Emerging Gene Therapy Approaches in the Management of Spinal Muscular Atrophy (SMA): An Overview of Clinical Trials and Patent Landscape
Abstract
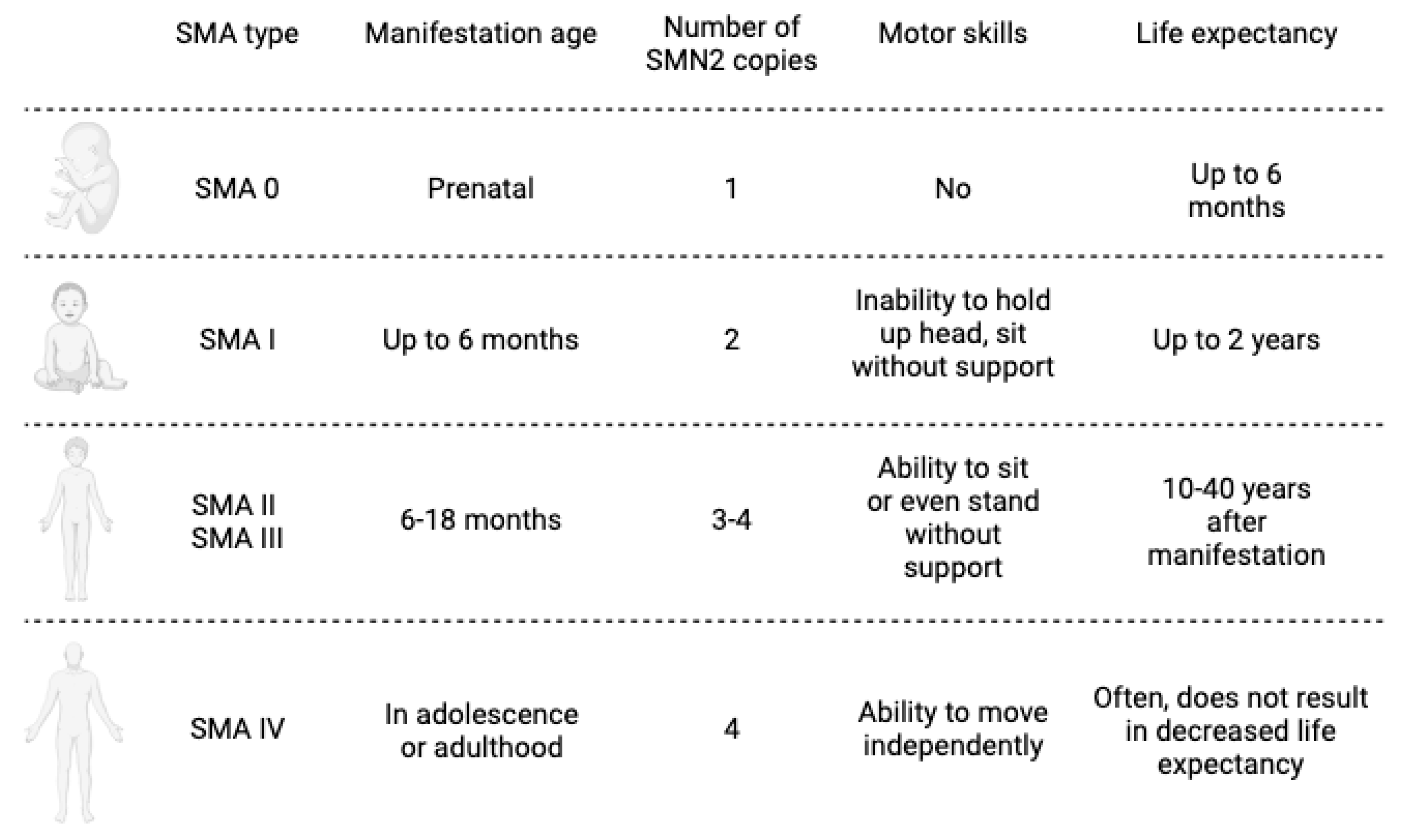
1. Introduction
2. Traditional Therapies for SMA Treatment
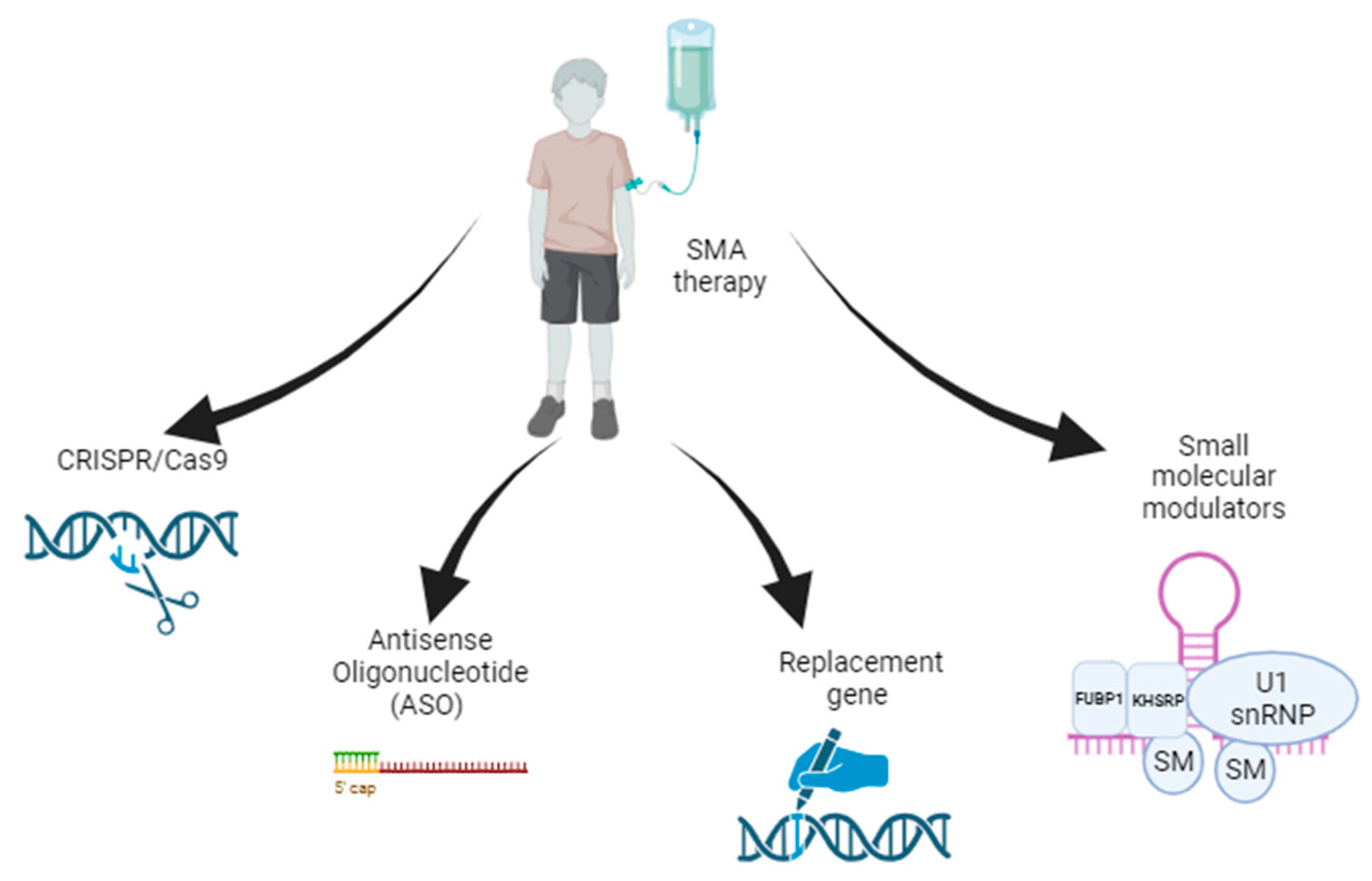
3. Gene Therapy Strategies for SMA
3.1. Gene Replacement Therapy
3.2. Antisense Oligonucleotide Therapy
3.3. Small-Molecule Modulators
3.4. CRISPR/Cas9-Based Gene Editing
3.5. Practical Difficulties of Current Treatment Approaches
4. Methods of Combined Gene Therapy
5. Intellectual Property in SMA Therapy: Patent Landscape
6. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lefebvre, S.; Burglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Chen, X.; Sanchis-Juan, A.; French, C.E.; Connell, A.J.; Delon, I.; Kingsbury, Z.; Chawla, A.; Halpern, A.L.; Taft, R.J.; BioResource, N.; et al. Spinal muscular atrophy diagnosis and carrier screening from genome sequencing data. Genet. Med. 2020, 22, 945–953. [Google Scholar] [CrossRef]
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2012, 20, 27–32. [Google Scholar] [CrossRef]
- Chiriboga, C.A. Pharmacotherapy for Spinal Muscular Atrophy in Babies and Children: A Review of Approved and Experimental Therapies. Paediatr. Drugs 2022, 24, 585–602. [Google Scholar] [CrossRef]
- Pearn, J.H.; Wilson, J. Acute Werdnig-Hoffmann disease: Acute infantile spinal muscular atrophy. Arch. Dis. Child. 1973, 48, 425–430. [Google Scholar] [CrossRef][Green Version]
- Audic, F.; Barnerias, C. Spinal muscular atrophy (SMA) type I (Werdnig-Hoffmann disease). Arch. Pediatr. 2020, 27, 7S15–7S17. [Google Scholar] [CrossRef]
- Hjartarson, H.T.; Nathorst-Boos, K.; Sejersen, T. Disease Modifying Therapies for the Management of Children with Spinal Muscular Atrophy (5q SMA): An Update on the Emerging Evidence. Drug Des. Dev. Ther. 2022, 16, 1865–1883. [Google Scholar] [CrossRef]
- Von Gontard, A.; Zerres, K.; Backes, M.; Laufersweiler-Plass, C.; Wendland, C.; Melchers, P.; Lehmkuhl, G.; Rudnik-Schoneborn, S. Intelligence and cognitive function in children and adolescents with spinal muscular atrophy. Neuromuscul. Disord. 2002, 12, 130–136. [Google Scholar] [CrossRef]
- Schmalbruch, H.; Haase, G. Spinal muscular atrophy: Present state. Brain Pathol. 2001, 11, 231–247. [Google Scholar] [CrossRef]
- Kaufmann, P.; McDermott, M.P.; Darras, B.T.; Finkel, R.; Kang, P.; Oskoui, M.; Constantinescu, A.; Sproule, D.M.; Foley, A.R.; Yang, M.; et al. Observational study of spinal muscular atrophy type 2 and 3: Functional outcomes over 1 year. Arch. Neurol. 2011, 68, 779–786. [Google Scholar] [CrossRef]
- Arnold, W.D.; Kassar, D.; Kissel, J.T. Spinal muscular atrophy: Diagnosis and management in a new therapeutic era. Muscle Nerve 2015, 51, 157–167. [Google Scholar] [CrossRef]
- Liu, Q.; Dreyfuss, G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 1996, 15, 3555–3565. [Google Scholar] [CrossRef] [PubMed]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef] [PubMed]
- Rochette, C.F.; Gilbert, N.; Simard, L.R. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum. Genet. 2001, 108, 255–266. [Google Scholar] [CrossRef]
- Harada, Y.; Sutomo, R.; Sadewa, A.H.; Akutsu, T.; Takeshima, Y.; Wada, H.; Matsuo, M.; Nishio, H. Correlation between SMN2 copy number and clinical phenotype of spinal muscular atrophy: Three SMN2 copies fail to rescue some patients from the disease severity. J. Neurol. 2002, 249, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sato, H.; Lai, P.S.; Nurputra, D.K.; Harahap, N.I.; Morikawa, S.; Nishimura, N.; Kurashige, T.; Ohshita, T.; Nakajima, H.; et al. Intragenic mutations in SMN1 may contribute more significantly to clinical severity than SMN2 copy numbers in some spinal muscular atrophy (SMA) patients. Brain Dev. 2014, 36, 914–920. [Google Scholar] [CrossRef]
