Arginine Is a Novel Drug Target for Arginine Decarboxylase in Human Colorectal Cancer Cells
Abstract
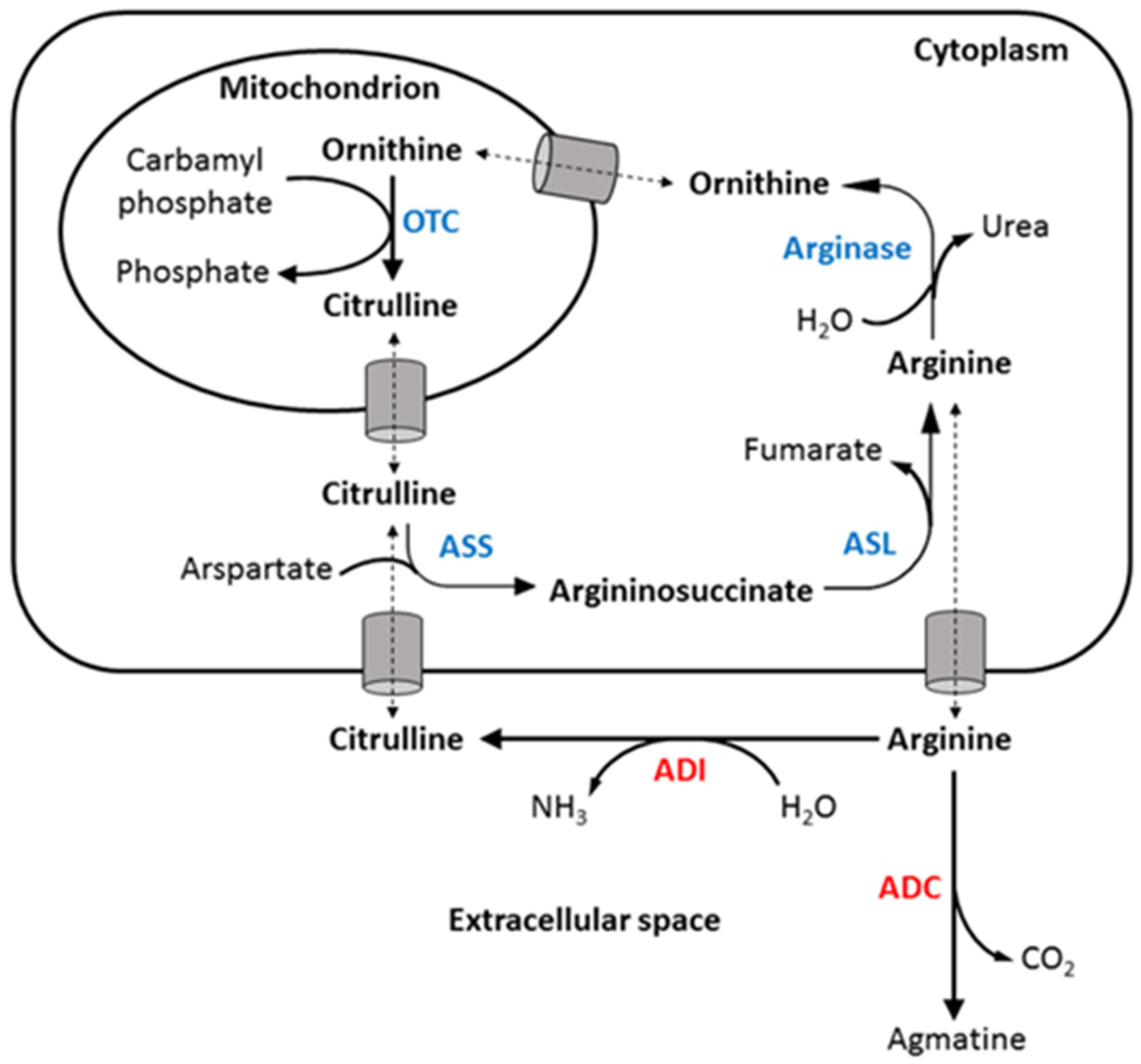
:1. Introduction
2. Results
2.1. A Single-Step Purification of Recombinant ADC Resulted in High Protein Yield
2.2. ADC Was Better Than ADI in Treating ASS-Positive Colorectal Cancer Cells
2.3. ADC Was Relatively Less Toxic to Non-Tumorous Cells
2.4. ADC Induced S and G2/M Arrest in Colorectal Cancer Cells
2.5. ADC Induced Apoptosis in Colorectal Cancer Cells in a Time- and Dose-Dependent Manner
2.6. ADC-Induced Apoptosis in HCT116 Cells Was Caspase-3-Dependent
3. Discussion
4. Materials and Methods
4.1. Construction of Expression Vector for ADC
- (a)
- 5′-GCATATGAGCAAGATGCTGCGTACTTACAATATTGCCTGGTGGGGC-3′
- (b)
- 5′-GCAGCCGGATCCTTAGTGATGGTGGTGGTGGTGCTCATCTTCA-3′
- (c)
- 5′-GAAGTGCAAAAGCAGCTGGACCCGCAAAACCG-3′
- (d)
- 5′-CGGTTTTGCGGGTCCAGCTGCTTTTGCACTTC-3′
4.2. Preparation of ADC
4.3. Specific Activity of ADC
4.4. Preparation of ADI
4.5. Culture of Cell Lines
4.6. Preparation and Culture of Rat Primary Hepatocytes
4.7. Cell Viability Assay
4.8. Cell-Cycle Analysis
4.9. Apoptosis Analysis by Flow Cytometry
4.10. Immunoblot Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, P.N.M.; Liu, A.M.; Bessudo, A.; Mussai, F. Safety, PK/PD and preliminary anti-tumor activities of pegylated recombinant human arginase 1 (BCT-100) in patients with advanced arginine auxotrophic tumors. Investig. New Drugs 2021, 39, 1633–1640. [Google Scholar] [CrossRef]
- Hall, P.E.; Lewis, R.; Syed, N.; Shaffer, R.; Evanson, J.; Ellis, S.; Williams, M.; Feng, X.; Johnston, A.; Thomson, J.A.; et al. A Phase I Study of Pegylated Arginine Deiminase (Pegargiminase), Cisplatin, and Pemetrexed in Argininosuccinate Synthetase 1-Deficient Recurrent High-grade Glioma. Clin. Cancer Res. 2019, 25, 2708–2716. [Google Scholar] [CrossRef]
- Yoon, C.-Y.; Shim, Y.-J.; Kim, E.-H.; Lee, J.-H.; Won, N.-H.; Kim, J.-H.; Park, I.-S.; Yoon, D.-K.; Min, B.-H. Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int. J. Cancer 2007, 120, 897–905. [Google Scholar] [CrossRef]
