The Role of Alarmins in Osteoarthritis Pathogenesis: HMGB1, S100B and IL-33
Abstract
:1. Introduction
2. Alarmins: HMGB1, IL-33, S100B
2.1. HMGB1
2.2. IL-33
2.3. S100B
3. Aim of the Study
4. Search Strategy
5. Results and Discussion
5.1. Role of HMGB1 in Osteoarthritis
5.2. Role of IL-33 in Osteoarthritis
5.3. Role of S100B in Osteoarthritis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lawrence, R.C.; Helmick, C.G.; Arnett, F.C.; Deyo, R.A.; Felson, D.T.; Giannini, E.H.; Heyse, S.P.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998, 41, 778–799. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.-P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Hill, C.L.; Hunter, D.J.; Niu, J.; Clancy, M.; Guermazi, A.; Genant, H.; Gale, D.; Grainger, A.; Conaghan, P.; Felson, D.T. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis. 2007, 66, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Triantafillou, S.; Parker, A.; Youssef, P.P.; Coleman, M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 1997, 24, 365–371. [Google Scholar]
- Bobinac, D.; Spanjol, J.; Zoricic, S.; Maric, I. Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans. Bone 2003, 32, 284–290. [Google Scholar] [CrossRef]
- Hussain, S.; Neilly, D.; Baliga, S.; Patil, S.; Meek, R. Knee osteoarthritis: A review of management options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef]
- Messier, S.P. Osteoarthritis of the knee and associated factors of age and obesity: Effects on gait. Med. Sci. Sports Exerc. 1994, 26, 1446–1452. [Google Scholar] [CrossRef]
- Powell, A.; Teichtahl, A.J.; E Wluka, A.; Cicuttini, F.M. Obesity: A preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Br. J. Sports Med. 2005, 39, 4–5. [Google Scholar] [CrossRef] [Green Version]
- Sacitharan, P.K. Ageing and Osteoarthritis. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science [Internet]; Harris, J.R., Korolchuk, V.I., Eds.; Subcellular Biochemistry; Springer: Singapore, 2019; Volume 91, pp. 123–159. Available online: http://link.springer.com/10.1007/978-981-13-3681-2_6 (accessed on 22 April 2023).
- Buckwalter, J.A. Osteoarthritis and articular cartilage use, disuse, and abuse: Experimental studies. J. Rheumatol. 1995, 43, 13–15. [Google Scholar]
- Smith, R.L.; Trindade, M.C.; Ikenoue, T.; Mohtai, M.; Das, P.; Carter, D.R.; Goodman, S.B.; Schurman, D.J. Effects of shear stress on articular chondrocyte metabolism. Biorheology 2000, 37, 95–107. [Google Scholar]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Guilak, F.; Abramson, S.B. Etiopathogenesis of osteoarthritis. Osteoarthr. Diagn. Med./Surg. Manag. 2007, 4, 27–49. [Google Scholar]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, Danger, and the Extended Family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Castiglioni, A.; Canti, V.; Rovere-Querini, P.; Manfredi, A.A. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 2011, 343, 189–199. [Google Scholar] [CrossRef]
- Danieli, M.G.; Antonelli, E.; Piga, M.A.; Claudi, I.; Palmeri, D.; Tonacci, A.; Allegra, A.; Gangemi, S. Alarmins in autoimmune diseases. Autoimmun. Rev. 2022, 21, 103142. [Google Scholar] [CrossRef] [PubMed]
- Štros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1799, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E. HMGB1, IL-1α, IL-33 and S100 proteins: Dual-function alarmins. Cell. Mol. Immunol. 2017, 14, 43–64. [Google Scholar] [CrossRef] [Green Version]
- Cleynen, I.; Van de Ven, W.J. The HMGA proteins: A myriad of functions (Review). Int. J. Oncol. 2008, 32, 289–305. Available online: http://www.spandidos-publications.com/10.3892/ijo.32.2.289 (accessed on 22 April 2023). [CrossRef] [Green Version]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; A Amoscato, A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and their role in Cancer and Inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musumeci, D.; Roviello, G.N.; Montesarchio, D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol. Ther. 2014, 141, 347–357. [Google Scholar] [CrossRef]