- Takarada, T.; Ar Rochmah, M.; Harahap, N.I.F.; Shinohara, M.; Saito, T.; Saito, K.; Lai, P.S.; Bouike, Y.; Takeshima, Y.; Awano, H.; et al. SMA mutations in SMN Tudor and C-terminal domains destabilize the protein. Brain Dev. 2017, 39, 606–612. [Google Scholar] [CrossRef]
- Ahn, E.J.; Yum, M.S.; Kim, E.H.; Yoo, H.W.; Lee, B.H.; Kim, G.H.; Ko, T.S. Genotype-Phenotype Correlation of SMN1 and NAIP Deletions in Korean Patients with Spinal Muscular Atrophy. J. Clin. Neurol. 2017, 13, 27–31. [Google Scholar] [CrossRef]
- Singh, N.N.; Hoffman, S.; Reddi, P.P.; Singh, R.N. Spinal muscular atrophy: Broad disease spectrum and sex-specific phenotypes. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166063. [Google Scholar] [CrossRef]
- Zilio, E.; Piano, V.; Wirth, B. Mitochondrial Dysfunction in Spinal Muscular Atrophy. Int. J. Mol. Sci. 2022, 23, 10878. [Google Scholar] [CrossRef]
- Fuller, H.R.; Gillingwater, T.H.; Wishart, T.M. Commonality amid diversity: Multi-study proteomic identification of conserved disease mechanisms in spinal muscular atrophy. Neuromuscul. Disord. 2016, 26, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Magri, F.; Vanoli, F.; Corti, S. miRNA in spinal muscular atrophy pathogenesis and therapy. J. Cell. Mol. Med. 2018, 22, 755–767. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, A.M.; Kariyawasam, D.; Venkat, P.; Mayoh, C.; Farrar, M.A. Identification of Novel CSF-Derived miRNAs in Treated Paediatric Onset Spinal Muscular Atrophy: An Exploratory Study. Pharmaceutics 2023, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Adami, R.; Bottai, D. NSC Physiological Features in Spinal Muscular Atrophy: SMN Deficiency Effects on Neurogenesis. Int. J. Mol. Sci. 2022, 23, 15209. [Google Scholar] [CrossRef] [PubMed]
- Torres-Benito, L.; Neher, M.F.; Cano, R.; Ruiz, R.; Tabares, L. SMN requirement for synaptic vesicle, active zone and microtubule postnatal organization in motor nerve terminals. PLoS ONE 2011, 6, e26164. [Google Scholar] [CrossRef]
- Rossoll, W.; Jablonka, S.; Andreassi, C.; Kroning, A.K.; Karle, K.; Monani, U.R.; Sendtner, M. Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J. Cell Biol. 2003, 163, 801–812. [Google Scholar] [CrossRef]
- Mentis, G.Z.; Blivis, D.; Liu, W.; Drobac, E.; Crowder, M.E.; Kong, L.; Alvarez, F.J.; Sumner, C.J.; O’Donovan, M.J. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron 2011, 69, 453–467. [Google Scholar] [CrossRef]
- Lefebvre, S.; Sarret, C. Pathogenesis and therapeutic targets in spinal muscular atrophy (SMA). Arch. Pediatr. 2020, 27, 7S3–7S8. [Google Scholar] [CrossRef]
- Will, C.L.; Luhrmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 2011, 3, a003707. [Google Scholar] [CrossRef]
- Carvalho, T.; Almeida, F.; Calapez, A.; Lafarga, M.; Berciano, M.T.; Carmo-Fonseca, M. The spinal muscular atrophy disease gene product, SMN: A link between snRNP biogenesis and the Cajal (coiled) body. J. Cell Biol. 1999, 147, 715–728. [Google Scholar] [CrossRef]
- Pellizzoni, L.; Kataoka, N.; Charroux, B.; Dreyfuss, G. A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell 1998, 95, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Jangi, M.; Fleet, C.; Cullen, P.; Gupta, S.V.; Mekhoubad, S.; Chiao, E.; Allaire, N.; Bennett, C.F.; Rigo, F.; Krainer, A.R.; et al. SMN deficiency in severe models of spinal muscular atrophy causes widespread intron retention and DNA damage. Proc. Natl. Acad. Sci. USA 2017, 114, E2347–E2356. [Google Scholar] [CrossRef] [PubMed]