- Scott, L.; Lamb, J.; Smith, S.; Wheatley, D.N. Single amino acid (arginine) deprivation: Rapid and selective death of cultured transformed and malignant cells. Br. J. Cancer 2000, 83, 800–810. [Google Scholar] [CrossRef]
- Kelly, M.P.; Jungbluth, A.A.; Wu, B.-W.; Bomalaski, J.; Old, L.J.; Ritter, G. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br. J. Cancer 2012, 106, 324–332. [Google Scholar] [CrossRef]
- Kim, R.H.; Coates, J.M.; Bowles, T.L.; McNerney, G.P.; Sutcliffe, J.; Jung, J.U.; Gandour-Edwards, R.; Chuang, F.Y.; Bold, R.J.; Kung, H.-J. Arginine Deiminase as a Novel Therapy for Prostate Cancer Induces Autophagy and Caspase-Independent Apoptosis. Cancer Res. 2009, 69, 700–708. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.-R.; Liu, X.; Chu, C.-Y.; Shen, L.-J.; Xu, J.; Gaur, S.; Forman, H.J.; Zhang, H.; Zheng, S.; et al. Arginine Starvation Impairs Mitochondrial Respiratory Function in ASS1-Deficient Breast Cancer Cells. Sci. Signal. 2014, 7, ra31. [Google Scholar] [CrossRef]
- Glazer, E.S.; Piccirillo, M.; Albino, V.; Di Giacomo, R.; Palaia, R.; Mastro, A.A.; Beneduce, G.; Castello, G.; De Rosa, V.; Petrillo, A.; et al. Phase II Study of Pegylated Arginine Deiminase for Nonresectable and Metastatic Hepatocellular Carcinoma. J. Clin. Oncol. 2010, 28, 2220–2226. [Google Scholar] [CrossRef]
- Izzo, F.; Marra, P.; Beneduce, G.; Castello, G.; Vallone, P.; De Rosa, V.; Cremona, F.; Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; et al. Pegylated Arginine Deiminase Treatment of Patients With Unresectable Hepatocellular Carcinoma: Results From Phase I/II Studies. J. Clin. Oncol. 2004, 22, 1815–1822. [Google Scholar] [CrossRef]
- Ott, P.A.; Carvajal, R.D.; Pandit-Taskar, N.; Jungbluth, A.A.; Hoffman, E.W.; Wu, B.-W.; Bomalaski, J.S.; Venhaus, R.; Pan, L.; Old, L.J.; et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Investig. New Drugs 2013, 31, 425–434. [Google Scholar] [CrossRef]
