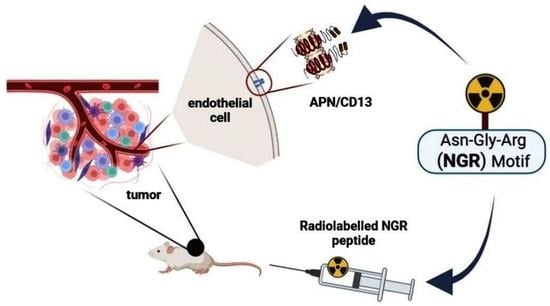
NGR-Based Radiopharmaceuticals for Angiogenesis Imaging: A Preclinical Review
Abstract
1. Introduction
2. Overview of Preclinical Studies with Radiolabelled NGR-Based Imaging Probes
2.1. Labelling with Gallium-68 (68Ga)
2.1.1. Tracer Uptake in Primary Tumours—First Results
2.1.2. Tracer Uptake in Primary Tumours and Related Metastases
2.1.3. Temporal Evolution of Radiopharmaceutical Uptake with Tumour Growth
2.1.4. Comparison of Various 68Ga-labelled NGR-Based Radiotracers
2.1.5. CendR Sequence-Containing NGR-Based Probe
2.1.6. Linear NGR Peptide-Based Probe
2.1.7. Dimeric NGR Peptide-Based Probes
2.2. Labelling with Copper-64 (64Cu)
2.3. Labelling with 99mTc-Pertecnetate (99mTc-Pertechnetate)
| Investigated Object | Investigated Phenomenon/Initiatives | Radiopharmaceutical | In Vitro and In Vivo Methods | Highlights | Reference |
|---|---|---|---|---|---|
| in vitro studies: HT1080 and HT29 cell lines in vivo studies: HT1080 and HT29 tumour-bearing athymic nude mice | in vitro properties, in vivo imaging capability, and APN/CD13 targeting ability | monomeric [64Cu]Cu-DOTA-NGR1 dimeric [64Cu]Cu-DOTA-NGR2 | in vitro studies: in vitro stability, cell uptake and efflux studies, cell binding assay in vivo studies: in vivo static microPET imaging, ex vivo gamma counter measurements for radioactivity calculation, blocking experiments | both the dimer and the monomer specifically target APN/CD13 overexpressing HT1080 tumour xenografts, improved tumour accumulation and retention were found for the dimeric compound compared to the monomer, and dimeric [64Cu]Cu-DOTA-NGR2 is a better PET imaging probe for the in vivo visualisation of the APN/CD13 pattern | [55] |
| HT1080 and MCF-7 cells, bilater tumour-bearing female athymic nude mice (HT1080 and MCF-7) | synthesis process, in vitro, and in vivo performance evaluation | [64Cu]Cu-Sar-NGR2 | in vitro cell binding assay, cell uptake, and efflux studies in vivo static microPET imaging, ex vivo uptake studies, and in vivo blocking examinations | Sarcophagine cage could be an alternative chelator for radiopharmaceutical development [64Cu]Cu-Sar-NGR2 is a useful diagnostic agent for the in vivo assessment of APN/CD13 expression | [31] |
| HepG2 cells, HepG2 hepatoma-bearing female BALB/c nude mice | APN/CD13 receptor binding specificity and comparison of monomer and dimer NGR derivatives | [99mTc]Tc-NGR1 monomer [99mTc]Tc-NGR2 dimer | in vitro cell binding assay and cell uptake studies, in vivo microSPECT imaging and blocking experiments, and ex vivo gamma counter measurements for organ distribution assessment | [99mTc]Tc-NGR2 dimer presented more improved in vitro and in vivo properties than the monomer (binding potential, cell uptake, tumour accumulation and retention, pharmacokinetics), [99mTc]Tc-NGR2 dimer is a valuable tracer for SPECT-based angiogenesis imaging | [6] |
| A549, PC-3, and OVCAR-3 cell lines and A549, PC-3, and OVCAR-3 tumour carrying athymic male nude mice | in vitro and in vivo angiogenesis imaging characteristic, organ distribution profile | [99mTc]Tc-MAG3-PEG8-c(NGRyk) | in vitro cell binding assay, in vivo planar gamma camera imaging, ex vivo gamma counting, in vitro and in vivo receptor blocking studies | [99mTc]Tc-MAG3-PEG8-c(NGRyk) appears to be an effective imaging probe, especially in cases of pulmonary malignancies | [49] |
| B16F10 and SKMEL28 cell lines and corresponding B16F10 and SKMEL28 xenografted nude mice | angiogenesis detection | [99mTc]Tc-MAG3-PEG8-c(NGRyk) | in vitro internalization studies, ex vivo organ distribution, and blocking experiments | The human melanoma cells (SKMEL28) exhibited higher [99mTc]Tc-MAG3-PEG8-c(NGRyk) uptake than the murine ones (B16F10) | [50] |
| in vitro studies: LLC and Colo 205 cells | in vitro studies: anti-angiogenic biological activity | in vitro studies: NGR-hPK5, wild-type hPK5 | in vitro studies: cell proliferation assay (MTT assay), cell migration assay (transwell assay, and the wound healing assay) cord morphogenesis assay, CAM assay | NGR-hPK5 exerted a more prominent antiangiogenic effect and response than wild-type hPK5 (inhibition of proliferation, migration, and cord formation of vascular endothelial cells), and the addition of the NGR sequence to the antiangiogenic molecules could enhance their therapeutic potential | [89] |
| LLC carrying female C57BL/6J mice, athymic nude mice bearing Colo 205 tumours | diagnostic feasibility: tumour-targeting ability, in vivo and ex vivo organ distribution therapeutic effect: effect of NGR on the antitumour activity of hPK5, tumour growth inhibition | imaging: [99mTc]Tc-hPK5, [99 mTc]Tc-NGR-hPK5 therapy: hPK5, NGR-hPK5, hPK5/NGR-hPK5 in combination with cisplatin | imaging: in vivo planar imaging with a gamma camera; ex vivo radioactivity determination by gamma counting therapy: calliper measurements for tumour volume determination; CI index calculation | [99 mTc]Tc-NGR-hPK5 showed greater tumour-homing ability than wild-type hPK5, NGR-hPK5 presented favourable anti-tumour effect, the NGR motif improved the anti-tumour potential of hPK5, and the addition of the NGR sequence to antiangiogenic molecules could enhance their therapeutic potential | [89] |
| cytotoxicity studies: B16F10 cell imaging: B16F10 tumour bearing C57BL6 mice | cytotoxicity studies: growth inhibition imaging: organ distribution pattern | cytotoxicity studies: cNGR-CLB complex, cNGR, CLB imaging: [99mTc]Tc-HYNIC-CLB-c(NGR) | in vitro cytotoxicity studies: colorimetric MTT assay imaging: ex vivo biodistribution and blocking studies | the peptide-drug conjugate (cNGR-CLB) exhibited higher cytotoxicity than the peptide (cNGR) or the drug (CLB), so the conjugation of target-specific peptides with CLB or other chemotherapeutic agents could be used for the achievement of increased therapeutic efficacy | [34] |
| nude mice bilaterally bearing HT1080 tumours | radiochemical synthesis process, in vivo detection of APN/CD13 expression, and organ distribution pattern | 99mTcO-N3S-PEG2-probestin | in vivo whole-body static planar imaging, microSPECT imaging, blocking experiments, and ex vivo radioactivity determination | 99mTcO-N3S-PEG2-probestin shows APN selective uptake and high affinity APN inhibitor conjugates could be effectively used for the targeting of APN | [90] |