- Cuartas, J.; Gangwani, L. R-loop Mediated DNA Damage and Impaired DNA Repair in Spinal Muscular Atrophy. Front. Cell. Neurosci. 2022, 16, 826608. [Google Scholar] [CrossRef]
- Karyka, E.; Berrueta Ramirez, N.; Webster, C.P.; Marchi, P.M.; Graves, E.J.; Godena, V.K.; Marrone, L.; Bhargava, A.; Ray, S.; Ning, K.; et al. SMN-deficient cells exhibit increased ribosomal DNA damage. Life Sci. Alliance 2022, 5, e202101145. [Google Scholar] [CrossRef]
- Simon, C.M.; Dai, Y.; Van Alstyne, M.; Koutsioumpa, C.; Pagiazitis, J.G.; Chalif, J.I.; Wang, X.; Rabinowitz, J.E.; Henderson, C.E.; Pellizzoni, L.; et al. Converging Mechanisms of p53 Activation Drive Motor Neuron Degeneration in Spinal Muscular Atrophy. Cell Rep. 2017, 21, 3767–3780. [Google Scholar] [CrossRef]
- Courtney, N.L.; Mole, A.J.; Thomson, A.K.; Murray, L.M. Reduced P53 levels ameliorate neuromuscular junction loss without affecting motor neuron pathology in a mouse model of spinal muscular atrophy. Cell Death Dis. 2019, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Rindt, H.; Feng, Z.; Mazzasette, C.; Glascock, J.J.; Valdivia, D.; Pyles, N.; Crawford, T.O.; Swoboda, K.J.; Patitucci, T.N.; Ebert, A.D.; et al. Astrocytes influence the severity of spinal muscular atrophy. Hum. Mol. Genet. 2015, 24, 4094–4102. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Meyer, O.H.; Simonds, A.K.; Schroth, M.K.; Graham, R.J.; Kirschner, J.; Iannaccone, S.T.; Crawford, T.O.; Woods, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 2018, 28, 197–207. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef]
- Chen, T.H. New and Developing Therapies in Spinal Muscular Atrophy: From Genotype to Phenotype to Treatment and Where Do We Stand? Int. J. Mol. Sci. 2020, 21, 3297. [Google Scholar] [CrossRef]
- Calder, A.N.; Androphy, E.J.; Hodgetts, K.J. Small Molecules in Development for the Treatment of Spinal Muscular Atrophy. J. Med. Chem. 2016, 59, 10067–10083. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, K.J.; Scott, C.B.; Crawford, T.O.; Simard, L.R.; Reyna, S.P.; Krosschell, K.J.; Acsadi, G.; Elsheik, B.; Schroth, M.K.; D’Anjou, G.; et al. SMA CARNI-VAL trial part I: Double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS ONE 2010, 5, e12140. [Google Scholar] [CrossRef] [PubMed]
- Mariot, V.; Joubert, R.; Hourde, C.; Feasson, L.; Hanna, M.; Muntoni, F.; Maisonobe, T.; Servais, L.; Bogni, C.; Le Panse, R.; et al. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nat. Commun. 2017, 8, 1859. [Google Scholar] [CrossRef]
- Russell, A.J.; Hartman, J.J.; Hinken, A.C.; Muci, A.R.; Kawas, R.; Driscoll, L.; Godinez, G.; Lee, K.H.; Marquez, D.; Browne, W.F.t.; et al. Activation of fast skeletal muscle troponin as a potential therapeutic approach for treating neuromuscular diseases. Nat. Med. 2012, 18, 452–455. [Google Scholar] [CrossRef]
- Russman, B.S.; Iannaccone, S.T.; Samaha, F.J. A phase 1 trial of riluzole in spinal muscular atrophy. Arch. Neurol. 2003, 60, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Bertini, E.; Dessaud, E.; Mercuri, E.; Muntoni, F.; Kirschner, J.; Reid, C.; Lusakowska, A.; Comi, G.P.; Cuisset, J.M.; Abitbol, J.L.; et al. Safety and efficacy of olesoxime in patients with type 2 or non-ambulatory type 3 spinal muscular atrophy: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017, 16, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Ogbonmide, T.; Rathore, R.; Rangrej, S.B.; Hutchinson, S.; Lewis, M.; Ojilere, S.; Carvalho, V.; Kelly, I. Gene Therapy for Spinal Muscular Atrophy (SMA): A Review of Current Challenges and Safety Considerations for Onasemnogene Abeparvovec (Zolgensma). Cureus 2023, 15, e36197. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef]
