Transcriptome Analysis Reveals Brassinolide Signaling Pathway Control of Foxtail Millet Seedling Starch and Sucrose Metabolism under Freezing Stress, with Implications for Growth and Development
Abstract
1. Introduction
2. Results
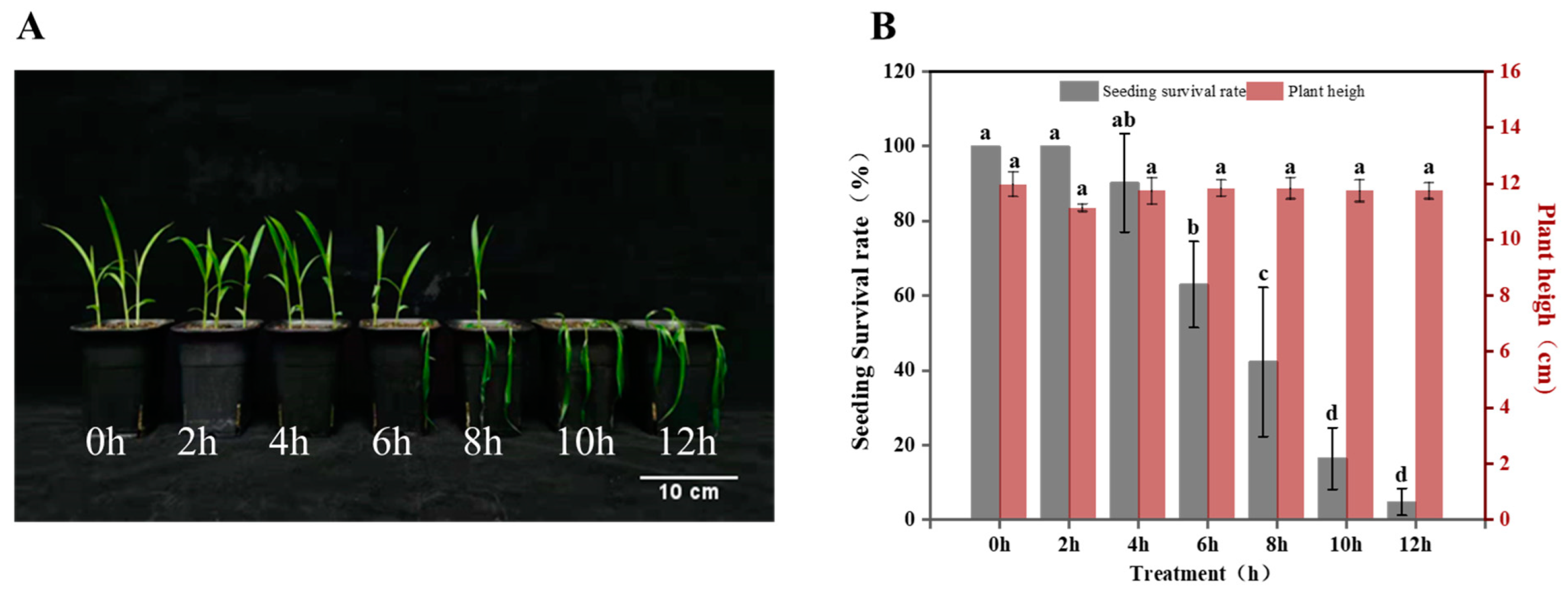
2.1. Seedling Survival Rate of Foxtail Millet Leave during Freezing Stress
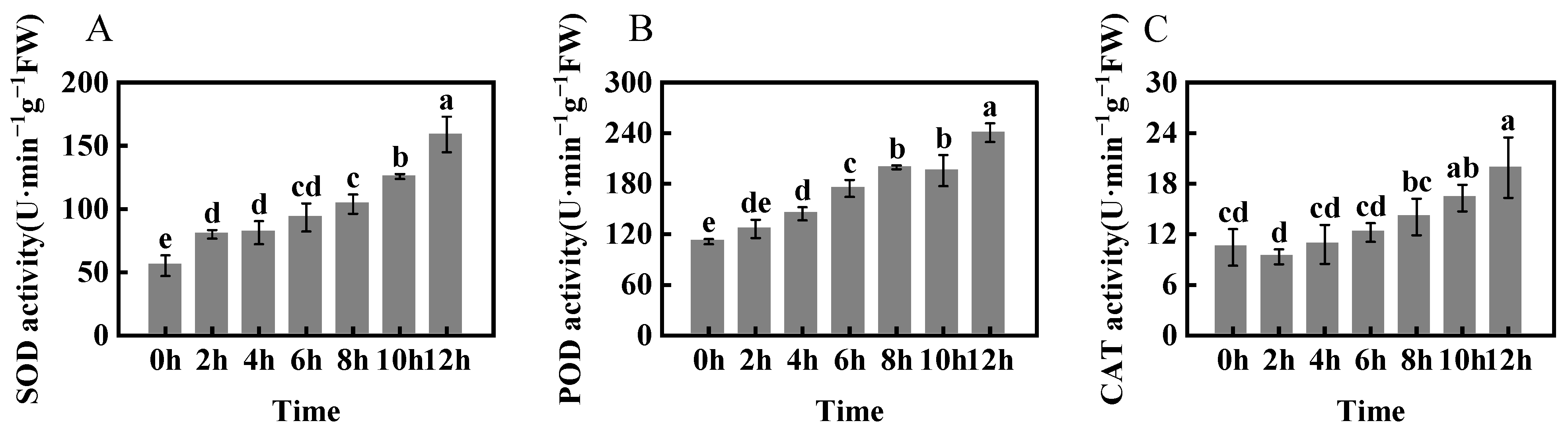
2.2. Antioxidant Enzymatic Activities of Foxtail Millet under Freezing Stress
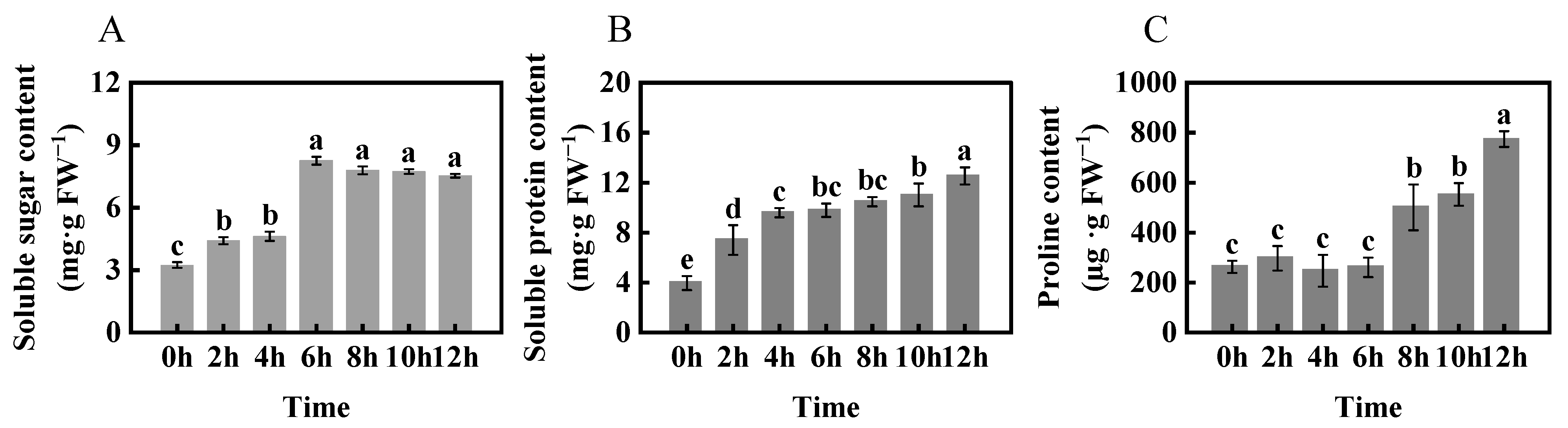
2.3. Changes in Osmotic Adjustment of Foxtail Millet under Freezing Stress
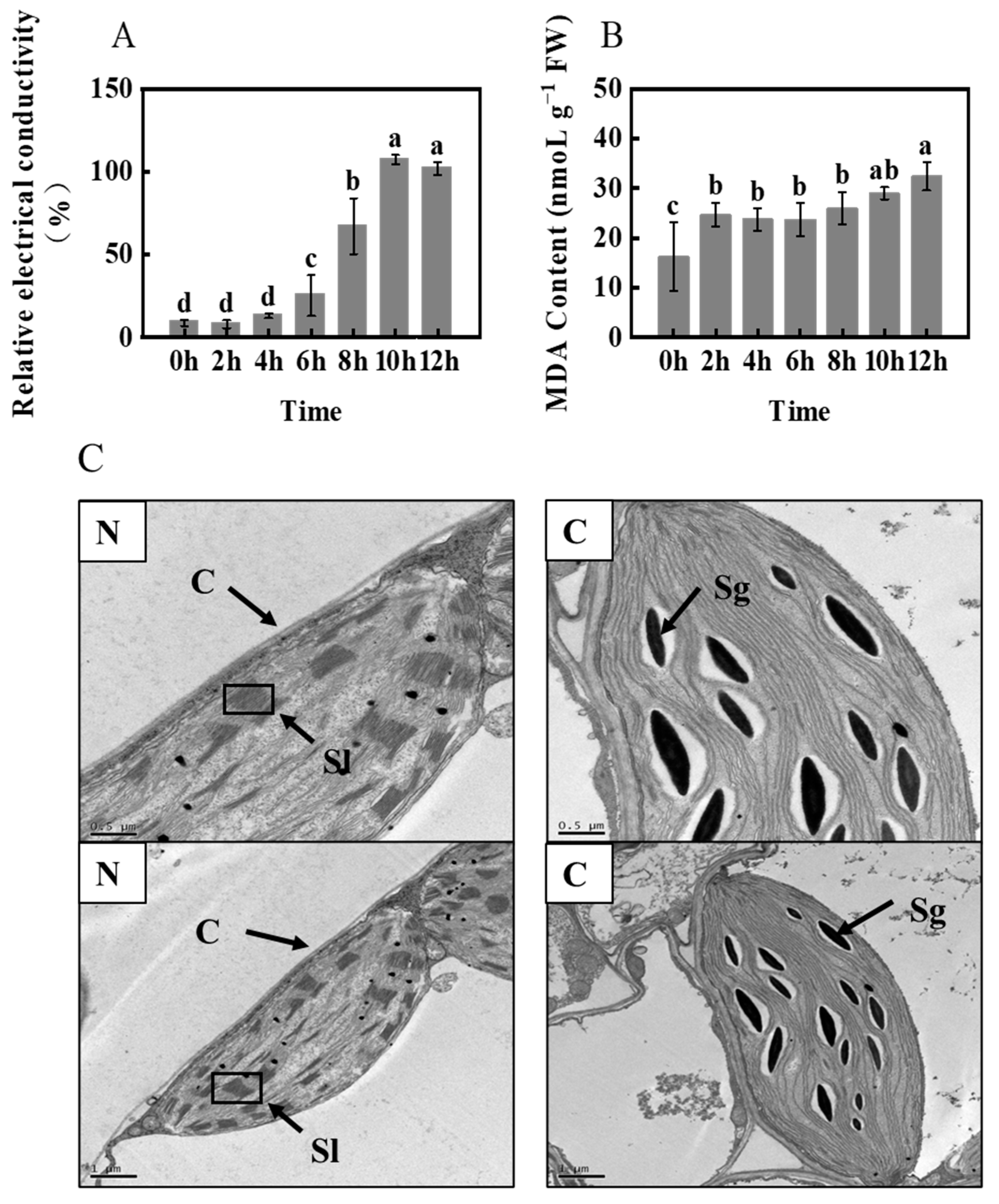
2.4. Membrane Injury and Chloroplast Ultrastructures of Foxtail Millet under Freezing Stress
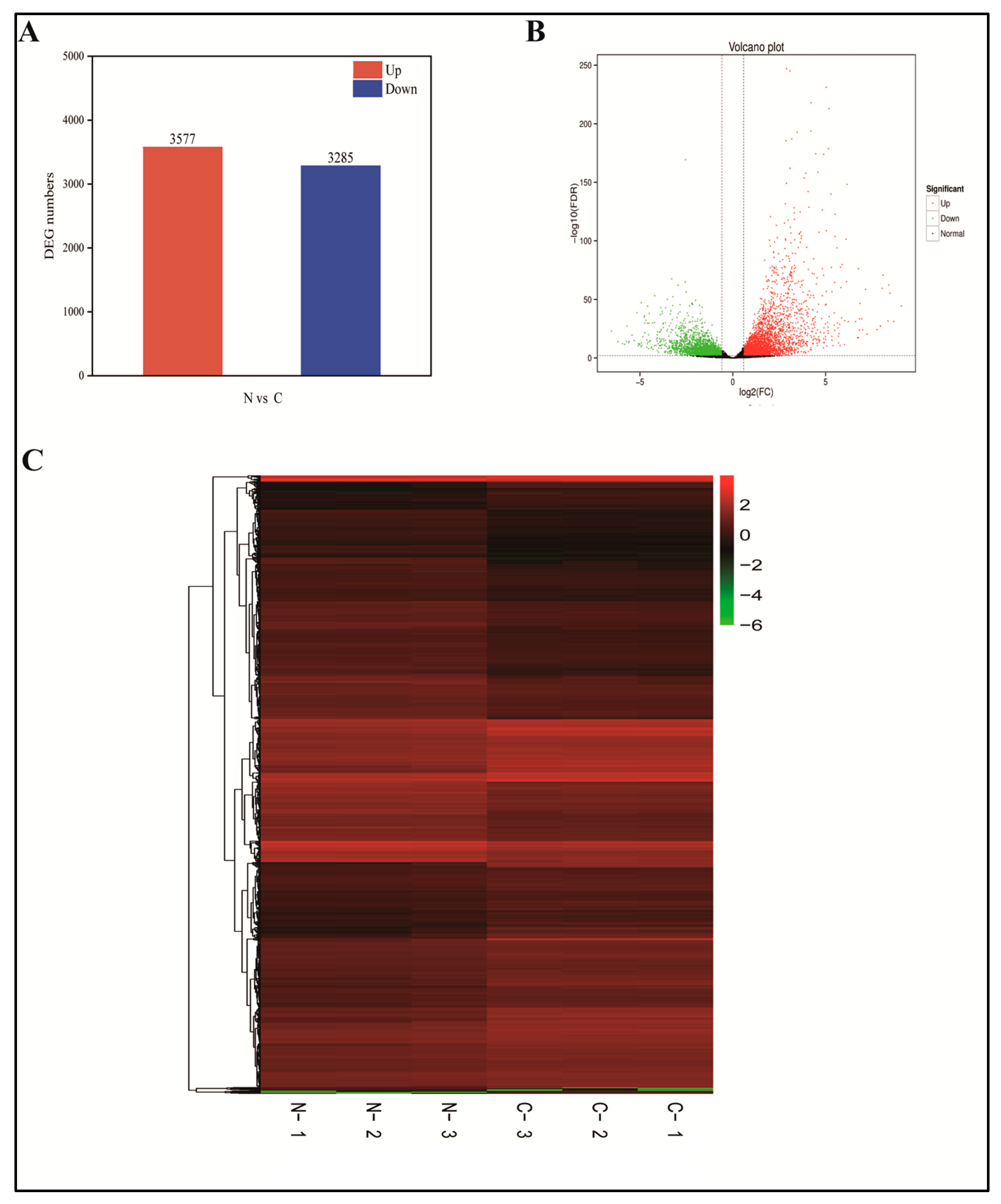
2.5. Transcriptome Sequencing and Correlation Analysis
2.6. Defining DEGs
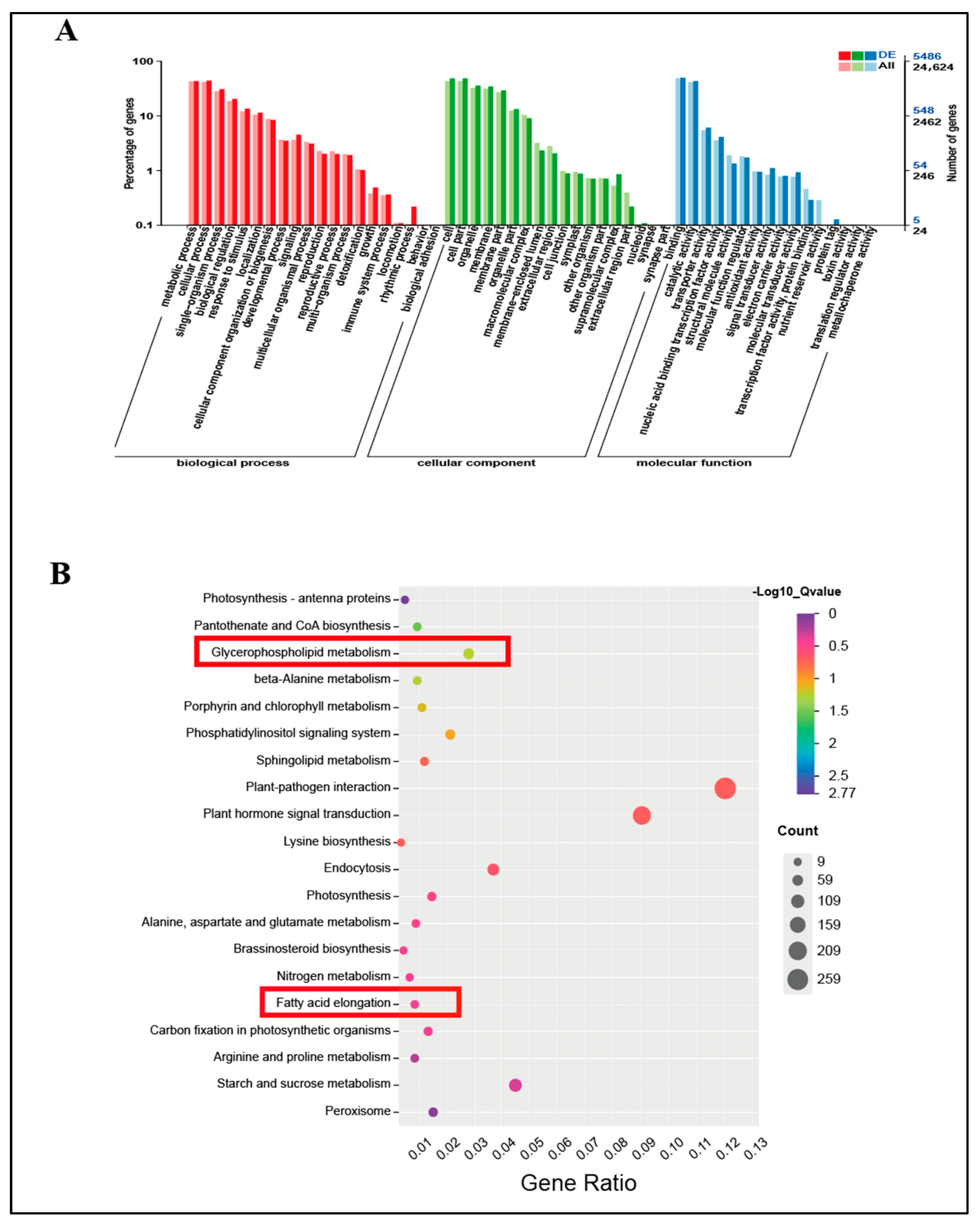
2.7. GO and KEGG Analyses of Freezing Stress DEGs
2.8. Starch and Sucrose Metabolism and the Antioxidant Defense Systema Related DEGs under Freezing Stress
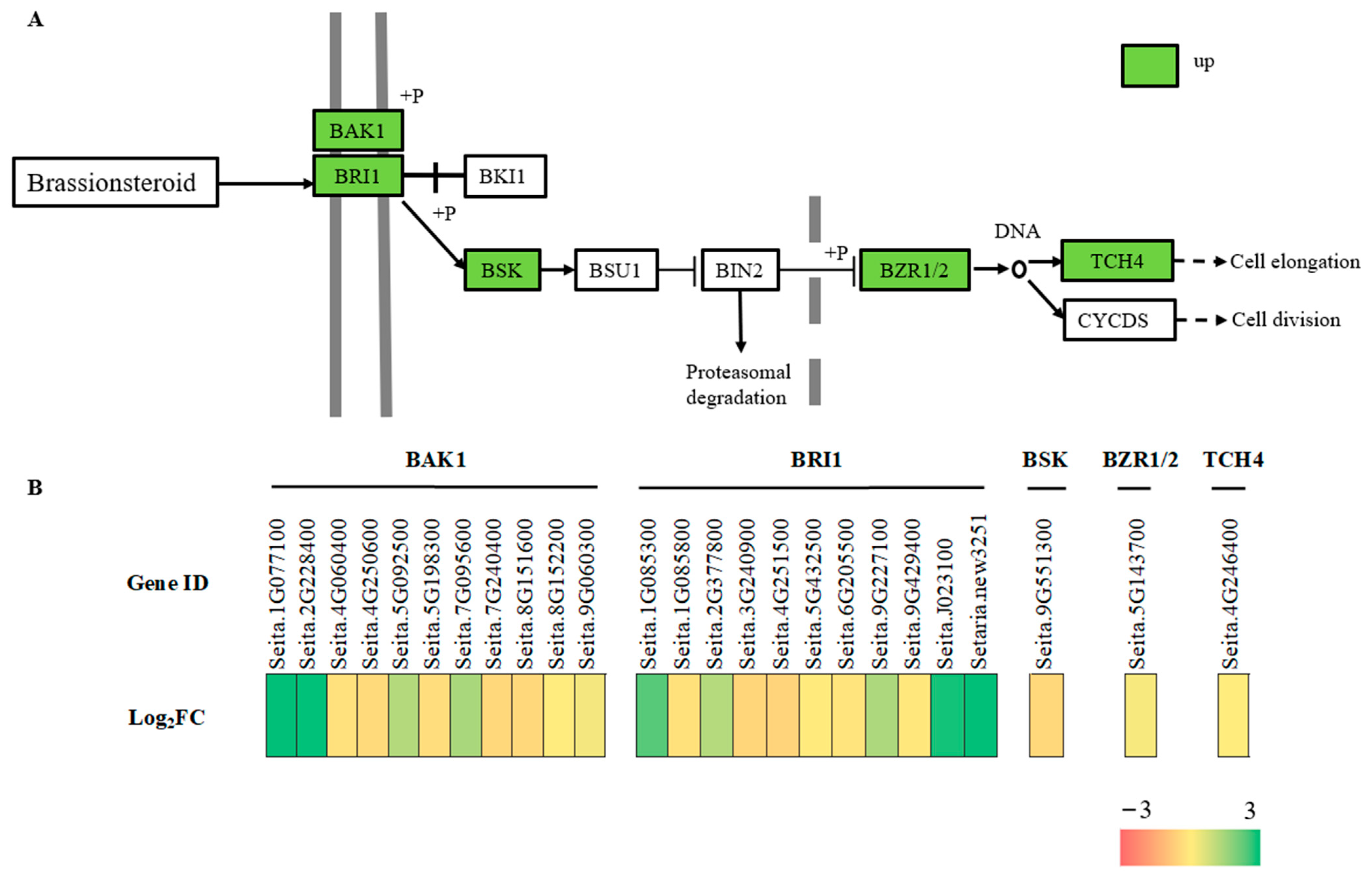
2.9. Brassinosteroids Signaling Pathway Related DEGs
2.10. Transcription Factors under Freezing Stress
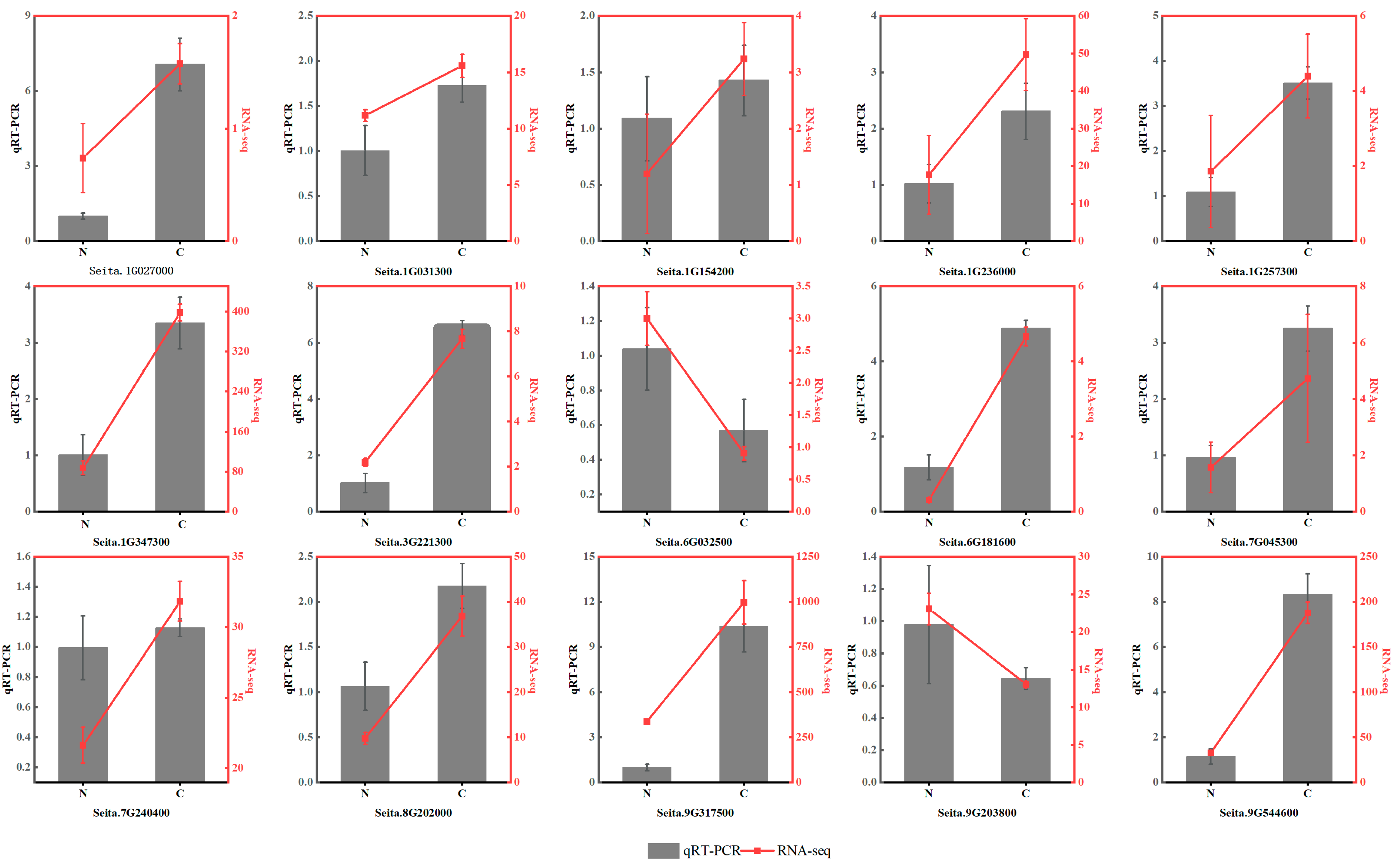
2.11. Quantitative Real-Time PCR (qRT–PCR) Analysis
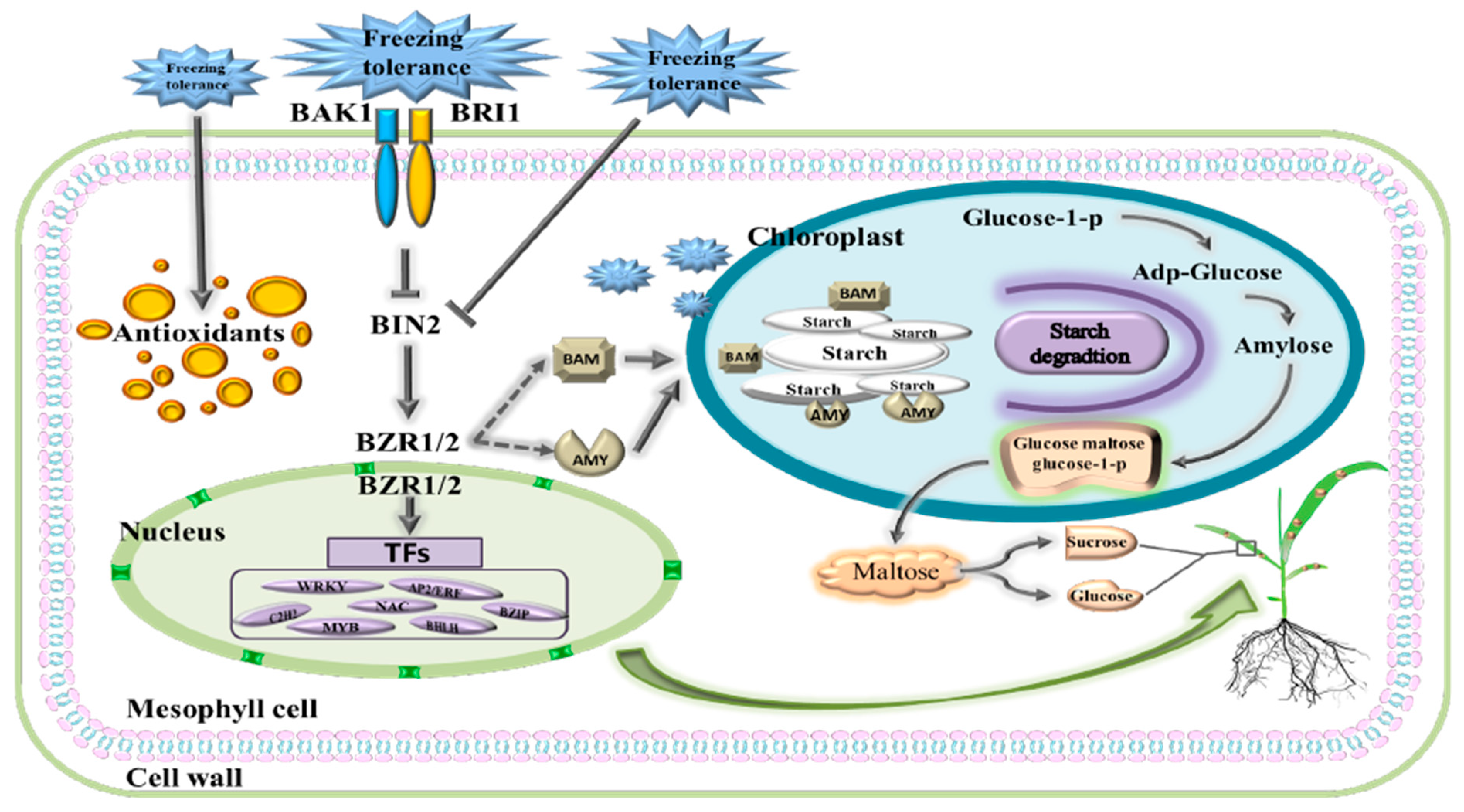
3. Discussion
3.1. Effects of Freezing Stress on Membrane Injury of Foxtail Millet Leaves
3.2. Metabolic Pathways of Starch and Sucrose under Freezing Stress
3.3. BR Signal Network Responses to Freezing Stress
3.4. TFs Responding to Freezing Stress
4. Materials and Methods
4.1. Plant Materials and Freezing Treatment
4.2. Determinations of Leaf Physiological Indicators
4.2.1. Growth Index Measurement
4.2.2. Physiological Indicators
4.2.3. Transmission Electron Microscopy
4.3. RNA Extraction, Library Preparation, RNA-Seq, and Sequence Assembly
4.4. qRT–PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.; Yang, S. Insights into the regulation of C-repeat binding factors in plant cold signaling. J. Integr. Plant Biol. 2018, 60, 780–795. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Barrero-Gil, J.; Salinas, J. Gene Regulatory Networks Mediating Cold Acclimation: The CBF Pathway. In Survival Strategies in Extreme Cold and Desiccation: Adaptation Mechanisms and Their Applications; Iwaya-Inoue, M., Sakurai, M., Uemura, M., Eds.; Springer: Singapore, 2018; pp. 3–22. [Google Scholar]