| HT1080 tumour-bearing CB17 SCID mice | 213Bi: APN/CD13 positive tumour-homing ability, anti-tumour potential 68Ga: in vivo organ distribution, tumour targeting competence | [213Bi]Bi-DOTAGA-cKNGRE [68Ga]Ga-DOTAGA-cKNGRE | cancer treatment studies, ex vivo gamma counting with 213Bi, calliper measurements for tumour growth determination, body weight and tumour volume measurements, and in vivo MiniPET imaging with 68Ga | [213Bi]Bi-DOTAGA-cKNGRE is a potential weapon in the targeted treatment of APN/CD13 expressing fibrosarcoma [68Ga]Ga-DOTAGA-cKNGRE is a potent PET diagnostic probe for the detection of APN/CD13 overexpressing primary tumours and metastases | [30] |
| HT1080 cells, HT1080 xenografted BALB/c nude mice | in vivo diagnostic and therapeutic feasibility, in vitro inhibitory effect | [188Re]Re-NGR-VEGI | in vivo SPECT imaging and radiotherapy studies; ex vivo radioactivity determination by gamma counter; in vitro apoptosis assay by flow cytometry | [188Re]Re-NGR-VEGI can be efficiently used in theranostic settings for both the diagnostics and the targeted therapy of APN/CD13 tumours | [32] |
| B16F10 tumour-bearing C57BL6 mice | drug delivery, in vivo imaging properties, ex vivo organ biodistribution, and in vivo anti-tumour effect | ([177Lu]Lu-DOTA-CNS-cNGR, ([177Lu]Lu-DOTA-cNGR, ([177Lu]Lu-DOTA-CNS) | in vivo uptake studies, blocking studies | [177Lu]Lu-DOTA-CNS-cNGR presented a higher tumour-to-background ratio than [177Lu]Lu-DOTA-CNS, CNS attached to target-specific molecules, can be used to design nanoprobes for targeted drug delivery | [35] |
2.4. Labelling with Bismuth-213 (213Bi)
2.5. Labelling with Rhenium-188 (188Re)
2.6. Labelling with Lutetium-177 (177Lu)
3. Radiolabelled NGR Peptides beyond Cancer Imaging
3.1. Radiolabelled NGR Peptides for Cardiac Imaging
3.2. Radiolabelled NGR Peptides for Ischaemia-Reperfusion Imaging
3.3. Radiolabelled NGR Peptides for Therapeutic Applications
4. Future Perspectives, Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APN/CD13 | Aminopeptidase N |
| ARA-SL[DXR] | mismatched peptide ARA-targeted liposomal doxorubicin |
| c(KNGRE)-NH2 | cyclic(lysyl-asparaginyl-glycyl-argininyl-glutamic acid amide |
| c(NGR) | cyclic NGR |
| CendR sequence | C-end rule peptide; R/KXXR/K |
| CLB | chlorambucil |
| CNS | carbon nanospheres |
| DAR | differential absorption ratio |
| DOTA | 1,4,7,10-teraazacyclododecane-N,N′,N″,N‴-teraacetic acid |
| DOTAGA | 1,4,7,10-tetrakis(carboxymethyl)-1,4,7,10-tetraazacyclododecane glutaric acid |
| DR | diabetic retinopathy |
| DXR | doxorubicin |
| Fe3O4 NPs | iron oxide nanoparticles |
| G3-NGR | Gly3-CNGRC |
| He/De | hepatocellular carcinoma |
| hPK5 | human plasminogen kringle 5 |
| HYNIC | hydrazinonicotinamide |
| IC50 | half-maximal inhibitory concentration |
| iNGR | internalizing NGR |
| IR | ischemia-reperfusion |
| LAD | left anterior descending coronary artery |
| LN peptide | linear peptide |
| MR | magnetic resonance |
| MR/NIRF | magnetic resonance/near-infrared fluorescence imaging |
| NB | neuroblastoma |
| Ne/De | mesoblastic nephroma tumors |
| NGR | Asparagine-Glycine-Arginine) |
| NGR-SL | NGR peptide-targeted liposome |
| NGR-SL[DXR] | NGR peptide-targeted liposomal doxorubicin |
| NODAGA | 1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid |
| NOTA | 1,4,7-triazacyclononane-triacetic acid |
| NRP-1 | neuropilin-1 |
| PBCA | poly(butyl cyanoacrylate |
| PET | positron emission tomography |
| PET/MRI | positron emission tomography/magnetic resonance imaging |
| RGD | arginine-glycine-aspartate |
| Sar | sarcophagine cage |
| SL[DXR] | liposomal doxorubicin (non-targeted) |
| SPECT | single-photon emission computed tomography |
| SRCA | subrenal capsule assay |
| SUV | standardized uptake value |
| T/M | tumor-to-muscle ratios |
| TETA | 1,4,8,11-tetraazacyclotetradecane-N,N′,N″,N‴-tetraacetic acid |
| TRNT | targeted radionuclide therapy |
| UH | ultrasound |
| USPIO | ultrasmall superparamagnetic iron oxide |
| VEGI | vascular endothelial growth inhibitor |
| YEVGHRC | tyrosyl-glutamyl-valyl-glycyl-histidyl-arginyl-cysteine |
References
- Corti, A.; Fiocchi, M.; Curnis, F. Targeting CD13 with Asn-Gly-Arg (NGR) peptide-drug conjugates. In Next-Generation Therapies and Technologies for Immune-Mediated Inflammatory Diseases, Progress in Inflammation Research; Mina-Osorio, P., Ed.; Springer International Publishing: Basel, Switzerland, 2017; pp. 101–122. [Google Scholar] [CrossRef]
- Look, A.T.; Ashmun, R.A.; Shapiro, L.H.; Peiper, S.C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase. N. J. Clin. Investig. 1989, 83, 1299–1307. [Google Scholar] [CrossRef]
- Ota, K.; Uzuka, Y. Clinical trials of bestatin for leukemia and solid tumors. Biotherapy 1992, 4, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends. Mol. Med. 2008, 14, 361371. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A. Aminopeptidases: Structure and function. FASEB. J. 1993, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Kang, F.; Wang, Z.; Yang, W.; Li, G.; Ma, X.; Li, G.; Chen, K.; Zhang, Y.; Wang, J. (99m)Tc-labeled monomeric and dimeric NGR peptides for SPECT imaging of CD13 receptor in tumor-bearing mice. Amino. Acids. 2013, 44, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Kaklamanis, L.; Turley, H.; Hickson, I.D.; Leek, R.D.; Harris, A.L.; Gatter, K.C. Expression of aminopeptidase-n (CD 13) in normal tissues and malignant neoplasms of epithelial and lymphoid origin. J. Clin. Pathol. 1994, 47, 43–47. [Google Scholar] [CrossRef]
- Hogg, N.; Horton, M.A. Myeloid antigens: New and previously defined clusters. In Leukocyte Typing III. Proceedings of the Third International Workshop on Human Leukocyte Differentiation Antigens; McMichael, A.J., Ed.; Oxford University Press: Oxford, UK, 1987; pp. 576–621. [Google Scholar]
- Bhagwat, S.V.; Lahdenranta, J.; Giordano, R.; Arap, W.; Pasqualini, R.; Shapiro, L.H. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 2001, 97, 652–659. [Google Scholar] [CrossRef]