- Kotin, R.M.; Linden, R.M.; Berns, K.I. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992, 11, 5071–5078. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, Y.; Noor, A.F.; Tran, J.; Li, R. Characteristics and advantages of adeno-associated virus vector-mediated gene therapy for neurodegenerative diseases. Neural Regen. Res. 2019, 14, 931–938. [Google Scholar] [CrossRef]
- Benkhelifa-Ziyyat, S.; Besse, A.; Roda, M.; Duque, S.; Astord, S.; Carcenac, R.; Marais, T.; Barkats, M. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol. Ther. 2013, 21, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, N.; Lattanzi, A.; Jeavons, M.; Van Wittenberghe, L.; Gjata, B.; Marais, T.; Martin, S.; Vignaud, A.; Voit, T.; Mavilio, F.; et al. Efficacy and biodistribution analysis of intracerebroventricular administration of an optimized scAAV9-SMN1 vector in a mouse model of spinal muscular atrophy. Mol. Ther. Methods Clin. Dev. 2016, 3, 16060. [Google Scholar] [CrossRef]
- Tanguy, Y.; Biferi, M.G.; Besse, A.; Astord, S.; Cohen-Tannoudji, M.; Marais, T.; Barkats, M. Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Front. Mol. Neurosci. 2015, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Valori, C.F.; Ning, K.; Wyles, M.; Mead, R.J.; Grierson, A.J.; Shaw, P.J.; Azzouz, M. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci. Transl. Med. 2010, 2, 35ra42. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009, 27, 59–65. [Google Scholar] [CrossRef]
- Hordeaux, J.; Wang, Q.; Katz, N.; Buza, E.L.; Bell, P.; Wilson, J.M. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Mol. Ther. 2018, 26, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Weber, T. Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions. Front. Immunol. 2021, 12, 658399. [Google Scholar] [CrossRef]
- Glanzman, A.M.; Mazzone, E.; Main, M.; Pelliccioni, M.; Wood, J.; Swoboda, K.J.; Scott, C.; Pane, M.; Messina, S.; Bertini, E.; et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): Test development and reliability. Neuromuscul. Disord. 2010, 20, 155–161. [Google Scholar] [CrossRef]
- Al-Zaidy, S.; Pickard, A.S.; Kotha, K.; Alfano, L.N.; Lowes, L.; Paul, G.; Church, K.; Lehman, K.; Sproule, D.M.; Dabbous, O.; et al. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr. Pulmonol. 2019, 54, 179–185. [Google Scholar] [CrossRef]
- Lowes, L.P.; Alfano, L.N.; Arnold, W.D.; Shell, R.; Prior, T.W.; McColly, M.; Lehman, K.J.; Church, K.; Sproule, D.M.; Nagendran, S.; et al. Impact of Age and Motor Function in a Phase 1/2A Study of Infants With SMA Type 1 Receiving Single-Dose Gene Replacement Therapy. Pediatr. Neurol. 2019, 98, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Pena, L.D.M.; Shieh, P.B.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Monine, M.; Norris, D.; Wang, Y.; Nestorov, I. A physiologically-based pharmacokinetic model to describe antisense oligonucleotide distribution after intrathecal administration. J. Pharmacokinet. Pharmacodyn. 2021, 48, 639–654. [Google Scholar] [CrossRef]
- Passini, M.A.; Bu, J.; Richards, A.M.; Kinnecom, C.; Sardi, S.P.; Stanek, L.M.; Hua, Y.; Rigo, F.; Matson, J.; Hung, G.; et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 2011, 3, 72ra18. [Google Scholar] [CrossRef]
- Wadman, R.I.; van der Pol, W.L.; Bosboom, W.M.; Asselman, F.L.; van den Berg, L.H.; Iannaccone, S.T.; Vrancken, A.F. Drug treatment for spinal muscular atrophy type I. Cochrane Database Syst. Rev. 2019, 12, CD006281. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef]
