Simultaneous Comparison of Aqueous Humor and Serum Metabolic Profiles of Diabetic and Nondiabetic Patients Undergoing Cataract Surgery—A Targeted and Quantitative Metabolomics Study
Abstract
1. Introduction
2. Results
2.1. Univariate Analysis
2.2. Correlation between Concentrations of Metabolites in AH and Serum
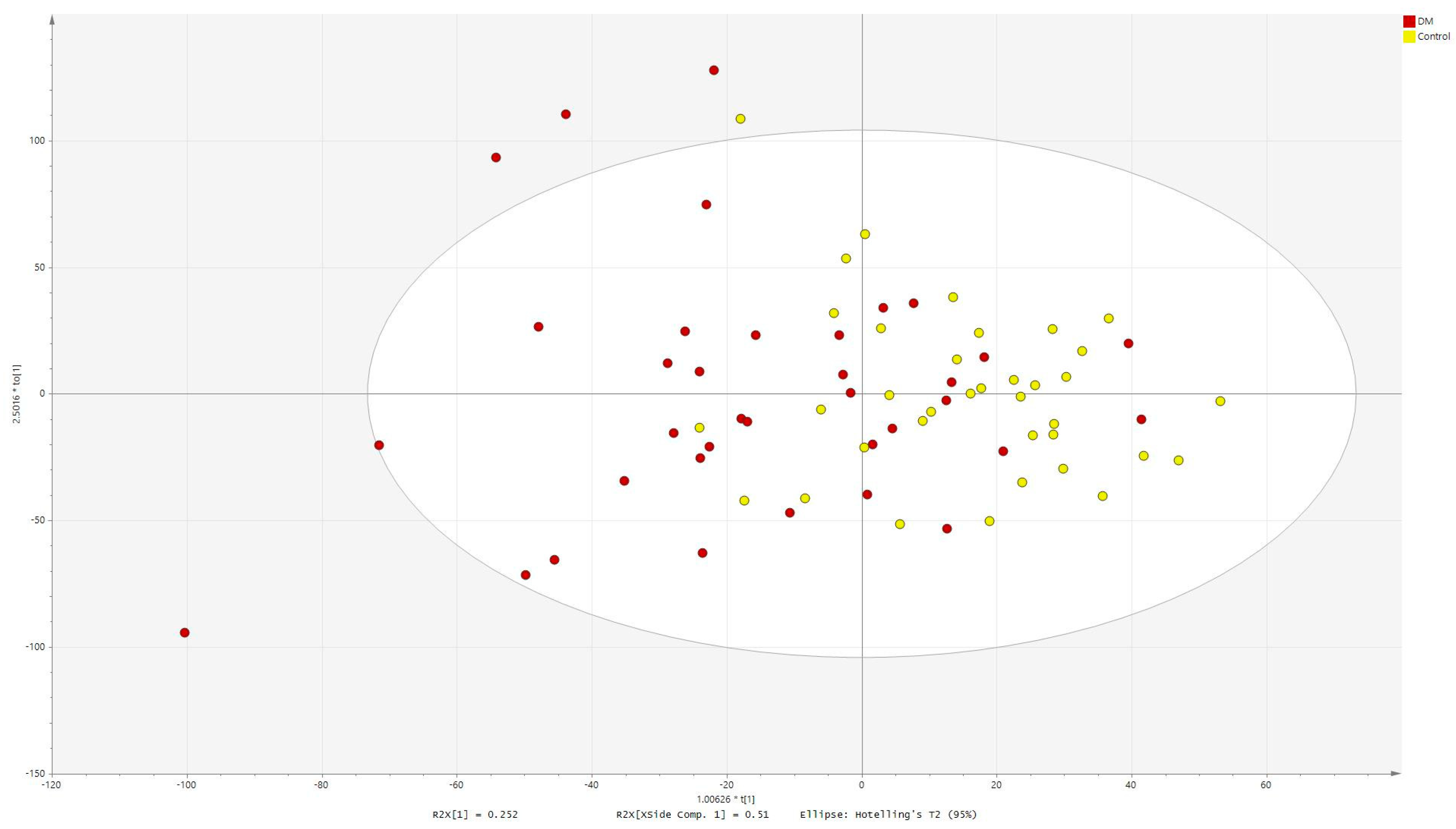
2.3. Multivariate Analysis
2.4. Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
Study Participants and Sample Collection
4.2. Glucose Metabolism Assessment
4.3. Metabolomic Analysis
4.4. Statistical Analysis
4.5. Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. National Diabetes Statistics Report Website. 2022. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 28 August 2022).