- Nayyar, H.; Bains, T.S.; Kumar, S.; Kaur, G. Chilling effects during seed filling on accumulation of seed reserves and yield of chickpea. Sci. Food Agric. 2005, 85, 1925–1930. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, D.; Chai, X.; Wu, Y.; Wang, Y.; Nan, Z.; Yang, Q.; Liu, W.; Liu, Z. Multiple Regulatory Networks Are Activated during Cold Stress in Medicago sativa L. Int. J. Mol. Sci. 2018, 19, 3169. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Zhou, X.; Muhammad, I.; Lan, H.; Xia, C. Recent Advances in the Analysis of Cold Tolerance in Maize. Front. Plant Sci. 2022, 13, 866034. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.E.; Bamberg, J.B. Screening the U.S. potato collection for frost hardiness. Am. Potato J. 1995, 72, 13–21. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Ji, K.; Wang, Y.; Sun, W.; Lou, Q.; Mei, H.; Shen, S.; Chen, H. Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012, 169, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Wei, Z.; Xiaokai, L.; Jing, N.; Honghong, H.; Xianghua, L.; Jinghua, X.; Lizhong, X. The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. J. Exp. Bot. 2012, 64, 569. [Google Scholar]
- Wang, M.; Zhang, X.; Liu, J.-H. Deep sequencing-based characterization of transcriptome of trifoliate orange (Poncirus trifoliata (L.) Raf.) in response to cold stress. BMC Genom. 2015, 16, 555. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Geng, J.C.; Miao, Y.J.; Xu, Y.M.; Hu, T.M.; Yang, P.Z. Transcriptomic analyses reveal genotype- and organ-specific molecular responses to cold stress in Elymus nutans. Biol. Plant. 2018, 62, 671–683. [Google Scholar] [CrossRef]
- Arfan, M.; Zhang, D.W.; Zou, L.J.; Luo, S.S.; Lin, H.H. Hydrogen Peroxide and Nitric Oxide Crosstalk Mediates Brassinosteroids Induced Cold Stress Tolerance in Medicago truncatula. Int. J. Mol. Sci. 2019, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Eremina, M.; Rozhon, W.; Poppenberger, B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2016, 73, 797–810. [Google Scholar] [CrossRef]
