The Variability of Tryptophan Metabolism in Patients with Mixed Type of Irritable Bowel Syndrome
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Participants
4.2. Diagnostic Procedures
4.3. Breathing Test
4.4. Laboratory Tests
4.5. Nutritional Intervention
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef]
- Palsson, O.S.; Baggish, J.S.; Turner, M.J.; Whitehead, W.E. IBS Patients Show Frequent Fluctuations between Loose/Watery and Hard/Lumpy Stools: Implications for treatment. Am. J. Gastroenterol. 2012, 107, 286–295. [Google Scholar] [CrossRef]
- Chira, A.; Filip, M.; Dumitrescu, D.L. Patterns of alternation in irritable bowel syndrome. Clujul Med. 2016, 89, 220–223. [Google Scholar] [CrossRef]
- Lacy, B.E. Update on Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2020, 16, 648. [Google Scholar]
- Mamieva, Z.; Poluektova, E.; Svistushkin, V.; Sobolev, V.; Shirin, O.; Gaurner, V.I. Antibiotics, gut microbiota, and irritable bowel syndrome: What are the relations? World J. Gastroenterol. 2022, 28, 1204–1219. [Google Scholar] [CrossRef]
- Vervier, K.; Moss, S.; Kumar, N.; Adoum, A.; Barne, M.; Browne, H.; Kase, A.; Kiely, C.J.; Neville, B.A.; Powel, N.; et al. Two microbiota subtypes identified in irritable bowel syndromewith distinct response to the low FODMAP diet. Gut 2022, 71, 1821–1830. [Google Scholar] [CrossRef]
- Awad, K.; Barmeyer, C.; Bojarski, C.; Nagel, O.; Lee, I.F.M.; Schweiger, M.R.; Schulzke, J.D.; Bücker, R. Impaired Intestinal Permeability of Tricellular Tight Junctions in Patients with Irritable Bowel Syndrome with mixed Bowel Habits (IBS-M). Cells 2023, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, H.; Ye, Z.; Qiu, X.; Zhang, H. Fecal microbiota proofing in irritable bowel syndrome and inflammatory bowel disease with irritable bowel syndrome-type symptoms. BMC Gastroenterol. 2021, 21, 433. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Patel, D.; Shah, H.; Hann, K.S.; Kaur, H.; Alazzeh, M.S.; Thandavaram, A.; Channar, A.; Purohit, A.; Venugopal, S. The Role of Gut-Microbiota in the Pathophysiology and Therapy of Irritable Bowel Syndrome: A Systematic Review. Cureus 2022, 14, e28064. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Nair, D.; Jain, S.; Montagbe, T.; Flores, D.V.; Nguyen, A.; Dietsche, S.; Gombar, S.; Cotter, P. Alternations in Gut Microbiome Composition and Function in Irritable Bowel Syndrome and Increase Probiotic Abudance with Daily Supplementation. mSystem 2021, 6, e01215-21. [Google Scholar] [CrossRef] [PubMed]
- Algera, J.; Colomier, E.; Simren, M. The Dietary Management of Patients with Irritable Bowel Syndrome: A Narrative Review of the Existing and Emerging Evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef]