- Klein, B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007, 14, 179–183. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Wang, W.; Yan, W.; Fotis, K.; Prasad, N.M.; Lansingh, V.C.; Taylor, H.R.; Finger, R.P.; Facciolo, D.; He, M. Cataract Surgical Rate and Socioeconomics: A Global Study. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5872–5881. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Chung, S.S.; Kador, P.F. Diabetic cataracts: Mechanisms and management. Diabetes Metab. Res. Rev. 2010, 26, 172–180. [Google Scholar] [CrossRef]
- Dmuchowska, D.A.; Pietrowska, K.; Krasnicki, P.; Grochowski, E.T.; Mariak, Z.; Kretowski, A.; Ciborowski, M. Letter to the Editor: Metabolomics of Aqueous Humor in Diabetes Mellitus. J. Ocul. Pharmacol. Ther. 2020, 36, 580–581. [Google Scholar] [CrossRef]
- Dmuchowska, D.A.; Pietrowska, K.; Kretowski, A.; Ciborowski, M. Comment on “Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy” Graefes Arch Clin Exp Ophthalmol. 2021 Jul 3. doi: 10.1007/s00417-021-05285-y. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1039–1040. [Google Scholar] [CrossRef]
- Midena, E.; Frizziero, L.; Midena, G.; Pilotto, E. Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3549–3560. [Google Scholar] [CrossRef]
- Pietrowska, K.; Dmuchowska, D.A.; Krasnicki, P.; Bujalska, A.; Samczuk, P.; Parfieniuk, E.; Kowalczyk, T.; Wojnar, M.; Mariak, Z.; Kretowski, A.; et al. An exploratory LC-MS-based metabolomics study reveals differences in aqueous humor composition between diabetic and non-diabetic patients with cataract. Electrophoresis 2018, 39, 1233–1240. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Zhu, B.; Hu, J.; Huang, J.; Zhu, W.; Miao, W.; Tang, J. Aqueous humor metabolomic profiles in association with diabetic mellitus. Int. J. Clin. Exp. Pathol. 2018, 11, 3479–3486. [Google Scholar]
- Kunikata, H.; Ida, T.; Sato, K.; Aizawa, N.; Sawa, T.; Tawarayama, H.; Murayama, N.; Fujii, S.; Akaike, T.; Nakazawa, T. Metabolomic profiling of reactive persulfides and polysulfides in the aqueous and vitreous humors. Sci. Rep. 2017, 7, 41984. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Chen, F.; Sun, Q.; Xu, X.; Lin, S.H.; Liu, K. Metabolomic profile of diabetic retinopathy: A GC-TOFMS-based approach using vitreous and aqueous humor. Acta Diabetol. 2020, 57, 41–51. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, B.; Liu, X.; Jin, J.; Zou, H. Metabolic characterization of diabetic retinopathy: An (1)H-NMR-based metabolomic approach using human aqueous humor. J. Pharm. Biomed. Anal. 2019, 174, 414–421. [Google Scholar] [CrossRef]