- Synakiewicz, A.; Stachowicz-Stencel, T.; Adamkiewicz-Drozynska, E. The role of arginine and the modified arginine deiminase enzyme ADI-PEG 20 in cancer therapy with special emphasis on Phase I/II clinical trials. Expert Opin. Investig. Drugs 2014, 23, 1517–1529. [Google Scholar] [CrossRef]
- Yau, T.; Cheng, P.N.; Chan, P.; Chan, W.; Chen, L.; Yuen, J.; Pang, R.; Fan, S.T.; Poon, R.T. A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Investig. New Drugs 2013, 31, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Cheng, P.N.; Liu, A.M.; Chan, L.L.; Li, L.; Chu, C.M.; Chong, C.C.; Lau, Y.M.; Yeo, W.; Ng, K.K.; et al. A phase II clinical study on the efficacy and predictive biomarker of pegylated recombinant arginase on hepatocellular carcinoma. Investig. New Drugs 2021, 39, 1375–1382. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.M.; Sargent, D.J.; Morton, R.F.; Fuchs, C.S.; Ramanathan, R.K.; Williamson, S.K.; Findlay, B.P.; Pitot, H.C.; Alberts, S.R. A Randomized Controlled Trial of Fluorouracil Plus Leucovorin, Irinotecan, and Oxaliplatin Combinations in Patients with Previously Untreated Metastatic Colorectal Cancer. J. Clin. Oncol. 2004, 22, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Alexandrou, C.; Al-Aqbi, S.S.; Higgins, J.A.; Boyle, W.; Karmokar, A.; Andreadi, C.; Luo, J.-L.; Moore, D.A.; Viskaduraki, M.; Blades, M.; et al. Sensitivity of Colorectal Cancer to Arginine Deprivation Therapy is Shaped by Differential Expression of Urea Cycle Enzymes. Sci. Rep. 2018, 8, 12096. [Google Scholar] [CrossRef]
- Huang, H.-L.; Chen, W.-C.; Hsu, H.-P.; Cho, C.-Y.; Hung, Y.-H.; Wang, C.-Y.; Lai, M.-D. Silencing of argininosuccinate lyase inhibits colorectal cancer formation. Oncol. Rep. 2017, 37, 163–170. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Liu, X.-X.; Jiang, C.-Y.; Lu, Y.; Liu, S.-L.; Bian, J.-F.; Wang, X.-M.; Geng, Z.; Zhang, Y.; Zhang, B. Ornithine decarboxylase gene is overexpressed in colorectal carcinoma. World J. Gastroenterol. 2005, 11, 2244–2248. [Google Scholar] [CrossRef]