- Altobelli, E.; Del Negr, V.; Angeletti, P.M.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef]
- Spiller, R. Impact of Diet on Symptoms of the Irritable Bowel Syndrome. Nutrients 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Tuck, C.; Gibson, P.R.; Chey, W.D. The Role of Food in the Treatment of Bowel Disorders: Focus on Irritable Bowel Syndrome and Functional Constipation. Am. J. Gastroenterol. 2022, 117, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bartoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adults in IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 6831912. [Google Scholar] [CrossRef] [PubMed]
- Nybacka, S.; Störsrud, S.; Lindqvist, H.M.; Törnblom, H.; Simren, M.; Winkvist, A. Habitual FODMAP Intake in Relation to Symptom Severity and Pattern in Patients with Irritable Bowel Syndrome. Nutrients 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef]
- Gonzalez Delgado, S.; Garza-Veloz, I.; Trejo-Vazquez, F.; Martinez-Fierro, M.L. Interplay between Serotonin, Immune Response, and Intestinal Dysbiosis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 15632. [Google Scholar] [CrossRef] [PubMed]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef]
- Bidel, M.R.; Hobbs, A.L.V.; Lodise, T. Gut microbiome health and dysbiosis: A clinical primer. Pharmacotherapy 2022, 42, 849–857. [Google Scholar] [CrossRef]
- Mishima, Y.; Ishihara, S. Enteric Microbiota- Mediated Serotonergic Signaling in Pathogenesis od Irritable Bowel Syndrome. Int. J. Mol. Sci. 2021, 22, 10235. [Google Scholar] [CrossRef]
- Bearcroft, C.P.; Perret, M.J.; Farthing, M.J.G. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominat irritable bowel syndrome: A pilot study. Gut 1998, 42, 42–46. [Google Scholar] [CrossRef]
- Houghton, L.A.; Atkinson, W.; Lockhart, C.; Whorwell, J.P.; Keevil, B. Sigmoid-colon motility in healthy and irritable bowel syndrome. Neurogastroenterol. Motil. 2007, 19, 724–731. [Google Scholar] [CrossRef]
- Chojnacki, C.; Błońska, A.; Kaczka, A.; Chojnacki, J.; Stępień, A.; Gąsiorowska, A. Evaluation of serotonin and dopaminę secretion and metabolism in patients with irritable bowel syndrome. Pol. Arch. Inteern. Med. 2018, 128, 711–713. [Google Scholar] [CrossRef]
- Miwa, J.; Echizen, H.; Matsueda, K.; Umeda, N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentration in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion 2001, 53, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alternation in 5-HT signalling and metabolism in human diseases. Neurogastroenterol. Motil. 2007, 19 (Suppl. S2), 25–31. [Google Scholar] [CrossRef]