- Taverna, S.; Tonacci, A.; Ferraro, M.; Cammarata, G.; Cuttitta, G.; Bucchieri, S.; Pace, E.; Gangemi, S. High Mobility Group Box 1: Biological Functions and Relevance in Oxidative Stress Related Chronic Diseases. Cells 2022, 11, 849. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Andersson, U.; Antoine, D.J.; Tracey, K.J. Expression of Concern: The functions of HMGB 1 depend on molecular localization and post-translational modifications. J. Intern. Med. 2014, 276, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Bell, C.W.; Pisetsky, D.S. The Relationship between Apoptosis and High-Mobility Group Protein 1 Release from Murine Macrophages Stimulated with Lipopolysaccharide or Polyinosinic-Polycytidylic Acid. J. Immunol. 2007, 178, 6495–6503. [Google Scholar] [CrossRef] [Green Version]
- Klune, J.R.; Dhupar, R.; Cardinal, J.; Billiar, T.R.; Tsung, A. HMGB1: Endogenous Danger Signaling. Mol. Med. 2008, 14, 476–484. [Google Scholar] [CrossRef]
- Willingham, S.B.; Allen, I.C.; Bergstralh, D.T.; Brickey, W.J.; Huang, M.T.-H.; Taxman, D.J.; Duncan, J.A.; Ting, J.P.-Y. NLRP3 (NALP3, Cryopyrin) Facilitates In Vivo Caspase-1 Activation, Necrosis, and HMGB1 Release via Inflammasome-Dependent and -Independent Pathways. J. Immunol. 2009, 183, 2008–2015. [Google Scholar] [CrossRef] [Green Version]
- Rouhiainen, A.; Kuja-Panula, J.; Wilkman, E.; Pakkanen, J.; Stenfors, J.; Tuominen, R.K.; Lepäntalo, M.; Carpén, O.; Parkkinen, J.; Rauvala, H. Regulation of monocyte migration by amphoterin (HMGB1). Blood 2004, 104, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, N.; Yoshida, K.; Ito, T.; Tsuda, M.; Mishima, Y.; Furumatsu, T.; Ronfani, L.; Abeyama, K.; Kawahara, K.-I.; Komiya, S.; et al. Stage-Specific Secretion of HMGB1 in Cartilage Regulates Endochondral Ossification. Mol. Cell. Biol. 2007, 27, 5650–5663. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, H.; Ju, Z.; Ragab, A.A.; Lundbäck, P.; Long, W.; Valdés-Ferrer, S.I.; He, M.; Pribis, J.P.; Li, J.; et al. MD-2 is required for disulfide HMGB1—Dependent TLR4 signaling. J. Exp. Med. 2015, 212, 5–14. [Google Scholar] [CrossRef]
- Yang, H.; Lundbäck, P.; Ottosson, L.; Erlandsson-Harris, H.; Venereau, E.; Bianchi, M.E.; Al-Abed, Y.; Andersson, U.; Tracey, K.J. Redox modifications of cysteine residues regulate the cytokine activity of HMGB1. Mol. Med. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Chavakis, E.; Hain, A.; Vinci, M.; Carmona, G.; Bianchi, M.E.; Vajkoczy, P.; Zeiher, A.M.; Chavakis, T.; Dimmeler, S. High-Mobility Group Box 1 Activates Integrin-Dependent Homing of Endothelial Progenitor Cells. Circ. Res. 2007, 100, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Yang, H.; Harris, H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin. Ther. Targets 2018, 22, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Venereau, E.; Casalgrandi, M.; Schiraldi, M.; Antoine, D.J.; Cattaneo, A.; De Marchis, F.; Liu, J.; Antonelli, A.; Preti, A.; Raeli, L.; et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012, 209, 1519–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Antoine, D.J.; Andersson, U.; Tracey, K.J. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 2013, 93, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Youn, J.H.; Oh, Y.J.; Kim, E.S.; Choi, J.E.; Shin, J.-S. High Mobility Group Box 1 Protein Binding to Lipopolysaccharide Facilitates Transfer of Lipopolysaccharide to CD14 and Enhances Lipopolysaccharide-Mediated TNF-α Production in Human Monocytes. J. Immunol. 2008, 180, 5067–5074. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Zmijewski, J.; Xu, Z.; Abraham, E. HMGB1 Develops Enhanced Proinflammatory Activity by Binding to Cytokines. J. Immunol. 2008, 180, 2531–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiraldi, M.; Raucci, A.; Muñoz, L.M.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; De Marchis, F.; Pedotti, M.; Bachi, A.; et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Avalos, A.M.; Mao, S.-Y.; Chen, B.; Senthil, K.; Wu, H.; Parroche, P.; Drabic, S.; Golenbock, D.; Sirois, C.; et al. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007, 8, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.-C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High Mobility Group 1 Protein (Hmg-1) Stimulates Proinflammatory Cytokine Synthesis in Human Monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef]
- Moussion, C.; Ortega, N.; Girard, J.P. The IL-1-Like Cytokine IL-33 Is Constitutively Expressed in the Nucleus of Endothelial Cells and Epithelial Cells In Vivo: A Novel ‘Alarmin’? Unutmaz D, curatore. PLoS ONE. 2008, 3, e3331. [Google Scholar] [CrossRef] [Green Version]
