Ceramide Risk Score in the Evaluation of Metabolic Syndrome: An Additional or Substitutive Biochemical Marker in the Clinical Practice?
Abstract
1. Introduction
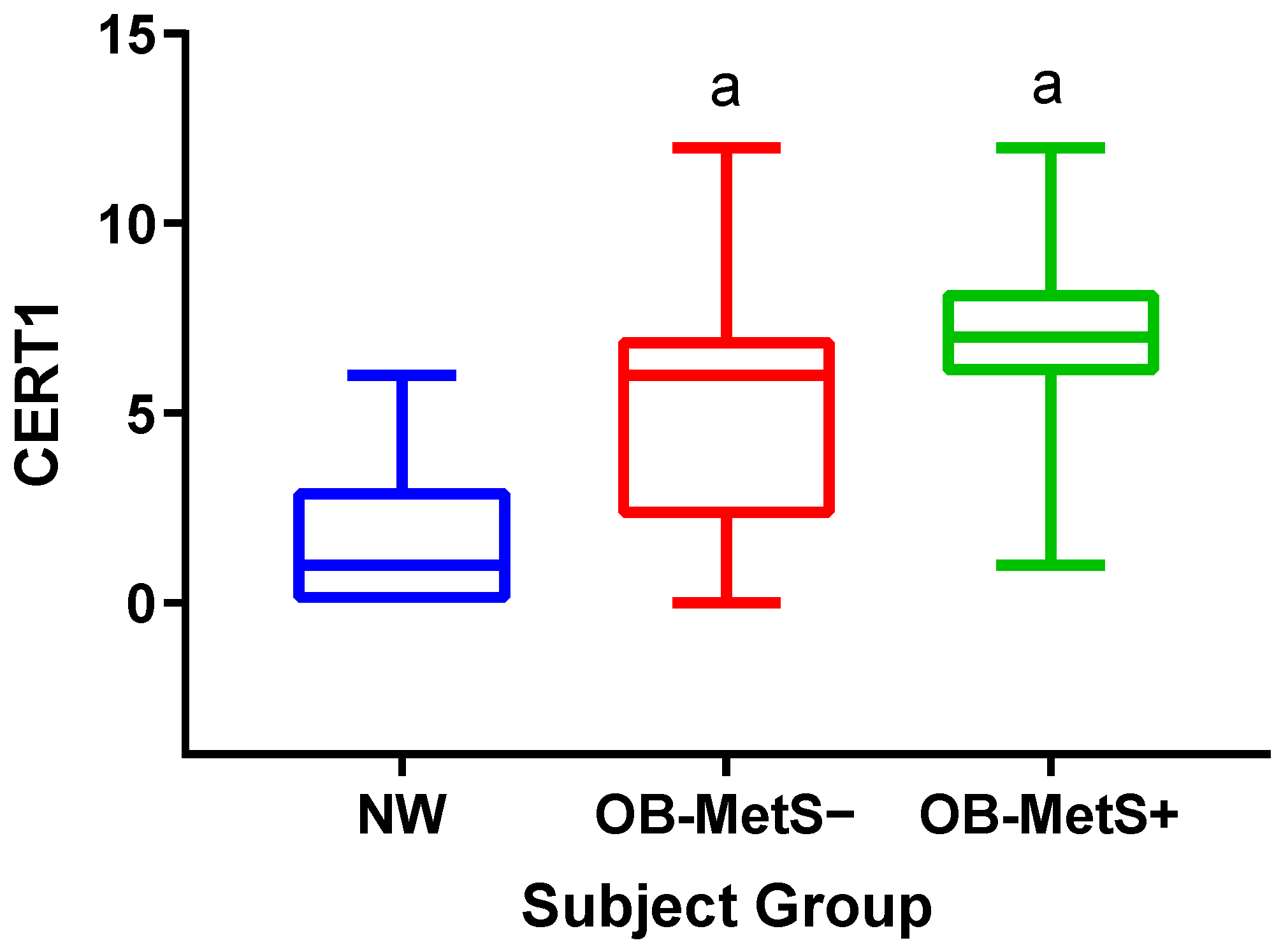
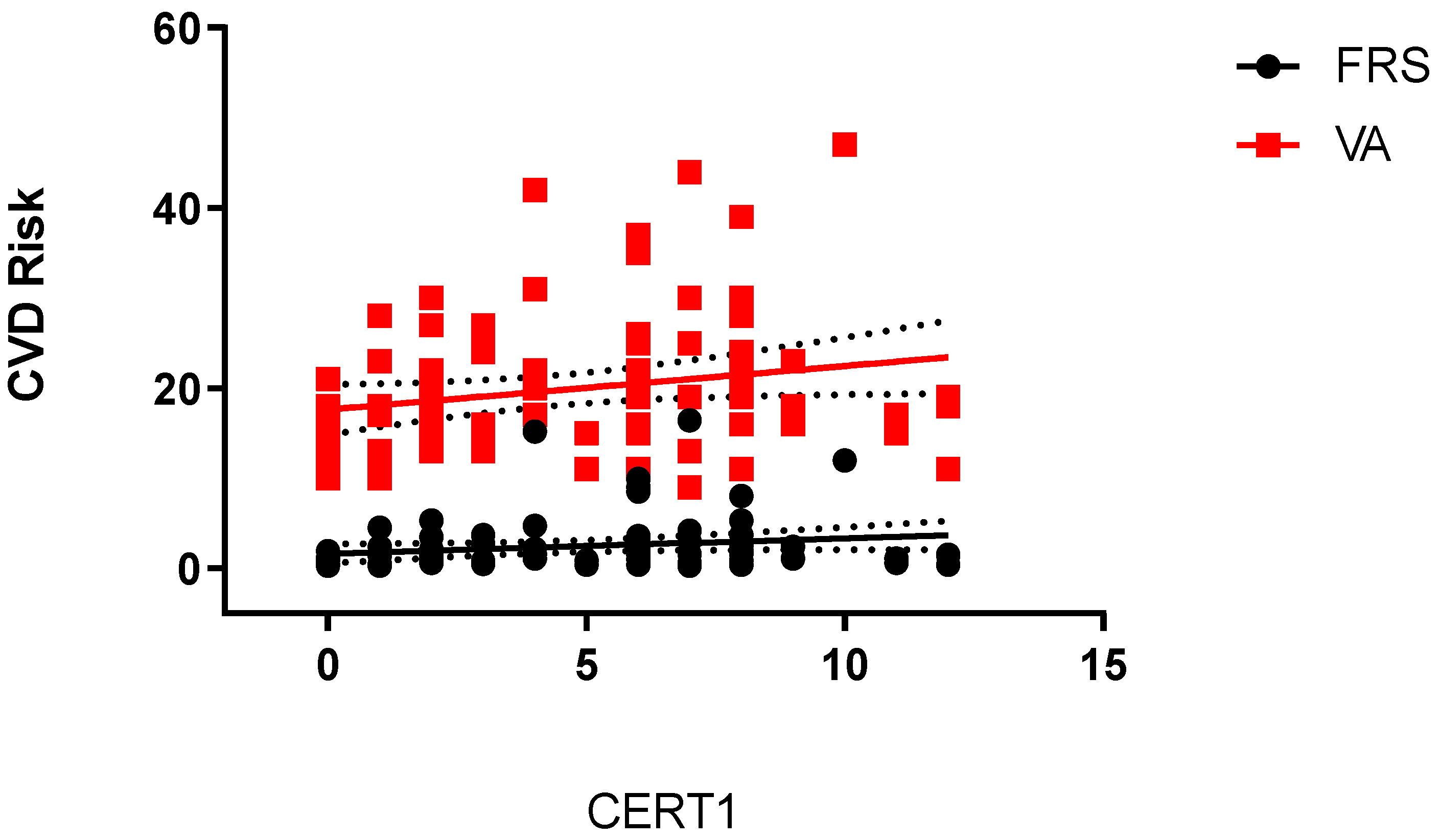
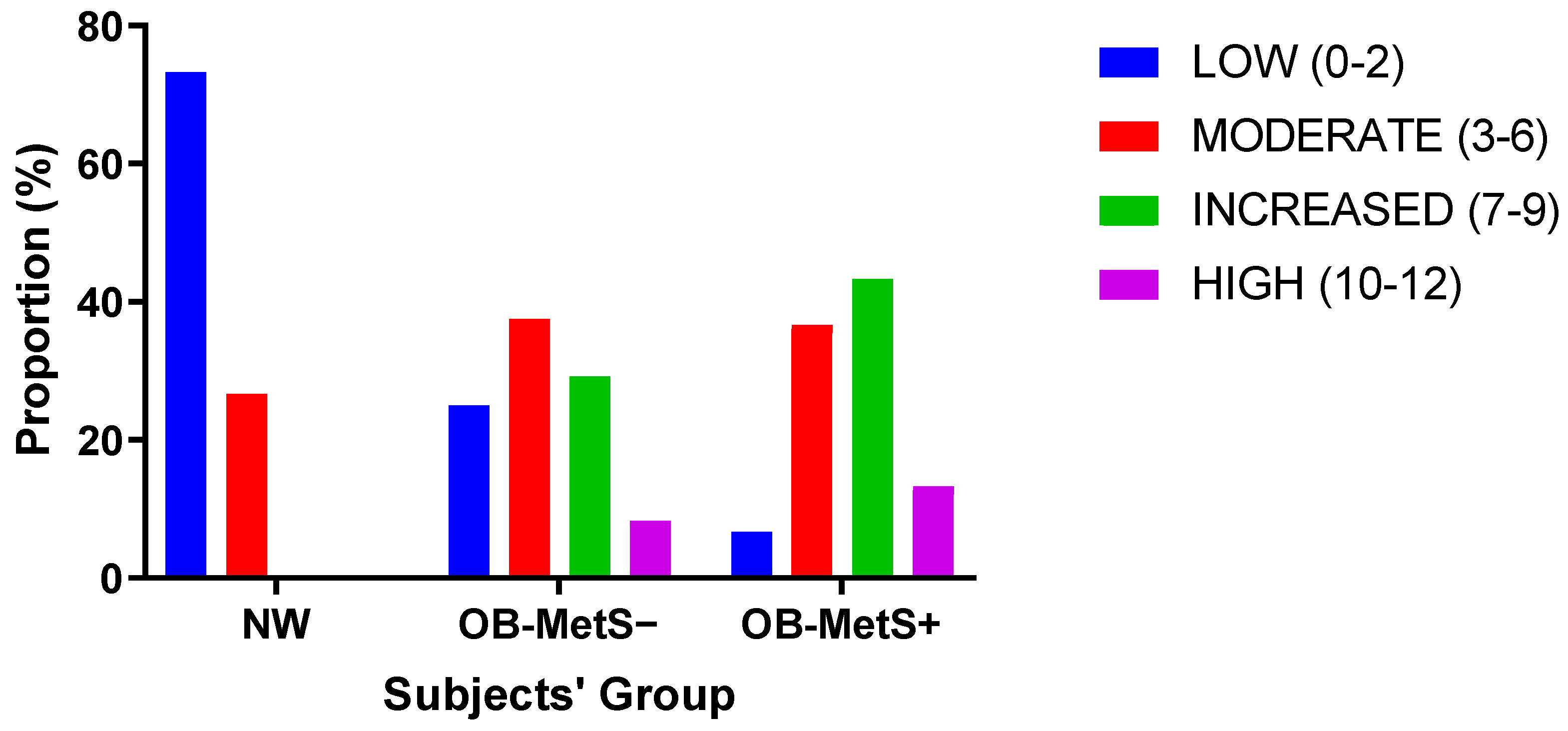
2. Results
3. Discussion
- (a)
- Being a cross-sectional study, CVD risk was calculated for each subject by means of FRS and VA, which mainly predict CVD risk related to CAD [29]; thus, the power of CERT1 in predicting CVD risk in metabolic syndrome is only presumptive; long-term, large-scale, and (importantly) prospective studies are mandatory for assessing the “real” hazard rate of CVD events in OB-MetS− and, particularly, OB-MetS+ subjects, categorized according to CERT1 groups;
- (b)
- Since there is the intriguing view that ceramides may better stratify (only residual?) CVD risk than the cholesterol-related lipids such as LDL-C and HDL-C [8], we cannot rule out that different factors, rather than visceral adiposity (WC), chronic low-grade inflammation (CRP), and insulin-resistance (HOMA-IR), might affect CERT1, being pathophysiologically involved in the dysregulation of ceramide metabolism in obesity and metabolic syndrome;
- (c)
- CERT2 has been proposed as an alternative to CERT1, with the former one being a more robust marker of CVD risk than the latter one [6]; unfortunately, in the present study, plasma levels of PCs, components of the algorithm used to calculate CERT2, were not measured; although we are willing to evaluate CERT2 in a future clinical study of ours, we believe that the new results will confirm the conclusions drawn by the use of only CERT1.
4. Materials and Methods
4.1. Subjects
4.2. Anthropometric Measurements
4.3. Metabolic Parameters
4.4. Blood Pressure
4.5. Definition of Metabolic Syndrome
4.6. Lipid Extraction and Ceramide Content Quantification
4.7. Ceramide Risk Score
4.8. Calculation of Framingham Risk Score and Vascular Age