- Atkinson, W.; Lockhart, S.; Whorwell, P.J.; Houghton, L.A. Altered 5-hydroxytryptamine signalling in patients with constipation and diarrhoea irritable bowel syndrome. Gastroenterology 2006, 30, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, C.; Monaghan, P.J.; Morris, J.; Issa, B.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Rome III Functional Constipation and Irritable Bowel Syndrome with Constipation Are Similar within a Spectrum of Sensitinization Regulated by Serotonin. Gastroenterology 2013, 145, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Costedio, M.M.; Coates, M.D.; Brooks, E.M.; Glass, L.M.; Ganguly, E.K.; Blaszyk, H.; Ciolino, A.L.; Wood, M.J.; Strader, D.; Hyman, N.H.; et al. Mucosal Serotonin Signalling Is Altered in Chronic Constipation but Not in Opiate-Induced Constipation. Am. J. Gastroenterol. 2010, 105, 1173–1180. [Google Scholar] [CrossRef]
- Stasi, C.; Bellini, M.; Bassotti, G.; Blandizzi, C.; Milani, S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech. Colproctol. 2014, 18, 613–621. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classfication of Receptors for 5-hydroxytryptamine. Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef]
- Koopman, N.; Katsavelis, D.; ten Hove, A.S.; Brul, S.; de Jonge, W.J. Rhe Multifaced Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2020, 26, 11–18. [Google Scholar] [CrossRef]
- So, D.; Loughman, A.; Staudacher, H.M. Effects of low FODMAP diet on the colonic microbiome in irritable bowel syndrome: A systematic review with meta-analysis. Am. J. Clin. Nutr. 2022, 116, 943–952. [Google Scholar] [CrossRef]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut Dysbiosis in Irritable Bowel Syndrome: A Narrative Review on Correlation with Disease Subtypes and Novel Therapeutic Implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Jackson, M.; Valesti, J.; Rao, S.S.C. Methanogenic Flora is associated with Altered Colonic Transit but Not Stool Characteristics in Constipation without IBS. Am. J. Gastroenterol. 2010, 105, 1407–1411. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Moir, Y.; Nara, M.; Kotani, Y.; Nagai, E.; Kawada, H. Gut bacterial aromatic amine production: Aromatic amino acid decarboxylase and its effects on peripheral serotonin production. Gut Microbes 2022, 14, e128605. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.-C.; Cao, H.-L.; Xu, M.-Q.; Wang, S.-N.; Wang, Y.-M.; Yan, F.; Wang, B.-M. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J. Gastroenterol. 2016, 22, 8137–8148. [Google Scholar] [CrossRef]