- Nakae, S.; Morita, H.; Ohno, T.; Arae, K.; Matsumoto, K.; Saito, H. Role of Interleukin-33 in Innate-Type Immune Cells in Allergy. Allergol. Int. 2013, 62, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Vladimir, T.; Sweet, M.J.; Xu, D. T1/ST2—An IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004, 15, 87–95. [Google Scholar]
- Tago, K.; Noda, T.; Hayakawa, M.; Iwahana, H.; Yanagisawa, K.; Yashiro, T.; Tominaga, S.-i. Tissue Distribution and Subcellular Localization of a Variant Form of the Human ST2 Gene Product, ST2V. Biochem. Biophys. Res. Commun. 2001, 285, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Espinassous, Q.; Garcia-De-Paco, E.; Garcia-Verdugo, I.; Synguelakis, M.; von Aulock, S.; Sallenave, J.-M.; McKenzie, A.N.J.; Kanellopoulos, J. IL-33 Enhances Lipopolysaccharide-Induced Inflammatory Cytokine Production from Mouse Macrophages by Regulating Lipopolysaccharide Receptor Complex. J. Immunol. 2009, 183, 1446–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.D.; Oak, S.R.; Hartigan, A.J.; Finn, W.G.; Kunkel, S.L.; Duffy, K.E.; Das, A.; Hogaboam, C.M. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Moulin, D.; Donzé, O.; Talabot-Ayer, D.; Mézin, F.; Palmer, G.; Gabay, C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 2007, 40, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Suzukawa, M.; Koketsu, R.; Iikura, M.; Nakae, S.; Matsumoto, K.; Nagase, H.; Saito, H.; Matsushima, K.; Ohta, K.; Yamamoto, K.; et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab. Investig. 2008, 88, 1245–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgeois, E.; Van, L.P.; Samson, M.; Diem, S.; Barra, A.; Roga, S.; Gombert, J.M.; Schneider, E.; Dy, M.; Gourdy, P.; et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-c production. Eur. J. Immunol. 2009, 39, 1046–1055. [Google Scholar] [CrossRef]
- Sorci, G.; Riuzzi, F.; Arcuri, C.; Tubaro, C.; Bianchi, R.; Giambanco, I.; Donato, R. S100B protein in tissue development, repair and regeneration. World J. Biol. Chem. 2013, 4, 1. [Google Scholar] [CrossRef]
- Sorci, G.; Bianchi, R.; Riuzzi, F.; Tubaro, C.; Arcuri, C.; Giambanco, I.; Donato, R. S100B Protein, a Damage-Associated Molecular Pattern Protein in the Brain and Heart, and Beyond. Cardiovasc. Psychiatry Neurol. 2010, 2010, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Terada, C.; Yoshida, A.; Nasu, Y.; Mori, S.; Tomono, Y.; Tanaka, M.; Takahashi, H.; Nishibori, M.; Ozaki, T.; Nishida, K. Gene expression and localization of high-mobility group box chromosomal protein-1 (HMGB-1)in human osteoarthritic cartilage. Acta Med. Okayama 2011, 65, 369–377. [Google Scholar]
- Heinola, T.; Kouri, V.-P.; Clarijs, P.; Ciferska, H.; Sukura, A.K.K.; Salo, J.; Konttinen, Y.T. High mobility group box-1 (HMGB-1) in osteoarthritic cartilage. Ann. Rheum. Dis. 2010, 28, 511–518. [Google Scholar]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Sekundiak, T.D.; Agrawal, D.K. Increased expression of damage-associated molecular patterns (DAMPs) in osteoarthritis of human knee joint compared to hip joint. Mol. Cell. Biochem. 2017, 436, 59–69. [Google Scholar] [CrossRef]
- Amin, A.R.; Islam, A.B.M.M.K. Genomic Analysis and Differential Expression of HMG and S100A Family in Human Arthritis: Upregulated Expression of Chemokines, IL-8 and Nitric Oxide by HMGB1. DNA Cell Biol. 2014, 33, 550–565. [Google Scholar] [CrossRef]
- Wagner, G.; Lehmann, C.; Bode, C.; Miosge, N.; Schubert, A. High Mobility Group Box 1 Protein in Osteoarthritic Knee Tissue and Chondrogenic Progenitor Cells: An Ex Vivo and In Vitro Study. Cartilage 2019, 12, 484–495. [Google Scholar] [CrossRef]
- Hwang, H.S.; Choi, M.H.; Kim, H.A. 29-kDa FN-f inhibited autophagy through modulating localization of HMGB1 in human articular chondrocytes. BMB Rep. 2018, 51, 508–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Lei, J.; Zhuang, Y.; Zhang, K.; Lu, D. Overexpression of HMGB1 A-box reduced IL-1β-induced MMP expression and the production of inflammatory mediators in human chondrocytes. Exp. Cell Res. 2016, 349, 184–190. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, G.; Si, Z.; Yu, L.; Hou, L. Glycyrrhizin, an HMGB1 inhibitor, Suppresses Interleukin-1β-Induced Inflammatory Responses in Chondrocytes from Patients with Osteoarthritis. CARTILAGE 2021, 13 (Suppl. 2), 947S–955S. [Google Scholar] [CrossRef]
