Role of SiPHR1 in the Response to Low Phosphate in Foxtail Millet via Comparative Transcriptomic and Co-Expression Network Analyses
Abstract
1. Introduction
2. Results
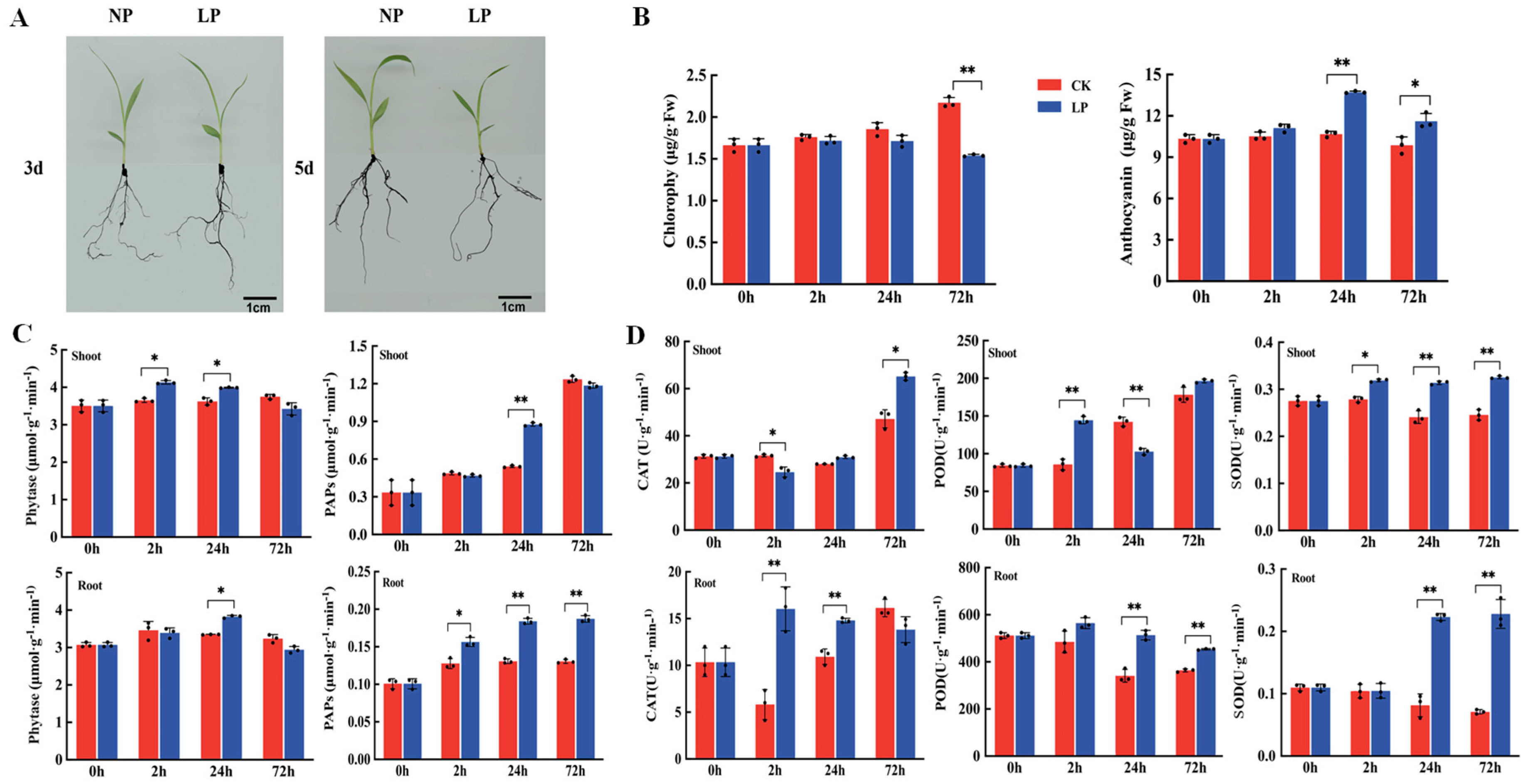
2.1. Chlorophyll and Anthocyanin Contents and Antioxidant Enzyme Activity under Phosphorus-Deficiency Treatment
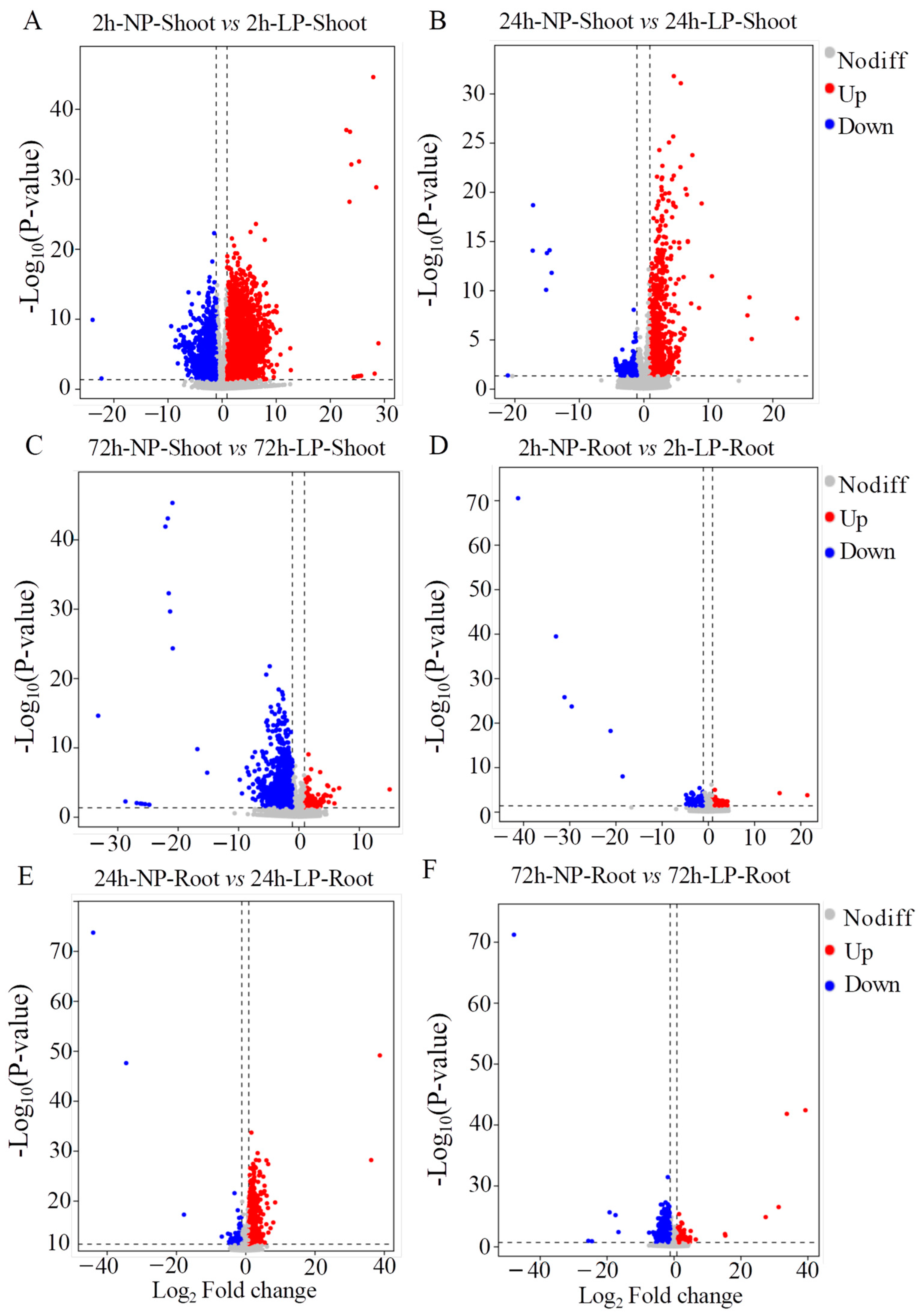
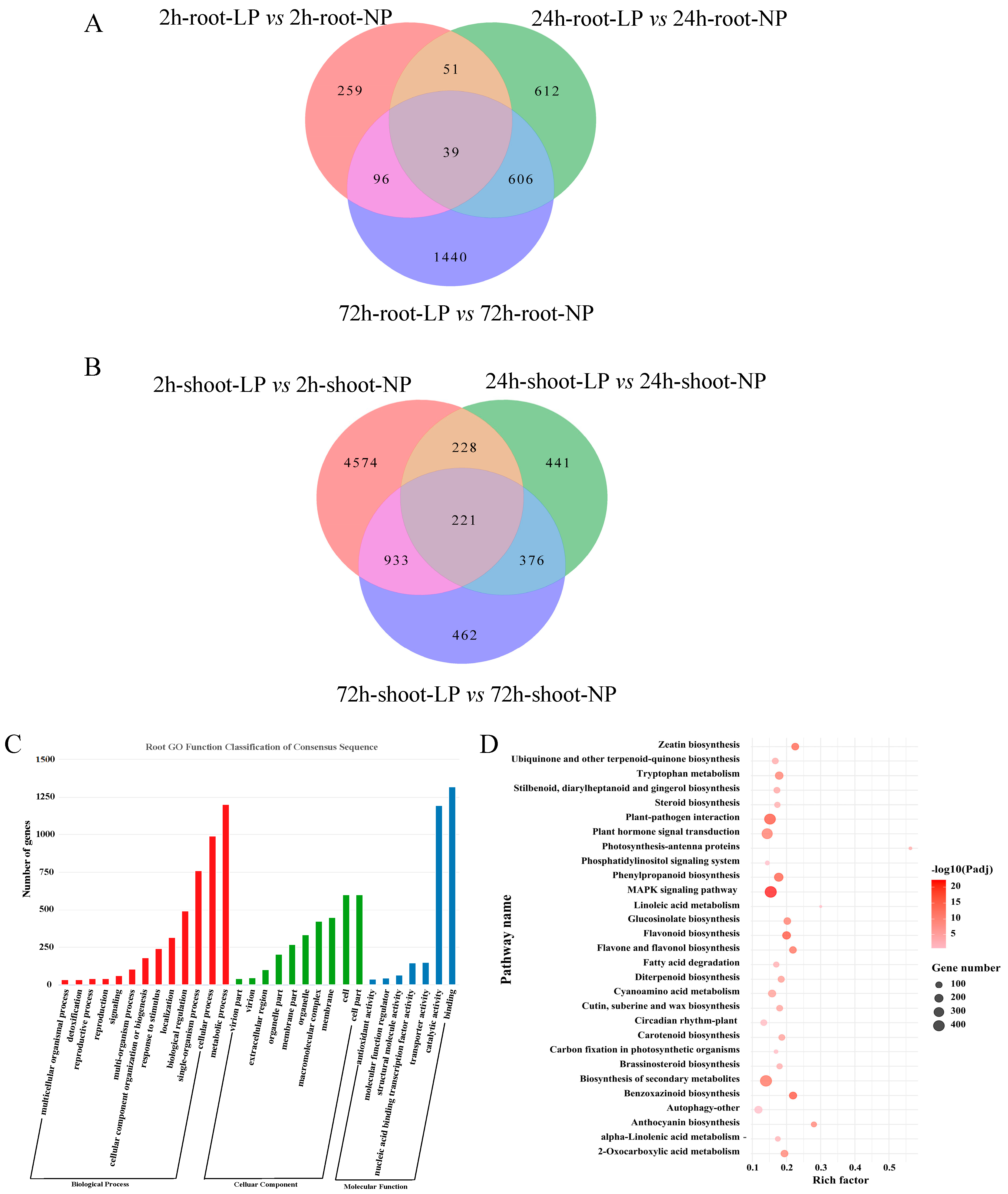
2.2. Transcriptome Analyses of Foxtail Millet Shoots and Roots Exposed to LP Treatment
2.3. Gene Co-Expression Network Analysis of Foxtail Millet Related to the Pi Response
2.4. Gene Structure, Protein Domains, and Analysis of SiPHR1 in Relation to LP Responsiveness
2.5. Subcellular Location and SiPHR1 Overexpression in Arabidopsis enhanced LP Tolerance Ability
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination Content of Chlorophyll and Anthocyanin Contents
4.3. Determination of Antioxidant Enzyme, Purple Acid Phosphatase, and Phytase Activities
4.4. RNA Extraction and RNA-seq
4.5. Analysis of RNA-seq Data and Differentially Expressed Genes
4.6. Functional Annotation and WGCNA Analysis of DEGs
4.7. qRT-PCR Analyses
4.8. Vector Construction and Genetic Transformation of Arabidopsis and Tobacco
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadeem, F.; Ahmad, Z.; Wang, R.; Han, J.; Shen, Q.; Chang, F.; Diao, X.; Zhang, F.; Li, X. Foxtail millet [Setaria italica (L.) Beauv.] grown under low nitrogen shows a smaller root system, enhanced biomass accumulation, and nitrate transporter expression. Front. Plant Sci. 2018, 9, 205. [Google Scholar] [CrossRef]
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate acquisition. Plant Soil 2005, 274, 37–49. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use. FAO Fertil. Plant Nutr. Bull. 2008, 18, 5–50. [Google Scholar]
- Péret, B.; Desnos, T.; Jost, R.; Kanno, S.; Berkowitz, O.; Nussaume, L. Root architecture responses: In search of phosphate. Plant Physiol. 2014, 166, 1713–1723. [Google Scholar] [CrossRef]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef]