- Hashida, H.; Takabayashi, A.; Kanai, M.; Adachi, M.; Kondo, K.; Kohno, N.; Yamaoka, Y.; Miyake, M. Aminopeptidase N is involved in cell motility and angiogenesis: Its clinical significance in human colon cancer. Gastroenterology 2002, 122, 376–386. [Google Scholar] [CrossRef]
- Ikeda, N.; Nakajima, Y.; Tokuhara, T.; Hattori, N.; Sho, M.; Kanehiro, H.; Miyake, M. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin. Cancer Res. 2003, 9, 1503–1508. [Google Scholar]
- Kehlen, A.; Lendeckel, U.; Dralle, H.; Langner, J.; Hoang-Vu, C. Biological significance of aminopeptidase N/CD13 in thyroid carcinomas. Cancer Res. 2003, 63, 8500–8506. [Google Scholar]
- Murakami, H.; Yokoyama, A.; Kondo, K.; Nakanishi, S.; Kohno, N.; Miyake, M. Circulating aminopeptidase N/CD13 is an independent prognostic factor in patients with non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 8674–8679. [Google Scholar] [CrossRef] [PubMed]
- Ranogajec, I.; Jakić-Razumović, J.; Puzović, V.; Gabrilovac, J. Prognostic value of matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9) and aminopeptidase N/CD13 in breast cancer patients. Med. Oncol. 2012, 29, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Surowiak, P.; Drag, M.; Materna, V.; Suchocki, S.; Grzywa, R.; Spaczyński, M.; Dietel, M.; Oleksyszyn, J.; Zabel, M.; Lage, H. Expression of aminopeptidase N/CD13 in human ovarian cancers. Int. J. Gynecol. Cancer 2006, 16, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Nakashima, T.; Hamada, H.; Takayama, Y.; Akita, S.; Masuda, T.; Horimasu, Y.; Miyamoto, S.; Iwamoto, H.; Fujitaka, K.; et al. Aminopeptidase N/CD13 as a potential therapeutic target in malignant pleural mesothelioma. Eur. Respir. J. 2018, 51, 1701610. [Google Scholar] [CrossRef] [PubMed]
- van Hensbergen, Y.; Broxterman, H.J.; Hanemaaijer, R.; Jorna, A.S.; van Lent, N.A.; Verheul, H.M.; Pinedo, H.M.; Hoekman, K. Soluble aminopeptidase N/CD13 in malignant and nonmalignant effusions and intratumoral fluid. Clin. Cancer Res. 2002, 8, 3747–3754. [Google Scholar]
- Wang, X.; Niu, Z.; Jia, Y.; Cui, M.; Han, L.; Zhang, Y.; Liu, Z.; Bi, D.; Liu, S. Ubenimex inhibits cell proliferation, migration and invasion by inhibiting the expression of APN and inducing autophagic cell death in prostate cancer cells. Oncol. Rep. 2016, 35, 2121–2130. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Zhang, H.; Zhao, D.; Zhang, Z.; Zhang, S. Expression and clinical significance of aminopeptidase N/CD13 in non-small cell lung cancer. J. Cancer Res. Ther. 2015, 11, 223–228. [Google Scholar] [CrossRef]
- Lendeckel, U.; Karimi, F.; Al Abdulla, R.; Wolke, C. The Role of the Ectopeptidase APN/CD13 in Cancer. Biomedicines 2023, 11, 724. [Google Scholar] [CrossRef]
- Curnis, F.; Arrigoni, G.; Sacchi, A.; Fischetti, L.; Arap, W.; Pasqualini, R.; Corti, A. Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells. Cancer Res. 2002, 62, 867–874. [Google Scholar]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Bieker, R.; Kessler, T.; Schwöppe, C.; Padró, T.; Persigehl, T.; Bremer, C.; Dreischalück, J.; Kolkmeyer, A.; Heindel, W.; Mesters, R.M.; et al. Infarction of tumor vessels by NGR-peptide-directed targeting of tissue factor: Experimental results and first-in-man experience. Blood 2009, 113, 5019–5027. [Google Scholar] [CrossRef]
- Zhu, L.; Ding, Z.; Li, X.; Wei, H.; Chen, Y. Research Progress of Radiolabeled Asn-Gly-Arg (NGR) Peptides for Imaging and Therapy. Mol. Imaging. 2020, 19, 1536012120934957. [Google Scholar] [CrossRef]
- Zucali, P.A.; Simonelli, M.; De Vincenzo, F.; Lorenzi, E.; Perrino, M.; Bertossi, M.; Finotto, R.; Naimo, S.; Balzarini, L.; Bonifacio, C.; et al. Phase I and pharmacodynamic study of high-dose NGR-hTNF in patients with refractory solid tumours. Br. J. Cancer 2013, 108, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Conti, P.S. Target-specific delivery of peptide-based probes for PET imaging. Adv. Drug. Deliv. Rev. 2010, 62, 1005–1022. [Google Scholar] [CrossRef]
- Corti, A.; Giovannini, M.; Belli, C.; Villa, E. Immunomodulatory Agents with Antivascular Activity in the Treatment of Non-Small Cell Lung Cancer: Focus on TLR9 Agonists, IMiDs and NGR-TNF. J. Oncol. 2010, 2010, 732680. [Google Scholar] [CrossRef]
- Meng, J.; Yan, Z.; Wu, J.; Li, L.; Xue, X.; Li, M.; Li, W.; Hao, Q.; Wan, Y.; Qin, X.; et al. High-yield expression, purification and characterization of tumor-targeted IFN-alpha2a. Cytotherapy 2007, 9, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ndinguri, M.W.; Solipuram, R.; Gambrell, R.P.; Aggarwal, S.; Hammer, R.P. Peptide targeting of platinum anti-cancer drugs. Bioconjug. Chem. 2009, 20, 1869–1878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Képes, Z.; Arató, V.; Szabó, J.P.; Gyuricza, B.; Szücs, D.; Hajdu, I.; Fekete, A.; Bruchertseifer, F.; Szikra, D.; Trencsényi, G. Therapeutic Performance Evaluation of 213Bi-Labelled Aminopeptidase N (APN/CD13)-Affine NGR-Motif ([213Bi]Bi-DOTAGA-cKNGRE) in Experimental Tumour Model: A Treasured Tailor for Oncology. Pharmaceutics 2023, 15, 491. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Zong, S.; Wang, J.; Conti, P.S.; Chen, K. MicroPET imaging of CD13 expression using a 64Cu-labeled dimeric NGR peptide based on sarcophagine cage. Mol. Pharm. 2014, 11, 3938–3946. [Google Scholar] [CrossRef]
- Ma, W.; Shao, Y.; Yang, W.; Li, G.; Zhang, Y.; Zhang, M.; Zuo, C.; Chen, K.; Wang, J. Evaluation of (188)Re-labeled NGR-VEGI protein for radioimaging and radiotherapy in mice bearing human fibrosarcoma HT-1080 xenografts. Tumour. Biol. 2016, 37, 9121–9129. [Google Scholar] [CrossRef]
- Máté, G.; Kertész, I.; Enyedi, K.N.; Mező, G.; Angyal, J.; Vasas, N.; Kis, A.; Szabó, É.; Emri, M.; Bíró, T.; et al. In vivo imaging of Aminopeptidase N (CD13) receptors in experimental renal tumors using the novel radiotracer 68Ga-NOTA-c(NGR). Eur. J. Pharm. Sci. 2015, 69, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Vats, K.; Satpati, D.; Sharma, R.; Kumar, C.; Sarma, H.D.; Dash, A. 99m Tc-labeled NGR-chlorambucil conjugate, 99m Tc-HYNIC-CLB-c(NGR) for targeted chemotherapy and molecular imaging. J. Labelled. Comp. Radiopharm. 2017, 60, 431–438. [Google Scholar] [CrossRef]