- Chu, K.O.; Chan, T.I.; Chan, K.P.; Yip, Y.W.; Bakthavatsalam, M.; Wang, C.C.; Pang, C.P.; Brelen, M.E. Untargeted metabolomic analysis of aqueous humor in diabetic macular edema. Mol. Vis. 2022, 28, 230–244. [Google Scholar]
- Lillo, A.; Marin, S.; Serrano-Marín, J.; Bernal-Casas, D.; Binetti, N.; Navarro, G.; Cascante, M.; Sánchez-Navés, J.; Franco, R. Biogenic Amine Levels Markedly Increase in the Aqueous Humor of Individuals with Controlled Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 12752. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, F.; Zhu, J. Bridging Targeted and Untargeted Mass Spectrometry-Based Metabolomics via Hybrid Approaches. Metabolites 2020, 10, 348. [Google Scholar] [CrossRef]
- Thompson, J.W.; Adams, K.J.; Adamski, J.; Asad, Y.; Borts, D.; Bowden, J.A.; Byram, G.; Dang, V.; Dunn, W.B.; Fernandez, F.; et al. International Ring Trial of a High Resolution Targeted Metabolomics and Lipidomics Platform for Serum and Plasma Analysis. Anal. Chem. 2019, 91, 14407–14416. [Google Scholar] [CrossRef]
- Grigsby, J.G.; Cardona, S.M.; Pouw, C.E.; Muniz, A.; Mendiola, A.S.; Tsin, A.T.; Allen, D.M.; Cardona, A.E. The Role of Microglia in Diabetic Retinopathy. J. Ophthalmol. 2014, 2014, 705783. [Google Scholar] [CrossRef]
- Midena, E.; Pilotto, E. Emerging Insights into Pathogenesis. Dev. Ophthalmol. 2017, 60, 16–27. [Google Scholar] [CrossRef]
- Oh, I.K.; Kim, S.W.; Oh, J.; Lee, T.S.; Huh, K. Inflammatory and angiogenic factors in the aqueous humor and the relationship to diabetic retinopathy. Curr. Eye Res. 2010, 35, 1116–1127. [Google Scholar] [CrossRef]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef]
- Park, J.W.; Piknova, B.; Jenkins, A.; Hellinga, D.; Parver, L.M.; Schechter, A.N. Potential roles of nitrate and nitrite in nitric oxide metabolism in the eye. Sci. Rep. 2020, 10, 13166. [Google Scholar] [CrossRef]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef]
- Grochowski, E.T.; Pietrowska, K.; Kowalczyk, T.; Mariak, Z.; Kretowski, A.; Ciborowski, M.; Dmuchowska, D.A. Omics in Myopia. J. Clin. Med. 2020, 9, 3464. [Google Scholar] [CrossRef]
- Riddell, N.; Crewther, S.G. Novel evidence for complement system activation in chick myopia and hyperopia models: A meta-analysis of transcriptome datasets. Sci. Rep. 2017, 7, 9719. [Google Scholar] [CrossRef] [PubMed]