4.9. Statistics
5. Conclusions
- (1)
- CERT1 is higher in obese than NW subjects, with no difference between the OB-MetS− and OB-MetS+ groups;
- (2)
- WC, HOMA-IR, and CRP are predictors of CERT1, with the contribution of the other IDF criteria such as arterial hypertension and dyslipidemia being negligible;
- (3)
- Adjustment for WC resulted in a loss of the difference in CERT1 between OB-MetS− and NW subjects, with the combination of WC and HOMA-IR or CRP as covariates being necessary to yield the same effect for the difference in CERT1 between OB-MetS+ and NW subjects;
- (4)
- CERT1 is associated with VA;
- (5)
- The proportions of NW, OB-MetS−, and OB-MetS+ subjects appeared to be distributed according to the CERT1-based risk groups (i.e., low, moderate, increased, and high risk).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Turpin-Nolan, S.M.; Brüning, J.C. The role of ceramics in metabolic disorders: When size and localization matters. Nat. Rev. Endocrinol. 2020, 16, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Albeituni, S.; Stiban, J. Roles of ceramics and other sphingolipids in immune cell function and inflammation. Adv. Exp. Med. Biol. 2019, 1161, 169–191. [Google Scholar] [PubMed]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Obeid, L.M. Ceramide and apoptosis: Exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med. Chem. 2012, 12, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Muñoz, A.; Presa, N.; Gomez-Larrauri, A.; Rivera, I.G.; Trueba, M.; Ordoñez, M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and creamed 1-phosphate. Prog. Lipid Res. 2016, 61, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front. Endocrinol. 2020, 11, 570628. [Google Scholar] [CrossRef]