- Aulin, C.; Lassacher, T.; Palmblad, K.; Harris, H.E. Early stage blockade of the alarmin HMGB1 reduces cartilage destruction in experimental OA. Osteoarthr. Cartil. 2020, 28, 698–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Buckwalter, J.A.; Martin, J.A. DAMPs Synergize with Cytokines or Fibronectin Fragment on Inducing Chondrolysis but Lose Effect When Acting Alone. Mediat. Inflamm. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.-H.; Liu, Y.; Han, Y.; Wang, J. Expression and Significance of High-Mobility Group Protein B1 (HMGB1) and the Receptor for Advanced Glycation End-Product (RAGE) in Knee Osteoarthritis. Experiment 2016, 22, 2105–2112. [Google Scholar] [CrossRef] [Green Version]
- García-Arnandis, I.; Guillén, M.I.; Gomar, F.; Pelletier, J.P.; Martel-Pelletier, J.; Alcaraz, M.J. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1b in osteoarthritic synoviocytes. Arthritis Res. Ther. 2010, 12, R165. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Jin, G.; Yang, Y.; Cao, X.; Fang, R.; Feng, X.; Lei, B. Synovial Fluid HMGB-1 Levels are Associated with Osteoarthritis Severity. Clin. Lab. 2015, 61, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Fang, W.; Li, C.; Guo, H.; Li, Y.; Long, X. The expression of high-mobility group box protein-1 in temporomandibular joint osteoarthritis with disc perforation. J. Oral Pathol. Med. 2015, 45, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Wähämaa, H.; Schierbeck, H.; Hreggvidsdottir, H.S.; Palmblad, K.; Aveberger, A.-C.; Andersson, U.; Harris, H. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Thromb. Haemost. 2011, 13, R136. [Google Scholar] [CrossRef] [Green Version]
- Hreggvidsdottir, H.S.; Östberg, T.; Wähämaa, H.; Schierbeck, H.; Aveberger, A.-C.; Klevenvall, L.; Palmblad, K.; Ottosson, L.; Andersson, U.; Harris, H.E. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J. Leukoc. Biol. 2009, 86, 655–662. [Google Scholar] [CrossRef]
- Li, Y.Y.; Feng, Y.P.; Liu, L.; Ke, J.; Long, X. Inhibition of HMGB1 suppresses inflammation and catabolism in temporomandibular joint osteoarthritis via NF-κB signaling pathway. Eur. J. Histochem. 2022, 66, 3357. Available online: https://www.ejh.it/index.php/ejh/article/view/3357 (accessed on 27 April 2023). [CrossRef] [PubMed]
- Qiu, M.; Liu, D.; Fu, Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021, 269, 118987. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, L.; Zhang, T.; Lu, H.; Wang, C.; Xue, B.; Xu, X.; Liu, Y.; Cai, Z.; Sang, W.; et al. BRD4 has dual effects on the HMGB1 and NF-κB signalling pathways and is a potential therapeutic target for osteoarthritis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 3001–3015. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, H.; Li, Y. LncRNA MCM3AP-AS1 regulates miR-142-3p/HMGB1 to promote LPS-induced chondrocyte apoptosis. BMC Musculoskelet. Disord. 2019, 20, 605. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shen, S.; Li, Z.; Li, W.; Weng, X. MIR-140-5p affects chondrocyte proliferation, apoptosis, and inflammation by targeting HMGB1 in osteoarthritis. Inflamm. Res. 2020, 69, 63–73. [Google Scholar] [CrossRef]
- Meng, Y.; Qiu, S.; Sun, L.; Zuo, J. Knockdown of exosome-mediated lnc-PVT1 alleviates lipopolysaccharide-induced osteoarthritis progression by mediating the HMGB1/TLR4/NF-κB pathway via miR-93-5p. Mol. Med. Rep. 2020, 22, 5313–5325. [Google Scholar] [CrossRef]
- Lin, S.S.; Yuan, L.J.; Niu, C.C.; Tu, Y.K.; Yang, C.Y.; Ueng, S.W.N. Hyperbaric oxygen inhibits the HMGB1/RAGE signaling pathway by upregulating Mir-107 expression in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2019, 27, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hu, S.; Liu, L.; Ke, J.; Long, X. HMGB1 contributes to osteoarthritis of temporomandibular joint by inducing synovial angiogenesis. J. Oral Rehabil. 2021, 48, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-C.; Cheng, G.-Q.; Hu, K.-Z.; Li, M.-Q.; Zang, W.-P.; Dong, Y.-Q.; Wang, W.-L.; Liu, Z.-D. Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clin. Invest. Med. 2011, 34, E298. [Google Scholar] [CrossRef] [Green Version]
- Aulin, C.; Larsson, S.; Vogl, T.; Roth, J.; Åkesson, A.; Swärd, P.; Heinbäck, R.; Erlandsson Harris, H.; Struglics, A. The alarmins high mobility group box protein 1 and S100A8/A9 display different inflammatory profiles after acute knee injury. Osteoarthr. Cartil. 2022, 30, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Amendola, A.; Wolf, B.; Bollier, M.; Albright, J.; Wang, Q.; Wu, M.; Wang, X.; Song, H.; Pedersen, D.; et al. Association of chemokine expression in anterior cruciate ligament deficient knee with patient characteristics: Implications for post-traumatic osteoarthritis. Knee 2020, 27, 36–44. [Google Scholar] [CrossRef]
