KLF10 Inhibits TGF-β-Mediated Activation of Hepatic Stellate Cells via Suppression of ATF3 Expression
Abstract
1. Introduction
2. Results
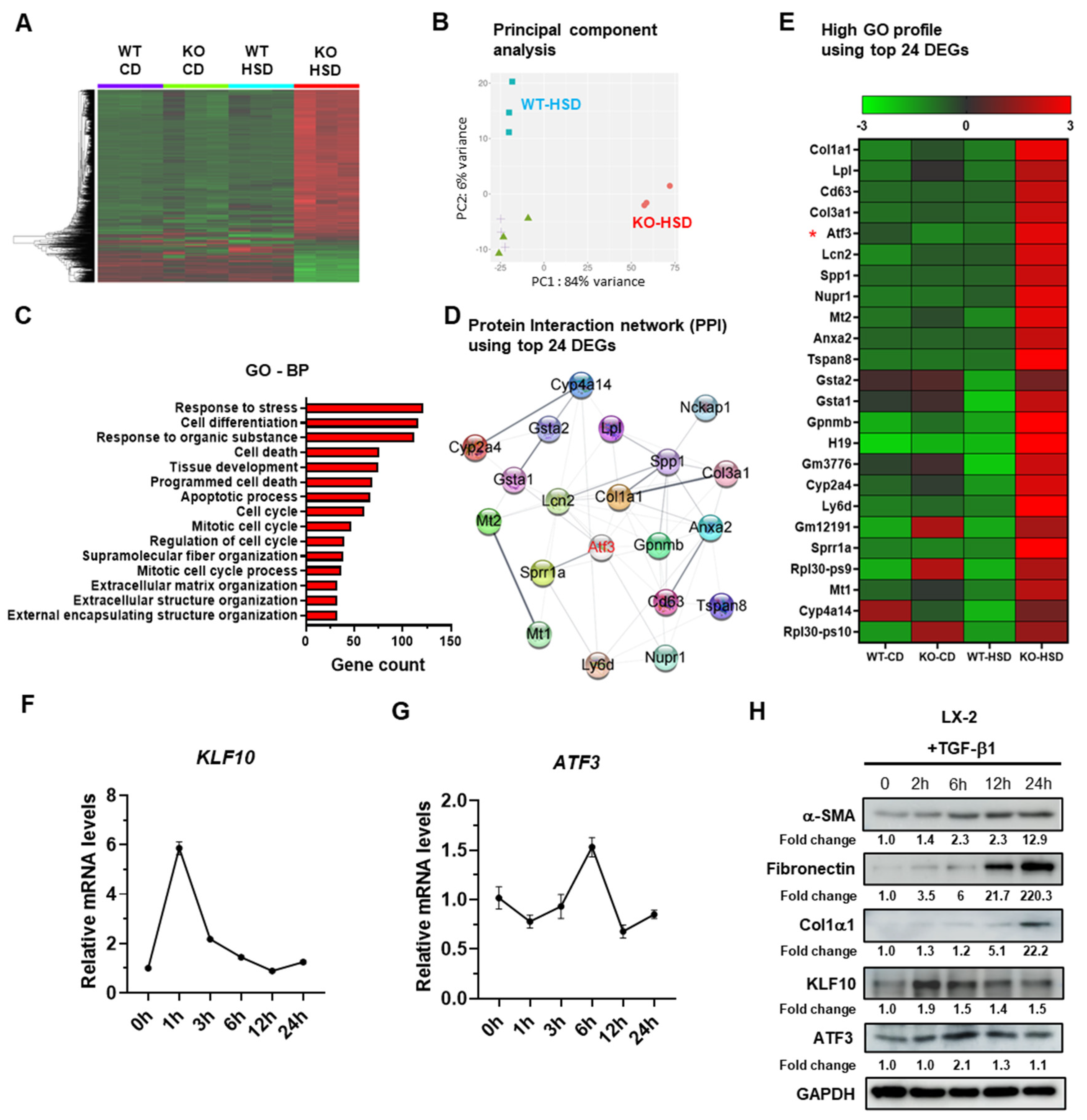
2.1. ATF3 Is Upregulated in Fibrotic Livers of HSD-Fed KLF10 KO Mice and in Activated HSCs
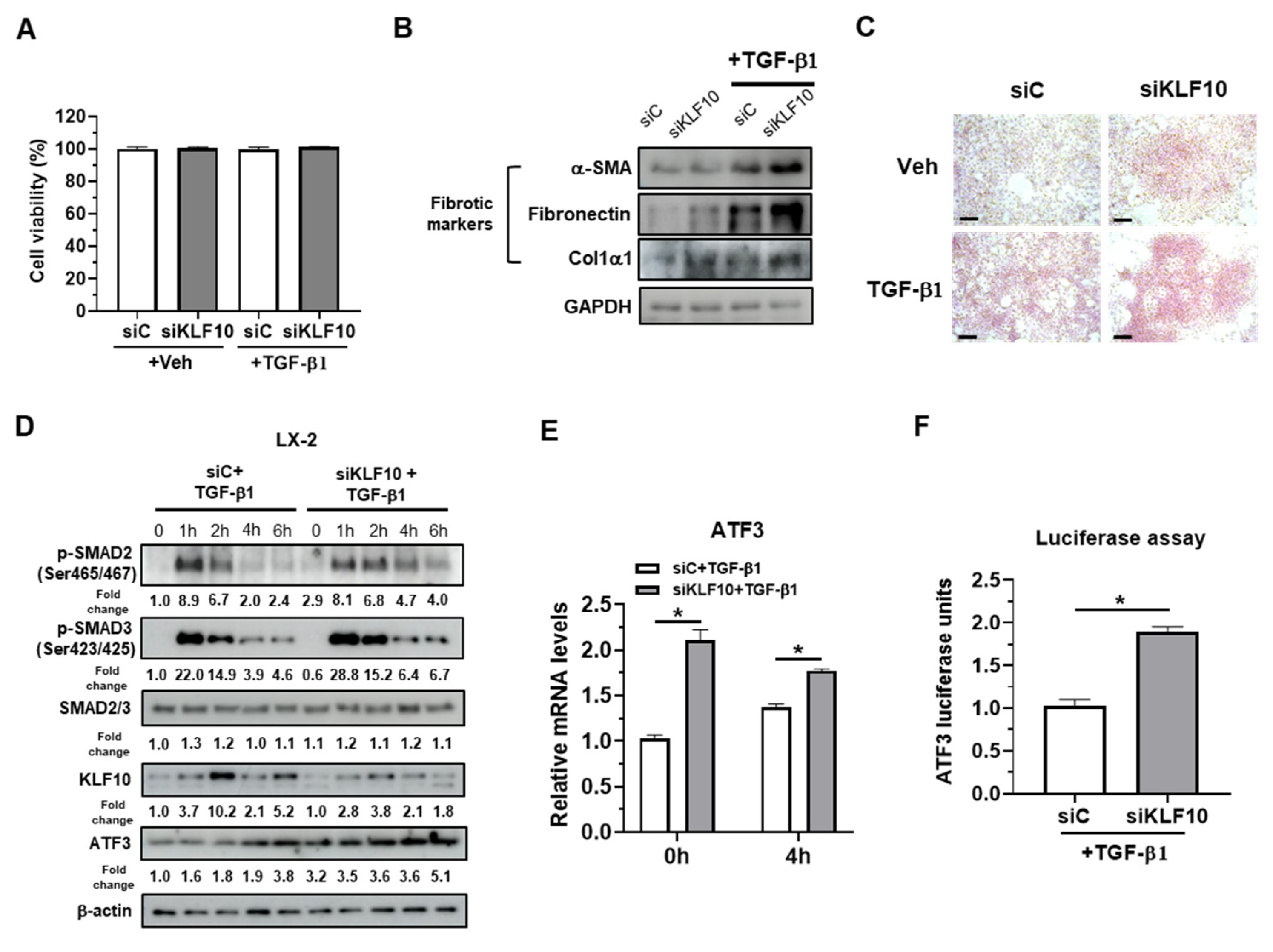
2.2. KLF10 Silencing Promotes TGF-β-Mediated Activation of HSCs via ATF3 Upregulation
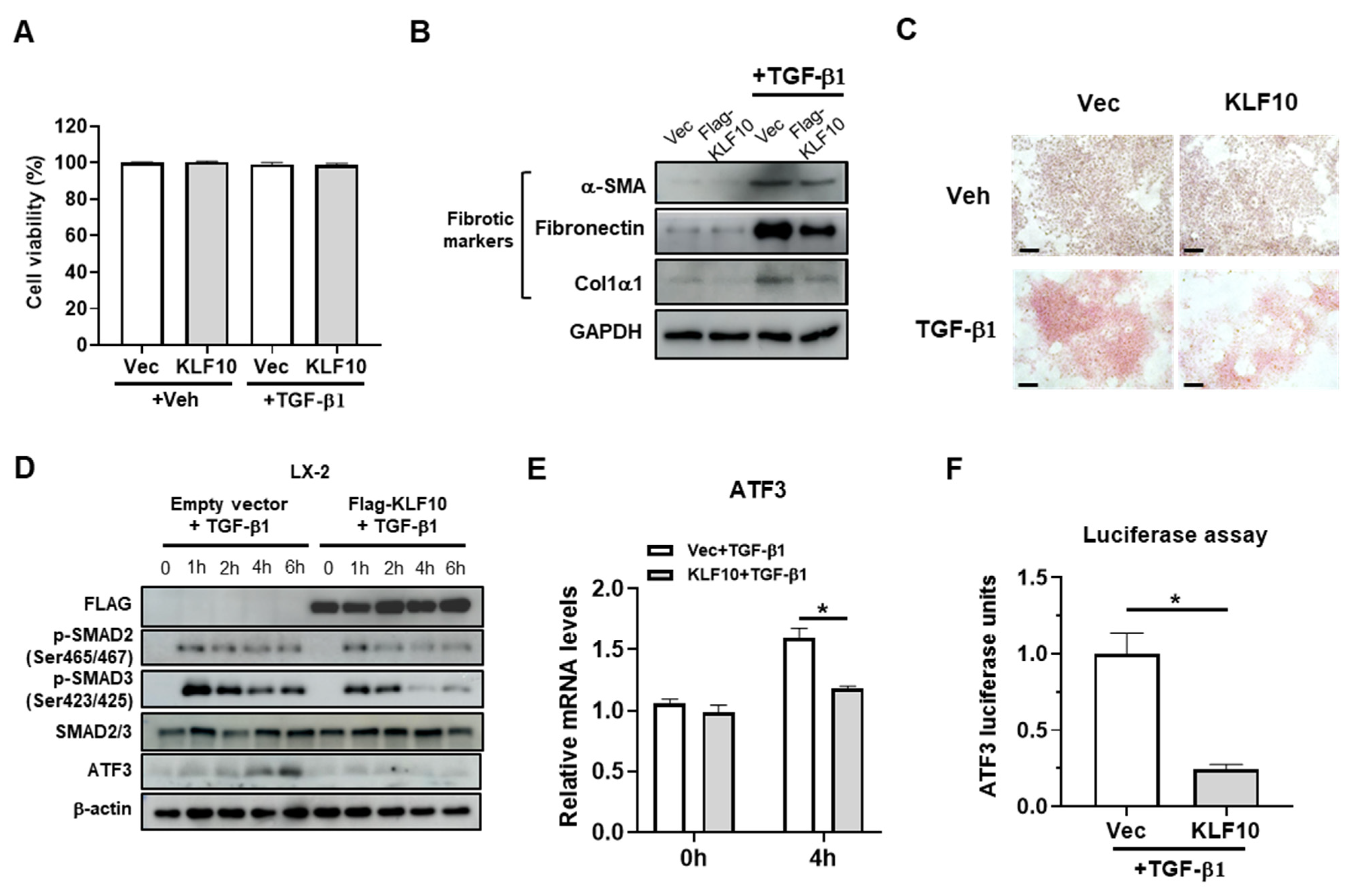
2.3. KLF10 Suppresses TGF-β-Mediated Activation in HSCs
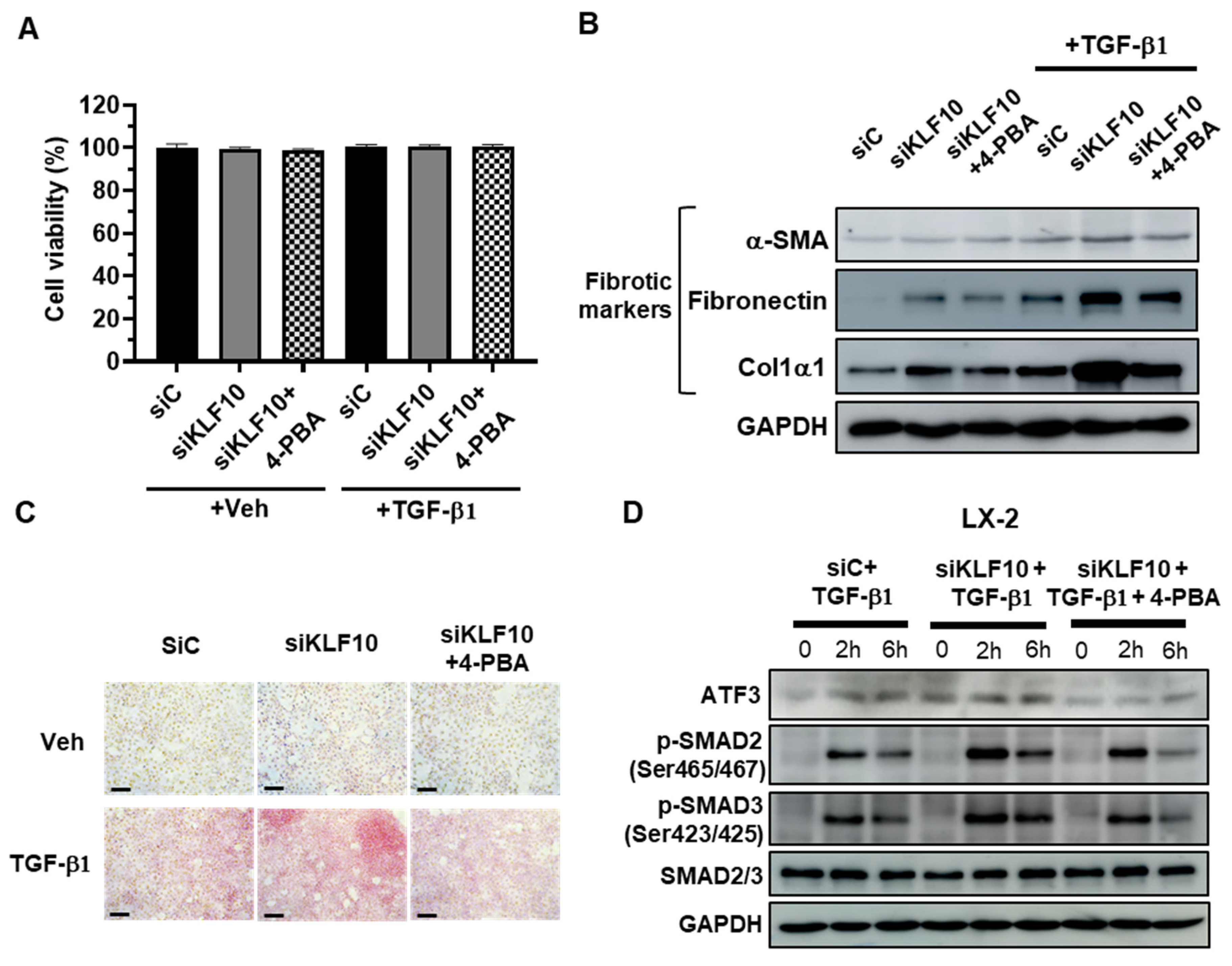
2.4. 4-PBA Treatment Suppresses siKLF10-Mediated Upregulation of ATF3 and Fibrogenic Responses in LX-2 Cells Treated with TGF-β
3. Discussion
4. Materials and Methods
4.1. RNA Sequencing and Gene Set Enrichment Analysis
4.2. Cell Culture
4.3. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR (qPCR)
4.4. Cell Transfection
4.5. Cell Viability Analysis
4.6. Western Blot Analysis
4.7. Antibodies
4.8. Sirius Red Staining
4.9. Luciferase Reporter Assays
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | Alpha smooth muscle actin |
| 4-PBA | 4-Phenylbutyric acid |
| ATF3 | Activating transcription factor 3 |
| Col1α1 | Collagen type I alpha 1 |
| DEG | Differentially expressed gene |
| GO | Gene ontology |
| HSC | Hepatic stellate cell |
| HSD | High-sucrose diet |