- Tarasov, K.; Ekroos, K.; Suoniemi, M.; Kauhanen, D.; Sylvänne, T.; Hurme, R.; Gouni-Berthold, I.; Berthold, H.K.; Kleber, M.E.; Laaksonen, R.; et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 2014, 99, E45–E52. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL- cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma ceramides independently predict coronary artery disease and major adverse cardiovascular events. J. Clin. Lipidol. 2016, 10, 656–657. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating ceramides predict cardiovascular outcomes in the population- based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef]
- Anroedh, S.; Hilvo, M.; Martijn Akkerhuis, K.; Kauhanen, D.; Koistinen, K.; Oemrawsingh, R.; Serruys, P.; van Geuns, R.J.; Boersma, E.; Laaksonen, R.; et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res. 2018, 59, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Völzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc. 2018, 7, 7931. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.K.; Li, Q.; Liu, L.; Zeng, P.; Ren, R.F.; Guo, Z.F.; Wang, G.H.; Song, J.G.; Zhang, P. Plasma levels of ceramides relate to ischemic stroke risk and clinical severity. Brain Res. Bull. 2020, 158, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, A.; Kozak-Sykała, A.; Bobak, Ł.; Kałas, W.; Strzadała, L. Ceramides and sphingosine-1-phosphate as potential markers in diagnosis of ischaemic stroke. Neurol. Neurochir. Pol. 2019, 53, 484–491. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Fretts, A.M.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circe Heart Fail. 2019, 12, e005708. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Bo, S.; Ruscica, M.; Sahebkar, A. Ceramides and diabetes mellitus: An update on the potential molecular relationships. Diabet. Med. 2020, 37, 11–19. [Google Scholar] [CrossRef]
- Wigger, L.; Cruciani-Guglielmacci, C.; Nicolas, A.; Denom, J.; Fernandez, N.; Fumeron, F.; Marques-Vidal, P.; Ktorza, A.; Kramer, W.; Schulte, A.; et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 2017, 18, 2269–2279. [Google Scholar] [CrossRef]