- Vats, K.; Satpati, A.K.; Sharma, R.; Sarma, H.D.; Satpati, D.; Dash, A. 177Lu-labeled cyclic Asn-Gly-Arg peptide tagged carbon nanospheres as tumor targeting radio-nanoprobes. J. Pharm. Biomed. Anal. 2018, 152, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Liang, W.; Kang, F.; Yang, W.; Ma, X.; Li, G.; Zong, S.; Chen, K.; Wang, J. 68Ga-labeled cyclic NGR peptide for microPET imaging of CD13 receptor expression. Molecules 2014, 19, 11600–11612. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Liang, W.; Kang, F.; Yang, W.; Ma, X.; Li, G.; Zong, S.; Chen, K.; Wang, J. A direct comparison of tumor angiogenesis with 68Ga-labeled NGR and RGD peptides in HT-1080 tumor xenografts using microPET imaging. Amino. Acids. 2014, 46, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Dénes, N.; Szabó, J.P.; Arató, V.; Jószai, I.; Enyedi, K.N.; Lakatos, S.; Garai, I.; Mező, G.; Kertész, I.; et al. In vivo assessment of aminopeptidase N (APN/CD13) specificity of different 68Ga-labelled NGR derivatives using PET/MRI imaging. Int. J. Pharm. 2020, 589, 119881. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Szabó, J.P.; Dénes, N.; Vágner, A.; Nagy, G.; Garai, I.; Fekete, A.; Szikra, D.; Hajdu, I.; Matolay, O.; et al. In Vivo Imaging of Hypoxia and Neoangiogenesis in Experimental Syngeneic Hepatocellular Carcinoma Tumor Model Using Positron Emission Tomography. Biomed. Res. Int. 2020, 2020, 4952372. [Google Scholar] [CrossRef]
- Dénes, N.; Kis, A.; Szabó, J.P.; Jószai, I.; Hajdu, I.; Arató, V.; Enyedi, K.N.; Mező, G.; Hunyadi, J.; Trencsényi, G.; et al. In Vivo preclinical assessment of novel 68Ga-labelled peptides for imaging of tumor associated angiogenesis using positron emission tomography imaging. Appl. Radiat. Isot. 2021, 174, 109778. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Wan, N.; Hua, Z.; Wang, Z.; Huang, H.; Yang, M.; Wang, F. 68Ga-DOTA-NGR as a novel molecular probe for APN-positive tumor imaging using MicroPET. Nucl. Med. Biol. 2014, 41, 268–275. [Google Scholar] [CrossRef]
- Szabo, J.P.; Denes, N.; Arato, V.; Racz, S.; Kis, A.; Opposits, G.; Kepes, Z.; Hajdu, I.; Joszai, I.; Emri, M.; et al. In Vivo Imaging of Neo-angiogenesis of Transplanted Metastases in Subrenal Capsule Assay Induced Rat Model. In Vivo 2022, 36, 1667–1675. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, W.; Zhang, M.; Li, G.; Wang, S.; Wang, Z.; Ma, X.; Kang, F.; Wang, J. Evaluation of 68Ga-labeled iNGR peptide with tumor-penetrating motif for microPET imaging of CD13-positive tumor xenografts. Tumour. Biol. 2016, 37, 12123–12131. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zou, H.; Shen, Y.; Deng, S.; Wu, Y. Synthesis and evaluation of 68Ga-labeled dimeric cNGR peptide for PET imaging of CD13 expression with ovarian cancer xenograft. J. Cancer 2021, 12, 244–252. [Google Scholar] [CrossRef]
- Satpati, D.; Sharma, R.; Kumar, C.; Sarma, H.D.; Dash, A. 68Ga-Chelation and comparative evaluation of N,N′-bis-[2-hydroxy-5-(carboxyethyl)benzyl] ethylenediamine-N,N′-diacetic acid (HBED-CC) conjugated NGR and RGD peptides as tumor targeted molecular imaging probes. Med. Chem. Comm. 2017, 8, 673–679. [Google Scholar] [CrossRef]
- Satpati, D.; Sharma, R.; Sarma, H.D.; Dash, A. Comparative evaluation of 68Ga-labeled NODAGA, DOTAGA, and HBED-CC-conjugated cNGR peptide chelates as tumor-targeted molecular imaging probes. Chem. Biol. Drug. Des. 2018, 91, 781–788. [Google Scholar] [CrossRef]
- Israel, I.; Elflein, K.; Schirbel, A.; Chen, K.; Samnick, S. A comparison of the monomeric [68Ga]NODAGA-NGR and dimeric [68Ga]NOTA-(NGR)2 as aminopeptidase N ligand for positron emission tomography imaging in tumor-bearing mice. Eur. J. Pharm. Sci. 2021, 166, 105964. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, C.E.; Sølling, K. Studies on renal tubular protein reabsorption: Partial and near complete inhibition by certain amino acids. Scand. J. Clin. Lab. Investig. 1977, 37, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Faintuch, B.L.; Oliveira, E.A.; Targino, R.C.; Moro, A.M. Radiolabeled NGR phage display peptide sequence for tumor targeting. Appl. Radiat. Isot. 2014, 86, 41–45. [Google Scholar] [CrossRef]
- Oliveira, É.A.; Faintuch, B.L.; Núñez, E.G.; Moro, A.M.; Nanda, P.K.; Smith, C.J. Radiotracers for different angiogenesis receptors in a melanoma model. Melanoma. Res. 2012, 22, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Negussie, A.H.; Miller, J.L.; Reddy, G.; Drake, S.K.; Wood, B.J.; Dreher, M.R. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J. Control. Release. 2010, 143, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Plesniak, L.A.; Salzameda, B.; Hinderberger, H.; Regan, E.; Kahn, J.; Mills, S.A.; Teriete, P.; Yao, Y.; Jennings, P.; Marassi, F.; et al. Structure and activity of CPNGRC: A modified CD13/APN peptidic homing motif. Chem. Biol. Drug. Des. 2010, 75, 551–562. [Google Scholar] [CrossRef][Green Version]
- Soudy, R.; Ahmed, S.; Kaur, K. NGR peptide ligands for targeting CD13/APN identified through peptide array screening resemble fibronectin sequences. ACS. Comb. Sci. 2012, 14, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Trencsenyi, G.; Kertai, P.; Bako, F.; Hunyadi, J.; Marian, T.; Hargitai, Z.; Pocsi, I.; Muranyi, E.; Hornyak, L.; Banfalvi, G. Renal capsule-parathymic lymph node complex, a new in vivo metastatic model in rats. Anticancer Res. 2009, 29, 2121–2126. [Google Scholar] [PubMed]
- Chen, K.; Ma, W.; Li, G.; Wang, J.; Yang, W.; Yap, L.P.; Hughes, L.D.; Park, R.; Conti, P.S. Synthesis and evaluation of 64Cu-labeled monomeric and dimeric NGR peptides for MicroPET imaging of CD13 receptor expression. Mol. Pharm. 2013, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Kim, T.I.; Kim, P.H.; Ryu, J.; Yun, C.O.; Kim, S.W. Active targeting of RGD-conjugated bioreducible polymer for delivery of oncolytic adenovirus expressing shRNA against IL-8 mRNA. Biomaterials 2011, 32, 5158–5166. [Google Scholar] [CrossRef]
- Borgstrom, P.; Oh, P.; Czarny, M.; Racine, B.; Schnitzer, J.E. Co-implanting orthotopic tissue creates stroma microenvironment enhancing growth and angiogenesis of multiple tumors. F1000Research 2013, 2, 129. [Google Scholar] [CrossRef]
- Kerbel, R.S. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: Better than commonly perceived-but they can be improved. Cancer Biol. Ther. 2003, 2, S134–S139. [Google Scholar] [CrossRef]