- Strock, C.F.; Morrow De La Riva, L.; Lynch, J.P. Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiol. 2018, 176, 691–703. [Google Scholar] [CrossRef]
- Luo, B.; Ma, P.; Nie, Z.; Zhang, X.; He, X.; Ding, X.; Feng, X.; Lu, Q.; Ren, Z.; Lin, H.; et al. Metabolite profiling and genome-wide association studies reveal response mechanisms of phosphorus deficiency in maize seedling. Plant J. 2018, 97, 947–969. [Google Scholar] [CrossRef]
- Peret, B.; Clement, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant Biol. 2014, 56, 192–220. [Google Scholar] [CrossRef]
- Prathap, V.; Kumar, A.; Maheshwari, C.; Tyagi, A. Phosphorus homeostasis: Acquisition, sensing, and long-distance signaling in plants. Mol. Biol. Rep. 2022, 49, 8071–8086. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, Y.; Pei, W.; Jain, A.; Sun, R.; Cao, Y.; Wu, X.; Jiang, T.; Zhang, L.; Fan, X.; et al. Involvement of OsPht1;4 in phosphate acquisition and mobilization facilitates embryo development in rice. Plant J. 2015, 82, 556–569. [Google Scholar] [CrossRef]
- Ngo, A.H.; Nakamura, Y. Phosphate starvation-inducible GLYCEROPHOSPHODIESTER PHOSPHODIESTERASE6 is involved in Arabidopsis root growth. J. Exp. Bot. 2022, 73, 2995–3003. [Google Scholar] [CrossRef]
- Liu, F.; Chang, X.J.; Ye, Y.; Xie, W.B.; Wu, P.; Lian, X.M. Comprehensive sequence and whole-life-cycle expression profile analysis of the phosphate transporter gene family in rice. Mol. Plant 2011, 4, 1105–1122. [Google Scholar] [CrossRef]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.I.; Rubio, V.; Perez-Perez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 2010, 6, e1001102. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Y.; Zhang, W.H. OsWRKY74, a WRKY transcription factor, modulates tolerance to phosphate starvation in rice. J. Exp. Bot. 2016, 67, 947–960. [Google Scholar] [CrossRef]
- Yang, W.T.; Baek, D.; Yun, D.J.; Hwang, W.H.; Park, D.S.; Nam, M.H.; Chung, E.S.; Chung, Y.S.; Yi, Y.B.; Kim, D.H. Overexpression of OsMYB4P, an R2R3-type MYB transcriptional activator, increases phosphate acquisition in rice. Plant Physiol. Biochem. 2014, 80, 259–267. [Google Scholar] [CrossRef]