- Soda, K. Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation. Cells 2022, 11, 164. [Google Scholar] [CrossRef]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Gerner, E.W.; Meyskens, F.L., Jr. Combination Chemoprevention for Colon Cancer Targeting Polyamine Synthesis and Inflammation. Clin. Cancer Res. 2009, 15, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.R.; Pardee, A.B. Multiple Pathways of Putrescine Biosynthesis in Escherichia coli. J. Biol. Chem. 1966, 241, 3129–3135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Y.; Iyo, A.; Piletz, J.E.; Regunathan, S. Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochim. Biophys. Acta 2004, 1670, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.; Campbell, E.; Wheatley, D.N. Arginine deprivation, growth inhibition and tumour cell death: 2. Enzymatic degradation of arginine in normal and malignant cell cultures. Br. J. Cancer 2003, 88, 613–623. [Google Scholar] [CrossRef]
- Gerner, E.W.; Bruckheimer, E.; Cohen, A. Cancer pharmacoprevention: Targeting polyamine metabolism to manage risk factors for colon cancer. J. Biol. Chem. 2018, 293, 18770–18778. [Google Scholar] [CrossRef]
- Cai, Y.; Chow, J.P.H.; Leung, Y.-O.; Lu, X.; Yuen, C.-H.; Lee, W.L.; Chau, K.-C.; Yang, L.L.; Wong, R.M.H.; Lam, J.Y.T.; et al. NEI-01-Induced Arginine Deprivation Has Potent Activity Against Acute Myeloid Leukemia Cells Both In Vitro and In Vivo. Mol. Cancer Ther. 2021, 20, 2218–2227. [Google Scholar] [CrossRef]
- Feng, Q.; Bian, X.; Liu, X.; Wang, Y.; Zhou, H.; Ma, X.; Quan, C.; Yao, Y.; Zheng, Z. Intracellular expression of arginine deiminase activates the mitochondrial apoptosis pathway by inhibiting cytosolic ferritin and inducing chromatin autophagy. BMC Cancer 2020, 20, 665. [Google Scholar] [CrossRef]
- Kim, R.H.; Bold, R.J.; Kung, H.-J. ADI, autophagy and apoptosis: Metabolic stress as a therapeutic option for prostate cancer. Autophagy 2009, 5, 567–568. [Google Scholar] [CrossRef]
- Métayer, L.E.; Brown, R.D.; Carlebur, S.; Burke, G.A.A.; Brown, G.C. Mechanisms of cell death induced by arginase and asparaginase in precursor B-cell lymphoblasts. Apoptosis 2019, 24, 145–156. [Google Scholar] [CrossRef]