- Wu, K.; Duan, X.; Zhu, Z.; Sang, Z.; Duan, J.; Jia, Z.; Ma, L. Physiological and transcriptome analysis of Magnolia denudata leaf buds during long-term cold acclimation. BMC Plant Biol. 2021, 21, 460. [Google Scholar] [CrossRef]
- Misra, V.; Mall, A.K. Plant sugar transporters and their role in abiotic stress. In Transporters and Plant Osmotic Stress; Roychoudhury, A., Tripathi, D.K., Deshmukh, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 101–112. [Google Scholar]
- Pollock, C.J.; Lloyd, E.J. The Effect of Low Temperature upon Starch, Sucrose and Fructan Synthesis in Leaves. Ann. Bot. 1987, 60, 231–235. [Google Scholar] [CrossRef]
- Savitch, L.V.; Harney, T.; Huner, N.P.A. Sucrose metabolism in spring and winter wheat in response to high irradiance, cold stress and cold acclimation. Physiol. Plant. 2000, 108, 270–278. [Google Scholar] [CrossRef]
- Li, H.S.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Principles and Techniques of Plant Physiological Biochemical Experimental; Higher Education: Beijing, China, 2000. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, L.; Wang, H.; Lu, J.; Wei, H.; Yu, S. Transcriptomic Profiling of Young Cotyledons Response to Chilling Stress in Two Contrasting Cotton (Gossypium hirsutum L.) Genotypes at the Seedling Stage. Int. J. Mol. Sci. 2020, 21, 5095. [Google Scholar] [CrossRef] [PubMed]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Stern, D.B. Increased Rubisco content in maize mitigates chilling stress and speeds recovery. Plant Biotechnol. J. 2020, 18, 1409–1420. [Google Scholar] [CrossRef]
- Augspurger, C.K. Reconstructing patterns of temperature, phenology, and frost damage ov er 124 years: Spring damage risk is increasing. Ecology 2013, 94, 41–50. [Google Scholar] [CrossRef]
- Gu, L.; Hanson, P.J.; Post, W.M.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 Eastern US Spring Freeze: Increased Cold Damage in a Warming World? BioScience 2008, 58, 253–262. [Google Scholar] [CrossRef]