- Yang, T.; Hao, L.; Yao, S.; Zhao, Y.; Lu, W.; Xiao, K. TabHLH1, a bHLH-type transcription factor gene in wheat, improves plant tolerance to Pi and N deprivation via regulation of nutrient transporter gene transcription and ROS homeostasis. Plant Physiol. Biochem. 2016, 104, 99–113. [Google Scholar] [CrossRef]
- Devaiah, B.N.; Nagarajan, V.K.; Raghothama, K.G. Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol. 2007, 145, 147–159. [Google Scholar] [CrossRef]
- Pant, B.D.; Musialak-Lange, M.; Nuc, P.; May, P.; Buhtz, A.; Kehr, J.; Walther, D.; Scheible, W.R. Identification of nutrient-responsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiol. 2009, 150, 1541–1555. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, H.; Shen, Y.; Gao, J.; Xiang, K.; Liu, L.; Ding, H.; Yuan, G.; Lan, H.; Zhou, S.; et al. Cloning and characterization of miRNAs from maize seedling roots under low phosphorus stress. Mol. Biol. Rep. 2012, 39, 8137–8146. [Google Scholar] [CrossRef]
- Xu, X.W.; Zhou, X.H.; Wang, R.R.; Peng, W.L.; An, Y.; Chen, L.L. Functional analysis of long intergenic non-coding RNAs in phosphate-starved rice using competing endogenous RNA network. Sci. Rep. 2016, 6, 20715. [Google Scholar] [CrossRef]
- Wang, X.; Du, G.; Wang, X.; Meng, Y.; Li, Y.; Wu, P.; Yi, K. The function of LPR1 is controlled by an element in the promoter and is independent of SUMO E3 Ligase SIZ1 in response to low Pi stress in Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 380–394. [Google Scholar] [CrossRef]
- Gao, W.; Lu, L.; Qiu, W.; Wang, C.; Shou, H. OsPAP26 encodes a major purple acid phosphatase and regulates phosphate remobilization in Rice. Plant Cell Physiol. 2017, 58, 885–889. [Google Scholar] [CrossRef]
- Aslam, M.M.; Waseem, M.; Weifeng, X.; Qamar, M. Identification and expression analysis of phosphate transporter (PHT) gene family in Lupinus albus cluster root under phosphorus stress. Int. J. Biol. Macromol. 2022, 205, 772–781. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, H.; Li, X.; Shen, H.; Gao, J.; Hou, S.; Zhang, B.; Mayes, S.; Bennett, M.; Ma, J.; et al. A mini foxtail millet with an Arabidopsis-like life cycle as a C4 model system. Nat. Plants 2020, 6, 1167–1178. [Google Scholar] [CrossRef]