- Bishop, K.M.; Montes, J.; Finkel, R.S. Motor milestone assessment of infants with spinal muscular atrophy using the hammersmith infant neurological Exam-Part 2: Experience from a nusinersen clinical study. Muscle Nerve 2018, 57, 142–146. [Google Scholar] [CrossRef]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Bishop, K.M.; Foster, R.; Liu, Y.; Ramirez-Schrempp, D.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: Final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child. Adolesc. Health 2021, 5, 491–500. [Google Scholar] [CrossRef]
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.J.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies. Neurology 2019, 92, e2492–e2506. [Google Scholar] [CrossRef]
- Acsadi, G.; Crawford, T.O.; Müller-Felber, W.; Shieh, P.B.; Richardson, R.; Natarajan, N.; Castro, D.; Ramirez-Schrempp, D.; Gambino, G.; Sun, P.; et al. Safety and efficacy of nusinersen in spinal muscular atrophy: The EMBRACE study. Muscle Nerve 2021, 63, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Schultz, P.G.; Johnson, K.A. Mechanistic studies of a small-molecule modulator of SMN2 splicing. Proc. Natl. Acad. Sci. USA 2018, 115, E4604–E4612. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Ebeling, M.; Baird, J.; Bendels, S.; Bylund, J.; Chen, K.S.; Denk, N.; Feng, Z.; Green, L.; Guerard, M.; et al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 6501–6517. [Google Scholar] [CrossRef] [PubMed]
- Darras, B.T.; Masson, R.; Mazurkiewicz-Beldzinska, M.; Rose, K.; Xiong, H.; Zanoteli, E.; Baranello, G.; Bruno, C.; Vlodavets, D.; Wang, Y.; et al. Risdiplam-Treated Infants with Type 1 Spinal Muscular Atrophy versus Historical Controls. N. Engl. J. Med. 2021, 385, 427–435. [Google Scholar] [CrossRef]
- Mercuri, E.; Baranello, G.; Boespflug-Tanguy, O.; De Waele, L.; Goemans, N.; Kirschner, J.; Masson, R.; Mazzone, E.S.; Pechmann, A.; Pera, M.C.; et al. Risdiplam in types 2 and 3 spinal muscular atrophy: A randomised, placebo-controlled, dose-finding trial followed by 24 months of treatment. Eur. J. Neurol. 2023, 30, 1945–1956. [Google Scholar] [CrossRef]
- Oskoui, M.; Day, J.W.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Saito, K.; Vuillerot, C.; Baranello, G.; Goemans, N.; Kirschner, J.; et al. Two-year efficacy and safety of risdiplam in patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA). J. Neurol. 2023, 270, 2531–2546. [Google Scholar] [CrossRef]
- Alemdaroglu, I.; Karaduman, A.; Iyigun-Yatar, G.; Tunca-Yilmaz, O.; Topaloglu, H. Turkish version of the Egen Klassifikation scale version 2: Validity and reliability in the Turkish population. Turk. J. Pediatr. 2014, 56, 643–650. [Google Scholar]
- Nungo Garzon, N.C.; Pitarch Castellano, I.; Sevilla, T.; Vazquez-Costa, J.F. Risdiplam in non-sitter patients aged 16 years and older with 5q spinal muscular atrophy. Muscle Nerve 2023, 67, 407–411. [Google Scholar] [CrossRef]
- Cheung, A.K.; Hurley, B.; Kerrigan, R.; Shu, L.; Chin, D.N.; Shen, Y.; O’Brien, G.; Sung, M.J.; Hou, Y.; Axford, J.; et al. Discovery of Small Molecule Splicing Modulators of Survival Motor Neuron-2 (SMN2) for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 11021–11036. [Google Scholar] [CrossRef]
- Li, J.J.; Lin, X.; Tang, C.; Lu, Y.Q.; Hu, X.; Zuo, E.; Li, H.; Ying, W.; Sun, Y.; Lai, L.L.; et al. Disruption of splicing-regulatory elements using CRISPR/Cas9 to rescue spinal muscular atrophy in human iPSCs and mice. Natl. Sci. Rev. 2020, 7, 92–101. [Google Scholar] [CrossRef]