- Xu, H.; Tong, Z.; He, F.; Li, X. Response of Alfalfa (Medicago sativa L.) to Abrupt Chilling as Reflected by Changes in Freezing Tolerance and Soluble Sugars. Agronomy 2020, 10, 255. [Google Scholar] [CrossRef]
- Florea, L.; Song, L.; Salzberg, S.L. Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues. F1000 Res. 2013, 2, 188. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, C.; Zhong, C.; Lu, T.; Gul, J.; Jin, X.; Zhang, Y.; Liu, Q. Transcriptome analysis of Sonneratia caseolaris seedlings under chilling stress. PeerJ 2021, 9, 11506. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Lyons, J.M. Chilling Injury in Plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, Z.; Li, H.; Yu, J.; Jiang, J.; Tang, Z.; Ma, D.; Zhang, B.; Han, Y.; Li, Z. High throughput sequencing identifies chilling responsive genes in sweet potato (Ipomoea batatas Lam.) during storage. Genomics 2019, 111, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, S.; Karadağoğlu, Ö.; Atici, Ö.; Nalbantoğlu, B. Protective role of salicylic acid applied before cold stress on antioxidative system and protein patterns in barley apoplast. Biol. Plant. 2013, 57, 507–513. [Google Scholar] [CrossRef]
- Wise, R.R.; Naylor, A.W. Chilling-Enhanced Photooxidation: The Peroxidative Destruction of Lip ids during Chilling Injury to Photosynthesis and Ultrastructure. Plant Physiol. 1987, 83, 272–277. [Google Scholar] [CrossRef]
- Deng, S.; Ma, J.; Zhang, L.; Chen, F.; Sang, Z.; Jia, Z.; Ma, L. De novo transcriptome sequencing and gene expression profiling of Magnolia wufengensis in response to cold stress. BMC Plant Biol. 2019, 19, 321. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Song, H.; Chen, M.; Cheng, S. Protective Role of Leaf Variegation in Pittosporum tobira under Low Temperature: Insights into the Physio-Biochemical and Molecular Mechanisms. Int. J. Mol. Sci. 2019, 20, 4857. [Google Scholar] [CrossRef]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K.; Arumugam, N. The FAD2 Gene in Plants: Occurrence, Regulation, and Role. Front. Plant Sci. 2017, 8, 1789. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-P.; Huang, L.-M.; Chen, L.-F.O.; Chan, M.-T.; Shaw, J.