- Doust, A.N.; Kellogg, E.A.; Devos, K.M.; Bennetzen, J.L. Foxtail millet: A sequence-driven grass model system. Plant Physiol. 2009, 149, 137–141. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, X.; Quan, Z.W.; Cheng, S.F.; Xu, X.; Pan, S.K.; Xie, M.; Zeng, P.; Yue, Z.; Wang, W.L.; et al. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat. Biotechnol. 2012, 30, 549–554. [Google Scholar] [CrossRef]
- Jia, G.Q.; Huang, X.H.; Zhi, H.; Zhao, Y.; Zhao, Q.; Li, W.J.; Chai, Y.; Yang, L.F.; Liu, K.Y.; Lu, H.Y.; et al. A haplotype map of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica). Nat. Genet. 2013, 45, 957–961. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Schmutz, J.; Wang, H.; Percifield, R.; Hawkins, J.; Pontaroli, A.C.; Estep, M.; Feng, L.; Vaughn, J.N.; Grimwood, J.; et al. Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 2012, 30, 555–561. [Google Scholar] [CrossRef]
- Ahmad, Z.; Nadeem, F.; Wang, R.F.; Diao, X.M.; Han, Y.H.; Wang, X.C.; Li, X.X. A larger root system is coupled with contrasting expression patterns of phosphate and nitrate transporters in foxtail millet [Setaria italica (L.) Beauv.] under phosphate limitation. Front. Plant Sci. 2018, 9, 1367. [Google Scholar] [CrossRef]
- Rose, T.J.; Rose, M.T.; Pariasca-Tanaka, J.; Heuer, S.; Wissuwa, M. The frustration with utilization: Why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Front. Plant Sci. 2011, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Ceasar, S.A.; Ramakrishnan, M.; Vinod, K.K.; Roch, G.V.; Upadhyaya, H.D.; Baker, A.; Ignacimuthu, S. Phenotypic responses of foxtail millet (Setaria italica) genotypes to phosphate supply under greenhouse and natural field conditions. PLoS ONE 2020, 15, e233896. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef]
