Cardiometabolic Risk: Characteristics of the Intestinal Microbiome and the Role of Polyphenols
Abstract
1. Introduction
2. Methodological Strategies
3. Particular Aspects of the Gut Microbiota of Patients at Cardiometabolic Risk
3.1. Bacterial Composition and Diversity
3.2. The Immune Response to Bacterial Components
3.3. Involvement of Bacterial Metabolites
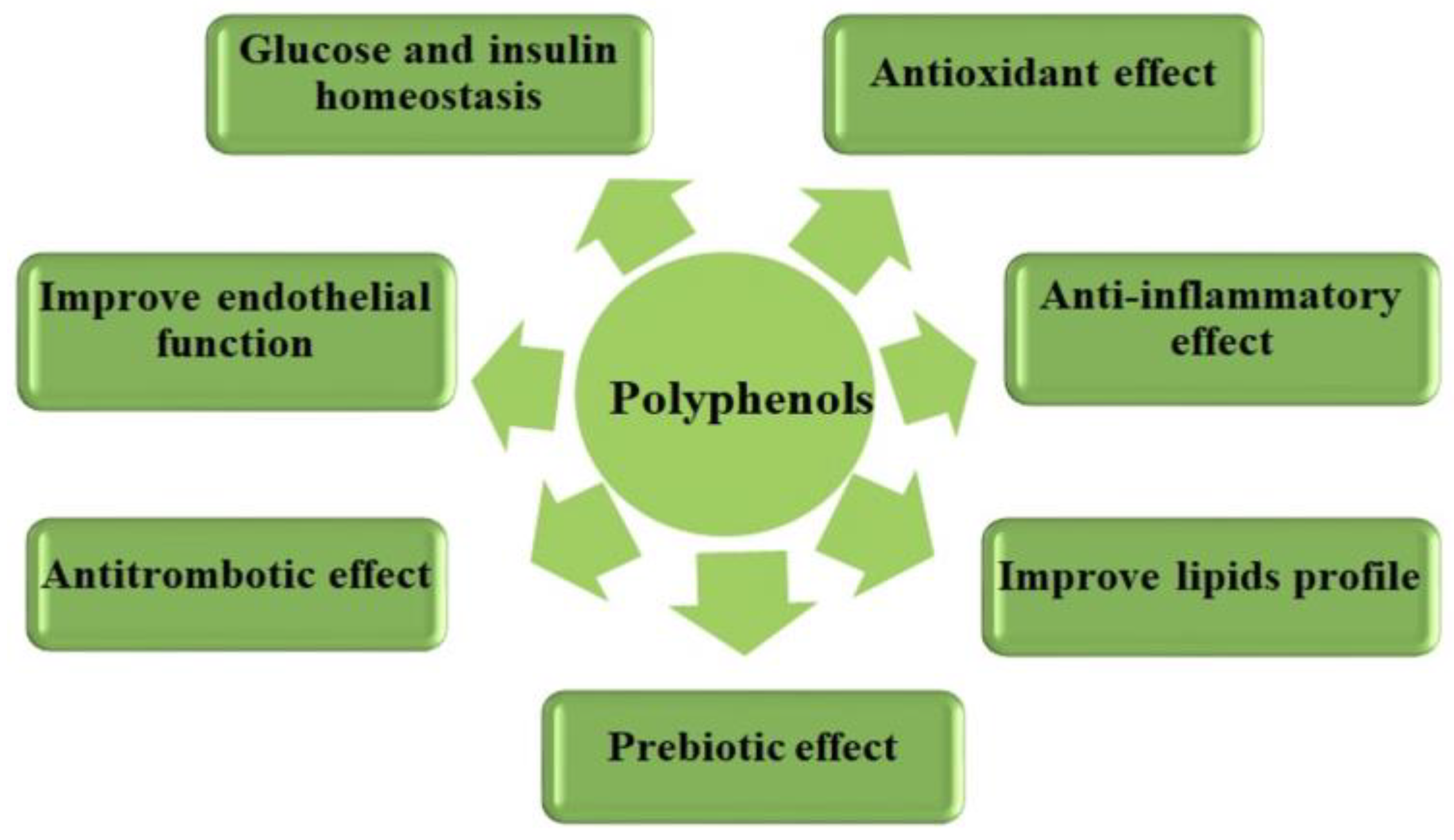
4. Beneficial Effects of the Polyphenols Action in Terms of Cardiometabolic Health
4.1. Antioxidant Effect
4.2. Anti-Inflammatory Effect
4.3. Anti-Atherosclerotic Effect
4.4. Beneficial Effect on Maintaining Glucose and Insulin Homeostasis
4.5. Prebiotic Effect on the Gut Microbiota
5. Crosstalk between Dietary Polyphenols and Gut Microbiota
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases: Mortality. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 30 April 2023).
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 May 2023).