- Barbas-Bernardos, C.; Armitage, E.G.; García, A.; Mérida, S.; Navea, A.; Bosch-Morell, F.; Barbas, C. Looking into aqueous humor through metabolomics spectacles—Exploring its metabolic characteristics in relation to myopia. J. Pharm. Biomed. Anal. 2016, 127, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Smiarowska, E.; Bondarczuk, K.; Bauer, W.; Niemira, M.; Szalkowska, A.; Raczkowska, J.; Kwasniewski, M.; Tarasiuk, E.; Dubatowka, M.; Lapinska, M.; et al. Gut Microbiome in Chronic Coronary Syndrome Patients. J. Clin. Med. 2021, 10, 5074. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska, K.; Dmuchowska, D.A.; Godlewski, A.; Grochowski, E.T.; Wojnar, M.; Gosk, W.; Konopinska, J.; Kretowski, A.; Ciborowski, M. Extent of interocular (a)symmetry based on the metabolomic profile of human aqueous humor. Front. Mol. Biosci. 2023, 10, 1166182. [Google Scholar] [CrossRef]




| Characteristics | DM | Control | p-Value |
|---|---|---|---|
| Number of patients | 36 | 36 | |
| Sex, female, n (%) | 22 (61) | 20 (56) | 0.4 |
| Age, mean ± SD (years) | 68.69 ± 10.08 | 70.56 ± 7.63 | 0.4 |
| BMI, mean ± SD | 29.23 ± 5.98 | 30.05 ± 4.88 | 0.5 |
| AXL, mean ± SD (mm) | 23.78 ± 1.12 | 23.54 ± 1.10 | 0.4 |
| Glu, median (IQR) (mg/dL) | 133 (50) | 110 (23) | 0.03 |
| INS, median (IQR) (µU/mL) | 15.76 (18.83) | 19.99 (13.15) | 0.2 |
| C-peptide, median (IQR) (ng/mL) | 2.60 (2.39) | 4.33 (1.93) | 0.02 |
| Metabolites in Serum | p-Value | pBH | Average Concentration DM (µM) | Average Concentration Control (µM) | Change |
|---|---|---|---|---|---|
| PC ae C36:4 | 0.00004 | 0.005 | 10,699.2 | 15,457.7 | −30.8 |
| PC ae C34:2 | 0.0006 | 0.03 | 6181.1 | 7958.9 | −22.3 |
| PC ae C36:3 | 0.0004 | 0.03 | 3959.8 | 5216.0 | −24.1 |
| PC ae C38:4 | 0.002 | 0.05 | 7859.8 | 9552.0 | −17.7 |
| PC ae C38:5 | 0.002 | 0.06 | 11,756.7 | 14,496.1 | −18.9 |
| Tetradecenoylcarnitine | 0.009 | 0.1 | 40.1 | 50.4 | −20.3 |
| Hexadecanoylcarnitine | 0.007 | 0.1 | 50.8 | 63.4 | −19.9 |
| Citrulline | 0.009 | 0.1 | 3878.9 | 6068.8 | −36.1 |
| PC aa C40:4 | 0.01 | 0.2 | 1862.2 | 2368.0 | −21.4 |
| Carnitine | 0.01 | 0.2 | 5575.7 | 6537.9 | −14.7 |
| Arginine | 0.02 | 0.2 | 15,544.1 | 18,524.8 | −16.1 |
| PC aa C38:3 | 0.02 | 0.2 | 33,204.1 | 40,364.0 | −17.7 |
| Octadecanoylcarnitine | 0.01 | 0.2 | 23.1 | 28.7 | −19.6 |
| PC ae C36:5 | 0.03 | 0.2 | 7439.4 | 9239.8 | −19.5 |
| SM C20:2 | 0.02 | 0.2 | 233.2 | 293.1 | −20.5 |
| lysoPC a C18:0 | 0.03 | 0.2 | 9027.2 | 10,725.3 | −15.8 |
| Decanoylcarnitine | 0.02 | 0.2 | 57.5 | 90.1 | −36.1 |
| PC ae C34:3 | 0.04 | 0.3 | 3825.6 | 4667.4 | −18.0 |
| PC ae C40:4 | 0.04 | 0.3 | 1236.4 | 1395.3 | −11.4 |
| Threonine | 0.05 | 0.3 | 13,506.6 | 15,304.5 | −11.7 |
| Metabolites in AH | p-Value | pBH | Average Concentration DM (µM) | Average Concentration Control (µM) | Change |
|---|---|---|---|---|---|
| Arginine | 0.008 | 0.06 | 15,381.3 | 17,997.5 | −14.5 |
| Isoleucine | 0.01 | 0.06 | 11,833.6 | 9650.9 | 22.6 |
| Leucine | 0.008 | 0.06 | 27,394.7 | 22,406.2 | 22.3 |