- Xu, H.X.; Weng, X.Y.; Yang, Y. Effect of phosphorus deficiency on the photosynthetic characteristics of rice plants. Russ. J. Plant Physiol. 2007, 54, 741–748. [Google Scholar] [CrossRef]
- Veronica, N.; Subrahmanyam, D.; Vishnu Kiran, T.; Yugandhar, P.; Bhadana, V.P.; Padma, V.; Jayasree, G.; Voleti, S.R. Influence of low phosphorus concentration on leaf photosynthetic characteristics and antioxidant response of rice genotypes. Photosynthetica 2016, 55, 285–293. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Chen, X.Y.; Li, M.M.; Li, P.; Jia, Y.T.; Han, Y.H.; Xing, G.F. Screening of germplasm tolerant to low phosphorus of seedling stage and response of root protective enzymes to low phosphorus in foxtail millet. Acta Agron. Sin. 2019, 45, 601–612. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, K.W.; Liu, H.H.; Song, J.L.; Wu, W.; Li, K.P.; Zhang, J.R. Physiological and comparative proteome analyses reveal low-phosphate tolerance and enhanced photosynthesis in a maize mutant owing to reinforced inorganic phosphate recycling. BMC Plant Biol. 2016, 16, 129. [Google Scholar] [CrossRef]
- Pei, L.M.; Liu, J.J.; Zhou, Y.Y.; Jiang, Y.H.; Li, H. Transcriptomic and metabolomic profiling reveals the protective role of anthocyanins in alleviating low phosphate stress in maize. Physiol. Mol. Biol. Plants 2021, 27, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Tian, J.; Lam, H.M.; Lim, B.L.; Yan, X.L.; Liao, H. Biochemical and molecular characterization of PvPAP3, a novel purple acid phosphatase isolated from common bean enhancing extracellular ATP utilization. Plant Physiol. 2010, 152, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Bhadouria, J.; Singh, A.P.; Mehra, P.; Verma, L.; Srivastawa, R.; Parida, S.K.; Giri, J. Identification of purple acid phosphatases in chickpea and potential roles of CaPAP7 in seed phytate accumulation. Sci. Rep. 2017, 7, 11012. [Google Scholar] [CrossRef]