- Müller, H.J.; Boos, J. Use of L-asparaginase in childhood ALL. Crit. Rev. Oncol. Hematol. 1998, 28, 97–113. [Google Scholar] [CrossRef]
- Narta, U.K.; Kanwar, S.S.; Azmi, W. Pharmacological and clinical evaluation of l-asparaginase in the treatment of leukemia. Crit. Rev. Oncol. Hematol. 2007, 61, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Beloussow, K.; Wang, L.; Wu, J.; Ann, D.; Shen, W.-C. Recombinant arginine deiminase as a potential anti-angiogenic agent. Cancer Lett. 2002, 183, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Isome, M.; Lortie, M.J.; Murakami, Y.; Parisi, E.; Matsufuji, S.; Satriano, J. The antiproliferative effects of agmatine correlate with the rate of cellular proliferation. Am. J. Physiol. Physiol. 2007, 293, C705–C711. [Google Scholar] [CrossRef] [PubMed]
- Molderings, G.J.; Kribben, B.; Heinen, A.; Schröder, D.; Brüss, M.; Göthert, M. Intestinal tumor and agmatine (decarboxylated arginine): Low content in colon carcinoma tissue specimens and inhibitory effect on tumor cell proliferation in vitro. Cancer 2004, 101, 858–868. [Google Scholar] [CrossRef]
- Wolf, C.; Brüss, M.; Hänisch, B.; Göthert, M.; von Kügelgen, I.; Molderings, G.J. Molecular Basis for the Antiproliferative Effect of Agmatine in Tumor Cells of Colonic, Hepatic, and Neuronal Origin. Mol. Pharmacol. 2007, 71, 276–283. [Google Scholar] [CrossRef]
- Mayeur, C.; Veuillet, G.; Michaud, M.; Raul, F.; Blottière, H.M.; Blachier, F. Effects of agmatine accumulation in human colon carcinoma cells on polyamine metabolism, DNA synthesis and the cell cycle. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2005, 1745, 111–123. [Google Scholar] [CrossRef]
- Delage, B.; Fennell, D.A.; Nicholson, L.; McNeish, I.; Lemoine, N.R.; Crook, T.; Szlosarek, P.W. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer 2010, 126, 2762–2772. [Google Scholar] [CrossRef]
- Shen, L.-J.; Lin, W.-C.; Beloussow, K.; Shen, W.-C. Resistance to the anti-proliferative activity of recombinant arginine deiminase in cell culture correlates with the endogenous enzyme, argininosuccinate synthetase. Cancer Lett. 2003, 191, 165–170. [Google Scholar] [CrossRef]