- Bismuth, J.; Lin, P.; Yao, Q.; Chen, C. Ceramide: A common pathway for atherosclerosis? Atherosclerosis 2008, 196, 497–504. [Google Scholar] [CrossRef]
- Zietzer, A.; Düsing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network with High Therapeutic Potential. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef]
- Guo, J.; Feng, J.; Qu, H.; Xu, H.; Zhou, H. Potential Drug Targets for Ceramide Metabolism in Cardiovascular Disease. J. Cardiovasc. Dev. Dis. 2022, 9, 434. [Google Scholar] [CrossRef]
- Khanji, M.Y.; van Waardhuizen, C.N.; Bicalho, V.V.S.; Ferket, B.S.; Hunink, M.G.M.; Petersen, S.E. Lifestyle advice and interventions for cardiovascular risk reduction: A systematic review of guidelines. Int. J. Cardiol. 2018, 263, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; McEvoy, J.W.; Blumenthal, R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019, 381, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. The metabolic syndrome: Is this diagnosis necessary? Am. J. Clin. Nutr. 2006, 83, 1237–1247. [Google Scholar] [CrossRef]
- Lemstra, M.; Bird, Y.; Nwankwo, C.; Rogers, M.; Moraros, J. Weight loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the metabolic syndrome. A joint interim statement of the International Diabetes Federation Task Force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef]
- Blüher, M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Cur. Opin. Lipidol. 2010, 21, 38–43. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Sr Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Vasile, V.C.; Meeusen, J.W.; Medina Inojosa, J.R.; Donato, L.J.; Scott, C.G.; Hyun, M.S.; Vinciguerra, M.; Rodeheffer, R.R.; Lopez-Jimenez, F.; Jaffe, A.S. Ceramide Scores Predict Cardiovascular Risk in the Community. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1558–1569. [Google Scholar] [CrossRef]
- Sokolowska, E.; Blachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Aburasayn, H.; Al Batran, R.; Ussher, J.R. Targeting ceramide metabolism in obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E423–E435. [Google Scholar] [CrossRef]