| KLF10 | Krüppel-like factor 10 |
| KO | Knockout |
| SMAD | Suppressor of mothers against decapentaplegic |
| TGF-β1 | Transforming growth factor-beta1 |
| WT | Wildtype |
References
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Castera, L.; Lammert, F.; Graupera, I.; Serra-Burriel, M.; Allen, A.M.; Wong, V.W.; Hartmann, P.; Thiele, M.; Caballeria, L.; et al. Population screening for liver fibrosis: Toward early diagnosis and intervention for chronic liver diseases. Hepatology 2021, 75, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Shi, Z.; Ren, M.; Rockey, D.C.; Jophlin, L.L.; Koutalos, Y.; Chen, C.; Shah, V.; Weaver, L.; Hamoud, A.-R.; et al. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Iwaisako, K.; Jiang, C.; Kisseleva, T. Cell Signals Influencing Hepatic Fibrosis. Int. J. Hepatol. 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Liu, C.; Gaca, M.D.; Swenson, E.S.; Vellucci, V.F.; Reiss, M.; Wells, R.G. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J. Biol. Chem. 2003, 278, 11721–11728. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-beta/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Subramaniam, M.; Harris, S.A.; Oursler, M.J.; Rasmussen, K.; Riggs, B.L.; Spelsberg, T.C. Identification of a novel TGF-beta-regulated gene encoding a putative zinc finger protein in human osteoblasts. Nucleic Acids Res. 1995, 23, 4907–4912. [Google Scholar] [CrossRef]