-F. Genome-wide analysis of GDSL-type esterases/lipases in Arabidopsis. Plant Mol. Biol. 2017, 95, 181–197. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, Y. Genome-wide identification of the trehalose-6-phosphate synthase gene family in sweet orange (Citrus sinensis) and expression analysis in response to phytohormones and abiotic stresses. PeerJ 2022, 10, e13934. [Google Scholar] [CrossRef]
- Wang, K.; Bai, Z.-Y.; Liang, Q.-Y.; Liu, Q.-L.; Zhang, L.; Pan, Y.-Z.; Liu, G.-L.; Jiang, B.-B.; Zhang, F.; Jia, Y. Transcriptome analysis of chrysanthemum (Dendranthema grandiflorum) in response to low temperature stress. BMC Genom. 2018, 19, 319. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhu, X.; Duan, N.; Liu, J.-H. PtrBAM1, a β-amylase-coding gene of Poncirus trifoliata, is a CBF regu lon member with function in cold tolerance by modulating soluble sugar levels. Plant Cell Environ. 2014, 37, 2754–2767. [Google Scholar] [CrossRef]
- Kaplan, F.; Guy, C.L. RNA interference of Arabidopsis beta-amylase8 prevents maltose accumul ation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J. Cell Mol. Biol. 2005, 44, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; Kong, F.; Zhang, S.; Meng, C.; Yang, M.; Liu, Z.; Wang, Y.; Ma, N.; Meng, Q. Whirly1 enhances tolerance to chilling stress in tomato via protection of photosystem II and regulation of starch degradation. New Phytol. 2019, 221, 1998–2012. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Pan, J.; Zhang, M.; Xing, X.; Zhou, Y.; Liu, Y.; Li, D.; Li, D. ZmMKK4, a novel group C mitogen-activated protein kinase kinase in mai ze (Zea mays), confers salt and cold tolerance in transgenic Arabidops is. Plant Cell Environ. 2011, 34, 1291–1303. [Google Scholar] [CrossRef]
- Peng, X.; Teng, L.; Yan, X.; Zhao, M.; Shen, S. The cold responsive mechanism of the paper mulberry: Decreased photosy nthesis capacity and increased starch accumulation. BMC Genom. 2015, 16, 898. [Google Scholar] [CrossRef] [PubMed]
- Filek, M.; Rudolphi-Skórska, E.; Sieprawska, A.; Kvasnica, M.; Janeczko, A. Regulation of the membrane structure by brassinosteroids and progesterone in winter wheat seedlings exposed to low temperature. Steroids 2017, 128, 37–45. [Google Scholar] [CrossRef]