- Billich, A.; Baumruker, T. Sphingolipid metabolizing enzymes as novel therapeutic targets. Subcell. Biochem. 2008, 49, 487–522. [Google Scholar] [PubMed]
- Park, J.-W.; Park, W.-J.; Futerman, A.H. Ceramide syntheses as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 2014, 1841, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.W.C.; Zheng, X.; Ali, Y. Ceramide Acyl Chain Length and Its Relevance to Intracellular Lipid Regulation. Int. J. Mol. Sci. 2022, 23, 9697. [Google Scholar] [CrossRef] [PubMed]
- Menuz, V.; Howell, K.S.; Gentina, S.; Epstein, S.; Riezman, I.; Fornallaz-mulhauser, M.; Hengartner, M.O.; Gomez, M.; Riezman, H.; Martinou, J. Protection of C. Elegance from Anoxia by HYL-2 Ceramide Synthase. Science 2009, 324, 381–384. [Google Scholar] [CrossRef]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Öhman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 haploinsufficiency inhibits b-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef]
- Nagao, M.; Nakajima, H.; Toh, R.; Hirata, K.I.; Ishida, T. Cardioprotective Effects of High-Density Lipoprotein beyond its Anti-Atherogenic Action. J. Atheroscler. Thromb. 2018, 25, 985–993. [Google Scholar] [CrossRef]
- Raichur, S. Ceramide Synthases Are Attractive Drug Targets for Treating Metabolic Diseases. Front. Endocrinol. 2020, 11, 483. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin-resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H.; Sullards, M.C.; Allegood, J.C.; Kelly, S.; Wang, E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005, 36, 207–224. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Dei Cas, M.; Cianciolo, S.; Fidilio, A.; Lazzara, F.; Paroni, R.; Pignatello, R.; Strettoi, E.; Ghidoni, R.; Drago, F.; et al. Novel ophthalmic formulation of myriocin: Implications in retinitis pigmentosa. Drug Deliv. 