- Fautsch, M.P.; Vrabel, A.; Subramaniam, M.; Hefferen, T.E.; Spelsberg, T.C.; Wieben, E.D. TGFbeta-inducible early gene (TIEG) also codes for early growth response alpha (EGRalpha): Evidence of multiple transcripts from alternate promoters. Genomics 1998, 51, 408–416. [Google Scholar] [CrossRef]
- Ribeiro, A.; Bronk, S.F.; Roberts, P.J.; Urrutia, R.; Gores, G.J. The transforming growth factor beta(1)-inducible transcription factor TIEG1, mediates apoptosis through oxidative stress. Hepatology 1999, 30, 1490–1497. [Google Scholar] [CrossRef]
- Subramaniam, M.; Hawse, J.R.; Rajamannan, N.M.; Ingle, J.N.; Spelsberg, T.C. Functional role of KLF10 in multiple disease processes. Biofactors 2010, 36, 8–18. [Google Scholar] [CrossRef]
- Miyake, M.; Hayashi, S.; Iwasaki, S.; Chao, G.; Takahashi, H.; Watanabe, K.; Ohwada, S.; Aso, H.; Yamaguchi, T. Possible role of TIEG1 as a feedback regulator of myostatin and TGF-beta in myoblasts. Biochem. Biophys. Res. Commun. 2010, 393, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Hayashi, S.; Iwasaki, S.; Uchida, T.; Watanabe, K.; Ohwada, S.; Aso, H.; Yamaguchi, T. TIEG1 negatively controls the myoblast pool indispensable for fusion during myogenic differentiation of C2C12 cells. J. Cell. Physiol. 