- Heitkemper, M.M.; Han, C.J.; Jarrett, M.E.; Gu, H.; Djukovic, D.; Shulman, R.J.; Raftery, D.; Henderson, W.A.; Cain, K.C. Serum Tryptophan Metabolite Levels During Sleep in Patients with and Without Irritable Bowel Syndrome (IBS). Biol. Res. Nurs. 2015, 18, 193–198. [Google Scholar] [CrossRef]
- Clarke, G.; Fitzgerald, P.; Cryan, J.F.; Cassidy, E.M.; Quigley, E.M.; Dinan, T.G. Tryptophan Degradation in Irritable Bowel Syndrome: Evidence of Indoleamine 2,3-Dioxygenase Activation in a Male Cohort. BMC Gastroenterol. 2009, 9, 6. [Google Scholar] [CrossRef]
- Fitzgerald, P.; Cassidy Eugene, M.; Clarke, G.; Scully, P.; Barry, S.; Quigley Eamonn, M.M.; Shanahan, F.; Cryan, J.; Dinan Timothy, G. Tryptophan Catabolism in Females with Irritable Bowel Syndrome: Relationship to Interferon-Gamma, Severity of Symptoms and Psychiatric Co-Morbidity. Neurogastroenterol. Motil. 2008, 20, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, D.; Troost, F.J.; Jonkers, D.M.; Kruimel, J.W.; Leue, C.; Masclee, A.A. Decreased Levels of Kynurenic Acid in the Intestinal Mucosa of IBS Patients: Relation to Serotonin and Psychological State. J. Psychosom. Res. 2013, 74, 501–504. [Google Scholar] [CrossRef]
- Haq, S.; Grondin, J.A.; Khan, W.I. Tryptophan-derived serotonin- kynurenine balance in immune activation and intestinal inflammation. FASEB J. 2021, 35, e21888. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Patney, N.L.; Mehrotra, M.P.; Verma, R.B.; Kumar, A. Urinary indicant in healthy Indian subjects. Indian J. Physiol. Pharmacol. 1977, 21, 342–346. [Google Scholar]
- Tews, H.C.; Elger, T.; Gunawan, S.; Fererberger, T.; Sommersberger, S.; Loibl, J.; Huss, M.; Liebisch, G.; Müller, M.; Kandulski, A.; et al. Fecal short chain fatty acids and urinary 3-indoxyl sulfate do not discriminate between patients with Crohn’s disease and ulcerative colitis and are not of diagnostic utility for predicting disease severity. Lipids Health Dis. 2023, 22, 164. [Google Scholar] [CrossRef]
- Choroszy, M.; Sobieszczańska, B.; Litwinowicz, K.; Łaczmański, Ł.; Chmielarz, M.; Walczuk, U.; Roleder, T.; Radziejewska, J.; Wawrzyńska, M. Co-toxity of Endoxin and Indoxyl Sulfate, Gut-Derived Bacterial Metabolites, to Vascular Endothelial Cells in Coronary Arterial Disease Accompanied by Gut Dysbiosis. Nutrients 2022, 14, 424. [Google Scholar] [CrossRef]
- Ye, X.; Li, H.; Anjum, K.; Zhong, X.; Miao, S.; Zheng, G.; Liu, W.; Li, L. Dual Role of Indoles Derivated from Intestinal Microbiota on Human Health. Front. Immunol. 2022, 13, 903526. [Google Scholar] [CrossRef] [PubMed]
- Kerckhoffs, A.P.M.; Linde, J.J.M.; Akkermans, L.M.A.; Samsom, M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G1053–G1060. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Mazmanian, S.K.; Hsiao, E.Y. Indogenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, P718–P726. [Google Scholar] [CrossRef]