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease A Scientific Statement From the American Heart Association. Circulation 2021, 143, E984–E1010. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and Cardiovascular Disease: Revisiting an Old Relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Heffron, S.P.; Parham, J.S.; Pendse, J.; Alemán, J.O. Treatment of Obesity in Mitigating Metabolic Risk. Circ. Res. 2020, 126, 1646–1665. [Google Scholar] [CrossRef] [PubMed]
- Telmisartan, T.; Assessment, R. Effects of the Angiotensin-Receptor Blocker Telmisartan on Cardiovascular Events in High-Risk Patients Intolerant to Angiotensin-Converting Enzyme Inhibitors: A Randomised Controlled Trial. Lancet 2008, 372, 1174–1183. [Google Scholar] [CrossRef]
- Dragana, S.; Milica, O. Cardiometabolic Risk Calculation in the Assessment of Cardiometabolic Risk Profiles. TEM J. 2013, 2, 224. [Google Scholar]
- Chatterjee, A.; Harris, S.B.; Leiter, L.A.; Fitchett, D.H.; Teoh, H.; Bhattacharyya, O.K. Managing Cardiometabolic Risk in Primary Care—Summary of the 2011 Consensus Statement. Can. Fam. Physician 2012, 58, 389–393. [Google Scholar] [PubMed]
- Brunzell, J.D.; Davidson, M.; Furberg, C.D.; Goldberg, R.B.; Howard, B.V.; Stein, J.H.; Witztum, J.L.; Kirkman, M.S. Lipoprotein Management in Patients with Cardiometabolic Risk: Consensus Statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008, 31, 811–822. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I. Abdominal Obesity and Metabolic Syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Farkas, G.J.; Burton, A.M.; McMillan, D.W.; Sneij, A.; Gater, D.R. The Diagnosis and Management of Cardiometabolic Risk and Cardiometabolic Syndrome after Spinal Cord Injury. J. Pers. Med. 2022, 12, 1088. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Zimmet, P.; Magliano, D.; Matsuzawa, Y.; Alberti, G.; Shaw, J. The Metabolic Syndrome: A Global Public Health Problem and a New Definition. J. Atheroscler. Thromb. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Guembe, M.J.; Fernandez-Lazaro, C.I.; Sayon-Orea, C.; Toledo, E.; Moreno-Iribas, C.; Cosials, J.B.; Reyero, J.B.; Martínez, J.D.; Diego, P.G.; Uche, A.M.G.; et al. Risk for Cardiovascular Disease Associated with Metabolic Syndrome and Its Components: A 13-Year Prospective Study in the RIVANA Cohort. Cardiovasc. Diabetol. 2020, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Regufe, V.M.G.; Pinto, C.M.C.B.; Perez, P.M.V.H.C. Metabolic Syndrome in Type 2 Diabetic Patients: A Review of Current Evidence. Porto Biomed. J. 2020, 5, e101. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Kavaric, N.; Klisic, A.; Ninic, A. Cardiovascular Risk Estimated by UKPDS Risk Engine Algorithm in Diabetes. Open Med. 2018, 13, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Sawyer, D.B. Encyclopedia of Cardiovascular Research and Medicine; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1–4, pp. 1–4799. [Google Scholar]
- Sofogianni, A.; Stalikas, N.; Antza, C.; Tziomalos, K. Cardiovascular Risk Prediction Models and Scores in the Era of Personalized Medicine. J. Pers. Med. 2022, 12, 1180. [Google Scholar] [CrossRef]
- Ridker, P.M.; Buring, J.E.; Rifai, N.; Cook, N.R. Development and Validation of Improved Algorithms for the Assessment of Global Cardiovascular Risk in Women. Am. Med. Assoc. 2007, 297, 611–620. [Google Scholar] [CrossRef]
- Cook, N.R.; Paynter, N.P.; Eaton, C.B.; Manson, J.E.; Martin, L.W.; Robinson, J.G.; Rossouw, J.E.; Wassertheil-Smoller, S.; Ridker, P.M. Comparison of the Framingham and Reynolds Risk Scores for Global Cardiovascular Risk Prediction in the Multiethnic Women’s Health Initiative. Circulation 2012, 125, 1748–1756. [Google Scholar] [CrossRef]
- Assmann, G. Calculating Global Risk: The Key to Intervention. Eur. Heart J. Suppl. 2005, 7, 9–14. [Google Scholar] [CrossRef][Green Version]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of Ten-Year Risk of Fatal Cardiovascular Disease in Europe: The SCORE Project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J.; D’Agostino, R.; Sullivan, L.; Elosua, R.; Wilson, P.; Ordovas, J.; Solanas, P.; Cordón, F.; Ramos, R.; Sala, J.; et al. An Adaptation of the Framingham Coronary Heart Disease Risk Function to European Mediterranean Areas. J. Epidemiol. Community Health 2003, 57, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E.; Lanti, M. Comparison of the Framingham Risk Function-Based Coronary Chart with Risk Function from an Italian Population Study. Eur. Heart J. 2000, 21, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Attaye, I.; Warmbrunn, M.V.; Boot, A.N.A.F.; van der Wolk, S.C.; Hutten, B.A.; Daams, J.G.; Herrema, H.; Nieuwdorp, M. A Systematic Review and Meta-Analysis of Dietary Interventions Modulating Gut Microbiota and Cardiometabolic Diseases—Striving for New Standards in Microbiome Studies. Gastroenterology 2022, 162, 1911–1932. [Google Scholar] [CrossRef] [PubMed]
- Aron, R.A.C.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia Muciniphila as a Key Gut Bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Pan, D.; Liu, H.; Yang, C.; Yang, X.; Wang, X.; Liu, F.; Feng, M.; Wu, Q.; Shen, Y.; et al. Improvement in Cardiometabolic Risk Markers Following an Oatmeal Diet Is Associated with Gut Microbiota in Mildly Hypercholesterolemic Individuals. Food Res. Int. 2022, 160, 111701. [Google Scholar] [CrossRef] [PubMed]
- Vesa, C.M.; Bungau, S.G. Novel Molecules in Diabetes Mellitus, Dyslipidemia and Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 4029. [Google Scholar] [CrossRef]
- Arora, A.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Sobarzo-Sanchez, E.; Bungau, S. Unravelling the Involvement of Gut Microbiota in Type 2 Diabetes Mellitus. Life Sci. 2021, 273, 119311. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Vodnar, D.-C.; Ștefănescu, B.E.; Tit, D.M.; Nițescu, M. Polyphenols and Cardiometabolic Health: Knowledge and Concern among Romanian People. Nutrients 2023, 15, 2281. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative Stress—Complex Pathological Issues Concerning the Hallmark of Cardiovascular and Metabolic Disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Vetrani, C.; Maukonen, J.; Bozzetto, L.; Della Pepa, G.; Vitale, M.; Costabile, G.; Riccardi, G.; Rivellese, A.A.; Saarela, M.; Annuzzi, G. Diets Naturally Rich in Polyphenols and/or Long-Chain n-3 Polyunsaturated Fatty Acids Differently Affect Microbiota Composition in High-Cardiometabolic-Risk Individuals. Acta Diabetol. 