| Valine | 0.008 | 0.06 | 36,702.9 | 31,379.4 | 17.0 |
| Alanine | 0.008 | 0.08 | 37,289.7 | 32,079.7 | 16.2 |
| Sugars | 0.007 | 0.08 | 1,176,402.0 | 756,224.1 | 55.6 |
| Propionylcarnitine | 0.02 | 0.1 | 105.4 | 79.7 | 32.3 |
| Lysine | 0.03 | 0.1 | 31,953.8 | 28,384.8 | 12.6 |
| Metabolite Category | Sample Identification | DM Group | Control Group | ||
|---|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | ||
| Acylcarnitines | Carnitine | 0.52 | 0.001 | 0.55 | 0.0005 |
| Acetylcarnitine | 0.28 | 0.01 | 0.20 | 0.2 | |
| Propionylcarnitine | 0.60 | 0.0001 | 0.53 | 0.001 | |
| Butyrylcarnitine | 0.25 | 0.1 | 0.66 | <0.0001 | |
| Valerylcarnitine | 0.29 | 0.09 | 0.41 | 0.01 | |
| Aminoacids | Alanine | 0.50 | 0.002 | 0.52 | 0.001 |
| Arginine | 0.38 | 0.02 | 0.47 | 0.004 | |
| Asparagine | 0.65 | <0.0001 | 0.59 | 0.0001 | |
| Citrulline | 0.84 | <0.0001 | 0.74 | <0.0001 | |
| Glutamine | 0.39 | 0.02 | 0.61 | 0.0001 | |
| Glutamate | 0.35 | 0.04 | −0.04 | 0.8 | |
| Glycine | 0.47 | 0.004 | 0.25 | 0.2 | |
| Histidine | 0.54 | 0.0007 | 0.34 | 0.04 | |
| Isoleucine | 0.54 | 0.0006 | 0.55 | 0.0005 | |
| Leucine | 0.45 | 0.006 | 0.45 | 0.006 | |
| Lysine | 0.42 | 0.01 | 0.41 | 0.01 | |
| Methionine | 0.54 | 0.0006 | 0.30 | 0.08 | |
| Ornithine | 0.63 | <0.0001 | 0.56 | 0.0004 | |
| Phenylalanine | 0.47 | 0.004 | 0.37 | 0.03 | |
| Proline | 0.62 | 0.0001 | 0.57 | 0.0003 | |
| Serine | 0.61 | 0.0001 | 0.52 | 0.001 | |
| Threonine | 0.72 | <0.0001 | 0.80 | <0.0001 | |
| Tryptophan | 0.39 | 0.02 | 0.28 | 0.09 | |
| Tyrosine | 0.63 | <0.0001 | 0.39 | 0.02 | |
| Valine | 0.54 | 0.0007 | 0.53 | 0.001 | |
| Biogenic Amines | alpha-Aminoadipic acid | 0.55 | 0.0005 | 0.18 | 0.3 |
| Creatinine | 0.88 | <0.0001 | 0.72 | <0.0001 | |
| Kynurenine | 0.59 | 0.0002 | 0.50 | 0.002 | |
| trans-4-Hydroxyproline | 0.83 | <0.0001 | 0.86 | <0.0001 | |
| Taurine | 0.06 | 0.7 | 0.09 | 0.6 | |
| Glycerophospholipids | PC aa C34:1 | 0.07 | 0.7 | 0.37 | 0.03 |
| PC aa C38:4 | 0.08 | 0.6 | 0.47 | 0.003 | |
| Sphingolipids | SM C16:1 | 0.29 | 0.08 | −0.11 | 0.5 |
| SM C18:1 | −0.03 | 0.8 | −0.02 | 0.9 | |
| Sugars | Hexoses | 0.67 | <0.0001 | 0.65 | <0.0001 |
| Pathway Name | Match Status | p | −log(p) | Holm p | FDR | Impact |
|---|---|---|---|---|---|---|
| 2/14 | 0.0011 | 2.95 | 0.094 | 0.094 | 0.30 |
| 2/48 | 0.013 | 1.89 | 1 | 0.4 | 0 |
| 1/4 | 0.015 | 1.81 | 1 | 0.4 | 0 |
| 1/5 | 0.019 | 1.71 | 1 | 0.4 | 0 |
| 1/8 | 0.03 | 1.51 | 1 | 0.51 | 0 |
| 1/13 | 0.049 | 1.3 | 1 | 0.69 | 0 |
| 1/33 | 0.12 | 0.92 | 1 | 1 | 0 |
| 1/36 | 0.13 | 0.88 | 1 | 1 | 0 |
| 1/36 | 0.13 | 0.88 | 1 | 1 | 0.095 |
| 1/38 | 0.14 | 0.86 | 1 | 1 | 0.058 |