- Altomare, A.; Di Rosa, C.; Imperia, E.; Emerenziani, S.; Cicala, M.; Guarino, M.P.L. Diarhhea Pradominant-Irritable Bowel Syndrome (IBS-D): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2021, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Joossens, M. Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganism 2020, 8, 1638. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome- Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Ghoi, S.-W. Dietary modulation of gut microbiota for the relief of irritable bowel syndrome. Nutr. Res. Pract. 2021, 15, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.R.; Tang, B.; Shi, Y.Z.; Peng, W.Y.; Ye, K.; Tao, Q.F.; Yu, S.G.; Zheng, H.; Chen, M. Low FODMAP Diet and Probiotics in Irritable Bowel Syndrome: A systematic Review with Network Meta-analysis. Front. Pharmacol. 2022, 13, 853011. [Google Scholar] [CrossRef]
- Turan, B.; Bengi, G.; Cehreli, R.; Akpina, H.; Soytürk, M. Clinical effectiveness of adding probiotics to a low FODMAP diet: Randomized double-blind placebo-contrlled study. World J. Clin. Cases 2021, 9, 7417–7432. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef]
- Chojnacki, C.; Poplawski, T.; Błońska, A.; Konrad, P.; Chojnacki, J.; Blasiak, J. The Usefuness of the Low-FODMAP Diet with Limited Tryptophan Intake in the Treatment of Diarrhea-Predominant Irritable Bowel Syndrome. Nutrients 2023, 15, 1837. [Google Scholar] [CrossRef]
- Błońska, A.; Chojnacki, M.; Macieja, A.; Błasiak, J.; Majsterek, I.; Chojnacki, J.; Poplawski, T. Tryptophan Metabolism in Postmenopausal Women with Functional Costipation. Int. J. Mol. Sci. 2024, 25, 273. [Google Scholar] [CrossRef] [PubMed]
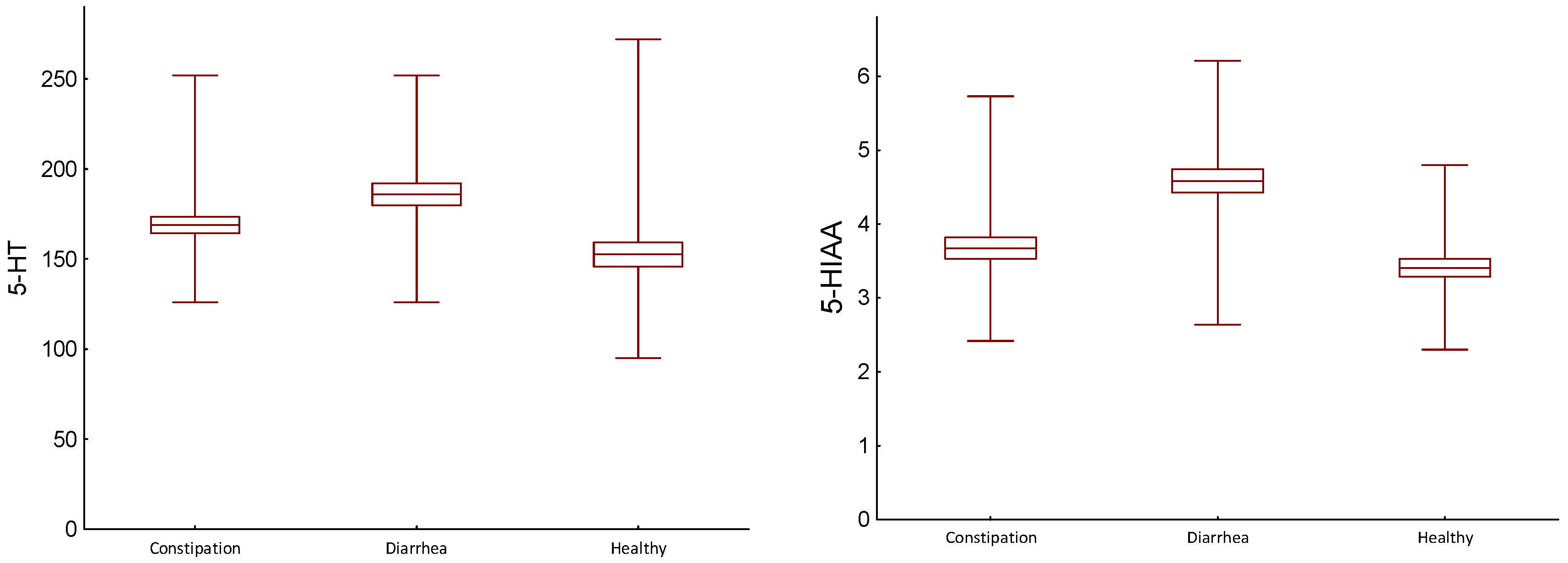
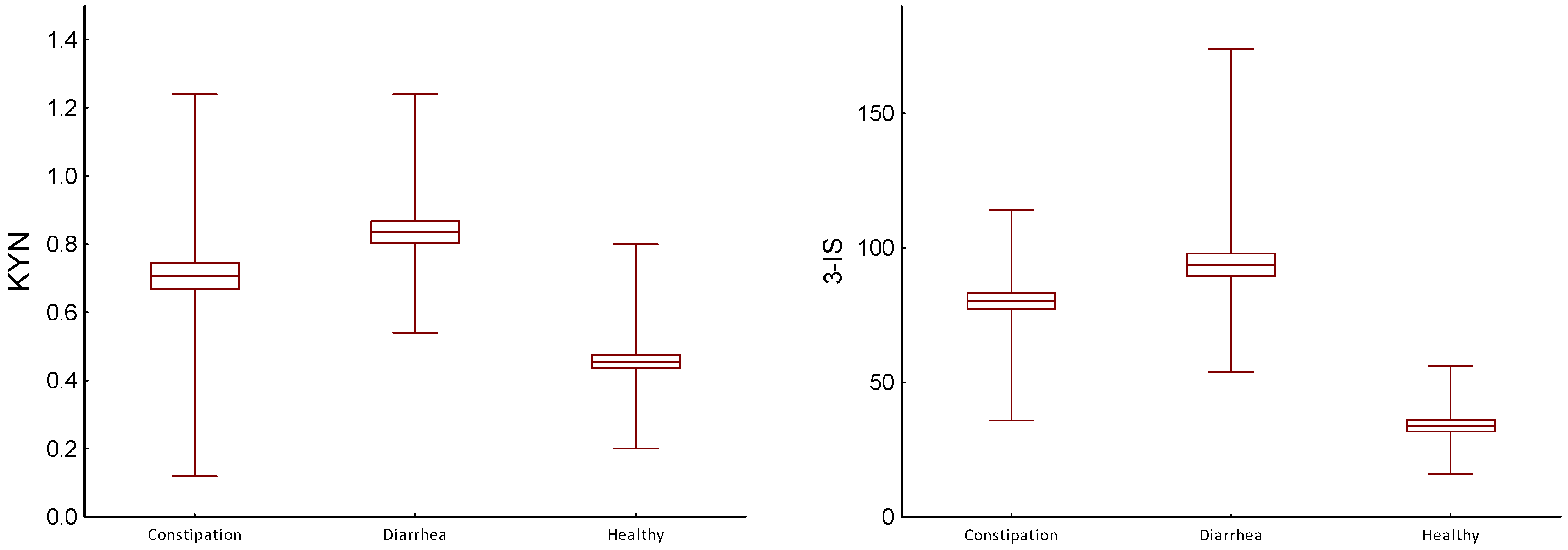
| Feature | Group I (n = 36) | Group IIa (n = 36) | Group IIb (n = 36) | p |
|---|---|---|---|---|
| Age (years) | 45.4 ± 9.4. | 44.7 ± 11.3 | 44.7 ± 11.3 | ns |
| Gender M/F | 8/28 | 7/29 | 7/29 | ns |
| BMI (kg/m2) | 23.8 ± 1.3 | 24.1 ± 2.2 | 24.0 ± 2.3 | ns |
| GFR (mL/min) | 99.8 ± 4.4 | 97.2 ± 6.8 | 98.5 ± 4.8 | ns |
| ALT (µ/L) | 13.5 ± 2.8 | 16.8 ± 3.7 | 16.2 ± 4.2 | ns |
| AST (µ/L) | 11.9 ± 1.8 | 12.1 ± 2.8 | 12.9 ± 4.1 | ns |
| CRP (mg/L) | 2.8 ± 1.9 | 3.4 ± 2.7 | 6.3 ± 3.2 | <0.01 bc |
| FC (µg/g) | 11.8 ± 7.5 | 25.6 ± 12.8 | 42.8 ± 15.8 | <0.01 abc |
| Ions (Time, min) | Group IIa (ppm) | Group IIb (ppm) | p |
|---|---|---|---|
| Hydrogen (0) | 6.46 ± 3.54 | 12.9 ± 11.86 | <0.05 |
| Hydrogen (90) | 23.1 ± 5.72 | 37.5 ± 11.2 | <0.01 |
| Hydrogen (150) | 91.4 ± 18.3 | 98.4 ± 26.7 | ns |
| Methane (0) | 4.5 ± 1.6 | 4.8 ± 1.4 | ns |