2019, 26, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Morano, C.; Zulueta, A.; Caretti, A.; Roda, G.; Paroni, R.; Dei Cas, M. An Update on Sphingolipidomics: Is Something Still Missing? Some Considerations on the Analysis of Complex Sphingolipids and Free-Sphingoid Bases in Plasma and Red Blood Cells. Metabolites 2022, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020, 41, 371–380. [Google Scholar] [CrossRef]
| Parameter | NW | OB-MetS− | OB-MetS+ |
|---|---|---|---|
| N. | (n = 30) | (n = 24) | (n = 30) |
| Sex (F/M) | 19F-11M | 18F-6M | 19F-11M |
| Age (years) | 29.15 [26.46; 33.14] | 27.38 [21.35; 35.65] | 30.43 [23.98; 41.18] |
| Smoking (yes/no) | 7/23 | 9/15 | 9/21 |
| BMI (kg/m2) | 22.85 [20.79; 24.70] | 42.88 [40.75; 119.25] a | 43.44 [41.53; 46.54] a |
| WC (cm) | 78.0 [76.3; 82.8] | 110.0 [106.0; 119.3] a | 120.0 [113.3; 126.5] a |
| SBP (mmHg) | 120 [110; 120] | 120 [120; 130] a | 130 [130; 140] a,b |
| DBP (mmHg) | 70 [70; 75] | 80 [77.50; 80] a | 80 [80; 90] a |
| Glucose (mg/dL) | 87 [82.25; 94.25] | 83 [80; 88.25] | 86 [82.25; 94.75] |
| Insulin (mU/L) | 6.65 [5.13; 8.80] | 15.85 [11; 23.55] a | 25.05 [19.08; 30.25] a |
| HOMA-IR | 1.54 [1.07; 1.84] | 3.23 [2.23; 4.68] a | 5.30 [4.32; 6.21] a |
| T-C (mg/dL) | 173 [158; 200.50] | 160.50 [133.25; 188.50] | 163 [148; 196] |
| HDL-C (mg/dL) | 65 [56.25; 70.75] | 45.50 [39.50; 50.25] a | 37.50 [32.50; 43.75] a |
| LDL-C (mg/dL) | 106.50 [86.25; 120.50] | 101.50 [77.75; 122.25] | 107.50 [96; 124.75] |
| TG (mg/dL) | 63 [53; 85.75] | 96 [85.75; 123.25] a | 125.50 [103.50; 159.25] a |
| CRP (mg/dL) | 0.10 [0; 0.20] | 0.50 [0.28; 1.03] a | 0.55 [0.40; 1.08] a |
| Ceramides (µmol/L) | |||
| Cer 16:0 | 0.4463 [0.3834; 0.5280] | 0.4359 [0.4025; 0.4727] | 0.4533 [0.3815; 0.5484] |
| Cer 18:0 | 0.0663 [0.0557; 0.0790] | 0.1121 [0.0794; 0.1377] a | 0.1242 [0.1053; 0.1714] a |
| Cer 24:1 | 0.7663 [0.5601; 0.9362] | 0.9581 [0.8408; 1.1890] | 1.1021 [0.8999; 1.3634] a |
| Cer 24:0 | 3.7086 [2.9225; 4.0040] | 2.1955 [1.7563; 3.1066] a | 2.3265 [1.9734; 3.0387] a |