2020, 57, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Simões, C.D.; Maganinho, M.; Sousa, A.S. FODMAPs, Inflammatory Bowel Disease and Gut Microbiota: Updated Overview on the Current Evidence. Eur. J. Nutr. 2022, 61, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of Gut Microbiota in Cardiovascular Diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Bouter, K.E.; van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Lopez Longo, M.N.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Kaur, G.; Behl, T.; Bungau, S.; Kumar, A.; Uddin, M.S.; Mehta, V.; Zengin, G.; Mathew, B.; Shah, M.A.; Arora, S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson’s Disease. Curr. Neuropharmacol. 2021, 19, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The Role of Intestinal Microbiota in Cardiovascular Disease. J. Cell Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Precup, G.; Pocol, C.B.; Teleky, B.-E.; Vodnar, D.C. Awareness, Knowledge, and Interest about Prebiotics—A Study among Romanian Consumers. Int. J. Environ. Res. Public Health 2022, 19, 1208. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Nemes, S.-A.; Szabo, K.; Teleky, B.-E.; Vodnar, D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association with Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef] [PubMed]
- Fitero, A.; Bungau, S.G.; Tit, D.M.; Endres, L.; Khan, S.A.; Bungau, A.F.; Romanul, I.; Vesa, C.M.; Radu, A.-F.; Tarce, A.G.; et al. Comorbidities, Associated Diseases, and Risk Assessment in COVID-19—A Systematic Review. Int. J. Clin. Pract. 2022, 2022, 1571826. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molina, F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with Early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The Resilience of the Intestinal Microbiota Influences Health and Disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.; Stanton, C. The Gut-Brain Axis Dietary, Probiotic, and Prebiotic Interventions on the Microbiota; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128025444. [Google Scholar]
- Kallassy, J.; Gagnon, E.; Rosenberg, D.; Silbart, L.K.; McManus, S.A. Strains of Faecalibacterium Prausnitzii and Its Extracts Reduce Blood Glucose Levels, Percent HbA1c, and Improve Glucose Tolerance without Causing Hypoglycemic Side Effects in Diabetic and Prediabetic Mice. BMJ Open Diabetes Res. Care 2023, 11, e003101. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Xu, W.; Ma, Y.; Wang, Q.; Eatman, D.; You, S.; Zou, J.; Champion, J.; Zhao, L.; et al. C-Reactive Protein Causes Adult-Onset Obesity Through Chronic Inflammatory Mechanism. Front. Cell Dev. Biol. 2020, 8, 18. [Google Scholar] [CrossRef]
- Pai, C.S.; Wang, C.Y.; Hung, W.W.; Hung, W.C.; Tsai, H.J.; Chang, C.C.; Hwang, S.J.; Dai, C.Y.; Ho, W.Y.; Tsai, Y.C. Interrelationship of Gut Microbiota, Obesity, Body Composition and Insulin Resistance in Asians with Type 2 Diabetes Mellitus. J. Pers. Med. 2022, 12, 617. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia Intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Hartvigsen, M.L.; Hedemann, M.S.; Hermansen, K. Mechanisms Whereby Whole Grain Cereals Modulate the Prevention of Type 2 Diabetes; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128015858. [Google Scholar]
- Sanchez-Alcoholado, L.; Castellano-Castillo, D.; Jordán-Martínez, L.; Moreno-Indias, I.; Cardila-Cruz, P.; Elena, D.; Muñoz-Garcia, A.J.; Queipo-Ortuño, M.I.; Jimenez-Navarro, M. Role of Gut Microbiota on Cardio-Metabolic Parameters and Immunity in Coronary Artery Disease Patients with and without Type-2 Diabetes Mellitus. Front. Microbiol. 2017, 8, 1936. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut Microbiome and Serum Metabolome Alterations in Obesity and after Weight-Loss Intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qin, Q.; Chen, J.; Yan, S.; Li, T.; Gao, X.; Yang, Y.; Li, A.; Ding, S. Gut Microbiome Alterations in Patients With Visceral Obesity Based on Quantitative Computed Tomography. Front. Cell Infect. Microbiol. 2022, 11, 823262. [Google Scholar] [CrossRef] [PubMed]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the Gut Microbiota Composition between Obese and Non-Obese Individuals in a Japanese Population, as Analyzed by Terminal Restriction Fragment Length Polymorphism and next-Generation Sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Kriss, M.; Nusbacherd, K.Z.H.M.; Martine, C.G.; Lozupone, C.A. Low Diversity Gut Microbiota Dysbiosis: Drivers, Functional Implications and Recovery Michael. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.Y.; Hu, X.M.; Zhang, Y.; Zhang, S.Y. Current Understanding of Gut Microbiota Alterations and Related Therapeutic Intervention Strategies in Heart Failure. Chin. Med. J. 2019, 132, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, C.C.K.; Kummen, M.; Holm, K.; Broch, K.; Awoyemi, A.; Vestad, B.; Storm-Larsen, C.; Seljeflot, I.; Ueland, T.; Bohov, P.; et al. Low Fibre Intake Is Associated with Gut Microbiota Alterations in Chronic Heart Failure. ESC Heart Fail. 2020, 7, 456–466. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in Food, Food Supplements, and Probiotics: Should We Be Worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef]
- Bowman, J.D.; Surani, S.; Horseman, M.A. Endotoxin, Toll-like Receptor-4, and Atherosclerotic Heart Disease. Curr. Cardiol. Rev. 2016, 13, 86–93. [Google Scholar] [CrossRef]
- André, P.; Laugerette, F.; Féart, C. Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans? Nutrients 2019, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Netto Candido, T.L.; Bressan, J.; Alfenas, R.d.C.G. Dysbiosis and Metabolic Endotoxemia Induced by High-Fat Diet. Nutr. Hosp. 2018, 35, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Lertwattanarak, R.; De Jesus Garduño, J.; Galeana, J.J.; Li, J.; Zamarripa, F.; Lancaster, J.L.; Mohan, S.; Hussey, S.; Musi, N. Elevated Muscle TLR4 Expression and Metabolic Endotoxemia in Human Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Pineau, G.; Drai, J.; Meugnier, E.; Pesenti, S.; Laville, M.; Laugerette, F.; Malpuech-Brugère, C.; Vidal, H.; Michalski, M.C. Postprandial Endotoxemia Linked with Chylomicrons and Lipopolysaccharides Handling in Obese versus Lean Men: A Lipid Dose-Effect Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Jimenez-Lucena, R.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Garcia-Carpintero, S.; Lopez-Moreno, J.; Blanco-Rojo, R.; Delgado-Lista, J.; Perez-Martinez, P.; van Ommen, B.; et al. Postprandial Endotoxemia May Influence the Development of Type 2 Diabetes Mellitus: From the CORDIOPREV Study. Clin. Nutr. 2019, 38, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.O.; Dix-Peek, T.; Duarte, R.; Dickens, C.; Naidoo, S.; Vachiat, A.; Grinter, S.; Manga, P.; Naicker, S. Association of Chronic Inflammation and Accelerated Atherosclerosis among an Indigenous Black Population with Chronic Kidney Disease. PLoS ONE 2020, 15, e0232741. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. The Gut Microbial Endocrine Organ: Bacterially Derived Signals Driving Cardiometabolic Diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The Role of Short-Chain Fatty Acids in the Interplay between Gut Microbiota and Diet in Cardio-Metabolic Health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. Conference on Diet and Digestive Disease Symposium 2: Sensing and Signalling of the Gut Environment: Scfa: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A Dietary Intervention with Functional Foods Reduces Metabolic Endotoxaemia and Attenuates Biochemical Abnormalities by Modifying Faecal Microbiota in People with Type 2 Diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Hwang, S.S.; Park, E.J.; Bae, J.W. Strict Vegetarian Diet Improves the Risk Factors Associated with Metabolic Diseases by Modulating Gut Microbiota and Reducing Intestinal Inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of Human Colonic Butyrate-Producing Bacteria Revealed by Analysis of the Butyryl-CoA:Acetate CoA-Transferase Gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Djekic, D.; Shi, L.; Brolin, H.; Carlsson, F.; Särnqvist, C.; Savolainen, O.; Cao, Y.; Bäckhed, F.; Tremaroli, V.; Landberg, R.; et al. Effects of a Vegetarian Diet on Cardiometabolic Risk Factors, Gut Microbiota, and Plasma Metabolome in Subjects with Ischemic Heart Disease: A Randomized, Crossover Study. J. Am. Heart Assoc. 2020, 9, e016518. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Al-Obaide, M.A.I.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; Al-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut Microbiota-Dependent Trimethylamine-N-Oxide and Serum Biomarkers in Patients with T2DM and Advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Li, X.S.; Fan, Y.; Li, D.S.; Wu, Y.; Hazen, S.L. Increased Trimethylamine N-Oxide Portends High Mortality Risk Independent of Glycemic Control in Patients with Type 2 Diabetes Mellitus. Clin. Chem. 2017, 63, 297–306. [Google Scholar] [CrossRef]
- Hui, D.Y. Intestinal Phospholipid and Lysophospholipid Metabolism in Cardiometabolic Disease. Curr. Opin. Lipidol. 2016, 27, 507–512. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Behl, T.; Bungau, S.G.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Ramassamy, C. Emerging Role of Polyphenolic Compounds in the Treatment of Neurodegenerative Diseases: A Review of Their Intracellular Targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary Polyphenols in the Prevention and Treatment of Allergic Diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial Roles of Honey Polyphenols against Some Human Degenerative Diseases: A Review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; Anti-Inflammatory/Antioxidant Effects of Polyphenols: A Comparative Review on the Parental Compounds and Their Metabolites. Food Rev. Int. 2021, 37, 759–811. [Google Scholar] [CrossRef]
- Mennen, L.I.; Sapinho, D.; De Bree, A.; Arnault, N.; Bertrais, S.; Galan, P.; Hercberg, S. Consumption of Foods Rich in Flavonoids Is Related to A Decreased Cardiovascular Risk in Apparently Healthy French Women. J. Nutr. 2004, 134, 923–926. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Thatcher, N.J.; Dargham, S.R.; Kilpatrick, E.S.; Atkin, S.L. Soy Isoflavones Improve Cardiovascular Disease Risk Markers in Women during the Early Menopause. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 691–697. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Jafari, T. Effect of Dietary Anthocyanins on Biomarkers of Glycemic Control and Glucose Metabolism: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Food Res. Int. 2020, 137, 109379. [Google Scholar] [CrossRef] [PubMed]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R. Flavonoid Intake and Cardiovascular Disease Mortality: A Prospective Study in Postmenopausal Women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. The Journal of Nutrition Nutrition and Disease Blood Pressure Is Reduced and Insulin Sensitivity Increased in Glucose-Intolerant, Hypertensive Subjects after 15 Days of Consuming High-Polyphenol Dark Chocolate 1–3. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol Improves Insulin Sensitivity, Reduces Oxidative Stress and Activates the Akt Pathway in Type 2 Diabetic Patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.A.; Yokoyama, W. Cinnamon Intake Lowers Fasting Blood Glucose: Meta-Analysis. J. Med. Food 2011, 14, 884–889. [Google Scholar] [CrossRef] [PubMed]
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic Potential of Dietary Polyphenols: A Mechanistic Review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zimmermann, D.; De Castro, C.A.; Actis-Goretta, L. Dose-Response Relationship between Cocoa Flavanols and Human Endothelial Function: A Systematic Review and Meta-Analysis of Randomized Trials. Food Funct. 2019, 10, 6322–6330. [Google Scholar] [CrossRef]
- Tanghe, A.; Heyman, E.; Vanden Wyngaert, K.; Van Ginckel, A.; Celie, B.; Rietzschel, E.; Calders, P.; Shadid, S. Evaluation of Blood Pressure Lowering Effects of Cocoa Flavanols in Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Funct. Foods 2021, 79, 104399. [Google Scholar] [CrossRef]
- Han, B.; Nazary-Vannani, A.; Talaei, S.; Clark, C.C.T.; Rahmani, J.; Rasekhmagham, R.; Kord-Varkaneh, H. The Effect of Green Coffee Extract Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phyther. Res. 2019, 33, 2918–2926. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Borges, C.V.; Borges, C.V.; Alonzo, H.; Hector, G.; Gomez, G.; Chen, C.O.; Chen, C.O.; Pace, G.; Lima, P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability, Phenolic Compounds—Biological Activity. Open Sci. 2017, 8, 1–24. [Google Scholar]
- Du, Y.; Guo, H.; Lou, H. Grape Seed Polyphenols Protect Cardiac Cells from Apoptosis via Induction of Endogenous Antioxidant Enzymes. J. Agric. Food Chem. 2007, 55, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, T.; Ichijo, Y.; Ito, M.; Kimura, M.; Katsuyama, M.; Iwata, K.; Miura, T.; Terada, T.; Yabe-Nishimura, C. Curcumin Activates Human Glutathione S-Transferase P1 Expression through Antioxidant Response Element. Toxicol. Lett. 2007, 170, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Pallag, A.; Bungau, S.G.; Tit, D.M.; Jurca, T.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative Study of Polyphenols, Flavonoids and Chlorophylls in Equisetum Arvense L. Populations. Rev. Chim. 2016, 67, 530–533. [Google Scholar]