- Olczak, M.; Morawiecka, B.; Watorek, W. Plant purple acid phosphatases—Genes, structures and biological function. Acta Biochim. Pol. 2003, 50, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Sega, P.; Kruszka, K.; Bielewicz, D.; Karlowski, W.; Nuc, P.; Szweykowska-Kulinska, Z.; Pacak, A. Pi-starvation induced transcriptional changes in barley revealed by a comprehensive RNA-Seq and degradome analyses. BMC Genom. 2021, 22, 165. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.G.; Wang, K.; Xu, C.; Zou, C.; Xie, C.X.; Xu, Y.B.; Li, W.X. Strand-specific RNA-Seq transcriptome analysis of genotypes with and without low-phosphorus tolerance provides novel insights into phosphorus-use efficiency in maize. BMC Plant Biol. 2016, 16, 222. [Google Scholar] [CrossRef]
- O’Rourke, J.A.; Yang, S.S.; Miller, S.S.; Bucciarelli, B.; Liu, J.; Rydeen, A.; Bozsoki, Z.; Uhde-Stone, C.; Tu, Z.J.; Allan, D.; et al. An RNA-Seq transcriptome analysis of orthophosphate-deficient white lupin reveals novel insights into phosphorus acclimation in plants. Plant Physiol. 2013, 161, 705–724. [Google Scholar] [CrossRef]
- He, M.J.; Li, X.L.; Mang, M.; Li, Z.; Ludewig, U.; Schulze, W.X. A systems-biology approach identifies co-expression modules in response to low phosphate supply in maize lines of different breeding history. Plant J. 2022, 109, 1249–1270. [Google Scholar] [CrossRef] [PubMed]
- Venkidasamy, B.; Selvaraj, D.; Ramalingam, S. Genome-wide analysis of purple acid phosphatase (PAP) family proteins in Jatropha curcas L. Int. J. Biol. Macromol. 2019, 123, 648–656. [Google Scholar] [CrossRef]
- Tian, H.N.; Wang, S.C. TRANSPARENT TESTA GLABRA1, a Key Regulator in Plants with Multiple Roles and Multiple Function Mechanisms. Int. J. Mol. Sci. 2020, 21, 4881. [Google Scholar] [CrossRef]
- Quan, C.Q.; Bai, Z.G.; Zheng, S.W.; Zhou, J.M.; Yu, Q.; Xu, Z.J.; Gao, X.L.; Li, L.H.; Zhu, J.Q.; Jia, X.M.; et al. Genome-wide analysis and environmental response profiling of phosphate-induced-1 family genes in rice (Oryza sativa). Biotechnol. Biotechnol. Equip. 2019, 33, 627–638. [Google Scholar] [CrossRef]