- García-Arnandis, I.; Guillén, M.I.; Castejón, M.A.; Gomar, F.; Alcaraz, M.J. Haem oxygenase-1 down-regulates high mobility group box 1 and matrix metalloproteinases in osteoarthritic synoviocytes. Rheumatology 2010, 49, 854–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Yu, W.; Huang, C.; Ding, Q.; Liang, C.; Wang, L.; Hou, Z.; Zhang, Z. Chrysin protects human osteoarthritis chondrocytes by inhibiting inflammatory mediator expression via HMGB1 suppression. Mol. Med. Rep. 2018, 19, 1222–1229. Available online: http://www.spandidos-publications.com/10.3892/mmr.2018.9724 (accessed on 27 April 2023). [CrossRef] [PubMed] [Green Version]
- Rai, V.; Dilisio, M.F.; Samadi, F.; Agrawal, D.K. Counteractive Effects of IL-33 and IL-37 on Inflammation in Osteoarthritis. Int. J. Environ. Res. Public Health 2022, 19, 5690. [Google Scholar] [CrossRef]
- Li, C.; Chen, K.; Kang, H.; Yan, Y.; Liu, K.; Guo, C.; Qi, J.; Yang, K.; Wang, F.; Guo, L.; et al. Double-stranded RNA released from damaged articular chondrocytes promotes cartilage degeneration via Toll-like receptor 3-interleukin-33 pathway. Cell Death Dis. 2017, 8, e3165. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Song, Y.; Yi, Y.; Qiu, F.; Wang, J.; Li, J.; Jin, Q.; Sacitharan, P.K. Blockade of IL-33 signalling attenuates osteoarthritis. Clin. Transl. Immunol. 2020, 9, e1185. Available online: https://onlinelibrary.wiley.com/doi/10.1002/cti2.1187 (accessed on 28 April 2023). [CrossRef]
- Hong, Y.-S.; Moon, S.-J.; Joo, Y.-B.; Jeon, C.-H.; Cho, M.-L.; Ju, J.H.; Oh, H.-J.; Heo, Y.-J.; Park, S.-H.; Kim, H.-Y.; et al. Measurement of Interleukin-33 (IL-33) and IL-33 Receptors (sST2 and ST2L) in Patients with Rheumatoid Arthritis. J. Korean Med. Sci. 2011, 26, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Weng, Z.; Shen, P.; Zhou, J.; Zeng, J.; Weng, F.; Zhang, X.; Yang, H. S100B regulates inflammatory response during osteoarthritis via fibroblast growth factor receptor 1 signaling. Mol. Med. Rep. 2018, 18, 4855–4864. Available online: http://www.spandidos-publications.com/10.3892/mmr.2018.9523 (accessed on 28 April 2023). [CrossRef] [PubMed] [Green Version]
- Loeser, R.F.; Yammani, R.R.; Carlson, C.S.; Chen, H.; Cole, A.; Im, H.-J.; Bursch, L.S.; Du Yan, S. Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum. 2005, 52, 2376–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyostio-Moore, S.; Nambiar, B.; Hutto, E.; Ewing, P.J.; Piraino, S.; Berthelette, P.; Sookdeo, C.; Matthews, G.; Armentano, D. STR/ort mice, a model for spontaneous osteoarthritis, exhibit elevated levels of both local and systemic inflammatory markers. Comp. Med. 2011, 61, 346–355. [Google Scholar]
- Pearle, A.; Scanzello, C.; George, S.; Mandl, L.; DiCarlo, E.; Peterson, M.; Sculco, T.; Crow, M. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr. Cartil. 2007, 15, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.K.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostberg, T.; Wahamaa, H.; Palmblad, K.; Ito, N.; Stridh, P.; Shoshan, M.; Lotze, M.T.; Harris, H.E.; Andersson, U. Oxaliplatin retains HMGB1 intranuclearly and ameliorates collagen type II-induced arthritis. Thromb. Haemost. 2008, 10, R1. [Google Scholar] [CrossRef] [Green Version]
- Kokkola, R.; Li, J.; Sundberg, E.; Aveberger, A.-C.; Palmblad, K.; Yang, H.; Tracey, K.J.; Andersson, U.; Harris, H.E. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003, 48, 2052–2058. [Google Scholar] [CrossRef]
- Schierbeck, H.; Lundbäck, P.; Palmblad, K.; Klevenvall, L.; Erlandsson-Harris, H.; Andersson, U.; Ottosson, L. Monoclonal Anti-HMGB1 (High Mobility Group Box Chromosomal Protein 1) Antibody Protection in Two Experimental Arthritis Models. Mol. Med. 2011, 17, 1039–1044. [Google Scholar] [CrossRef] [Green Version]
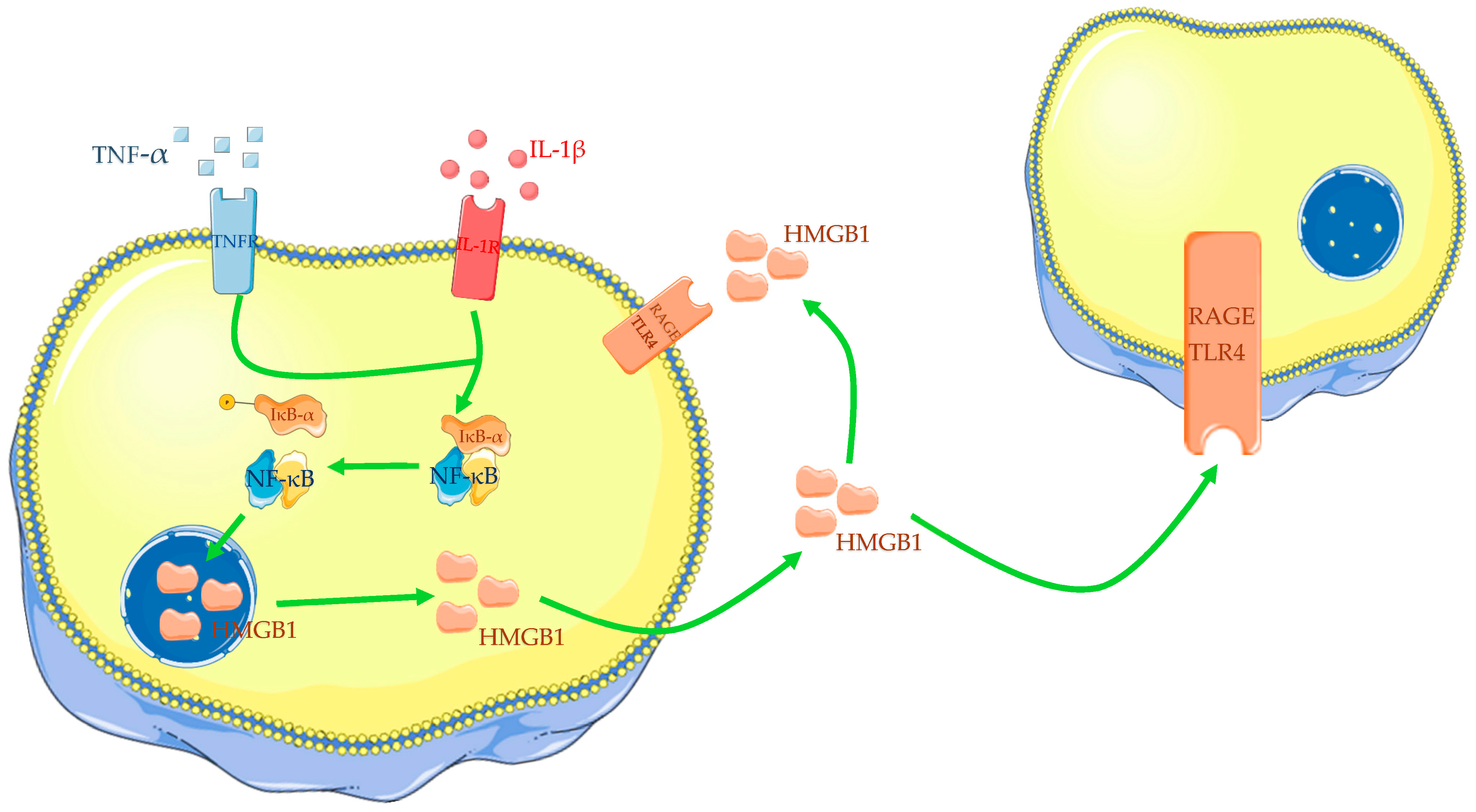
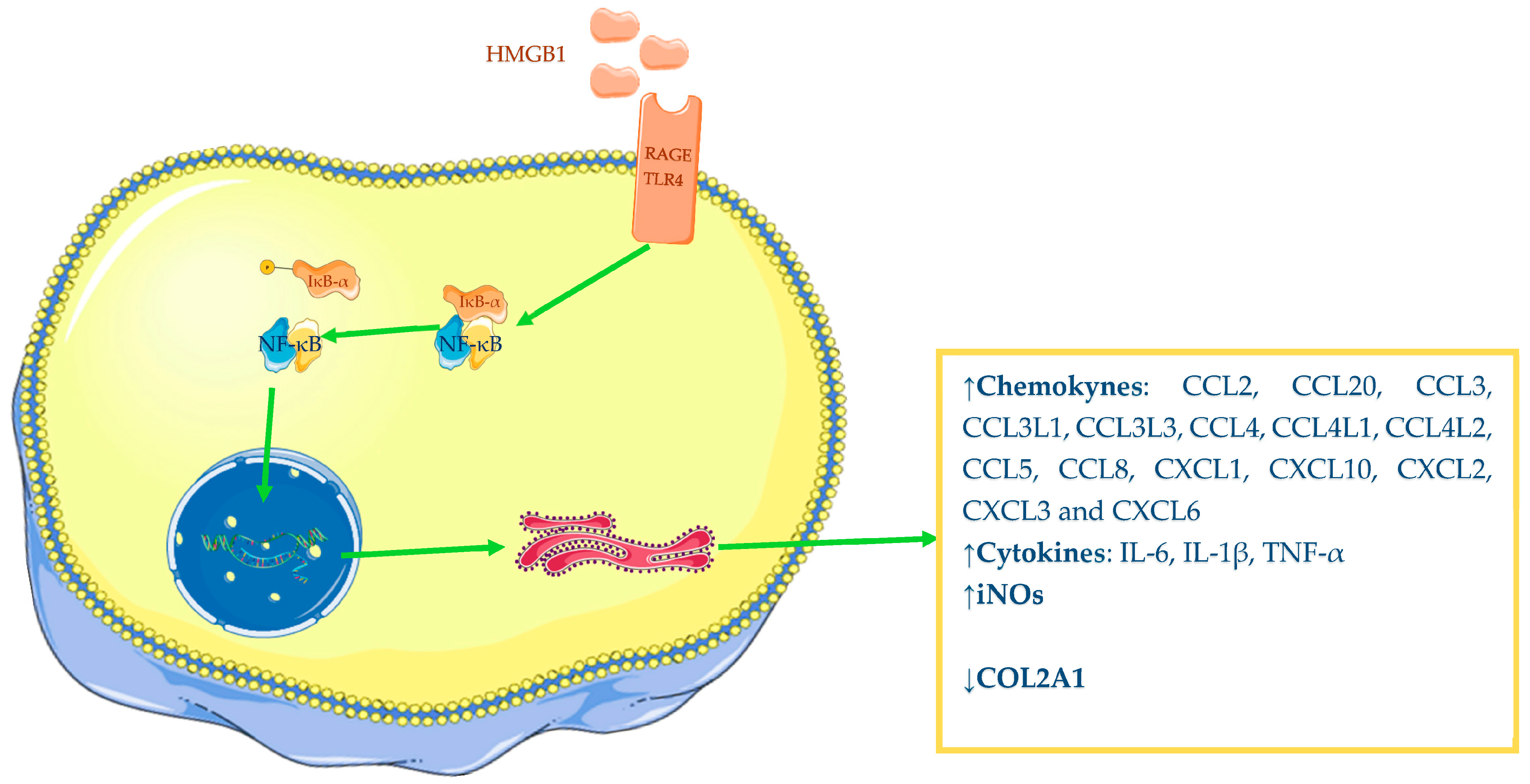

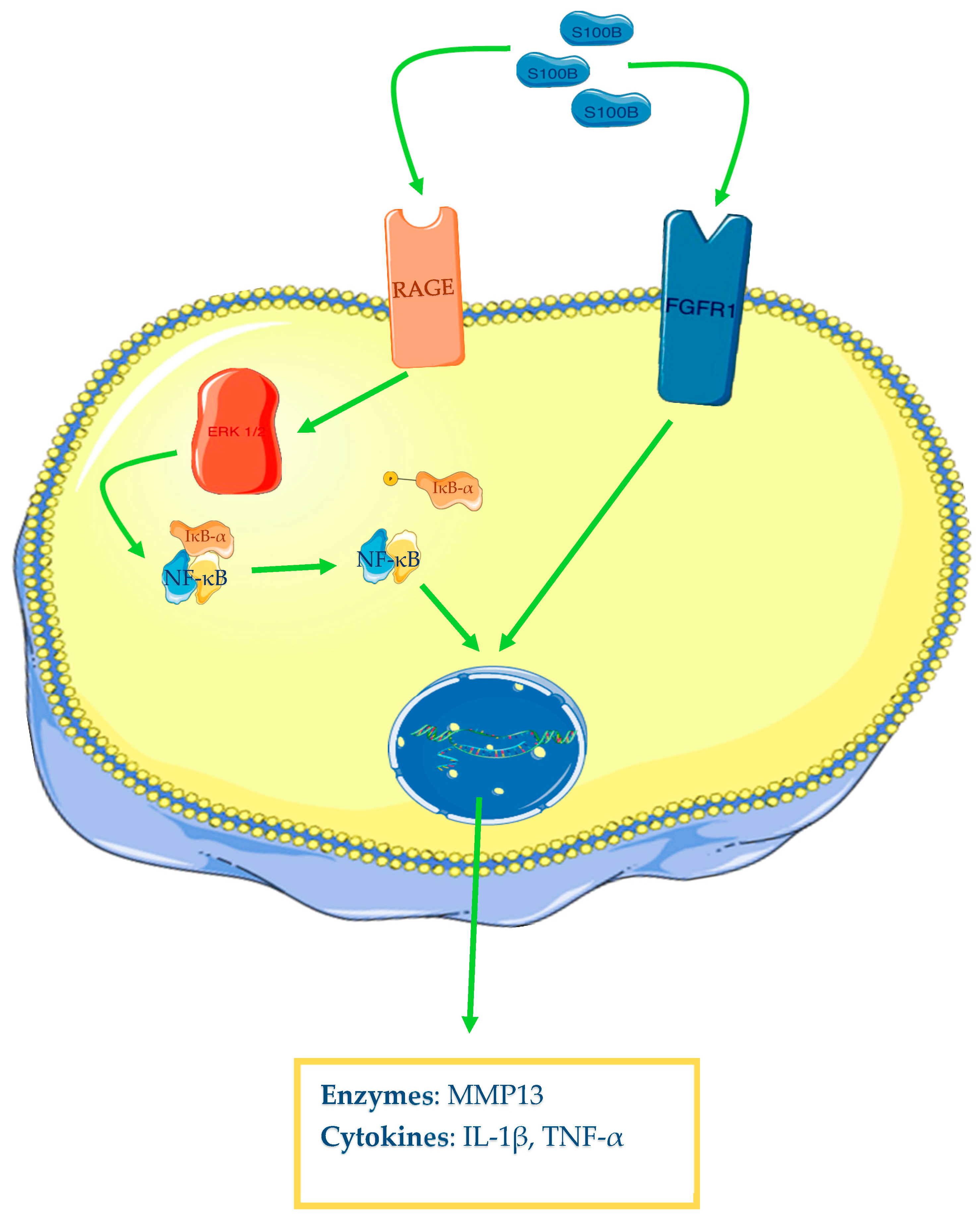
| Authors | Year | Cell Cultures | Biological Fluid | Outcomes |
|---|---|---|---|---|