- Kirschner, J.; Butoianu, N.; Goemans, N.; Haberlova, J.; Kostera-Pruszczyk, A.; Mercuri, E.; van der Pol, W.L.; Quijano-Roy, S.; Sejersen, T.; Tizzano, E.F.; et al. European ad-hoc consensus statement on gene replacement therapy for spinal muscular atrophy. Eur. J. Paediatr. Neurol. 2020, 28, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, C.; Katz, N.; Buza, E.L.; Dyer, C.; Goode, T.; Bell, P.; Richman, L.K.; Wilson, J.M. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018, 29, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Nidetz, N.F.; McGee, M.C.; Tse, L.V.; Li, C.; Cong, L.; Li, Y.; Huang, W. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol. Ther. 2020, 207, 107453. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Ottesen, E.W.; Singh, N.N.; Luo, D.; Kaas, B.; Gillette, B.J.; Seo, J.; Jorgensen, H.J.; Singh, R.N. Diverse targets of SMN2-directed splicing-modulating small molecule therapeutics for spinal muscular atrophy. Nucleic Acids Res. 2023, 51, 5948–5980. [Google Scholar] [CrossRef]
- Waldrop, M.A.; Karingada, C.; Storey, M.A.; Powers, B.; Iammarino, M.A.; Miller, N.F.; Alfano, L.N.; Noritz, G.; Rossman, I.; Ginsberg, M.; et al. Gene Therapy for Spinal Muscular Atrophy: Safety and Early Outcomes. Pediatrics 2020, 146, e20200729. [Google Scholar] [CrossRef]
- Mirea, A.; Shelby, E.S.; Axente, M.; Badina, M.; Padure, L.; Leanca, M.; Dima, V.; Sporea, C. Combination Therapy with Nusinersen and Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy Type I. J. Clin. Med. 2021, 10, 5540. [Google Scholar] [CrossRef]
- Harada, Y.; Rao, V.K.; Arya, K.; Kuntz, N.L.; DiDonato, C.J.; Napchan-Pomerantz, G.; Agarwal, A.; Stefans, V.; Katsuno, M.; Veerapandiyan, A. Combination molecular therapies for type 1 spinal muscular atrophy. Muscle Nerve 2020, 62, 550–554. [Google Scholar] [CrossRef]
| Name of Drug, Company | NCT Number | Phase | Participants Number | Summary Description | Status |
|---|---|---|---|---|---|
| Onasemnogene Abeparvovec (Zolgensma) by Novartis Gene Therapies | NCT02122952 | 1 | 15, with age <6 months | Dose-escalation trial for safety and efficacy evaluation of intravenous delivery of AVXS-101-CL-101 as a treatment of spinal muscular atrophy Type 1 (SMN1). | Completed 15 December 2017 |
| NCT03381729 | 1 | 32, with age ≥ 6 and <24 months; ≥24 and <60 months | Evaluation of the safety and tolerability of intrathecal administration in infants and children with SMA with bi-allelic deletion in SMN1, with 3 copies of SMN2 and deletion of SMN1. Single-dose administration. | Completed 18 November 2021 | |
| NCT03306277 | 3 | 22, with age <6 months | Open-label, single-arm, single-dose trial, intravenous administration of onasemnogene abeparvovec-xioi in SMA Type 1 participants. | Completed 12 November 2019 | |
| NCT03461289 | 3 | 33, with age <6 months | Open-label, single-arm, single-dose, trial in patients with SMA Type 1, with bi-allelic pathogenic mutation of SMN1 and 1 or 2 copies of SMN2. | Completed 11 September 2020 | |
| NCT03837184 | 3 | 2, with age <6 months | Open-label, single-arm, single-dose, efficacy trial in patients with SMA Type 1, with bi-allelic pathogenic mutation of SMN1 and 1 or 2 copies of SMN2. | Completed 29 June 2021 | |
| NCT03505099 | 3 | 30, with age <6 weeks | Open-label, single-arm, single-dose safety and efficacy trial in patients with SMA with bi-allelic deletion of SMN1 and 2 or 3 copies of SMN2. | Completed 15 June 2021 | |
| NCT03421977 | 13, Child, Adult, Older Adult | Long-term, safety follow-up study of patients in the NCT02122952 gene replacement therapy. | Completion estimated December 2033 | ||
| NCT04042025 | 85, Child, Adult, Older Adult | Long-term, safety and efficacy follow-up study of patients in the AVXS-101 gene replacement therapy. | Completion estimated 29 December 2035 | ||
| Nusinersen (spinraza), by Biogen | NCT01494701 | 1 | 28 | Evaluation of the safety, tolerability, and pharmacokinetics of a single dose of nusinersen (ISIS 396443) administered intrathecally to participants with SMA. | Completed 31 January 2013 |