| 1/39 | 0.14 | 0.85 | 1 | 1 | 0 |
| Pathway Name | Match Status | p | −log(p) | Holm p | FDR | Impact |
|---|---|---|---|---|---|---|
| 6/48 | <0.0001 | 6.58 | <0.0001 | <0.0001 | 0 |
| 3/8 | <0.0001 | 4.83 | 0.0012 | 0.0006 | 0 |
| 3/40 | 0.0023 | 2.64 | 0.19 | 0.064 | 0 |
| 2/27 | 0.015 | 1.84 | 1 | 0.31 | 0.052 |
| 2/37 | 0.027 | 1.57 | 1 | 0.39 | 0 |
| 1/4 | 0.028 | 1.55 | 1 | 0.39 | 0 |
| 1/8 | 0.056 | 1.26 | 1 | 0.67 | 0 |
| 1/10 | 0.069 | 1.16 | 1 | 0.72 | 0 |
| 1/14 | 0.095 | 1.02 | 1 | 0.89 | 0.076 |
| 1/19 | 0.13 | 0.9 | 1 | 1 | 0 |
| 1/20 | 0.13 | 0.87 | 1 | 1 | 0 |
| 1/25 | 0.16 | 0.78 | 1 | 1 | 0 |
| 1/26 | 0.17 | 0.77 | 1 | 1 | 0.0002 |
| 1/28 | 0.18 | 0.74 | 1 | 1 | 0 |
| 1/28 | 0.18 | 0.74 | 1 | 1 | 0.037 |
| 1/30 | 0.19 | 0.71 | 1 | 1 | 0.13 |
| 1/38 | 0.24 | 0.62 | 1 | 1 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochowski, E.T.; Pietrowska, K.; Godlewski, A.; Gosk, W.; Buczynska, A.; Wojnar, M.; Konopinska, J.; Kretowski, A.; Ciborowski, M.; Dmuchowska, D.A. Simultaneous Comparison of Aqueous Humor and Serum Metabolic Profiles of Diabetic and Nondiabetic Patients Undergoing Cataract Surgery—A Targeted and Quantitative Metabolomics Study. Int. J. Mol. Sci. 2023, 24, 12671. https://doi.org/10.3390/ijms241612671
Grochowski ET, Pietrowska K, Godlewski A, Gosk W, Buczynska A, Wojnar M, Konopinska J, Kretowski A, Ciborowski M, Dmuchowska DA. Simultaneous Comparison of Aqueous Humor and Serum Metabolic Profiles of Diabetic and Nondiabetic Patients Undergoing Cataract Surgery—A Targeted and Quantitative Metabolomics Study. International Journal of Molecular Sciences. 2023; 24(16):12671. https://doi.org/10.3390/ijms241612671
Chicago/Turabian StyleGrochowski, Emil Tomasz, Karolina Pietrowska, Adrian Godlewski, Wioleta Gosk, Angelika Buczynska, Malgorzata Wojnar, Joanna Konopinska, Adam Kretowski, Michal Ciborowski, and Diana Anna Dmuchowska. 2023. "Simultaneous Comparison of Aqueous Humor and Serum Metabolic Profiles of Diabetic and Nondiabetic Patients Undergoing Cataract Surgery—A Targeted and Quantitative Metabolomics Study" International Journal of Molecular Sciences 24, no. 16: 12671. https://doi.org/10.3390/ijms241612671
APA StyleGrochowski, E. T., Pietrowska, K., Godlewski, A., Gosk, W., Buczynska, A., Wojnar, M., Konopinska, J., Kretowski, A., Ciborowski, M., & Dmuchowska, D. A. (2023). Simultaneous Comparison of Aqueous Humor and Serum Metabolic Profiles of Diabetic and Nondiabetic Patients Undergoing Cataract Surgery—A Targeted and Quantitative Metabolomics Study. International Journal of Molecular Sciences, 24(16), 12671. https://doi.org/10.3390/ijms241612671