- Trencsenyi, G.; Marian, T.; Bako, F.; Emri, M.; Nagy, G.; Kertai, P.; Banfalvi, G. Metastatic hepatocarcinoma he/de tumor model in rat. J. Cancer 2014, 5, 548–558. [Google Scholar] [CrossRef]
- Cao, M.; Liang, Y.; Shen, C.; Miller, K.D.; Stantz, K.M. Developing DCE-CT to quantify intra-tumor heterogeneity in breast tumors with differing angiogenic phenotype. IEEE. Trans. Med. Imaging 2009, 28, 861–871. [Google Scholar] [CrossRef]
- Deshpande, N.; Ren, Y.; Foygel, K.; Rosenberg, J.; Willmann, J.K. Tumor angiogenic marker expression levels during tumor growth: Longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology 2011, 258, 804–811. [Google Scholar] [CrossRef]
- Trencsényi, G.; Dénes, N.; Nagy, G.; Kis, A.; Vida, A.; Farkas, F.; Szabó, J.P.; Kovács, T.; Berényi, E.; Garai, I.; et al. Comparative preclinical evaluation of 68Ga-NODAGA and 68Ga-HBED-CC conjugated procainamide in melanoma imaging. J. Pharm. Biomed. Anal. 2017, 139, 54–64. [Google Scholar] [CrossRef]
- Kis, A.; Dénes, N.; Szabó, J.P.; Arató, V.; Beke, L.; Matolay, O.; Enyedi, K.N.; Méhes, G.; Mező, G.; Bai, P.; et al. In Vivo Molecular Imaging of the Efficacy of Aminopeptidase N (APN/CD13) Receptor Inhibitor Treatment on Experimental Tumors Using 68Ga-NODAGA-c(NGR) Peptide. Biomed. Res. Int. 2021, 2021, 6642973. [Google Scholar] [CrossRef]
- Feron, O. Tumor-penetrating peptides: A shift from magic bullets to magic guns. Sci. Transl. Med. 2010, 2, 34ps26. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Tumor-penetrating peptides. Front. Oncol. 2013, 3, 216. [Google Scholar] [CrossRef] [PubMed]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Alberici, L.; Roth, L.; Sugahara, K.N.; Agemy, L.; Kotamraju, V.R.; Teesalu, T.; Bordignon, C.; Traversari, C.; Rizzardi, G.P.; Ruoslahti, E. De novo design of a tumor-penetrating peptide. Cancer Res. 2013, 73, 804–812. [Google Scholar] [CrossRef]
- Yang, Z.; Xiang, B.; Dong, D.; Wang, Z.; Li, J.; Qi, X. Dual receptor-specific peptides modified liposomes as VEGF siRNA vector for tumor-targeting therapy. Curr. Gene. Ther. 2014, 14, 289–299. [Google Scholar] [CrossRef]
- Chen, X.; Tohme, M.; Park, R.; Hou, Y.; Bading, J.R.; Conti, P.S. Micro-PET imaging of alphavbeta3-integrin expression with 18F-labeled dimeric RGD peptide. Mol. Imaging 2004, 3, 96–104. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Kim, Y.S.; Zhai, S.; Liu, Z.; Chen, X.; Liu, S. Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic arginine-glycine-aspartic (RGD) dimers with triglycine linkers. J. Med. Chem. 2008, 51, 7980–7990. [Google Scholar] [CrossRef]
- Wu, H.; Chen, H.; Pan, D.; Ma, Y.; Liang, S.; Wan, Y.; Fang, Y. Imaging integrin αvβ 3 and NRP-1 positive gliomas with a novel fluorine-18 labeled RGD-ATWLPPR heterodimeric peptide probe. Mol. Imaging Biol. 2014, 16, 781–792. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Yang, Y.; Zhang, H.; Mei, X. Photo-responsive and NGR-mediated multifunctional nanostructured lipid carrier for tumor-specific therapy. J. Pharm. Sci. 2015, 104, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Meinwald, Y.C.; Stimson, E.R.; Scheraga, H.A. Deamidation of the asparaginyl-glycyl sequence. Int. J. Pept. Protein Res. 1986, 28, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Han, Q.; Wang, Z.; Qian, Y.; Jia, Y.; Wang, W.; Hu, Z. Targeting peptide functionalized liposomes towards aminopeptidase N for precise tumor diagnosis and therapy. Biomater. Sci. 2017, 5, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Ruffini, F.; Tentori, L.; Scimeca, M.; Dorio, A.S.; Atzori, M.G.; Failla, C.M.; Morea, V.; Bonanno, E.; D’Atri, S.; et al. Antitumor activity of a novel anti-vascular endothelial growth factor receptor-1 monoclonal antibody that does not interfere with ligand binding. Oncotarget 2016, 7, 72868–72885. [Google Scholar] [CrossRef]
- Guzman-Rojas, L.; Rangel, R.; Salameh, A.; Edwards, J.K.; Dondossola, E.; Kim, Y.G.; Saghatelian, A.; Giordano, R.J.; Kolonin, M.G.; Staquicini, F.I.; et al. Cooperative effects of aminopeptidase N (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2012, 109, 1637–1642. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Z.; Liu, K.; Ye, L.; Liang, Y.; Gu, W. Aminopeptidase N (CD13) targeted MR and NIRF dual-modal imaging of ovarian tumor xenograft. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 968–974. [Google Scholar] [CrossRef]
- Fu, H.; Du, B.; Chen, Z.; Li, Y. Radiolabeled Peptides for SPECT and PET Imaging in the Detection of Breast Cancer: Preclinical and Clinical Perspectives. Curr. Med. Chem. 2020, 27, 6987–7002. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Z.; Cai, J.; Chen, X.; Zhou, Y.; Ma, X.; Dong, Q.; Li, F.; Xi, L. Primary Preclinical and Clinical Evaluation of 68Ga-DOTA-TMVP1 as a Novel VEGFR-3 PET Imaging Radiotracer in Gynecological Cancer. Clin. Cancer Res. 2020, 26, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.D.; Smith, S.V.; DiBartolo, N.; McIntosh, L.J.; Cyr, E.M.; Bonab, A.A.; Dearling, J.L.; Carter, E.A.; Fischman, A.J.; Treves, S.T.; et al. Positron emission tomography (PET) imaging of neuroblastoma and melanoma with 64Cu-SarAr immunoconjugates. Proc. Natl. Acad. Sci. USA 2007, 104, 17489–17493. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Xiong, Z.; Cheng, Z.; Fisher, D.R.; Liu, S.; Gambhir, S.S.; Chen, X. microPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J. Nucl. Med. 2005, 46, 1707–1718. [Google Scholar]
- Cai, H.; Li, Z.; Huang, C.W.; Shahinian, A.H.; Wang, H.; Park, R.; Conti, P.S. Evaluation of copper-64 labeled AmBaSar conjugated cyclic RGD peptide for improved microPET imaging of integrin alphavbeta3 expression. Bioconjug. Chem. 2010, 21, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, Z.; Huang, C.W.; Park, R.; Shahinian, A.H.; Conti, P.S. An improved synthesis and biological evaluation of a new cage-like bifunctional chelator, 4-((8-amino-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane-1-ylamino)methyl)benzoic acid, for 64Cu radiopharmaceuticals. Nucl. Med. Biol. 2010, 37, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Multimodality imaging of tumor integrin alphavbeta3 expression. Mini. Rev. Med. Chem. 2006, 6, 227–234. [Google Scholar] [CrossRef]