- Miura, K.; Rus, A.; Sharkhuu, A.; Yokoi, S.; Karthikeyan, A.S.; Raghothama, K.G.; Baek, D.; Koo, Y.D.; Jin, J.B.; Bressan, R.A.; et al. The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc. Natl. Acad. Sci. USA 2005, 102, 7760–7765. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Wu, X.Q.; Wang, E.H.; Liu, Y.N.; Wang, Y.; Zheng, Q.H.; Han, Y.Z.; Chen, Z.Z.; Zhang, Y.Q. PHR1 positively regulates phosphate starvation-induced anthocyanin accumulation through direct upregulation of genes F3’H and LDOX in Arabidopsis. Planta 2022, 256, 4. [Google Scholar] [CrossRef]
- Zhang, K.W.; Liu, H.H.; Tao, P.L.; Chen, H. Comparative proteomic analyses provide new insights into low phosphorus stress responses in maize leaves. PLoS ONE 2014, 9, e98215. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Tsai, P.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.R.; Gui, H.P.; Zhang, H.H.; Zhang, X.L.; Song, M.Z. High nitrogen enhance drought tolerance in Cotton through antioxidant enzymatic activities, nitrogen metabolism and osmotic adjustment. Plants 2020, 9, 178. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Shumate, A.; Wong, B.; Pertea, G.; Pertea, M. Improved transcriptome assembly using a hybrid of long and short reads with StringTie. PLoS Comput. Biol. 2022, 18, e1009730. [Google Scholar] [CrossRef]
- Ghosh, S.; Chan, C.K. Analysis of RNA-Seq Data Using TopHat and Cufflinks. Methods Mol. Biol. 2016, 1374, 339–361. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative Toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
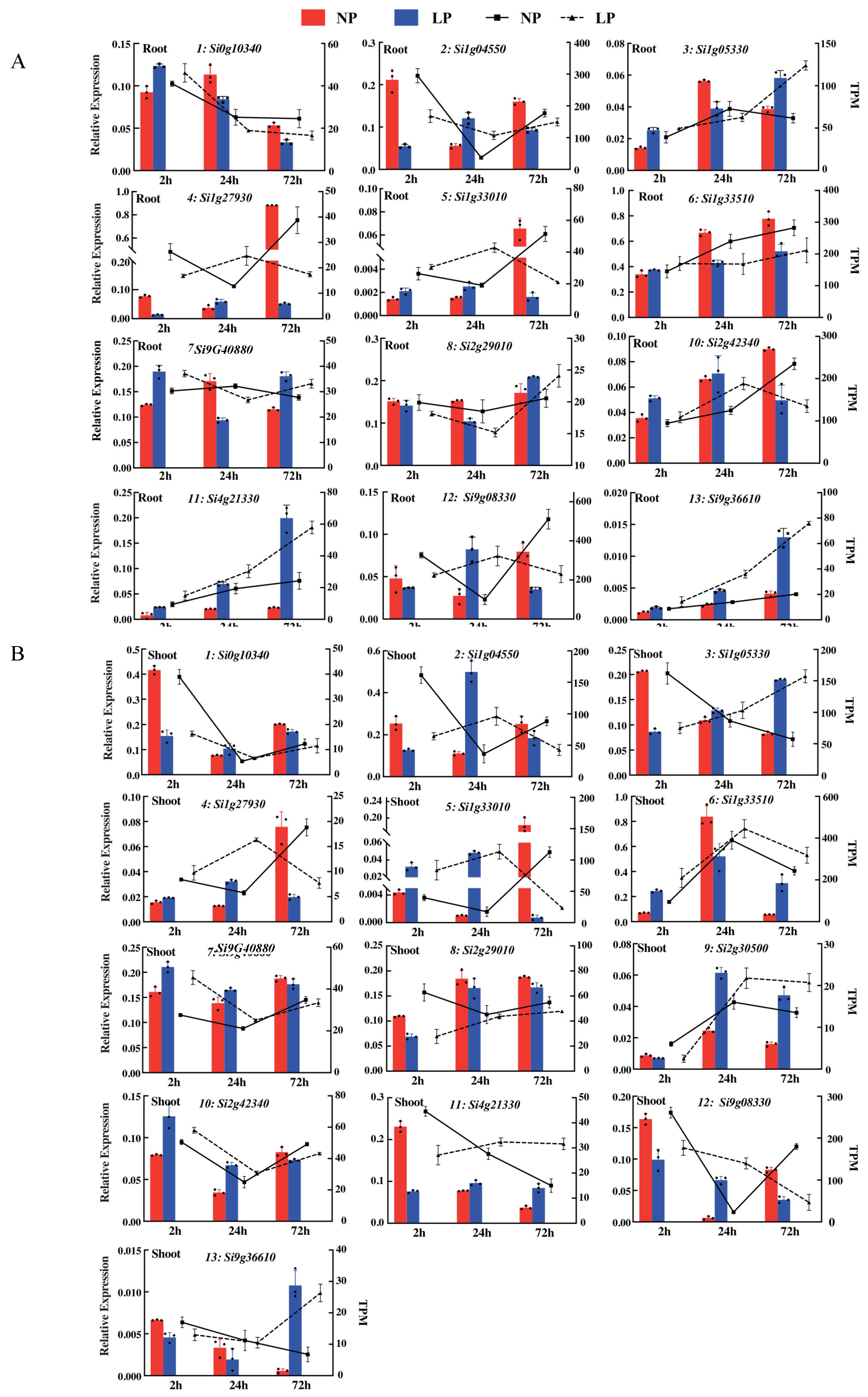

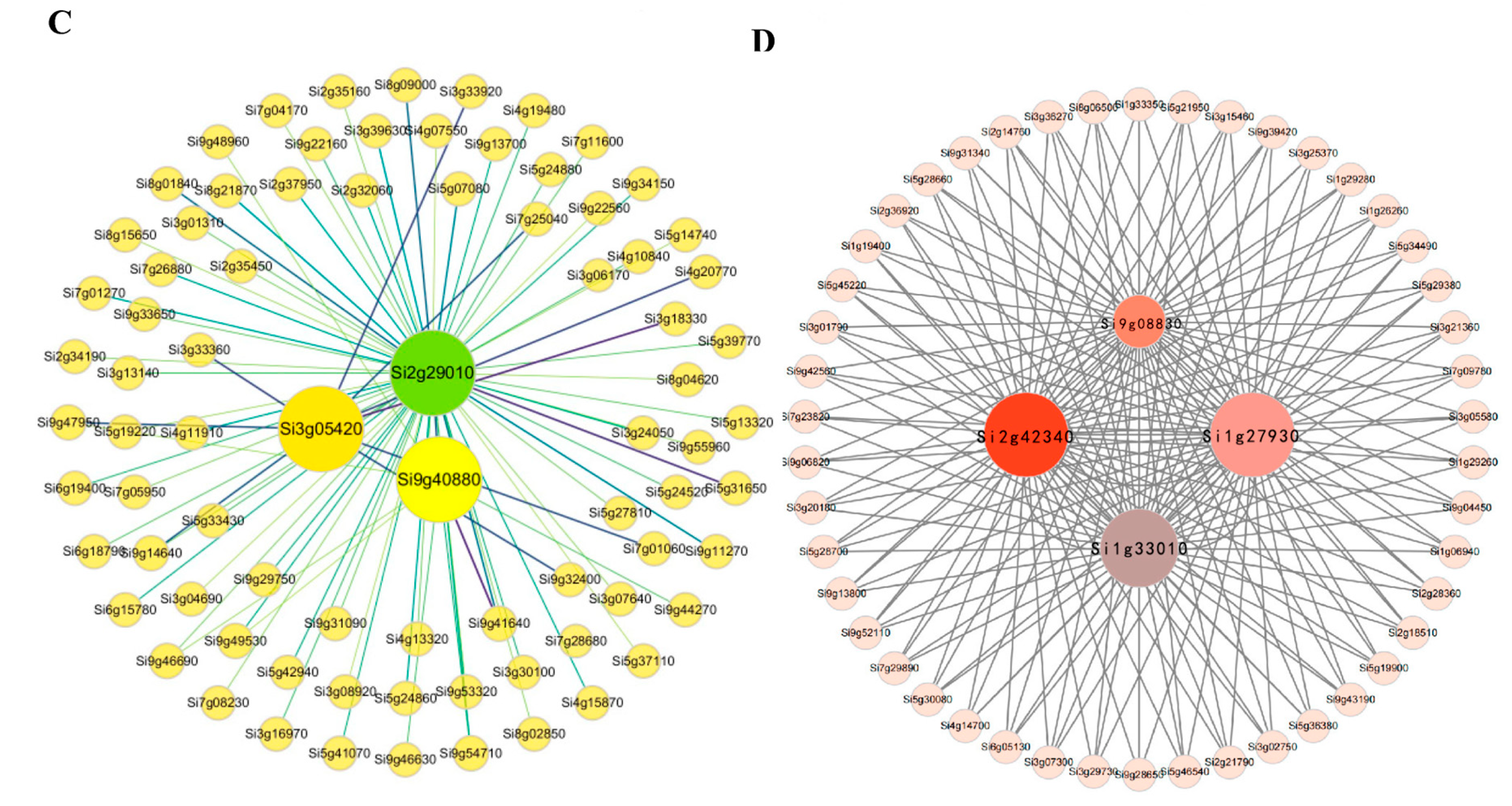
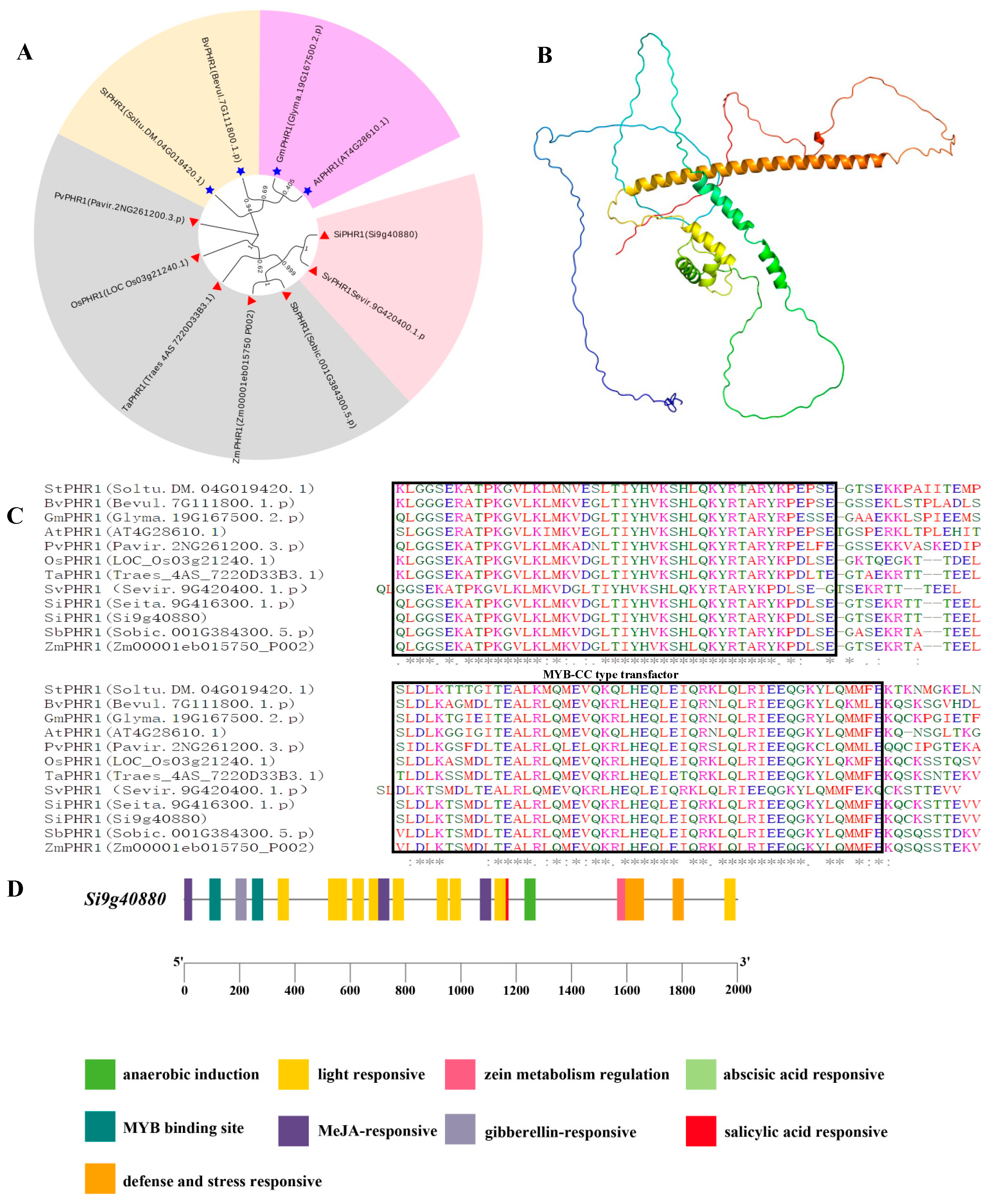

| Gene ID | Gene Length (bp) | Gene Expression Level (FC) | Comparison Combination (NP vs. LP at Three Time Points) | Functional Annotation (NR/Homologous Genes in Arabidopsis) |
|---|---|---|---|---|
| Si0g10340 | 1209 | −2.04, −1.26 | A | Nicotianamine aminotransferase A |
| Si1g04550 | 516 | −3.14, −1.65, 1.39 | A, B, E | Uncharacterized protein |
| Si1g05330 | 849 | 2.58, 1.36, −1.00, 1.13, 0.15 | A, C, D, E, F | SPX domain-containing protein 1 |
| Si1g27930 | 1080 | −0.04, 1.48, 1.36, −1.18 | B, E, D, F | TRANSPARENT TESTA GLABRA 1 (AT5G24520) |
| Si1g33010 | 939 | 6.7, 1.74, 2.74, −2.65, −1.66 | A, B, C, D, E | Phosphate-induced protein 1 (AT4G08950) |