- Fu, J.; Sun, P.; Luo, Y.; Zhou, H.; Gao, J.; Zhao, D.; Pubu, Z.; Liu, J.; Hu, T. Brassinosteroids enhance cold tolerance in Elymus nutans via mediating redox homeostasis and proline biosynthesis. Environ. Exp. Bot. 2019, 167, 103831. [Google Scholar] [CrossRef]
- Zhang, G.; Song, X.; Guo, H.; Wu, Y.; Chen, X.; Fang, R. A Small G Protein as a Novel Component of the Rice Brassinosteroid Signal Transduction. Mol. Plant 2016, 9, 1260–1271. [Google Scholar] [CrossRef]
- Nolan, T.M.; Nemanja, V.; Liu, D.; Eugenia, R.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2019, 32, 295–318. [Google Scholar] [CrossRef]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 Positively Regulates Freezing Tolerance via CBF-Dependent and CBF-Independent Pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef]
- Reinhold, H.; Soyk, S.; Šimková, K.; Hostettler, C.; Marafino, J.; Mainiero, S.; Vaughan, C.K.; Monroe, J.D.; Zeeman, S.C. β-Amylase–Like Proteins Function as Transcription Factors in Arabidopsis, Controlling Shoot Growth and Development. Plant Cell 2011, 23, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Henderson, D.A.; Zhu, J.-K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 2005, 17, 3155–3175. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Katiyar, A.; Smita, S.; Lenka, S.K.; Rajwanshi, R.; Chinnusamy, V.; Bansal, K.C. Genome-wide classification and expression analysis of MYB transcriptio n factor families in rice and Arabidopsis. BMC Genom. 2012, 13, 544. [Google Scholar] [CrossRef]
- Zou, C.; Jiang, W.; Yu, D. Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J. Exp. Bot. 2010, 61, 3901–3914. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, N.; Jiao, Y.; Li, R.; Xiao, D.; Wang, Z. The grapevine basic helix-loop-helix (bHLH) transcription factor posit ively modulates CBF-pathway and confers tolerance to cold-stress in Ar abidopsis. Mol. Biol. Rep. 2014, 41, 5329–5342. [Google Scholar] [CrossRef]
- Hou, X.-M.; Zhang, H.-F.; Liu, S.-Y.; Wang, X.-K.; Zhang, Y.-M.; Meng, Y.-C.; Luo, D.; Chen, R.-G. The NAC transcription factor CaNAC064 is a regulator of cold stress tolerance in peppers. Plant Sci. 2020, 291, 110346. [Google Scholar] [CrossRef]