| Methane (90) | 4.6 ± 1.8 | 5.2 ± 1.6 | ns |
| Methane (150) | 11.1 ± 4.2 | 15.2 ± 5.7 | <0.05 |
| Ammonia (0) | 5.2 ± 3.1 | 8.3 ± 2.8 | <0.001 |
| Ammonia (90) | 6.1 ± 2.9 | 9.4 ± 3.9 | <0.01 |
| Ammonia (150) | 10.3 ± 3.1 | 16.6 ± 4.1 | <0.001 |
| Parameter | Group (H) (D) (C) | Mean/SD/ Median/IQR | Statistical Analysis, p, Wilcoxon Test | |
|---|---|---|---|---|
| 5-HT (ng/mL) | Constipation (C) | 168.86/27.404 161.50/29.00 | D/C | 0.003 |
| Diarrhea (D) | 185.83/36.773 170.00/65.00 | H/D | 0.002 | |
| Healthy (H) | 152.56/40.841 147.00/57.00 | H/C | 0.045 | |
| TRP (mg/gCr) | Constipation (C) | 11.62/1.654 11.80/2.250 | D/C | 0.273 |
| Diarrhea (D) | 11.64/1.625 11.80/2.25 | H/D | 0.001 | |
| Healthy (H) | 13.82/1.857 14.20/2.65 | H/C | 0.001 | |
| 5-HIAA (mg/gCr) | Constipation (C) | 3.67/0.862 3.64/1.035 | D/C | 0.001 |
| Diarrhea (D) | 4.59/0.953 4.47/1.51 | H/D | 0.001 | |
| Healthy (H) | 3.41/0.735 3.20/0.80 | H/C | 0.081 | |
| KYN (mg/gCr) | Constipation (C) | 0.71/0.235 0.72/0.255 | H/C | 0.005 |
| Diarrhea (D) | 0.84/0.189 0.82/0.25 | H/D | 0.001 | |
| Healthy (H) | 0.46/0.113 0.50/0.10 | H/C | 0.001 | |
| 3-IS (mg/gCr) | Constipation (C) | 80.28/17.491 81.50/17.50 | D/C | 0.001 |
| Diarrhea (D) | 93.75/25.166 86.00/29.50 | H/D | 0.001 | |
| Healthy (H) | 33.97/12.557 35.00/22.50 | H/C | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacki, J.; Konrad, P.; Mędrek-Socha, M.; Kaczka, A.; Błońska, A.; Zajdel, R.; Chojnacki, C.; Gąsiorowska, A. The Variability of Tryptophan Metabolism in Patients with Mixed Type of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2024, 25, 2550. https://doi.org/10.3390/ijms25052550
Chojnacki J, Konrad P, Mędrek-Socha M, Kaczka A, Błońska A, Zajdel R, Chojnacki C, Gąsiorowska A. The Variability of Tryptophan Metabolism in Patients with Mixed Type of Irritable Bowel Syndrome. International Journal of Molecular Sciences. 2024; 25(5):2550. https://doi.org/10.3390/ijms25052550
Chicago/Turabian StyleChojnacki, Jan, Paulina Konrad, Marta Mędrek-Socha, Aleksandra Kaczka, Aleksandra Błońska, Radosław Zajdel, Cezary Chojnacki, and Anita Gąsiorowska. 2024. "The Variability of Tryptophan Metabolism in Patients with Mixed Type of Irritable Bowel Syndrome" International Journal of Molecular Sciences 25, no. 5: 2550. https://doi.org/10.3390/ijms25052550
APA StyleChojnacki, J., Konrad, P., Mędrek-Socha, M., Kaczka, A., Błońska, A., Zajdel, R., Chojnacki, C., & Gąsiorowska, A. (2024). The Variability of Tryptophan Metabolism in Patients with Mixed Type of Irritable Bowel Syndrome. International Journal of Molecular Sciences, 25(5), 2550. https://doi.org/10.3390/ijms25052550