| NCT01780246 | 1 | 18 | Safety and tolerability examination of ISIS 396443 administered intrathecally to participants SMA who previously participated in NCT02865109. Examination of the plasma pharmacokinetics of a single dose administered intrathecally to participants with SMA who previously participated in NCT02865109. | Completed 28 February 2014 | |
| NCT01839656 | 2 | 21 | Clinical examination of efficacy, safety and tolerability of multiple doses of nusinersen administered intrathecally to participants with Infantile-Onset SMA and cerebral spinal fluid and plasma pharmacokinetic examination. | Completed 21 August 2017 | |
| NCT01703988 | 1, 2 | 34 | Testing safety, tolerability, and pharmacokinetics of escalating doses of nusinersen administered into the spinal fluid either 2 or 3 times in participants with SMA. | Completed 31 January 2015 | |
| NCT02052791 | 1 | 47 | Testing safety, tolerability, and cerebrospinal fluid and plasma pharmacokinetics in participants with SMA who previously participated in NCT01703988 or NCT01780246. | Completed 31 January 2017 | |
| NCT02193074 | 3 | 122 | Examination of clinical efficacy, safety and tolerability of nusinersen administered intrathecally to participants with infantile-onset SMA. | Completed 21 November 2016 | |
| NCT02292537 | 3 | 126 | Examination of clinical efficacy, safety and tolerability of nusinersen administered intrathecally to participants with later-onset SMA. | Completed 20 February 2017 | |
| NCT02386553 | 2 | 25 | Examination of the efficacy and effects of multiple doses in preventing or delaying the need for respiratory intervention or death in infants with genetically diagnosed and presymptomatic SMA. | Completion estimated 27 January 2025 | |
| NCT04089566 | 3 | 145 | Examination of clinical efficacy, safety and tolerability of nusinersen in higher doses to participants with SMA. | Completion estimated 2 August 2024 | |
| NCT04729907 | 3 | 172 | Evaluation of the long-term safety, long-term efficacy and tolerability of nusinersen administered intrathecally at higher doses to participants with SMA who previously participated in NCT04089566. | Completion estimated 30 May 2026 | |
| NCT02594124 | 3 | 292 | Evaluation of the long-term safety, long-term efficacy and tolerability in participants who previously participated in investigational studies of nusinersen. | Completion estimated 29 August 2023 | |
| NCT04488133 | 4 | 60 | Evaluation of the clinical outcomes following treatment in participants with SMA. | Completion estimated 4 September 2024 | |
| Risdiplam (Evrysdi), by Hoffmann-La Roche | NCT02240355 | 1 | 9 | Multicenter, randomized, double-blind, 12-week, placebo-controlled multiple dose study will investigate the safety and tolerability of RO6885247 in adult and pediatric patients with SMA. The study was put on hold and eventually terminated. | Completed July 2015 |
| NCT02913482 | 2, 3 | 62 | Open-label, multi-center clinical study to assess the safety, tolerability, pharmacokinetic, pharmacodynamics, and efficacy of Risdiplam in infants with Type 1 SMA. The study is divided in two parts. | Completed 14 November 2019 Completion estimated 17 November 2023 | |
| NCT02908685 | 2, 3 | 231 | Multi-center, randomized, double-blind, placebo-controlled study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of risdiplam in adult and pediatric participants with Type 2 and Type 3 SMA. The study consists of two parts. | Completed 6 September 2019 Completion estimated 2 September 2023 | |
| NCT03032172 | 2 | 174 | Multi-center, exploratory, non-comparative, and open-label study to investigate the safety, tolerability, PK, and PK/PD relationship of risdiplam in adults, children and infants with SMA previously enrolled in NCT02240355 or previously treated with nusinersen, olesoxime or AVXS-101. | Completion estimated 27 December 2024 | |