- Haines, R.J.; Pendleton, L.C.; Eichler, D.C. Argininosuccinate synthase: At the center of arginine metabolism. Int. J. Biochem. Mol. Biol. 2011, 2, 8–23. [Google Scholar]
- Agrawal, V.; Woo, J.H.; Mauldin, J.P.; Jo, C.; Stone, E.M.; Georgiou, G.; Frankel, A.E. Cytotoxicity of human recombinant arginase I (Co)-PEG5000 in the presence of supplemental L-citrulline is dependent on decreased argininosuccinate synthetase expression in human cells. Anti-Cancer Drugs 2012, 23, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.N.-M.; Lam, T.-L.; Lam, W.-M.; Tsui, S.-M.; Cheng, A.W.-M.; Lo, W.-H.; Leung, Y.-C. Pegylated Recombinant Human Arginase (rhArg-peg5000mw) Inhibits the In vitro and In vivo Proliferation of Human Hepatocellular Carcinoma through Arginine Depletion. Cancer Res. 2007, 67, 309–317. [Google Scholar] [CrossRef]
- Glazer, E.S.; Kaluarachchi, W.D.; Massey, K.L.; Zhu, C.; Curley, S.A. Bioengineered arginase I increases caspase-3 expression of hepatocellular and pancreatic carcinoma cells despite induction of argininosuccinate synthetase-1. Surgery 2010, 148, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.-L.; Wong, G.K.Y.; Chow, H.-Y.; Chong, H.-C.; Chow, T.-L.; Kwok, S.-Y.; Cheng, P.N.M.; Wheatley, D.N.; Lo, W.-H.; Leung, Y.-C. Recombinant human arginase inhibits the in vitro and in vivo proliferation of human melanoma by inducing cell cycle arrest and apoptosis. Pigment. Cell Melanoma Res. 2011, 24, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.-H.; Zoncu, R.; Kim, D.; Sabatini, D.M. Defective Regulation of Autophagy upon Leucine Deprivation Reveals a Targetable Liability of Human Melanoma Cells In Vitro and In Vivo. Cancer Cell 2011, 19, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kang, H.; Park, I.S.; Choi, S.H.; Shin, K.H.; Chun, Y.S.; Chun, B.G.; Min, B.H. Cytoprotective effect of arginine deiminase on taxol-induced apoptosis in DU145 human prostate cancer cells. Mol. Cells 2000, 10, 331–337. [Google Scholar]