- Sharma, V.; Nath, D.; Gautam, S.; Radu, A.-F.; Behl, T.; Bungau, S.G.; Vesa, C.M. Reviewing the Traditional/Modern Uses, Phytochemistry, Essential Oils/Extracts and Pharmacology of Embelia Ribes Burm. Antioxidants 2022, 11, 1359. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and Cardiovascular Disease Mechanisms.—PubMed—NCBI. Am. J. Clin. Nutr. 2006, 83, 456–460. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Kengne, A.P.; Batty, G.D.; Hamer, M.; Stamatakis, E.; Czernichow, S. Association of C-Reactive Protein with Cardiovascular Disease Mortality According to Diabetes Status: Pooled Analyses of 25,979 Participants from Four U.K. Prospective Cohort Studies. Diabetes Care 2012, 35, 396–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gitea, M.A.; Bungau, S.G.; Gitea, D.; Pasca, B.M.; Purza, A.L.; Radu, A.-F. Evaluation of the Phytochemistry-Therapeutic Activity Relationship for Grape Seeds Oil. Life 2023, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, D.; Bungau, S.; Boscencu, R.; Tit, D.M.; Copolovici, L. The Fatty Acids Composition and Antioxidant Activity of Walnut Cold Press Oil. Rev. Chim. 2017, 68, 507–509. [Google Scholar] [CrossRef]
- Csiszar, A. Anti-Inflammatory Effects of Resveratrol: Possible Role in Prevention of Age-Related Cardiovascular Disease. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Mohar, S.D. The Sirtuin System: The Holy Grail of Resveratrol? J. Clin. Exp. Cardiolog. 2012, 3, 216–219. [Google Scholar] [CrossRef]
- Wang, M.; Weng, X.; Chen, H.; Chen, Z.; Liu, X. Resveratrol Inhibits Tnf-α-Induced Inflammation to Protect against Renal Ischemia/Reperfusion Injury in Diabetic Rats1. Acta Cir. Bras. 2020, 35, e202000506. [Google Scholar] [CrossRef]
- Xia, N.; Förstermann, U.; Li, H. Resveratrol and Endothelial Nitric Oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef]
- Floyd, Z.E.; Wang, Z.Q.; Kilroy, G.; Cefalu, W.T. Modulation of Peroxisome Proliferator-Activated Receptor γ Stability and Transcriptional Activity in Adipocytes by Resveratrol. Metabolism 2008, 57, 32–38. [Google Scholar] [CrossRef]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin Ameliorates Metabolic Syndrome and Improves the Inflammatory Status in Obese Zucker Rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Feskens, E.J.M.; Kromhout, D.; Hertog, M.G.L.; Hollman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Dietary Antioxidant Flavonoids and Risk of Coronary Heart Disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.; Miron, A.; Trifan, A.; Luca, V.; Costache, I.-I. The Cardiovascular Effects of Cocoa Polyphenols—An Overview. Diseases 2016, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Casas, R.; Urpí-Sardà, M.; Llorach, R.; Lamuela-Raventós, R.M.; Estruch, R. Effect of Cocoa Powder on the Modulation of Inflammatory Biomarkers in Patients at High Risk of Cardiovascular Disease. Am. J. Clin. Nutr. 2009, 90, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.-F.; Vodnar, D.-C.; Precup, G. Characterization of Grape and Apple Peel Wastes’ Bioactive Compounds and Their Increased Bioavailability After Exposure to Thermal Process. Bull. UASVM Food Sci. Technol. 2014, 73, 55–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ono-Moore, K.D.; Snodgrass, R.G.; Huang, S.; Singh, S.; Freytag, T.L.; Burnett, D.J.; Bonnel, E.L.; Woodhouse, L.R.; Zunino, S.J.; Peerson, J.M.; et al. Postprandial Inflammatory Responses and Free Fatty Acids in Plasma of Adults Who Consumed a Moderately High-Fat Breakfast with and without Blueberry Powder in a Randomized Placebo-Controlled Trial. J. Nutr. 2016, 146, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Kashi, D.S.; Shabir, A.; Boit, M.D.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef] [PubMed]
- Loo, B.M.; Erlund, I.; Koli, R.; Puukka, P.; Hellström, J.; Wähälä, K.; Mattila, P.; Jula, A. Consumption of Chokeberry (Aronia Mitschurinii) Products Modestly Lowered Blood Pressure and Reduced Low-Grade Inflammation in Patients with Mildly Elevated Blood Pressure. Nutr. Res. 2016, 36, 1222–1230. [Google Scholar] [CrossRef]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Gálvez, J.; Medina, F.S.d.; Zarzuelo, A. Inhibition of Pro-Inflammatory Markers in Primary Bone Marrow-Derived Mouse Macrophages by Naturally Occurring Flavonoids: Analysis of the Structure-Activity Relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of Flavonoids and Other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary Polyphenols: Structures, Bioavailability and Protective Effects against Atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.; Behl, T.; Behera, R.K.; Chigurupati, S.; Chauhan, M.; Singh, S.; Sharma, N.; Aldubayan, M.; Felemban, S.G.; Farasani, A.; et al. Effects of nitric oxide modulators and antioxidants on endocrine and cellular markers of acute stress in rats. Biochem. Biophys. Res. Commun. 2022, 589, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing By-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Stoclet, J.C.; Chataigneau, T.; Ndiaye, M.; Oak, M.H.; El Bedoui, J.; Chataigneau, M.; Schini-Kerth, V.B. Vascular Protection by Dietary Polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Precup, G.; Calinoiu, L.-F.; Mitrea, L.; Bindea, M.; Rusu, B.; Stefanescu, B.E.; Vodnar, D.C. The Molecular Restructuring of Classical Desserts by Using Food Industry By-Products. Bull. UASVM Food Sci. Technol. 2017, 74, 58–64. [Google Scholar] [CrossRef][Green Version]
- Pan, W.; Chang, M.J.; Booyse, F.M.; Grenett, H.E.; Bradley, K.M.; Wolkowicz, P.E.; Shang, Q.; Tabengwa, E.M. Quercetin Induced Tissue-Type Plasminogen Activator Expression Is Mediated through Sp1 and P38 Mitogen-Activated Protein Kinase in Human Endothelial Cells. J. Thromb. Haemost. 2008, 6, 976–985. [Google Scholar] [CrossRef]
- Olszanecki, R.; Bujak-Gizycka, B.; Madej, J.; Suski, M.; Wołkow, P.P.; Jawień, J.; Korbut, R. Kaempferol, but Not Resveratrol Inhibits Angiotensin Converting Enzyme. J. Physiol. Pharmacol. 2008, 59, 387–392. [Google Scholar]
- Davison, K.; Berry, N.M.; Misan, G.; Coates, A.M.; Buckley, J.D.; Howe, P.R.C. Dose-Related Effects of Flavanol-Rich Cocoa on Blood Pressure. J. Hum. Hypertens. 2010, 24, 568–576. [Google Scholar] [CrossRef]
- Nemes, S.A.; Florina, L.; Dulf, F.V.; Corina, A.; Vodnar, D.C. Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach. Antioxidants 2022, 11, 2159. [Google Scholar] [CrossRef]