- Haubner, R.; Wester, H.J.; Weber, W.A.; Mang, C.; Ziegler, S.I.; Goodman, S.L.; Senekowitsch-Schmidtke, R.; Kessler, H.; Schwaiger, M. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001, 61, 1781–1785. [Google Scholar]
- Shi, J.; Kim, Y.S.; Zhai, S.; Liu, Z.; Chen, X.; Liu, S. Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly3 and PEG4 linkers. Bioconjug. Chem. 2009, 20, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wuest, M.; Weisman, G.R.; Wong, E.H.; Reed, D.P.; Boswell, C.A.; Motekaitis, R.; Martell, A.E.; Welch, M.J.; Anderson, C.J. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J. Med. Chem. 2002, 45, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jin, G.; Ma, D.; Wang, F.; Fu, T.; Chen, X.; Chen, X.; Jia, K.; Marikar, F.M.; Hua, Z. Modification of cyclic NGR tumor neovasculature-homing motif sequence to human plasminogen kringle 5 improves inhibition of tumor growth. PLoS ONE 2012, 7, e37132. [Google Scholar] [CrossRef]
- Pathuri, G.; Hedrick, A.F.; Disch, B.C.; Doan, J.T.; Ihnat, M.A.; Awasthi, V.; Gali, H. Synthesis and evaluation of novel Tc-99m labeled probestin conjugates for imaging APN/CD13 expression in vivo. Bioconjug. Chem. 2012, 23, 115–124. [Google Scholar] [CrossRef]
- Adar, L.; Shamay, Y.; Journo, G.; David, A. Pro-apoptotic peptide-polymer conjugates to induce mitochondrial-dependent cell death. Polym. Adv. Technol. 2010, 22, 199–208. [Google Scholar] [CrossRef]
- Dijkgraaf, I.; Yim, C.B.; Franssen, G.M.; Schuit, R.C.; Luurtsema, G.; Liu, S.; Oyen, W.J.; Boerman, O.C. PET imaging of αvβ3 integrin expression in tumours with 68Ga-labelled mono-, di- and tetrameric RGD peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 128–137. [Google Scholar] [CrossRef]
- Däpp, S.; García Garayoa, E.; Maes, V.; Brans, L.; Tourwé, D.A.; Müller, C.; Schibli, R. PEGylation of (99m)Tc-labeled bombesin analogues improves their pharmacokinetic properties. Nucl. Med. Biol. 2011, 38, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Samli, K.N.; McGuire, M.J.; Newgard, C.B.; Johnston, S.A.; Brown, K.C. Peptide-mediated targeting of the islets of Langerhans. Diabetes 2005, 54, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cao, R.; Veitonmäki, N. Kringle structures and antiangiogenesis. Curr. Med. Chem. Anticancer Agents 2002, 2, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Antiangiogenic cancer therapy. Semin. Cancer Biol. 2004, 14, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Yoshida, S.; Nakamura, Y.; Shigihara, Y.; Hamada, M.; Takeuchi, T. Probestin, a new inhibitor of aminopeptidase M, produced by Streptomyces azureus MH663-2F6. I. Taxonomy, production, isolation, physico-chemical properties and biological activities. J. Antibiot. 1990, 43, 143–148. [Google Scholar] [CrossRef]
- Smith, C.J.; Gali, H.; Sieckman, G.L.; Higginbotham, C.; Volkert, W.A.; Hoffman, T.J. Radiochemical investigations of (99m)Tc-N(3)S-X-BBN[7-14]NH(2): An in vitro/in vivo structure-activity relationship study where X = 0-, 3-, 5-, 8-, and 11-carbon tethering moieties. Bioconjug. Chem. 2003, 14, 93–102. [Google Scholar] [CrossRef]
- Das, T.; Pillai, M.R. Options to meet the future global demand of radionuclides for radionuclide therapy. Nucl. Med. Biol. 2013, 40, 23–32. [Google Scholar] [CrossRef]
- Silindir-Gunay, M.; Karpuz, M.; Ozer, A.Y. Targeted Alpha Therapy and Nanocarrier Approach. Cancer Biother. Radiopharm. 2020, 35, 446–458. [Google Scholar] [CrossRef]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef]
- Liu, F.; Li, M.; Liu, C.; Liu, Y.; Liang, Y.; Wang, F.; Zhang, N. Tumor-specific delivery and therapy by double-targeted DTX-CMCS-PEG-NGR conjugates. Pharm. Res. 2014, 31, 475–488. [Google Scholar] [CrossRef]
- Persigehl, T.; Ring, J.; Bremer, C.; Heindel, W.; Holtmeier, R.; Stypmann, J.; Claesener, M.; Hermann, S.; Schäfers, M.; Zerbst, C.; et al. Non-invasive monitoring of tumor-vessel infarction by retargeted truncated tissue factor tTF-NGR using multi-modal imaging. Angiogenesis 2014, 17, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Lin, A.H.; Fang, Y. Advance in studies on NGR peptide modified liposome and its anti-tumor performance. Zhongguo Zhong Yao Za Zhi 2013, 38, 2041–2045. [Google Scholar]
- Argyrou, M.; Valassi, A.; Andreou, M.; Lyra, M. Rhenium-188 production in hospitals, by W-188/Re-188 generator, for easy use in radionuclide therapy. Int. J. Mol. Imaging 2013, 2013, 290750. [Google Scholar] [CrossRef] [PubMed]
- Jansen, D.R.; Krijger, G.C.; Kolar, Z.I.; Zonnenberg, B.A.; Zeevaart, J.R. Targeted radiotherapy of bone malignancies. Curr. Drug. Discov. Technol. 2010, 7, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Pillai, M.R.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, G.; De Saint-Hubert, M.; Dijkgraaf, I.; Bauwens, M.; Douma, K.; Wierts, R.; Pooters, I.; Van den Akker, N.M.; Hackeng, T.M.; Post, M.J.; et al. Molecular imaging of angiogenesis after myocardial infarction by (111)In-DTPA-cNGR and (99m)Tc-sestamibi dual-isotope myocardial SPECT. EJNMMI. Res. 2015, 5, 2. [Google Scholar] [CrossRef]
- Sander, H.M.; Strijkers, G.J.; Mulder, W.J.; Huinink, H.P.; Erich, S.J.; Adan, O.C.; Sommerdijk, N.A.; Merkx, M.; Nicolay, K. Morphology, binding behavior and MR-properties of paramagnetic collagen-binding liposomes. Contrast. Media. Mol. Imaging 2009, 4, 81–88. [Google Scholar] [CrossRef]
- Oostendorp, M.; Douma, K.; Wagenaar, A.; Slenter, J.M.; Hackeng, T.M.; van Zandvoort, M.A.; Post, M.J.; Backes, W.H. Molecular magnetic resonance imaging of myocardial angiogenesis after acute myocardial infarction. Circulation 2010, 121, 775–783. [Google Scholar] [CrossRef][Green Version]
- Farkasinszky, G.; Dénes, N.; Rácz, S.; Kis, A.; Péliné, J.S.; Opposits, G.; Veres, G.; Balkay, L.; Kertész, I.; Mező, G.; et al. In Vivo Imaging of Ischemia/Reperfusion-mediated Aminopeptidase N Expression in Surgical Rat Model Using 68Ga-NOTA-c(NGR). In Vivo 2022, 36, 657–666. [Google Scholar] [CrossRef]
- Fujii, H.; Nakajima, M.; Saiki, I.; Yoneda, J.; Azuma, I.; Tsuruo, T. Human melanoma invasion and metastasis enhancement by high expression of amino-peptidase N/CD13. Clin. Exp. Metastasis 1995, 13, 337–344. [Google Scholar] [CrossRef]