2010, 226, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, I.; Imoto, M.; Adjei, P.N.; Gores, G.J.; Subramaniam, M.; Spelsberg, T.C.; Urrutia, R. Overexpression of the TGFbeta-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J. Clin. Investig. 1997, 99, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, A.-R.; Lee, H.-Y.; Moon, Y.-A.; Lee, H.-J.; Cha, J.-Y. Deletion of KLF10 Leads to Stress-Induced Liver Fibrosis upon High Sucrose Feeding. Int. J. Mol. Sci. 2020, 22, 331. [Google Scholar] [CrossRef]
- Leclere, P.S.; Rousseau, D.; Patouraux, S.; Guerin, S.; Bonnafous, S.; Grechez-Cassiau, A.; Ruberto, A.A.; Luci, C.; Subrama-niam, M.; Tran, A.; et al. MCD diet-induced steatohepatitis generates a diurnal rhythm of associated biomarkers and worsens liver injury in Klf10 deficient mice. Sci. Rep. 2020, 10, 12139. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, K.; Chen, T.; Zhang, Y.; Du, X.; Zhao, Y.; Shao, S.; Zheng, L.; Han, T.; Hong, W. Transcriptional factor ATF3 promotes liver fibrosis via activating hepatic stellate cells. Cell Death Dis. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Xu, L.; Hui, A.Y.; Albanis, E.; Arthur, M.J.; O’byrne, S.M.; Blaner, W.S.; Mukherjee, P.; Friedman, S.L.; Eng, F.J. Human hepatic stellate cell lines, LX-1 and LX-2: New tools for analysis of hepatic fibrosis. Gut 2005, 54, 142–151. [Google Scholar] [CrossRef]