- Lu, T.M.; Chiu, H.F.; Shen, Y.C.; Chung, C.C.; Venkatakrishnan, K.; Wang, C.K. Hypocholesterolemic Efficacy of Quercetin Rich Onion Juice in Healthy Mild Hypercholesterolemic Adults: A Pilot Study. Plant Foods Hum. Nutr. 2015, 70, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Zhan, Z.; Luo, R.; Guo, X.; Guo, Q.; Zhou, J.; Kong, J.; Davis, P.A.; Stoecker, B.J. Cinnamon Extract Lowers Glucose, Insulin and Cholesterol in People with Elevated Serum Glucose. J. Tradit. Complement. Med. 2016, 6, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, C. Targeting Platelet in Atherosclerosis Plaque Formation: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9760. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable Effects of Berry Consumption on Platelet Function, Blood Pressure, and HDL Cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [CrossRef]
- Carnevale, R.; Loffredo, L.; Pignatelli, P.; Nocella, C.; Bartimoccia, S.; di Santo, S.; Martino, F.; Catasca, E.; Perri, L.; Violi, F. Dark Chocolate Inhibits Platelet Isoprostanes via NOX2 Down-Regulation in Smokers. J. Thromb. Haemost. 2012, 10, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ed Nignpense, B.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of Platelet Function and Platelet Microparticle Generation? Int. J. Mol. Sci. 2020, 21, 146. [Google Scholar] [CrossRef]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Flammer, A.J.; Sudano, I. Polyphenols: Anti-Platelet Nutraceutical? Curr. Pharm. Des. 2018, 24, 146–157. [Google Scholar] [CrossRef]
- Lai, H.T.M.; Threapleton, D.E.; Day, A.J.; Williamson, G.; Cade, J.E.; Burley, V.J. Fruit Intake and Cardiovascular Disease Mortality in the UK Women’s Cohort Study. Eur. J. Epidemiol. 2015, 30, 1035–1048. [Google Scholar] [CrossRef]
- Rivera, K.; Salas-Pérz, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; Pérez, D.; De La Cerda, P.; González, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. [Google Scholar] [CrossRef]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-Rich Diets Improve Glucose Metabolism in People at High Cardiometabolic Risk: A Controlled Randomised Intervention Trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in Blueberries Improve Insulin Sensitivity in Obese, Insulin-Resistant Men and Women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green Tea Extract Ingestion, Fat Oxidation, and Glucose Tolerance in Healthy Humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-Term Administration of Dark Chocolate Is Followed by a Significant Increase in Insulin Sensitivity and a Decrease in Blood Pressure in Healthy Persons. Am. J. Clin. Nutr. 2018, 81, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wedick, N.M.; Tworoger, S.S.; Pan, A.; Townsend, M.K.; Cassidy, A.; Franke, A.A.; Rimm, E.B.; Hu, F.B.; Van Dam, R.M. Urinary Excretion of Select Dietary Polyphenol Metabolites Is Associated with a Lower Risk of Type 2 Diabetes in Proximate but Not Remote Follow-up in a Prospective Investigation in 2 Cohorts of US Women. J. Nutr. 2015, 145, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P.M.; Henderson, T.R.; Debelo, H.; Ferruzzi, M.G.; Baer, D.J.; Novotny, J.A. An Anthocyanin-Rich Mixed-Berry Intervention May Overweight and Obese Adults. Nutrients 2019, 11, 2876. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhu, L.; Edirisinghe, I.; Fareed, J.; Brailovsky, Y.; Burton-Freeman, B. Attenuation of Postmeal Metabolic Indices with Red Raspberries in Individuals at Risk for Diabetes: A Randomized Controlled Trial. Obesity 2019, 27, 542–550. [Google Scholar] [CrossRef]
- Clapa, D.; Hârta, M.; Szabo, K.; Teleky, B.-E.; Pamfi, D. The Use of Wheat Starch as Gelling Agent for In Vitro Proliferation of Blackberry (Rubus fruticosus L.) Cultivars and the Evaluation of Genetic Fidelity after Repeated Subcultures. Horticulture 2023, 9, 902. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic Effect of Dietary Polyphenols: A Systematic Review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Precup, G.; Teleky, B.-E.; Ranga, F.; Vodnar, D.C. Assessment of Physicochemical and Rheological Properties of Xylo-Oligosaccharides and Glucose-Enriched Doughs Fermented with BB-12. Biology 2022, 11, 553. [Google Scholar] [CrossRef]
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent Advances on Dietary Polyphenol’s Potential Roles in Celiac Disease. Trends Food Sci. Technol. 2021, 107, 213–225. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary Polyphenols to Combat the Metabolic Diseases via Altering Gut Microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The Effects of Grape and Red Wine Polyphenols on Gut Microbiota—A Systematic Review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cheng, D.; Huang, C.; Li, Y.; Lao, C.; Xia, Y.; Liu, W.; Gong, X.; Hu, D.; Li, B.; et al. Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats. Molecules 2019, 24, 1139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Li, X.Y.; Shen, L. Modulation Effect of Tea Consumption on Gut Microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 981–987. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Romo-Vaquero, M.; García-Villalba, R.; Cortés-Martín, A.; Selma, M.V.; Espín, J.C. The Endotoxemia Marker Lipopolysaccharide-Binding Protein Is Reduced in Overweight-Obese Subjects Consuming Pomegranate Extract by Modulating the Gut Microbiota: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2018, 62, e1800160. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Y. Polyphenol Supplementation Benefits Human Health via Gut Microbiota: A Systematic Review via Meta-Analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(Vinyl Alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Piekarska-Radzik, L.; Klewicka, E. Mutual Influence of Polyphenols and Lactobacillus Spp. Bacteria in Food: A Review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Pascuta, M.S.; Varvara, R.; Teleky, B.-E.; Szabo, K.; Plamada, D.; Nemes, S.-A.; Mitrea, L.; Mărtau, G.A.; Ciont, C.; Călinoiu, L.-F.; et al. Polysaccharide-Based Edible Gels as Functional Ingredients:Characterization, Applicability, and Human Health Benefit. Gels 2022, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Nabil-Adam, A.; Elnosary, M.E.; Ashour, M.L.; El-Moneam, N.M.A.; Shreadah, M.A. Flavonoids Biosynthesis in Plants as a Defense Mechanism: Role and Function Concerning Pharmacodynamics and Pharmacokinetic Properties. In Flavonoid Metabolism—Recent Advances and Applications in Crop Breeding 3-deoxyanthocyanins; IntechOpen: London, UK, 2016; Volume I, p. 13. [Google Scholar]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxidative Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Campos, E.M.; Stehle, P.; Simon, M.C. Microbial Metabolites of Flavan-3-Ols and Their Biological Activity. Nutrients 2019, 11, 2260. [Google Scholar] [CrossRef]
