. Clin. Oncol. 2022, 40 (Suppl. 16), 6046. [Google Scholar]
- Ivanov, D. Notch Signaling-Induced Oscillatory Gene Expression May Drive Neurogenesis in the Developing Retina. Front. Mol. Neurosci. 2019, 12, 226. [Google Scholar] [CrossRef]
- Sant, D.W.; Tao, W.; Field, M.G.; Pelaez, D.; Jin, K.; Capobianco, A.; Dubovy, S.R.; Tse, D.T.; Wang, G. Whole exome sequencing of lacrimal gland adenoid cystic carcinoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO240–BIO246. [Google Scholar] [CrossRef] [PubMed]
- Piana, S.; Zanetti, E.; Bisagni, A.; Ciarrocchi, A.; Giordano, D.; Torricelli, F.; Rossi, T.; Ragazzi, M. Expression of NOTCH1 in thyroid cancer is mostly restricted to papillary carcinoma. Endocr. Connect. 2019, 8, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Tang, Q.; You, Q.; Liu, Z.; Wang, G.; Chen, Y.; Sun, Y.; Muhammad, S.; Wang, X. Disparity Expression of Notch1 in Benign and Malignant Colorectal Diseases. PLoS ONE 2013, 8, e81005. [Google Scholar] [CrossRef]
- Huang, J.; Song, H.; Liu, B.; Yu, B.; Wang, R.; Chen, L. Expression of Notch-1 and its clinical significance in different histological subtypes of human lung adenocarcinoma. J. Exp. Clin. Cancer Res. 2013, 32, 84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broustas, C.G.; Lieberman, H.B. DNA Damage Response Genes and the Development of Cancer Metastasis. Radiat. Res. 2014, 181, 111–130. [Google Scholar] [CrossRef]
- Peng, L.; Liang, J.; Wang, Q.; Chen, G. A DNA Damage Repair Gene Signature Associated With Immunotherapy Response and Clinical Prognosis in Clear Cell Renal Cell Carcinoma. Front. Genet. 2022, 13, 798846. [Google Scholar] [CrossRef]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer. 2012, 12, 801–817. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Gourley, C.; Balmaña, J.; Ledermann, J.A.; Serra, V.; Dent, R.; Loibl, S.; Pujade-Lauraine, E.; Boulton, S.J. Moving from poly (ADP-ribose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. J. Clin. Oncol. 2019, 37, 2257–2269. [Google Scholar] [CrossRef]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S.; et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018, 23, 239–254.e6. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Frierson, H.F., Jr.; Moskaluk, C.A. Mutation signature of adenoid cystic carcinoma: Evidence for transcriptional and epigenetic reprogramming. J. Clin. Investig. 2013, 123, 2783–2785. [Google Scholar] [CrossRef]
- Moskaluk, C.A. Adenoid Cystic Carcinoma: Clinical and Molecular Features. Head Neck Pathol. 2013, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.A.; da Silva, L.P.; de Sousa Lopes, M.L.D.; Sobral, A.P.V.; de Almeida Freitas, R.; de Souza, L.B.; Barboza, C.A.G. DNA base excision repair and nucleotide excision repair proteins in malignant salivary gland tumors. Arch. Oral Biol. 2021, 121, 104987. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, H.; García-Muse, T.; Aguilera, A. Replication stress and cancer. Nat. Rev. Cancer. 2015, 15, 276–289. [Google Scholar] [CrossRef]
- Lecona, E.; Fernandez-Capetillo, O. Targeting ATR in cancer. Nat. Rev. Cancer 2018, 18, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Laev, S.S.; Salakhutdinov, N.F.; Lavrik, O.I. Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1). Bioorg. Med. Chem. 2017, 25, 2531–2544. [Google Scholar] [CrossRef]
- Mandal, J.; Mandal, P.; Wang, T.-L.; Shih, I.-M. Treating ARID1A mutated cancers by harnessing synthetic lethality and DNA damage response. J. Biomed. Sci. 2022, 29, 71. [Google Scholar] [CrossRef]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Lu, Y.; Chan, Y.-T.; Tan, H.-Y.; Li, S.; Wang, N.; Feng, Y. Epigenetic regulation in human cancer: The potential role of epi-drug in cancer therapy. Mol. Cancer 2020, 19, 79. [Google Scholar] [CrossRef]
- Urnov, F.D.; Wolffe, A.P. Chromatin remodeling and transcriptional activation: The cast (in order of appearance). Oncogene 2001, 20, 2991–3006. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, S.K.; Kulakova, K.; Kennedy, S. A Review of the Molecular Landscape of Adenoid Cystic Carcinoma of the Lacrimal Gland. Int. J. Mol. Sci. 2023, 24, 13755. https://doi.org/10.3390/ijms241813755
Powell SK, Kulakova K, Kennedy S. A Review of the Molecular Landscape of Adenoid Cystic Carcinoma of the Lacrimal Gland. International Journal of Molecular Sciences. 2023; 24(18):13755. https://doi.org/10.3390/ijms241813755
Chicago/Turabian StylePowell, Sarah Kate, Karina Kulakova, and Susan Kennedy. 2023. "A Review of the Molecular Landscape of Adenoid Cystic Carcinoma of the Lacrimal Gland" International Journal of Molecular Sciences 24, no. 18: 13755. https://doi.org/10.3390/ijms241813755
APA StylePowell, S. K., Kulakova, K., & Kennedy, S. (2023). A Review of the Molecular Landscape of Adenoid Cystic Carcinoma of the Lacrimal Gland. International Journal of Molecular Sciences, 24(18), 13755. https://doi.org/10.3390/ijms241813755