| Terada et al. [60] | 2011 | (a) OA cartilage samples; (b) OA chondrocytes incubated with IL-1β and TNF-α; (c) OA chondrocytes incubated with HMGB1. | (a) ↑HMGB1 mRNA levels; gradually increased HMGB1 and RAGE protein expression and HMGB1 cytoplasmic positivity with progression of OARSI grade; (b) ↑HMGB1 translocation from the nucleus to the cytoplasm; (c) ↑TNF-α and IL-1β release in a dose-dependent manner for 12 h and 24 h, respectively, after stimulation. | |
| Heinola et al. [61] | 2010 | Human bone marrow-derived mesenchymal stem cells differentiated in vitro into chondrocytes and then stimulated with TNF- α. | ↑HMGB1 expression and cytoplasmic positivity; gradual increase of HMGB1 positivity with histological severity of OA. | |
| Rosenberg et al. [62] | 2017 | (a) Normal human articular chondrocytes vs. OA chondrocytes; cartilage tissues from (b) OA knee vs. (b) OA hip. | (a)↑ HMGB1, RAGE, S1008, and S1009 protein levels, and HMGB1, RAGE, and S1008 mRNA expression in OA chondrocytes; HMGB1, RAGE, S1008, and S1009 protein levels, and HMGB1, RAGE, and S1008 mRNA expression in OA knee in (b) > (a). | |
| Amin et al. [63] | 2014 | (a) Cartilage of the deep region from OA knees; (b) cartilage from OA knees incubated with HMGB1. | (a) ↑HMGB1 positivity compared with normal, in the cytoplasm and extracellular milieu; spontaneous release of HMGB1 from OA cartilage in ex vivo conditions; (b) ↑mRNA of CCL2, CCL20, CCL3, CCL3L1, CCL3L3, CCL4, CCL4L1, CCL4L2, CCL5, CCL8, CXCL1, CXCL10, CXCL2, CXCL3 and CXCL6, NF-κB1, NF-κB2; ↑mRNA and protein levels of IL-8, iNOs; HMGB1 induces paracrine effects on OA cartilage. | |
| Wagner et al. [64] | 2021 | (a) OA cartilage samples; (b) chondrogenic progenitor cells incubated with different concentrations of HMGB1. | (a) ↑HMGB1 protein level; HMGB1 positivity in the cytoplasm and extracellular medium; (b) ↑Migration rate (time- and dose-dependent). | |
| Hwang et al. [65] | 2018 | Cartilage from OA knee | ↑Nucleus to cytoplasm translocation and extracellular release of HMGB1; ↑p-mTOR; ↑Bcl-2/Beclin-1; ↓HMGB1/Beclin-1. | |
| Fu et al. [66] | 2016 | (a) Normal human chondrocytes pretreated with IL-1β and (b) then with HMGB1 A-box overexpression. | (a) ↑ mRNA and protein levels of HMGB1, ADAMTS-4, ADAMTS-5, and TLR4, ↑secretion of MMP-1, MMP-3 and MMP-9, ↑mRNA levels iNO and COX-2; ↑production NO and PGE2, ↑p-p65 levels; (b) ↓mRNA and protein levels of HMGB1, ADAMTS-4, ADAMTS-5 and TLR4, ↓secretion of MMP-1, MMP-3 and MMP-9, ↓mRNA levels iNO and COX-2; ↓production NO and PGE2, ↓p-p65 levels. | |
| Zhou et al. [67] | 2021 | (a) Chondrocytes from OA patients; (b) chondrocytes from OA patients incubated with IL-1β and glycyrrhizin. | (c) synovial fluid from OA patients | (a) ↑HMGB1 protein levels; (b) ↑cell viability, ↓mRNA and protein levels of HMGB1, TNF-α, IL-6, MMP-1, MMP-3, and MMP-13; ↓PGE2 and NO production (c) ↑HMGB1 compared with normal. |
| Aulin et al. [68] | 2020 | OA knees chondrocytes incubated with dsHMGB1 | ↑IL-6, IL-8 protein levels and ↓mRNA of COL2A1. | |
| Ding et al. [69] | 2017 | Normal human chondrocytes from weight-bearing joints incubated with IL-1β and (a) HMGB1 or (b) FN-f. | MMP-3, MMP-13, ADAMTS-5, ADAM-8, and iNOS synthesis in (a) > (b). | |
| Sun et al. [70] | 2016 | Synovial tissues from OA knees | ↑mRNA and protein levels of HMGB1 and RAGE; HMGB1 and RAGE expression is positively correlated with X-ray severity. | |
| García-Arnandis et al. [71] | 2010 | (a) OA synoviocytes; OA synoviocytes incubated with HMGB1 (b) with or (c) without IL-1β. | (a) ↑HMGB1 expression and cytoplasmic positivity compared with normal (statistical significance not reached); mRNA and protein expression IL-6, IL-8, CCL2, CCL20, MMP1, MMP3 in (b) > (c); mRNA and protein expression MMP13 in (b) > (c) but statistical significance not reached; p-ERK1/2, p-p38 and p-Akt in (b) > (c). | |
| Ke et al. [72] | 2015 | (a) Synovium from knee OA patients. | (b) Synovial fluid from OA patients | (a) ↑HMGB1 expression and cytoplasmic positivity compared with normal; (a,b) ↑HMGB1 levels compared with normal; (b) ↑HMGB1 in the radiological KL 2/3 group compared with KL 4; HMGB1 positively correlated with OA clinical findings: pain, synovitis, and daily activities. |
| Feng et al. [73] | 2016 | Synovial membrane from TMJOA patients. | ↑mRNA and protein levels HMGB1. | |
| Wähämaa et al. [74] | 2011 | Synovial fibroblast from OA patients incubated with HMGB1 in complex with (a) LPS or (b) IL-1β. | (a,b)↑TNF-α, IL-6, IL-8 and MMP-3; cytokine production in (a) > (b); (a) HMGB1-LPS complex interacts with TLR4; (b) HMGB1- IL-1β complex interacts with IL-1RI. | |