- Guo, L.; Yang, H.; Zhang, X.; Yang, S. Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J. Exp. Bot. 2013, 64, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, J.; Zhao, P.; Guo, X.; Chen, S.; Qi, D.; Cheng, L.; Liu, G. LcMYB4, an unknown function transcription factor gene from sheepgrass, as a positive regulator of chilling and freezing tolerance in transgenic Arabidopsis. BMC Plant Biol. 2020, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Q.; Huang, Q.-X.; Lin, P.; Zeng, Q.-H.; Li, Y.; Liu, Q.-L.; Zhang, L.; Pan, Y.-Z.; Jiang, B.-B.; Zhang, F. The Overexpression of a Transcription Factor Gene VbWRKY32 Enhances the Cold Tolerance in Verbena bonariensis. Front. Plant Sci. 2020, 10, 1746. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xiang, X.; Liu, D.; Yang, A.; Wang, Y. Tobacco Transcription Factor NtbHLH123 Confers Tolerance to Cold Stress by Regulating the NtCBF Pathway and Reactive Oxygen Species Homeostasis. Front. Plant Sci. 2018, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.T.S.; Schlögl, P.S.; Camargo, S.R.; Fernandez, J.H.; De Rosa, V.E.; Pompermayer, P.; Arruda, P. SsNAC23, a member of the NAC domain protein family, is associated with cold, herbivory and water stress in sugarcane. Plant Sci. 2005, 169, 93–106. [Google Scholar] [CrossRef]
- Zhou, W.; Leul, M. Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regul. 1998, 26, 41–47. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2–R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Yi, G.-X.; Wang, B.-M.; Deng, A.-X.; Nan, T.-G.; Li, Z.-H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked imm unosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79–85. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S.; et al. A comprehensive evolutionary classification of proteins encoded in com plete eukaryotic genomes. Genome Biol. 2004, 5, R7. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32 (Suppl. 1), D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Sample | Clean Reads | Clean Bases | GC Content | % ≥ Q30 | Mapped Reads | Uniq Mapped Reads |