- Liu, S.; He, F.; Zheng, T.; Wan, S.; Chen, J.; Yang, F.; Xu, X.; Pei, X. Ligustrum Robustum Alleviates Atherosclerosis by Decreasing Serum TMAO, Modulating Gut Microbiota, and Decreasing Bile Acid and Cholesterol Absorption in Mice. Mol. Nutr. Food Res. 2021, 65, 2100014. [Google Scholar] [CrossRef]
- Yuan, X.; Long, Y.; Ji, Z.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X.; et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018, 62, e1800178. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic Effects of Almonds and Almond Skins on Intestinal Microbiota in Healthy Adult Humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lipan, L.; Rusu, B.; Sendra, E.; Hernández, F.; Vázquez-araújo, L.; Vodnar, C. Spray Drying and Storage of Probiotic- Enriched Almond Milk: Probiotic Survival and Physicochemical Properties. J. Sci. Food Agric. 2020, 100, 3697–3708. [Google Scholar] [CrossRef] [PubMed]
- Teleky, B.E.; Martău, A.G.; Ranga, F.; Chețan, F.; Vodnar, D.C.; Gheorghe, A.; Chet, F. Exploitation of Lactic Acid Bacteria and Baker’s Yeast as Single or Multiple Starter Cultures of Wheat Flour Dough Enriched with Soy Flour. Biomolecules 2020, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Hu, L.; Wu, Y.; Chen, L.; Huang, K.; Wang, Z.; Liang, M. Daidzein Suppresses TGF-Β1-Induced Cardiac Fibroblast Activation via the TGF-Β1/SMAD2/3 Signaling Pathway. Eur. J. Pharmacol. 2022, 919, 174805. [Google Scholar] [CrossRef] [PubMed]
- Khorasanian, A.S.; Fateh, S.T.; Gholami, F.; Rasaei, N.; Gerami, H.; Khayyatzadeh, S.S.; Shiraseb, F.; Asbaghi, O. The Effects of Hesperidin Supplementation on Cardiovascular Risk Factors in Adults: A Systematic Review and Dose–Response Meta-Analysis. Front. Nutr. 2023, 9, 1177708. [Google Scholar] [CrossRef] [PubMed]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Zorița, M.D.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Villalba, K.J.O.; Barka, F.V.; Pasos, C.V.; Rodríguez, P.E. Food Ellagitannins: Structure, Metabolomic Fate, and Biological Properties. In Tannins—Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2016; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Toney, A.M.; Fox, D.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Immunomodulatory Role of Urolithin a on Metabolic Diseases. Biomedicines 2021, 9, 192. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on Their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. [Google Scholar] [CrossRef]
- Chen, P.; Guo, Z.; Chen, F.; Wu, Y.; Zhou, B. Recent Advances and Perspectives on the Health Benefits of Urolithin B, A Bioactive Natural Product Derived From Ellagitannins. Front. Pharmacol. 2022, 13, 917266. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Matthan, N.R.; Liu, J.; de la Torre, R.; Chen, C.Y.O. Cranberries Attenuate Animal-Based Diet-Induced Changes in Microbiota Composition and Functionality: A Randomized Crossover Controlled Feeding Trial. J. Nutr. Biochem. 2018, 62, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ştefanescu, B.E.; Szabo, K.; Mocan, A.; Crisan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Zhong, C.B.; Wu, W. Resveratrol and Diabetic Cardiomyopathy: Focusing on the Protective Signaling Mechanisms. Oxid. Med. Cell Longev. 2020, 2020, 7051845. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red Wine Polyphenols Modulate Fecal Microbiota and Reduce Markers of the Metabolic Syndrome in Obese Patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Romero, S.; Martínez-Maqueda, D.; Hereu, M.; Amézqueta, S.; Torres, J.L.; Pérez-Jiménez, J. Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-over Controlled Clinical Trial. Foods 2020, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo®) in Reducing Tmao Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-over Study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of Red Wine Polyphenols and Ethanol on the Gut Microbiota Ecology and Biochemical Biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Tucsek, Z.; Radnai, B.; Racz, B.; Debreceni, B.; Priber, J.K.; Dolowschiak, T.; Palkovics, T.; Gallyas, F.; Sumegi, B.; Veres, B. Suppressing LPS-Induced Early Signal Transduction in Macrophages by a Polyphenol Degradation Product: A Critical Role of MKP-1. J. Leukoc. Biol. 2010, 89, 105–111. [Google Scholar] [CrossRef]
- Beloborodova, N.V.; Olenin, A.Y.; Fedotcheva, N.I.; Shubina, V.; Teplova, V.V. Effect of Phenolic Acids Originating from Microbes on Mitochondria and Neutrophils. Crit. Care 2012, 16, 1–58. [Google Scholar] [CrossRef]
- Song, M.Y.; Wang, J.H.; Eom, T.; Kim, H. Schisandra Chinensis Fruit Modulates the Gut Microbiota Composition in Association with Metabolic Markers in Obese Women: A Randomized, Double-Blind Placebo-Controlled Study. Nutr. Res. 2015, 35, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Liu, Z.; Plimmer, G. Evaluation of the Effect of Blackcurrant Products on Gut Microbiota and on Markers of Risk for Colon Cancer in Humans. Phyther. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Reider, S.; Watschinger, C.; Längle, J.; Pachmann, U.; Przysiecki, N.; Pfister, A.; Zollner, A.; Tilg, H.; Plattner, S.; Moschen, A.R. Short- and Long-Term Effects of a Prebiotic Intervention with Polyphenols Extracted from European Black Elderberry—Sustained Expansion of Akkermansia spp. J. Pers. Med. 2022, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; Bashmakov, Y.; Chalyk, N.; Nielsen, D.S.; Krych, Ł.; Kot, W.; Klochkov, V.; Pristensky, D.; Bandaletova, T.; Chernyshova, M.; et al. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons. Biomed. Res. Int. 2019, 2019, 4625279. [Google Scholar] [CrossRef] [PubMed]
- Brasili, E.; Hassimotto, N.M.A.; Del Chierico, F.; Marini, F.; Quagliariello, A.; Sciubba, F.; Miccheli, A.; Putignani, L.; Lajolo, F. Daily Consumption of Orange Juice from Citrus Sinensis L. Osbeck Cv. Cara Cara and Cv. Bahia Differently Affects Gut Microbiota Profiling as Unveiled by an Integrated Meta-Omics Approach. J. Agric. Food Chem. 2019, 67, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Wijayabahu, A.T.; Waugh, S.G.; Ukhanova, M.; Mai, V. Dietary Raisin Intake Has Limited Effect on Gut Microbiota Composition in Adult Volunteers. Nutr. J. 2019, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of Aronia Berry (Poly)Phenols on Vascular Function and Gut Microbiota: A Double-Blind Randomized Controlled Trial in Adult Men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]


| Cardiometabolic Risk Components | Special Aspects of the Gut Microbiota | Refs. |
|---|---|---|
| Hypertension | ↑ Firmicutes/Bacteroides ratio, ↓ bacterial diversity ↑ Klebsiella, Prevotella, Desulfovibrio, Clostridium, Oscillibacter ↓ Faecalibacterium, Akkermansia, Roseburia, Butyrivibrio, Lactobacillus ↓SCFA | [57,64] |