- Hsu, C.-F.; Sui, C.-L.; Wu, W.-C.; Wang, J.-J.; Yang, D.H.; Chen, Y.-C.; Yu, W.C.; Chang, H.-S. Klf10 induces cell apoptosis through modulation of BI-1 expression and Ca2+ homeostasis in estrogen-responding adenocarcinoma cells. Int. J. Biochem. Cell Biol. 2011, 43, 666–673. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Zhang, H.; Liu, H.; Yao, H.; Wang, X.; Zhang, W.; Zhao, Y.; Yang, N. KLF10 inhibits cell growth by regulating PTTG1 in multiple myeloma under the regulation of microRNA-106b-5p. Int. J. Biol. Sci. 2020, 16, 2063–2071. [Google Scholar] [CrossRef]
- Lu, S.; Wang, X.-Z.; He, C.; Wang, L.; Liang, S.-P.; Wang, C.-C.; Li, C.; Luo, T.-F.; Feng, C.-S.; Wang, Z.-C.; et al. ATF3 contributes to brucine-triggered glioma cell ferroptosis via promotion of hydrogen peroxide and iron. Acta Pharmacol. Sin. 2021, 42, 1690–1702. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 1–24. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Salwinski, L.; Eisenberg, D. The MiSink Plugin: Cytoscape as a graphical interface to the Database of Interacting Proteins. Bioinformatics 2007, 23, 2193–2195. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, R.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Iny Stein, T.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinformatics 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Park, S.; Yaseen, U.; Lee, H.-J.; Cha, J.-Y. KLF10 Inhibits TGF-β-Mediated Activation of Hepatic Stellate Cells via Suppression of ATF3 Expression. Int. J. Mol. Sci. 2023, 24, 12602. https://doi.org/10.3390/ijms241612602
Hwang S, Park S, Yaseen U, Lee H-J, Cha J-Y. KLF10 Inhibits TGF-β-Mediated Activation of Hepatic Stellate Cells via Suppression of ATF3 Expression. International Journal of Molecular Sciences. 2023; 24(16):12602. https://doi.org/10.3390/ijms241612602
Chicago/Turabian StyleHwang, Soonjae, Sangbin Park, Uzma Yaseen, Ho-Jae Lee, and Ji-Young Cha. 2023. "KLF10 Inhibits TGF-β-Mediated Activation of Hepatic Stellate Cells via Suppression of ATF3 Expression" International Journal of Molecular Sciences 24, no. 16: 12602. https://doi.org/10.3390/ijms241612602
APA StyleHwang, S., Park, S., Yaseen, U., Lee, H.-J., & Cha, J.-Y. (2023). KLF10 Inhibits TGF-β-Mediated Activation of Hepatic Stellate Cells via Suppression of ATF3 Expression. International Journal of Molecular Sciences, 24(16), 12602. https://doi.org/10.3390/ijms241612602