| Hreggvidsdottir et al. [75] | 2009 | Synovial fibroblasts from OA patients incubated with (a) HMGB1 alone, (b) IL-1β alone, or with (c) HMGB1 and IL-1β. | IL-6 levels in (c) > (a,b) | |
| Li et al. [76] | 2022 | Human synovial fibroblasts from OA temporomandibular joint treated with HMGB1. | ↑MMP13, ADAMTS5, IL-1β, IL-6 and p-NF-κB p65; ↑nuclear localisation of p-NF-κB p65. | |
| Qiu et al. [77] | 2021 | Chondrocytes incubated with IL-1β and (a) exosomes poor in miR-129-5p compared with (b) exosomes rich in miR-129-5p. | (c) Synovial fluid from OA patients | Protein levels of HMGB1, TLR4, p-NF-κB, COX2, iNOS and MMP13 in (a) > (b); apoptosis rate in (a) > (b); collagen 2 in (a) < (b); (c) ↑HMGB1 and IL-1β and ↓miR-129-5p; miR-129-5p levels are negatively correlated with HMGB1. |
| Jiang et al. [78] | 2017 | (a) OA human chondrocytes; (b) OA human chondrocytes incubated with IL-1β and JQ1. | (a) ↑mRNA and protein levels BRD4; BRD4 levels positively correlated with histological grade of OA (OARSI); (b) ↓mRNA and protein levels of HMGB1, IL-6, TNF-α, MMP3, MMP9, and MMP13; ↓HMGB1 from the nucleus to cytoplasm translocation; ↓p65 from the cytoplasm to the nucleus. | |
| Gao et al. [79] | 2019 | (a) Cartilage from OA knee or hip; (b) chondrocyte from OA patients with MCM3AP-AS1 overexpression; (c) chondrocyte from OA patients with miR-142-3p overexpression; (d) chondrocyte from OA patients incubated with crescent doses of LPS (0-2000 ng/mL) for 24 h. | (a) ↑MCM3AP-AS1 (b) ↑HMGB1 mRNA and protein levels; ↑apoptotic rate; (c) ↓HMGB1 mRNA and protein levels; ↓apoptotic rate; (d) ↑MCM3AP-AS1 and HMGB1 mRNA; ↓miR-142-3p. | |
| Wang et al. [80] | 2020 | (a) Cartilage from OA knee; (b) normal human chondrocytes C28/I2 incubated with IL-1β and then transfected with miR-140-5p mimics. | (a) ↓miR-140-5 and ↑HMGB1 mRNA levels; (b) ↓HMGB1, p-PI3K, p-AKT, MMP-1, MMP-3, TNF-α and IL-6 protein levels; ↓apoptosis rate; ↑cell viability (CCK-8). | |
| Meng et al. [81] | 2020 | (a) Human normal chondrocytes C28/I2 stimulated with LPS and (b) then with si-PVT1; (c) human normal chondrocytes C28/I2 stimulated with LPS and then with miR-93-5p | (d) Serum from OA patients | (a) ↑PVT1, ↓miR-93-5p, ↑apoptosis rate, IL-6, IL-1β, TNF-α, MMP13, p-p65, TLR4, and p-IκB-α expression; (b) ↓PVT1, ↑miR-93-5p, ↓apoptosis rate, IL-6, IL-1β, TNF-α, MMP13, p-p65, TLR4, and p-IκB-α expression; (c) ↓mRNA and protein HMGB1; (d) ↑PVT1 and ↓miR-93-5p compared with controls. |
| Lin et al. [82] | 2019 | (a) OA articular cartilage specimens transfected with miR-107 mimics; (b) OA chondrocytes exposed to hyperbaric oxygen treatment. | (a) ↓HMGB1; (b) ↑miR-170, ↓HMGB1, TLR2, TLR4, RAGE and iNOS mRNA and protein levels, ↓HMGB1, MMP-9, and MMP- 13 extracellular release, ↓p38 MAPK, ERK and JNK phosphorylation, ↑IκBα protein synthesis. | |
| Feng et al. [83] | 2021 | (a) Human synovial fibroblasts from TMJOA stimulated with HMGB1; (b) human umbilical vein endothelial cells (HUVEC) incubated with conditioned medium from fibroblasts stimulated with HMGB1; (c) human umbilical vein endothelial cells (HUVEC) incubated with conditioned medium from fibroblasts stimulated with HMGB1 and anti-VEGF. | (a) ↑VEGF, HIF-1α, p-Erk, p-JNK; (b) ↑migration and tube formation of HUVEC; (c) ↓migration and tube formation of HUVEC. | |
| Zhan-Chun et al. [84] | 2011 | Synovial fluid from OA patients | ↑HMGB1 compared with controls; HMGB1 levels are positively correlated with X-ray severity. | |
| Aulin et al. [85] | 2022 | Synovial fluid from patients with recent or old knee injuries, OA knee, and a healthy knee | Expressions of HMGB1 in recent injury > old injury > OA; HMGB1 is associated with cartilage biomarkers. | |
| Ding et al. [86] | 2020 | Synovial fluid from patients with acute or chronic anterior cruciate ligament (ACL) injuries | HMGB1 in chronic group > acute group (p=0,075 statistical significance not reached). | |
| García-Arnandis et al. [87] | 2010 | OA patients’ synoviocytes incubated with IL-1β and overexpression of haem oxygenase-1. | ↓mRNA and protein levels of HMGB1, MMP-1 and MMP-3; ↓ from the nucleus to cytoplasm translocation of HMGB1. | |
| Zhang et al. [88] | 2018 | (a) Human chondrocytes pretreated with IL-1β and (b) then incubated with chrysin. | (a) ↑HMGB1, MMP-13, collagenase, and IL-6; ↓COL2A1; ↑apoptotic rate (b) ↓HMGB1, MMP-13, collagenase, and IL-6; ↑COL2A1; ↓apoptotic rate. |