| Si1g33510 | 1782 | 1.09 | A | Ferredoxin-nitrite reductase (AT2G15620) |
| Si9g40880 | 1347 | −0.65, −0.52 | A, D | PHOSPHATE STARVATION RESPONSE 1 (AT4G28610) |
| Si2g29010 | 1200 | −1.23 | A | Purple acid phosphatase 29 (AT5G63140) |
| Si2g30500 | 954 | −2.49, −1.31 | A, D | Phosphate carrier protein (AT2G17270) |
| Si2g42340 | 2355 | −1.30, 1.28 | B, C | Potassium transporter 7 (AT2G30070) |
| Si4g21330 | 912 | 2.33, 1.21, 0.69 | C, D, E | SPX domain-containing protein 1 (AT5G20150) |
| Si9g08330 | 1548 | 1.74, −2.00, 2.56, −0.56 | B, C, D, E | Zinc finger protein 1 (AT5G04340) |
| Si9g36610 | 759 | 1.67, 2.21, 0.75 | C, D, E | SPX domain-containing protein 5 (AT2G45130) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, G.; Jin, M.; Yue, P.; Ren, C.; Hao, J.; Zhao, Y.; Zhao, X.; Sun, Z.; Hou, S. Role of SiPHR1 in the Response to Low Phosphate in Foxtail Millet via Comparative Transcriptomic and Co-Expression Network Analyses. Int. J. Mol. Sci. 2023, 24, 12786. https://doi.org/10.3390/ijms241612786
Xing G, Jin M, Yue P, Ren C, Hao J, Zhao Y, Zhao X, Sun Z, Hou S. Role of SiPHR1 in the Response to Low Phosphate in Foxtail Millet via Comparative Transcriptomic and Co-Expression Network Analyses. International Journal of Molecular Sciences. 2023; 24(16):12786. https://doi.org/10.3390/ijms241612786
Chicago/Turabian StyleXing, Guofang, Minshan Jin, Peiyao Yue, Chao Ren, Jiongyu Hao, Yue Zhao, Xiongwei Zhao, Zhaoxia Sun, and Siyu Hou. 2023. "Role of SiPHR1 in the Response to Low Phosphate in Foxtail Millet via Comparative Transcriptomic and Co-Expression Network Analyses" International Journal of Molecular Sciences 24, no. 16: 12786. https://doi.org/10.3390/ijms241612786
APA StyleXing, G., Jin, M., Yue, P., Ren, C., Hao, J., Zhao, Y., Zhao, X., Sun, Z., & Hou, S. (2023). Role of SiPHR1 in the Response to Low Phosphate in Foxtail Millet via Comparative Transcriptomic and Co-Expression Network Analyses. International Journal of Molecular Sciences, 24(16), 12786. https://doi.org/10.3390/ijms241612786