- Hossain, A.; Heron, D.; Davenport, I.; Huckaba, T.; Graves, R.; Mandal, T.; Muniruzzaman, S.; Wang, S.; Bhattacharjee, P.S. Protective effects of bestatin in the retina of streptozotocin-induced diabetic mice. Exp. Eye. Res. 2016, 149, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, H. Electroretinogram in unilateral vascular stress in nondiabetic and diabetic subjects. Optom. Vis. Sci. 1993, 70, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye. Res. 2012, 31, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, F.; Brignole, C.; Marimpietri, D.; Cilli, M.; Gambini, C.; Ribatti, D.; Longhi, R.; Allen, T.M.; Corti, A.; Ponzoni, M. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003, 63, 7400–7409. [Google Scholar] [PubMed]
- Kunjachan, S.; Jayapaul, J.; Mertens, M.E.; Storm, G.; Kiessling, F.; Lammers, T. Theranostic systems and strategies for monitoring nanomedicine-mediated drug targeting. Curr. Pharm. Biotechnol. 2012, 13, 609–622. [Google Scholar] [CrossRef]
- Palmowski, M.; Huppert, J.; Ladewig, G.; Hauff, P.; Reinhardt, M.; Mueller, M.M.; Woenne, E.C.; Jenne, J.W.; Maurer, M.; Kauffmann, G.W.; et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: Early assessment of antiangiogenic therapy effects. Mol. Cancer Ther. 2008, 7, 101–109. [Google Scholar] [CrossRef]
- Zhang, C.; Jugold, M.; Woenne, E.C.; Lammers, T.; Morgenstern, B.; Mueller, M.M.; Zentgraf, H.; Bock, M.; Eisenhut, M.; Semmler, W.; et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007, 67, 1555–1562. [Google Scholar] [CrossRef][Green Version]
- Gai, Y.; Jiang, Y.; Long, Y.; Sun, L.; Liu, Q.; Qin, C.; Zhang, Y.; Zeng, D.; Lan, X. Evaluation of an Integrin αvβ3 and Aminopeptidase N Dual-Receptor Targeting Tracer for Breast Cancer Imaging. Mol. Pharm. 2020, 17, 349–358. [Google Scholar] [CrossRef]

| Investigated Object | Investigated Phenomenon/Initiatives | Radiopharmaceutical | In Vitro and In Vivo Methods | Highlights | Reference |
|---|---|---|---|---|---|
| in vitro: HT1080 and HT29 cells in vivo: female nude BALB/c mice bearing HT1080 or HT29 tumours | in vitro properties, in vivo diagnostic potential, and APN/CD13 positive tumour-homing capability | [68Ga]Ga-NOTA-G3-NGR | in vitro: stability, lipophilicity, cell-based competitive assay (binding affinity and specificity), tumour cell uptake and efflux in vivo: microPET/CT acquisition, blocking studies, and ex vivo gamma counter-based radioactivity determination | [68Ga]Ga-NOTA-G3-NGR is applicable in the diagnostics of CD13-targeted tumour angiogenesis, [68Ga]Ga-NOTA-G3-NGR is a valuable CD13-affine PET probe | [36] |
| in vitro: HT1080 and HT29 cells in vivo: female nude BALB/c mice xenografted with HT1080 and HT29 | in vitro and in vivo comparative characterisation and comparison of angiogenesis imaging ability | [68Ga]Ga-NOTA-G3-NGR2 | in vitro: stability, competitive cell binding assay (binding affinity and specificity), in vivo: microPET studies, ex vivo biodistribution | [68Ga]Ga-NOTA-G3-NGR2 is useful for angiogenesis imaging in preclinical HT1080 tumour models [68Ga]Ga-NOTA-G3-NGR2 seems to be valuable for the PET diagnostics of patients with fibrosarcoma [68Ga]Ga-NOTA-G3-NGR2 could be used to track CD13-targeted treatment response in a non-invasive manner | [37] |
| adult male Fischer-344 rats bearing orthotopic and heterotopic transplanted Ne/De tumours | radiochemical synthesis, APN/CD13 positive primer tumour targeting competence, metastasis identification, and comparative characterisation of the imaging properties of NGR and RGD peptides | [68Ga]Ga-NOTA-c(NGR) | in vitro: stability in vivo: whole-body miniPET examinations, ex vivo gamma counting, in vivo and ex vivo blocking studies | [68Ga]Ga-NOTA-c(NGR) selectively binds to APN/CD13 positive ortho- and heterotopic transplanted NeDe tumours [68Ga]Ga-NOTA-c(NGR) is a promising radionuclide for the in vivo imaging of APN/CD13 overexpressing primary tumours and related metastatic lesions | [33] |
| chemically induced He/De and Ne/De tumour-bearing Fischer-344 rats | evaluation and comparison of APN/CD13 selectivity | [68Ga]Ga-NOTA-c(NGR) [68Ga]Ga -NODAGA-c(NGR) [68Ga]Ga-NODAGA-c(NGR) (MG1) [68Ga]Ga -NODAGA-c(NGR) (MG2) | in vitro: stability in vivo: PET/MRI imaging, ex vivo uptake pattern, in vivo and ex vivo blocking studies | APN/CD13 is a valuable biomarker for PET diagnostic purposes, the careful choice of the 68Ga-NGR radiotracer is pivotal to correctly representing tumour-related angiogenesis and to following treatment response | [38] |
| chemically induced He/De tumour carrying male Fischer-344 rats | detection of the temporal changes of APN/CD13 expression, hypoxia imaging | [68Ga]Ga-NOTA-c(NGR) | in vivo: whole-body PET/MRI acquisition, blocking experiments | [68Ga]Ga-NOTA-c(NGR) has the potential to identify the temporal changes of hypoxic regions and the presence of APN/CD13 in hepatocellular carcinoma xenografts | [39] |
| in vitro: B16F10 cells in vivo: C57BL/6 mice bearing B16F10 tumours | imaging of neo-vascularisation and performance evaluation of angiogenesis-selective radiolabelled peptide porbes APN/CD13 selective LN peptide (YEVGHRC) VEGFR-1 selective (APRPG) | [68Ga]Ga-NODAGA-YEVGHRC [68Ga]Ga-NODAGA-APRPG-NH2 [68Ga]Ga-NODAGA-APRPG-COOH [68Ga]Ga-NOTA-cNGR (reference compound) | in vitro: stability determination in vivo: static microPET scans, ex vivo radioactivity determination | [68Ga]Ga-NODAGA-YEVGHRC, [68Ga]Ga-NODAGA-APRPG-NH2, and [68Ga]Ga-NODAGA-APRPG-COOH exhibited specific uptake in B16F10 tumours, [68Ga]Ga-NODAGA-APRPG-COOH displayed similar characteristics to those of [68Ga]Ga-NOTA-cNGR | [40] |
| in vitro: A549 and MDA-MB231 cells in vivo: A549 and MDA-MB231 carcinoma bearing female Balb/c nude mice | synthesis and evaluation of the efficacy of the detection of APN/CD13 and APN/CD13 expressing tumours | [68Ga]Ga-DOTA-NGR | in vitro: stability, cell uptake and binding studies in vivo: in vivo static and dynamic microPET acquisition, ex vivo organ distribution, in vivo blocking examinations | [68Ga]Ga-DOTA-NGR shows high affinity for APN/CD13 receptors both in vitro and in vivo, [68Ga]Ga-DOTA-NGR could be a promising diagnostic probe for the imaging of APN/CD13 expressing tumours and vasculature | [41] |