| Authors | Year | Cell Cultures | Biological Fluid | Outcomes |
|---|---|---|---|---|
| Rai et al. [89] | 2022 | (a) Osteoarthritic human chondrocytes: (b) OA knee and (c) OA hip chondrocytes; (d) NHAC incubated with IL-33; (e) NHAC incubated with IL-37; (f) NHAC incubated with IL-37 followed by IL-33, LPS, or rHMGB1 and (g) NHAC incubated only with IL-33, LPS or rHMGB1. | (a) ↑mRNA of IL-33, IL-37, TLR2, TLR4, NF-κB, IL-6, TNF-α, MMP2, and MMP9; ↑protein expression IL-37, TLR2, TLR4, NF-κB, IL-6, TNF-α, MMP2, and MMP9; mRNA and protein levels of IL-33, TLR2, TLR4, NF-κB, MMP2, and MMP9 in (b) > (c); protein level of IL-37 in (c) > (b); (d) ↑mRNA of IL-33, TNF-α, IL-6, TLR2, TLR4, MMP2, MMP9, NF-κB, HMGB1, RAGE, and ↑M1 macrophage; (e) ↓mRNA levels of IL-37, TLR2, TLR4, IL-6, TNF-α, NF-κB, MMP, MMP9, RAGE and HMGB1; ↓TLR2, TLR4, IL-6, TNF-α, NF-κB, MMP2, MMP9, RAGE, and HMGB1 mRNA levels in (f) < (g). | |
| Li et al. [90] | 2017 | (a) OA knee cartilage samples: (c) chondrocytes of weight-bearing areas; (d) normal human primary chondrocytes incubated with supernatant from healthy and (e) injured cartilage lysates; (f) normal human primary chondrocytes incubated with supernatant from injured cartilage lysates with and (g) without RNase A; (h) normal human primary chondrocytes incubated with supernatant from injured cartilage lysates with TLR3 silencing and (i) with TLR7 knocking down; (l) IL-33-stimulated human chondrocytes in vitro; human chondrocytes incubated with commercial dsRNA analogue poly(I:C) and (m) with or (n) without IL-33 siRNA. | (b) Synovial fluid from OA patients | (a)↑mRNA and protein levels of IL-33, MMP1 and MMP13; (b)↑protein levels of IL-33, MMP1 and MMP13; (c)↑mRNA levels of IL-33, MMP1 and MMP13; ↑mRNA and protein levels of IL-33, MMP1 and MMP13 and ↓ collagen II in (e) > (d); ↑mRNA and protein levels of IL-33, MMP1 and MMP13, and ↓collagen II in (g) > (f); ↑mRNA and protein levels of IL-33, MMP1 and MMP13, and ↓collagen II in (i) > (h); (l) ↑mRNA and protein levels of MMP1 and MMP13 and ↓collagen II; mRNA of MMP1 and MMP13 in (n) > (m). |
| He et al. [91] | 2020 | (a) OA human chondrocytes | (b) Serum and synovial fluid of OA patients | (a) ↑mRNA and protein levels of IL-33 and ST2; (b) ↑levels of IL-33 only in synovial fluid. |
| Hong et al. [92] | 2011 | Serum of OA patients | ↑Levels of IL-33 |
| Authors | Year | Cell Cultures | Biological Fluid | Outcomes |
|---|---|---|---|---|
| Zhu et al. [93] | 2018 | (a) OA cartilage samples: human synovial fibroblast from the normal knee (b) transfected with S100B overexpression or (c) knockdown siRNA and incubated with LPS; (d) human synovial fibroblast transfected with S100B overexpression, incubated with LPS, and exposed to FGFR1 siRNA. | (a) ↑S100B, TNF-α and IL-1β levels; strong correlation between S100B levels and TNF-α and IL-1β expression; (b) ↑TNF-α and IL-1β protein levels and FGFR1 mRNA and protein expression in medium; (c) ↓TNF-α and IL-1β levels and FGFR1 mRNA and protein expression in medium; (d)↓TNF-α and IL-1β levels. | |
| Loeser et al. [94] | 2005 | (a) chondrocytes incubated with HMGB1 and S100B; (b) chondrocytes incubated with HMGB1 and S100B and soluble RAGE. | (a) ↑p-ERK-1/2, p-p65 and MMP-13 protein levels; (b) ↓p-ERK-1/2, p-p65 and MMP-13 protein levels. |
| Key Points |
|---|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, A.; Atzeni, F.; Murdaca, G.; Gangemi, S. The Role of Alarmins in Osteoarthritis Pathogenesis: HMGB1, S100B and IL-33. Int. J. Mol. Sci. 2023, 24, 12143. https://doi.org/10.3390/ijms241512143
Palumbo A, Atzeni F, Murdaca G, Gangemi S. The Role of Alarmins in Osteoarthritis Pathogenesis: HMGB1, S100B and IL-33. International Journal of Molecular Sciences. 2023; 24(15):12143. https://doi.org/10.3390/ijms241512143
Chicago/Turabian StylePalumbo, Antonino, Fabiola Atzeni, Giuseppe Murdaca, and Sebastiano Gangemi. 2023. "The Role of Alarmins in Osteoarthritis Pathogenesis: HMGB1, S100B and IL-33" International Journal of Molecular Sciences 24, no. 15: 12143. https://doi.org/10.3390/ijms241512143
APA StylePalumbo, A., Atzeni, F., Murdaca, G., & Gangemi, S. (2023). The Role of Alarmins in Osteoarthritis Pathogenesis: HMGB1, S100B and IL-33. International Journal of Molecular Sciences, 24(15), 12143. https://doi.org/10.3390/ijms241512143