| Heart failure | ↓ Firmicutes/Bacteroides ratio, ↓ bacterial diversity ↑ Escherichia, Klebsiella, Streptococcus, Rumnicoccus ↓ Faecalibacterium, Eubacterium | [71,72] |
| Atherosclerosis | ↓ bacterial diversity ↑ Streptococcus, Pseudomonas, Klebsiella, Veillonella spp., ↓ Roseburia, Faecalibacterium, Bacteroides spp. ↑ TMAO, ↓ SCFA | [61,73] |
| T2DM and obesity | ↑ Firmicutes/Bacteroides ratio, ↓bacterial diversity ↑ Lactobacillus reuteri, Proteobacteria ↓ Faecalibacterium, Akkermansia, Roseburia, Methanobrevibacter smithii ↓ SCFA, ↑ LPS | [56,60,61,62,63,64] |
| Polyphenol Source | Method | Outcome | Ref. |
|---|---|---|---|
| Blackcurrant | Clinical trial (30 healthy human volunteers): consumption of blackcurrant extract First Leaf 1500 mg/day and Cassis Anthomix 672 mg/day for 4 week and 2-week washout. | ↑ Lactobacillus and Bifidobacteria ↓ Clostridium spp. and Bacteroides spp. | [219] |
| Elderberry | Clinical trial (30 healthy human volunteers): consumption of purified extract from European black elderberries with a high and standardized content of polyphenols and anthocyanins (600 mg/day). Nine week, three distinct phases, three weeks each (baseline, intervention, and washout). | ↑ Akkermansia spp. and Bacteroides cellulosolyticus | [220] |
| Blueberry | Double-blind randomized clinical study (20 healthy male individuals): consumption of a wild blueberry drink (25 g of wild blueberry powder in 250 mL of water) every day for 6 weeks. | ↑ Bifidobacterium spp. | [203] |
| Cocoa | Randomized, controlled, double-blind, crossover intervention (22 healthy human volunteers): consumption of cocoa flavanols (high quantity–494 mg cocoa flavanols/d, and low quantity–23 mg cocoa flavanols/d) for 4 weeks each, with a 4 week washout period. | ↑ Bifidobacteria and Lactobacillus ↓ Clostridium spp., triacylglycerol, and C-reactive protein concentrations | [185] |
| Randomized clinical trial (30 moderately obese with BMI between 30 and 35 kg/m2) consumption of 10 g fark chocolate (4 weeks). | ↑ Lactobacillus ↓ Bacteroidetes | [221] | |
| Almond | Randomized, crossover-controlled intervention (48 healthy subjects): almond (56 g) and almond skin (10 g) ingestion, with commercial fructo-oligosaccharides (8 g) as positive control (6 weeks). | ↑ Bifidobacterium spp., Lactobacillus spp. and fecal β-galactosidase activity ↓ Clostridum perfringens, fecal β-glucuronidase, nitro reductase and azo-reductase activities | [197] |
| Green tea | Randomized, crossover-controlled intervention (12 healthy volunteers): green tea liquid consumption (400 mL/day 2 weeks). | ↑ Firmicutes: Bacteroidetes ratio, SCFA producing genera ↓ bacterial LPS synthesis | [196] |
| Red wine polyphenols | Randomized, crossover-controlled intervention (10 metabolic syndrome patients and 10 healthy subjects): consumption of red wine or dealcoholized red wine (272 mL per day), 30 days each after a washout period. | In the metabolic syndrome patients, ↑ Bifidobacteria, Lactobacillus and butyrate-producing bacteria (Faecalibacterium prausnitzii and Roseburia) ↓ LPS producers (Escherichia coli and Enterobacter cloacae) | [211] |
| Randomized, crossover, controlled intervention (10 healthy male volunteers): consumption of red wine (272 mL/d) and dealcoholized red wine (272 mL/d) and gin (100 mL/d), 20 days each after a washout period. | ↑ Enterococcus, Bifidobacterium, Bacteroides, Prevotella, Bacteroides uniformis, Eggerthella lenta, and Blautia coccoides. ↓ blood pressures, total cholesterol, triglyceride, and C-reactive protein concentrations | [215] | |
| Pomegranate | Double-blind, cross-over, dose-response, randomized, and placebo controlled clinical trial (49 overweight obese subjects with mild hyperlipidemia): consumption two doses (D1 = 164 mg and D2 = 656 mg lasting 3 weeks each) of pomegranate extract or placebo alternating with 3 weeks of washout periods. | ↑ Bacteroides, Faecalibacterium, Butyricicoccus, Odoribacter, and Bacteroides, Faecalibacterium, Butyricicoccus, Odoribacter, and Butyricimonas. ↓ Parvimonas, Methanobrevibacter, and Methanosphaera. ↓ high-sensitivity C-reactive protein and lipopolysaccharide-binding protein | [181] |
| Orange | Randomized crossover design (21 healthy volunteers): 500 mL of orange juice from Citrus sinensis L. Osbeck cv. Cara Cara, Bahia, or isocaloric control drink. Seven days with a one-week washout period between consecutive interventions. | ↑ Mogibacteriaceae, Tissierellaceae, Veillonellaceae, Odoribacteraceae, and Ruminococcaceae | [222] |
| Phenolic acids, Dietary raisin | Exploratory feeding study (13 healthy volunteers): consumption of three servings (28.3 g each) of sun-dried raisins daily for 14 days (on days 7 and 14). | ↑ Faecalibacterium prausnitzii, Bacteroidetes spp., Ruminococcus spp.; Decrease Klebsiella spp., Prevotella spp., Bifidobacterium spp. | [223] |
| Aronia berry | Double-blind randomized controlled trial (66 healthy men): consumption of (poly)phenol-rich extract (116 mg, 75 g berries), a whole fruit powder (12 mg, 10 g berries), or placebo (maltodextrin) for 12 wk. | extract ↑ Anaerostipes whole fruit Bacteroides | [224] |
| Grape pomace | Randomized cross-over clinical trial (49 subjects at cardiometabolic risk exhibiting at least two metabolic syndrome factors): consumption of a daily dose of 8 g of grape pomace for 6 weeks with an equivalent control period. | ↓ Lactobacilliales and Insulin in responders | [212] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haș, I.M.; Tit, D.M.; Bungau, S.G.; Pavel, F.M.; Teleky, B.-E.; Vodnar, D.C.; Vesa, C.M. Cardiometabolic Risk: Characteristics of the Intestinal Microbiome and the Role of Polyphenols. Int. J. Mol. Sci. 2023, 24, 13757. https://doi.org/10.3390/ijms241813757
Haș IM, Tit DM, Bungau SG, Pavel FM, Teleky B-E, Vodnar DC, Vesa CM. Cardiometabolic Risk: Characteristics of the Intestinal Microbiome and the Role of Polyphenols. International Journal of Molecular Sciences. 2023; 24(18):13757. https://doi.org/10.3390/ijms241813757
Chicago/Turabian StyleHaș, Ioana Mariana, Delia Mirela Tit, Simona Gabriela Bungau, Flavia Maria Pavel, Bernadette-Emoke Teleky, Dan Cristian Vodnar, and Cosmin Mihai Vesa. 2023. "Cardiometabolic Risk: Characteristics of the Intestinal Microbiome and the Role of Polyphenols" International Journal of Molecular Sciences 24, no. 18: 13757. https://doi.org/10.3390/ijms241813757
APA StyleHaș, I. M., Tit, D. M., Bungau, S. G., Pavel, F. M., Teleky, B.-E., Vodnar, D. C., & Vesa, C. M. (2023). Cardiometabolic Risk: Characteristics of the Intestinal Microbiome and the Role of Polyphenols. International Journal of Molecular Sciences, 24(18), 13757. https://doi.org/10.3390/ijms241813757