| NCT03779334 | 2 | 25, age up to 6 weeks | A global study of oral risdiplam in pre-symptomatic participants with SMA to investigate the efficacy, safety, pharmacokinetics, and pharmacodynamics of risdiplam. | Completion estimated 21 January 2029 |
| Title | Applicant | Patent Number | Date of Publication |
|---|---|---|---|
| Gene therapy for neurodegenerative disorders | GENZYME CORPORATION | WO2010129021A1 | 11 November 2010 |
| ARGETING PEPTIDES FOR DIRECTING ADENO-ASSOCIATED VIRUSES (AAVs) | CALIFORNIA INSTITUTE OF TECHNOLOGY | WO2017100671A1 | 15 June 2017 |
| Methods of treating spinal muscular atrophy | BIOGEN MA INC. [US]. | WO2019147960 | 1 August 2019 |
| Compositions and methods for treating spinal muscular atrophy | ACCELERON PHARMA INC. [US] | WO2018187209 | 11 October 2018 |
| Means and method for preparing viral vectors and uses of same | AVEXIS INC. | WO2019094253 | 16 May 2019 |
| AAV viral vectors and uses thereof | NOVARTIS GENE THERAPIES, INC. | WO2020113034 | 4 June 2020 |
| Treatment for spinal muscular atrophy | ACADEMIA SINICA | WO2008095357 | 30 August 2006 |
| Composition useful in treatment of spinal muscular atrophy | THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA | WO2018160585 | 17 January 2018 |
| Lentiviral vectors with tropism to motor neurons comprising an antibody that binds to a pre-synaptic terminal receptor on the neuromuscular junction and a fusogenic protein | IMPERIAL INNOVATIONS LIMITED | WO2014184562 | 20 November 2014 |
| Compositions and methods for treating motor neuron diseases | THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK | WO2019118734 | 20 June 2019 |
| ERK inhibitors for use in treating spinal muscular atrophy | UNIVERSITE PARIS DESCARTES; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE Paris | WO2012160130A1 | 29 November 2012 |
| Mesenchymal stem cell therapy for spinal muscular atrophy | CELL MEDICINEINC. | US20190136192 | 9 May 2019 |
| Treatment of spinal muscular atrophy | GENETHON | WO2019011817 | 17 January 2019 |
| Codon-optimized nucleic acid encoding smn1 protein | Joint Stock Company “Biocad” | WO 2021/246909 | 9 December 2021 |
| Combination therapy for spinal muscular atrophy | BIOGEN MA INC. | WO2021030766 | 18 February 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponomarev, A.S.; Chulpanova, D.S.; Yanygina, L.M.; Solovyeva, V.V.; Rizvanov, A.A. Emerging Gene Therapy Approaches in the Management of Spinal Muscular Atrophy (SMA): An Overview of Clinical Trials and Patent Landscape. Int. J. Mol. Sci. 2023, 24, 13743. https://doi.org/10.3390/ijms241813743
Ponomarev AS, Chulpanova DS, Yanygina LM, Solovyeva VV, Rizvanov AA. Emerging Gene Therapy Approaches in the Management of Spinal Muscular Atrophy (SMA): An Overview of Clinical Trials and Patent Landscape. International Journal of Molecular Sciences. 2023; 24(18):13743. https://doi.org/10.3390/ijms241813743
Chicago/Turabian StylePonomarev, Aleksei S., Daria S. Chulpanova, Lina M. Yanygina, Valeriya V. Solovyeva, and Albert A. Rizvanov. 2023. "Emerging Gene Therapy Approaches in the Management of Spinal Muscular Atrophy (SMA): An Overview of Clinical Trials and Patent Landscape" International Journal of Molecular Sciences 24, no. 18: 13743. https://doi.org/10.3390/ijms241813743
APA StylePonomarev, A. S., Chulpanova, D. S., Yanygina, L. M., Solovyeva, V. V., & Rizvanov, A. A. (2023). Emerging Gene Therapy Approaches in the Management of Spinal Muscular Atrophy (SMA): An Overview of Clinical Trials and Patent Landscape. International Journal of Molecular Sciences, 24(18), 13743. https://doi.org/10.3390/ijms241813743