| in vivo: female Fischer-344 rats bearing primary and metastatic mesoblastic nephroma (Ne/De) tumours | detection of serially transplanted metastases and assessment of the change in APN/CD13 and αvβ3 integrin receptor expression | [68Ga]Ga-NOTA-c(NGR) | in vivo: static microPET imaging | [68Ga]Ga-NOTA-c(NGR) is a useful tracer to detect primary Ne/De tumours and their thoracic parathymic lymph node metastases, the upregulation of the presence of APN/CD13 during serial metastasis transplantation projects increased malignancy | [42] |
| in vitro: HT1080 and HT29 cell lines in vivo: Female Balb/c nude mice with HT1080 and HT29 xenografts | imaging of APN/CD13 overexpressing tumours, comparative performance evaluation, and role of NRP-1 | [68Ga]Ga-DOTA-iNGR with CendR (R/KXXR/K) penetrating motif, [68Ga]Ga-DOTA-NGR | in vitro: stability, cell binding affinity studies, cell uptake analysis, cell blocking studies with neutralising NRP-1 antibody in vivo: microPET imaging, ex vivo organ uptake studies, in vivo blocking studies with unlabelled cyclic NGR peptide or neutralising NRP-1 antibody | the use of the CendR motif led to higher tumour accumulation and increased tumour retention, indicating its capability to enhance the in vivo behaviour of NGR peptides | [43] |
| in vitro ES2 and SKOV3 cell lines in vivo: ES2 and SKOV3 tumourous female Balb/c nude mice | investigation of imaging efficacy | [68Ga]Ga-DOTA-c(NGR)2 | in vitro: cell binding (affinity and specificity) assay; cell-based competitive cell binding assay; in vivo: static microPET examinations; ex vivo gamma counter-based measurements; in vivo receptor blocking studies | [68Ga]Ga-DOTA-c(NGR)2 demonstrated specific binding to APN/CD13 both in vitro and in vivo in ovarian tumour models, and [68Ga]Ga-DOTA-c(NGR)2 could be effectively applied in the PET diagnostics of APN/CD13 overexpressing tumours | [44] |
| in vitro: B16F10 and HT1080 cell lines in vivo: B16F10 tumour-bearing C57BL6 mice, athymic nude mice bearing HT-1080 tumours | in vivo imaging behaviour, tumour targeting ability | [68Ga]Ga-HBED-CC-c(NGR) | in vitro: cell uptake studies in vivo: biodistribution assessment, blocking experiments | [68Ga]Ga-HBED-CC-c(NGR) shows APN/CD13 target specificity and could be a promising diagnostic radiopharmaceutical for the imaging of receptor positive malignancies, chelator HBED-CC seems feasible for the complexation with imaging vectors other than NGR, and for the design of peptide-based radiotraces | [45] |
| in vitro: HT1080 tumour cells in vivo: nude mice xenografted with HT1080 tumours | exploring the influence of chelators on chemical properties, target selectivity, and distribution pattern | [68Ga]Ga-DOTAGA-c(NGR) [68Ga]Ga-NODAGA-c(NGR) [68Ga]Ga-HBED-CC-c(NGR) | in vitro: cellular uptake studies, uptake inhibition studies, efflux studies in vivo: ex vivo biodistribution studies, blocking studies | [68Ga]Ga-DOTAGA-c(NGR), [68Ga]Ga-NODAGA-c(NGR), and [68Ga]Ga-HBED-CC-c(NGR) are applicable molecular agents for the in vivo identification of tumours with APN/CD13 expression, [68Ga]Ga-NODAGA-c(NGR) exhibited more improved imaging performance compared to the other two probes | [46] |
| in vitro A549, SKHep-1, MDA-MB-231 cell lines in vivo: CD1-Foxn1nu-mice bearing A549 or SKHep-1 tumours | evaluation of the effect of NGR dimerisation and comparison of the target specificity of NGR monomer and dimer | [68Ga]Ga-NODAGA-NGR (NGR monomer) [68Ga]Ga-NOTA-(NGR)2 (NGR dimer) | in vitro: cellular uptake studies; in vivo: microPET imaging; in vivo uptake inhibition with APN/CD13 inhibitor bestatin; ex vivo accumulation profile | the monomer and the dimer tracers showed similar imaging behviour; both [68Ga]Ga-NODAGA-NGR and [68Ga]Ga-NOTA-(NGR)2 are favourable to detect APN/CD13 positive tumours without the superiority of the dimer to the monomer | [47] |
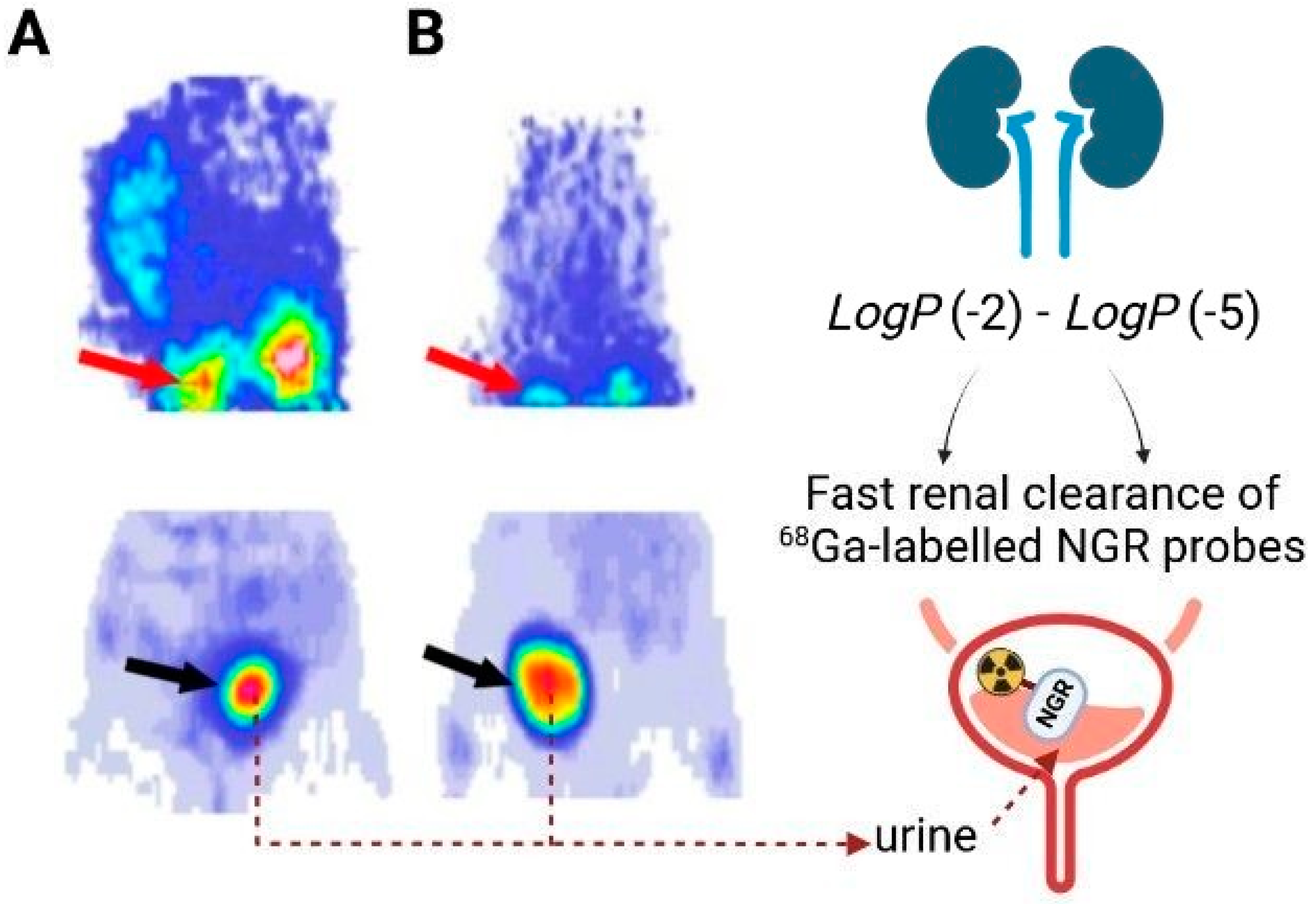
| Radiopharmaceutical | LogP |
|---|---|
| [68Ga]Ga-NOTA-c(NGR) | −2.77 ± 0.12 |
| [68Ga]Ga-NODAGA-c(NGR) | −4.07 ± 0.13 |
| [68Ga]Ga-NODAGA-c(NGR) (MG1) | −2.33 ± 0.14 |
| [68Ga]Ga-NODAGA-c(NGR) (MG2) | −2.29 ± 0.13 |
| [68Ga]Ga-NODAGA-YEVGHRC | −4.421 |
| [68Ga]Ga-NOTA-G3-NGR | −2.25 ± 0.17 |
| [68Ga]Ga-NOTA-G3-NGR2 | −2.76 ± 0.20 |
| Isotope | Half-Life (h) | Production | Decay Method (%) | Decay Product | β+ Endpoint Energy, keV (%) | Principal γ Energies, keV (Abs. %) | Positron Range in Water (mm) | Delayed Imaging (>3 h) | Application |
|---|---|---|---|---|---|---|---|---|---|
| 68Ga | 1.13 | 68Ge/68Ga generator and cyclotron | EC (11.1) ß+ (88.9) | 64Ni 64Zn | 1899 (87.7) 822 (1.2) | 511 (177.8) 1077 (3.2) 1261 (0.1) 1883 (0.1) | 1.2 | not possible | PET diagnostics For radiopharmaceuticals requiring short circulation times for targeting; e.g., peptides |
| 64Cu | 12.7 | cyclotron | EC (43.9) ß+ (17.6) ß− (38.5) | 68 Zn | 653 (17.6) | 511 (35.2) 1346 (0.5) | 0.2 | possible | PET imaging/therapy (theranostic) For radiopharmaceuticals requiring long circulation times, e.g., slowly localising antibodies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trencsényi, G.; Enyedi, K.N.; Mező, G.; Halmos, G.; Képes, Z. NGR-Based Radiopharmaceuticals for Angiogenesis Imaging: A Preclinical Review. Int. J. Mol. Sci. 2023, 24, 12675. https://doi.org/10.3390/ijms241612675
Trencsényi G, Enyedi KN, Mező G, Halmos G, Képes Z. NGR-Based Radiopharmaceuticals for Angiogenesis Imaging: A Preclinical Review. International Journal of Molecular Sciences. 2023; 24(16):12675. https://doi.org/10.3390/ijms241612675
Chicago/Turabian StyleTrencsényi, György, Kata Nóra Enyedi, Gábor Mező, Gábor Halmos, and Zita Képes. 2023. "NGR-Based Radiopharmaceuticals for Angiogenesis Imaging: A Preclinical Review" International Journal of Molecular Sciences 24, no. 16: 12675. https://doi.org/10.3390/ijms241612675
APA StyleTrencsényi, G., Enyedi, K. N., Mező, G., Halmos, G., & Képes, Z. (2023). NGR-Based Radiopharmaceuticals for Angiogenesis Imaging: A Preclinical Review. International Journal of Molecular Sciences, 24(16), 12675. https://doi.org/10.3390/ijms241612675