|---|---|---|---|---|---|---|
| N-1 | 36,475,245 | 10,900,981,318 | 53.90% | 94.82% | 94.76% | 91.27% |
| N-2 | 38,642,791 | 11,547,424,178 | 53.67% | 94.75% | 94.65% | 91.17% |
| N-3 | 38,450,107 | 11,483,404,412 | 53.33% | 94.06% | 94.46% | 91.32% |
| C-1 | 28,449,360 | 8,503,541,058 | 52.94% | 94.65% | 95.00% | 92.38% |
| C-2 | 28,701,001 | 8,585,680,730 | 53.39% | 95.03% | 95.22% | 92.54% |
| C-3 | 37,159,829 | 11,117,086,398 | 52.79% | 94.84% | 94.65% | 92.12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Ma, K.; Li, Z.; Li, W.; Zhang, X.; Liu, S.; Meng, R.; Lu, B.; Li, X.; Ren, J.; et al. Transcriptome Analysis Reveals Brassinolide Signaling Pathway Control of Foxtail Millet Seedling Starch and Sucrose Metabolism under Freezing Stress, with Implications for Growth and Development. Int. J. Mol. Sci. 2023, 24, 11590. https://doi.org/10.3390/ijms241411590
Zhao X, Ma K, Li Z, Li W, Zhang X, Liu S, Meng R, Lu B, Li X, Ren J, et al. Transcriptome Analysis Reveals Brassinolide Signaling Pathway Control of Foxtail Millet Seedling Starch and Sucrose Metabolism under Freezing Stress, with Implications for Growth and Development. International Journal of Molecular Sciences. 2023; 24(14):11590. https://doi.org/10.3390/ijms241411590
Chicago/Turabian StyleZhao, Xiatong, Ke Ma, Zhong Li, Weidong Li, Xin Zhang, Shaoguang Liu, Ru Meng, Boyu Lu, Xiaorui Li, Jianhong Ren, and et al. 2023. "Transcriptome Analysis Reveals Brassinolide Signaling Pathway Control of Foxtail Millet Seedling Starch and Sucrose Metabolism under Freezing Stress, with Implications for Growth and Development" International Journal of Molecular Sciences 24, no. 14: 11590. https://doi.org/10.3390/ijms241411590
APA StyleZhao, X., Ma, K., Li, Z., Li, W., Zhang, X., Liu, S., Meng, R., Lu, B., Li, X., Ren, J., Zhang, L., & Yuan, X. (2023). Transcriptome Analysis Reveals Brassinolide Signaling Pathway Control of Foxtail Millet Seedling Starch and Sucrose Metabolism under Freezing Stress, with Implications for Growth and Development. International Journal of Molecular Sciences, 24(14), 11590. https://doi.org/10.3390/ijms241411590