- Kim, J.-E.; Kim, S.Y.; Lee, K.W.; Lee, H.J. Arginine deiminase originating from Lactococcus lactis ssp. lactis American Type Culture Collection (ATCC) 7962 induces G1-phase cell-cycle arrest and apoptosis in SNU-1 stomach adenocarcinoma cells. Br. J. Nutr. 2009, 102, 1469–1476. [Google Scholar] [CrossRef]
- Gong, H.; Zölzer, F.; von Recklinghausen, G.; Rössler, J.; Breit, S.; Havers, W.; Fotsis, T.; Schweigerer, L. Arginine Deiminase Inhibits Cell Proliferation by Arresting Cell Cycle and Inducing Apoptosis. Biochem. Biophys. Res. Commun. 1999, 261, 10–14. [Google Scholar] [CrossRef]
- Gong, H.; Zölzer, F.; von Recklinghausen, G.; Havers, W.; Schweigerer, L. Arginine deiminase inhibits proliferation of human leukemia cells more potently than asparaginase by inducing cell cycle arrest and apoptosis. Leukemia 2000, 14, 826–829. [Google Scholar] [CrossRef]
- Lam, T.L.; Wong, G.K.; Chong, H.C.; Cheng, P.N.; Choi, S.C.; Chow, T.L.; Kwok, S.; Poon, R.; Wheatley, D.; Lo, W.; et al. Recombinant human arginase inhibits proliferation of human hepatocellular carcinoma by inducing cell cycle arrest. Cancer Lett. 2009, 277, 91–100. [Google Scholar] [CrossRef]
- Roeksomtawin, S.; Navasumrit, P.; Waraprasit, S.; Parnlob, V.; Sricharunrat, T.; Bhudhisawasdi, V.; Savaraj, N.; Ruchirawat, M. Decreased argininosuccinate synthetase expression in Thai patients with cholangiocarcinoma and the effects of ADI-PEG20 treatment in CCA cell lines. Oncol. Lett. 2018, 16, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-S.; Lu, S.-N.; Chao, Y.; Sheen, I.-S.; Lin, C.-C.; Wang, T.-E.; Chen, S.-C.; Wang, J.-H.; Liao, L.-Y.; A Thomson, J.; et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br. J. Cancer 2010, 103, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Misawa, S.; Aoshima, M.; Takaku, H.; Matsumoto, M.; Hayashi, H. High-level expression of Mycoplasma arginine deiminase in Escherichia coli and its efficient renaturation as an anti-tumor enzyme. J. Biotechnol. 1994, 36, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Boyde, T.R.; Rahmatullah, M. Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Anal. Biochem. 1980, 107, 424–431. [Google Scholar] [CrossRef] [PubMed]