| Ceramide Ratios | |||
| Cer 16:0/24:0 | 0.1296 [0.1116; 0.1438] | 0.1896 [0.1520; 0.2371] a | 0.1859 [0.1706; 0.2285] a |
| Cer 18:0/24:0 | 0.0187 [0.0158; 0.0241] | 0.0454 [0.0355; 0.0655] a | 0.0557 [0.0480; 0.0654] a |
| Cer 24:1/24:0 | 0.2190 [0.1928; 0.2375] | 0.4044 [0.3280; 0.5424] a | 0.4660 [0.4076; 0.5187] a |
| CVD Risk | |||
| FRS (%) | 1.000 [0.675; 1.350] | 1.150 [0.600; 1.675] | 2.450 [1.100; 4.325] a,b |
| VA (years) | 16.000 [13.750; 18.000] | 16.000 [13.250; 19.750] | 23.500 [17.750; 30.000] a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigamonti, A.E.; Dei Cas, M.; Caroli, D.; Bondesan, A.; Cella, S.G.; Paroni, R.; Sartorio, A. Ceramide Risk Score in the Evaluation of Metabolic Syndrome: An Additional or Substitutive Biochemical Marker in the Clinical Practice? Int. J. Mol. Sci. 2023, 24, 12452. https://doi.org/10.3390/ijms241512452
Rigamonti AE, Dei Cas M, Caroli D, Bondesan A, Cella SG, Paroni R, Sartorio A. Ceramide Risk Score in the Evaluation of Metabolic Syndrome: An Additional or Substitutive Biochemical Marker in the Clinical Practice? International Journal of Molecular Sciences. 2023; 24(15):12452. https://doi.org/10.3390/ijms241512452
Chicago/Turabian StyleRigamonti, Antonello E., Michele Dei Cas, Diana Caroli, Adele Bondesan, Silvano G. Cella, Rita Paroni, and Alessandro Sartorio. 2023. "Ceramide Risk Score in the Evaluation of Metabolic Syndrome: An Additional or Substitutive Biochemical Marker in the Clinical Practice?" International Journal of Molecular Sciences 24, no. 15: 12452. https://doi.org/10.3390/ijms241512452
APA StyleRigamonti, A. E., Dei Cas, M., Caroli, D., Bondesan, A., Cella, S. G., Paroni, R., & Sartorio, A. (2023). Ceramide Risk Score in the Evaluation of Metabolic Syndrome: An Additional or Substitutive Biochemical Marker in the Clinical Practice? International Journal of Molecular Sciences, 24(15), 12452. https://doi.org/10.3390/ijms241512452