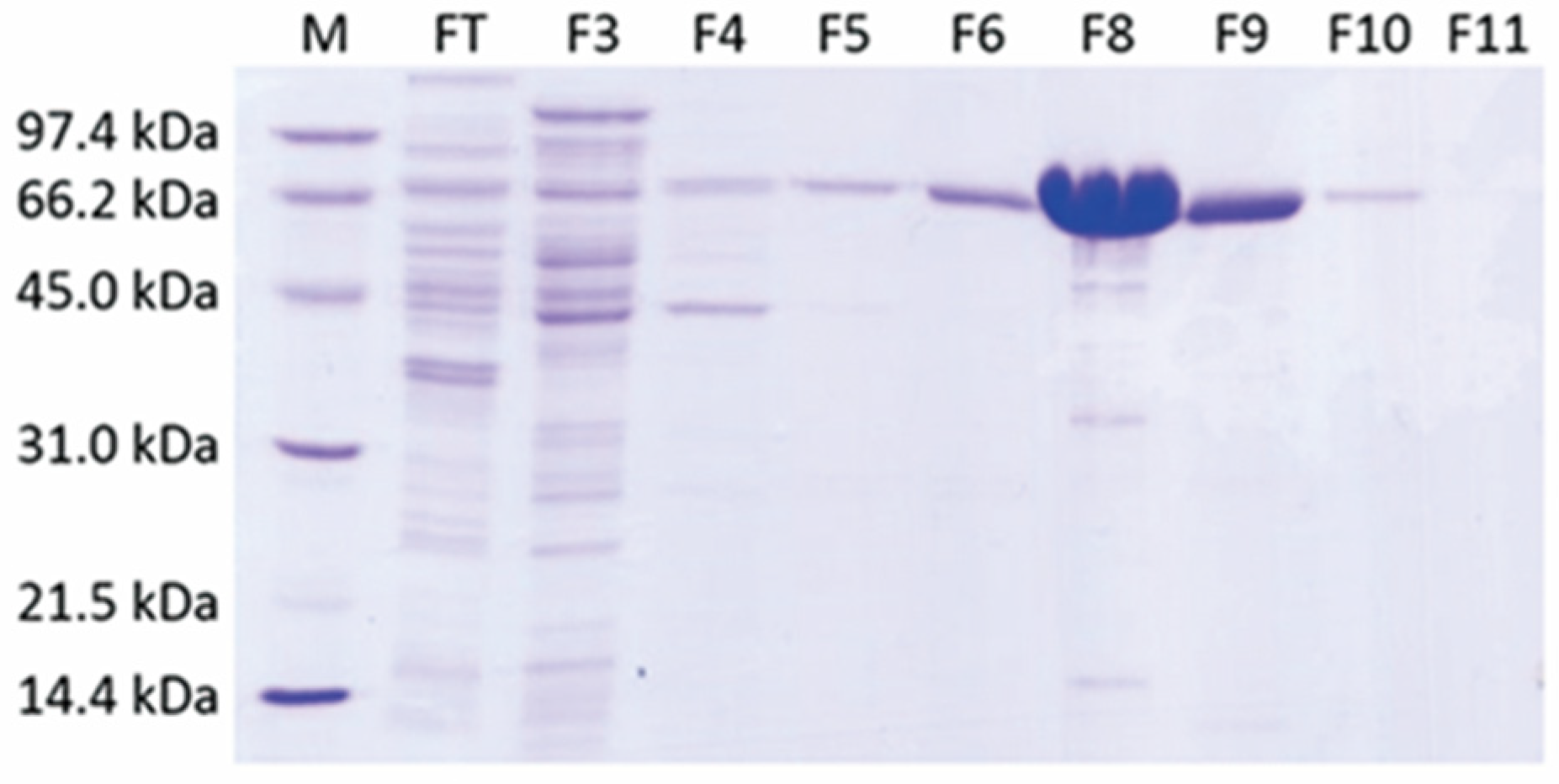
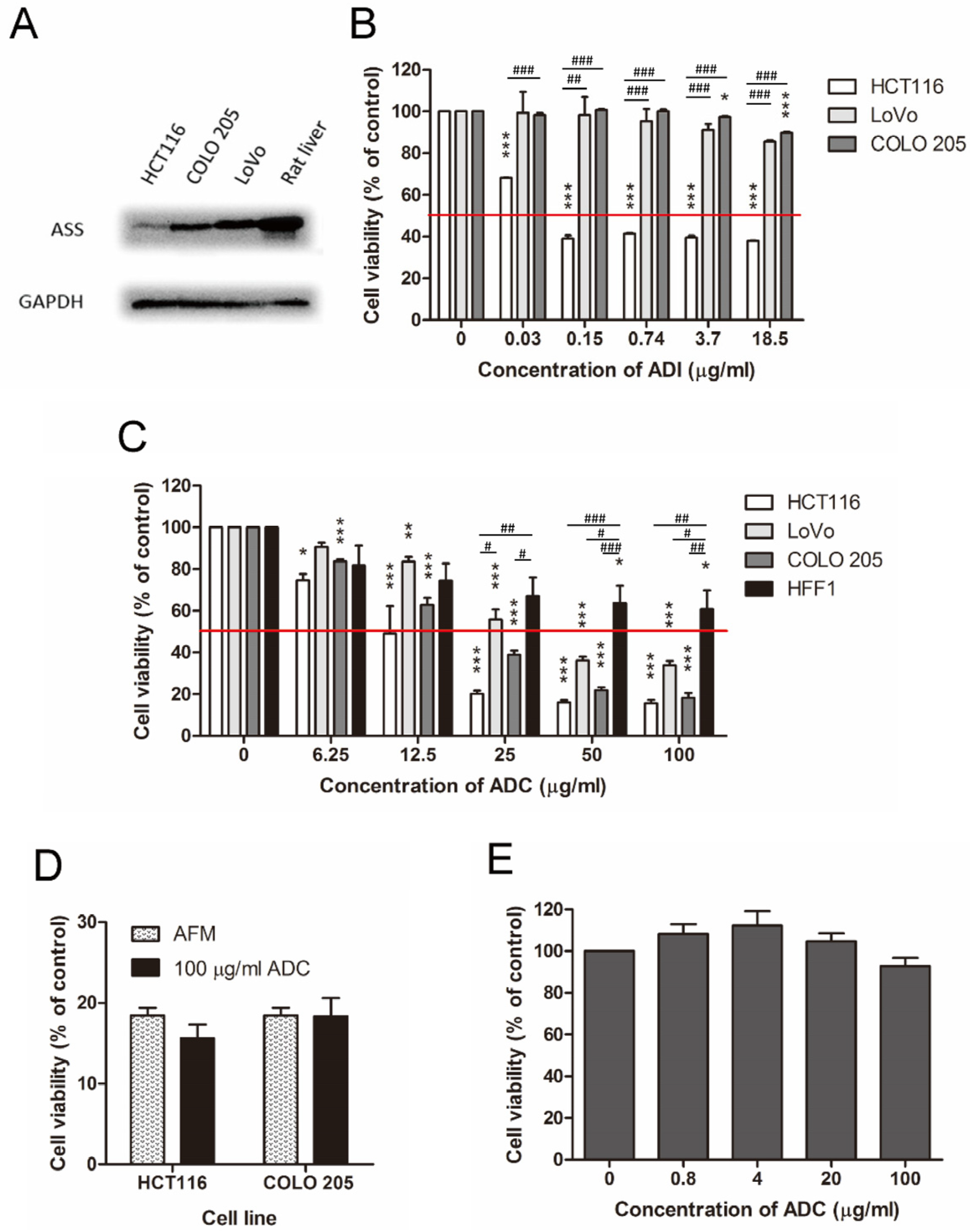
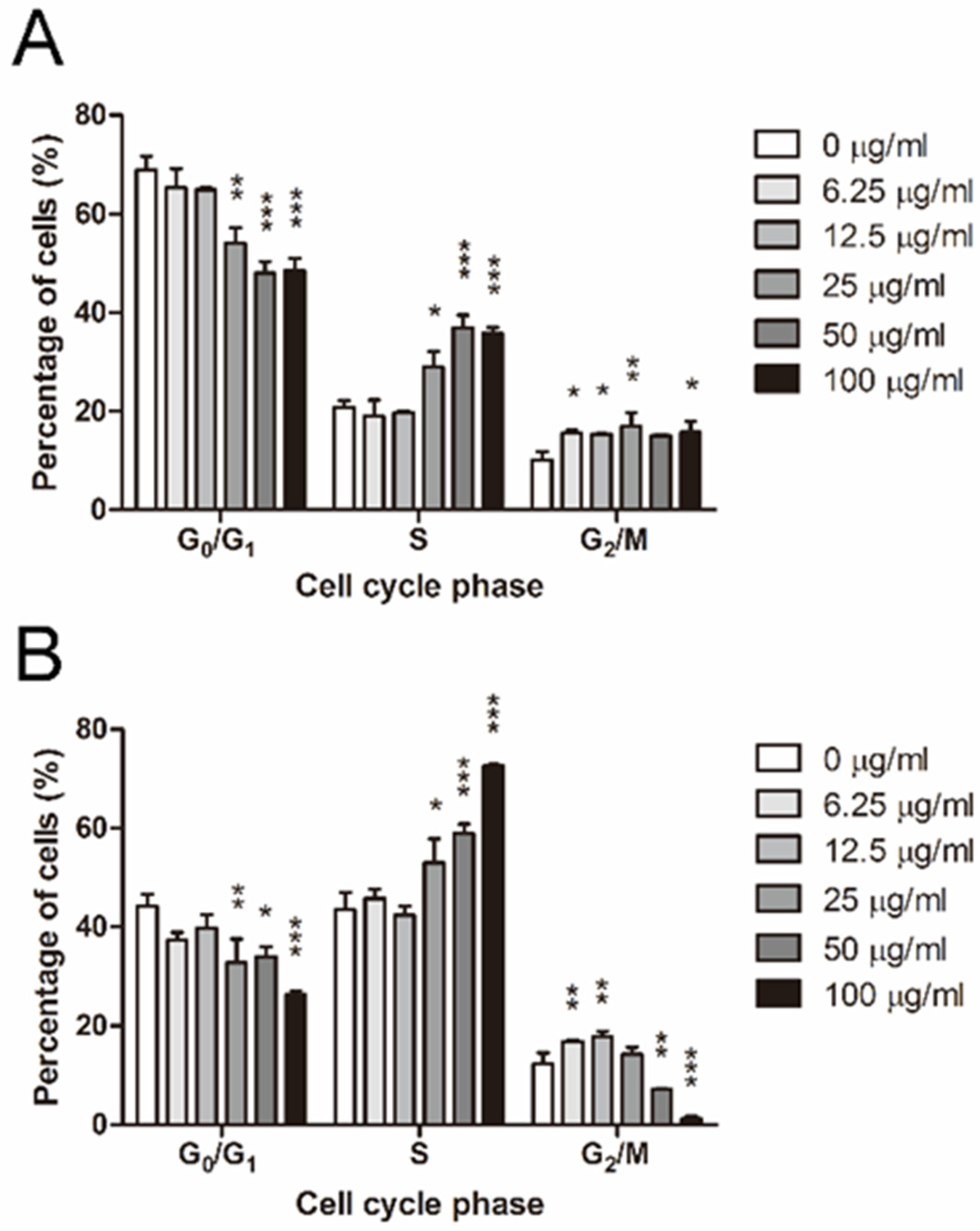

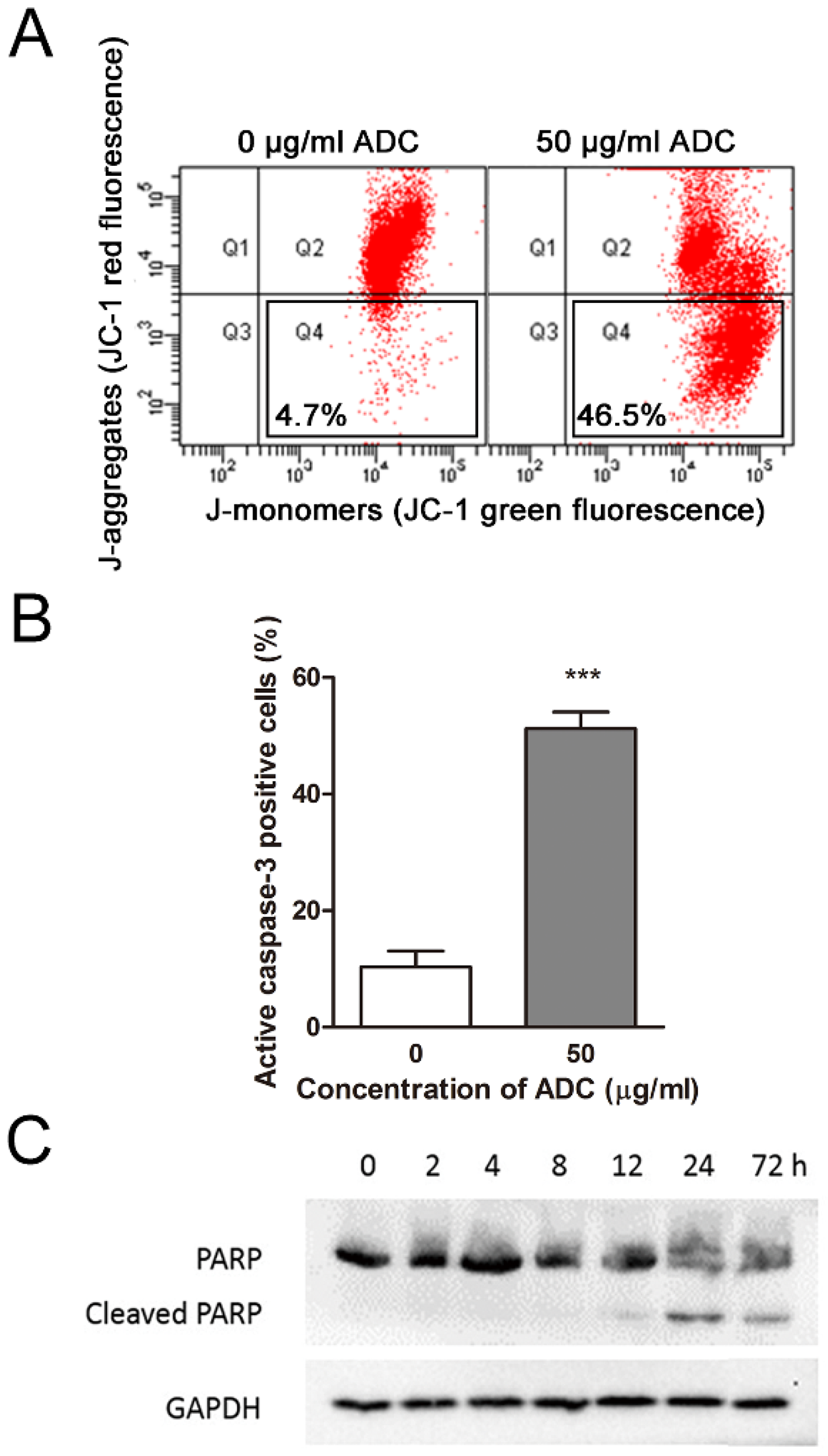
| Stage | Total Enzyme Activity (U) | Total Protein (mg) | Specific Enzyme Activity (U/mg) | Fold Purification | Recovery (%) |
|---|---|---|---|---|---|
| Whole cell lysate | 4113.5 | 304.7 | 13.5 | 1.00 | 100.00% |
| Soluble proteins | 3757.0 | 221.0 | 17.0 | 1.26 | 91.33% |
| Affinity column pooled fractions | 3144.1 | 111.1 | 28.3 | 2.10 | 76.43% |
| Final Product | 3005.7 | 110.1 | 27.3 | 2.02 | 73.07% |
| Cell Types | Cell Lines | IC50 of ADC (μg/mL) |
|---|---|---|
| HCT116 | 12.23 | |
| Human colorectal cancer | COLO205 | 19.40 |
| LoVo | 38.09 | |
| Human foreskin fibroblast | HFF | >100 |
| Rat primary hepatocyte | - | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Chow, H.-Y.; Chong, H.-C.; Leung, S.-L.; Ho, M.-K.; Lee, M.-Y.; Leung, Y.-C. Arginine Is a Novel Drug Target for Arginine Decarboxylase in Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 13741. https://doi.org/10.3390/ijms241813741
Wei X, Chow H-Y, Chong H-C, Leung S-L, Ho M-K, Lee M-Y, Leung Y-C. Arginine Is a Novel Drug Target for Arginine Decarboxylase in Human Colorectal Cancer Cells. International Journal of Molecular Sciences. 2023; 24(18):13741. https://doi.org/10.3390/ijms241813741
Chicago/Turabian StyleWei, Xinlei, Ho-Yin Chow, Hiu-Chi Chong, Siu-Lun Leung, Mei-Ki Ho, Man-Yuen Lee, and Yun-Chung Leung. 2023. "Arginine Is a Novel Drug Target for Arginine Decarboxylase in Human Colorectal Cancer Cells" International Journal of Molecular Sciences 24, no. 18: 13741. https://doi.org/10.3390/ijms241813741
APA StyleWei, X., Chow, H.-Y., Chong, H.-C., Leung, S.-L., Ho, M.-K., Lee, M.-Y., & Leung, Y.-C. (2023). Arginine Is a Novel Drug Target for Arginine Decarboxylase in Human Colorectal Cancer Cells. International Journal of Molecular Sciences, 24(18), 13741. https://doi.org/10.3390/ijms241813741





