Deciphering Molecular Mechanisms Governing the Reproductive Molt of Macrobrachium nipponense: A Transcriptome Analysis of Ovaries across Various Molting Stages
Abstract
1. Introduction
2. Results
2.1. Sequencing Data and Comparative Efficiency
2.2. Identification and Annotation of Differentially Expressed Genes (DEGs)
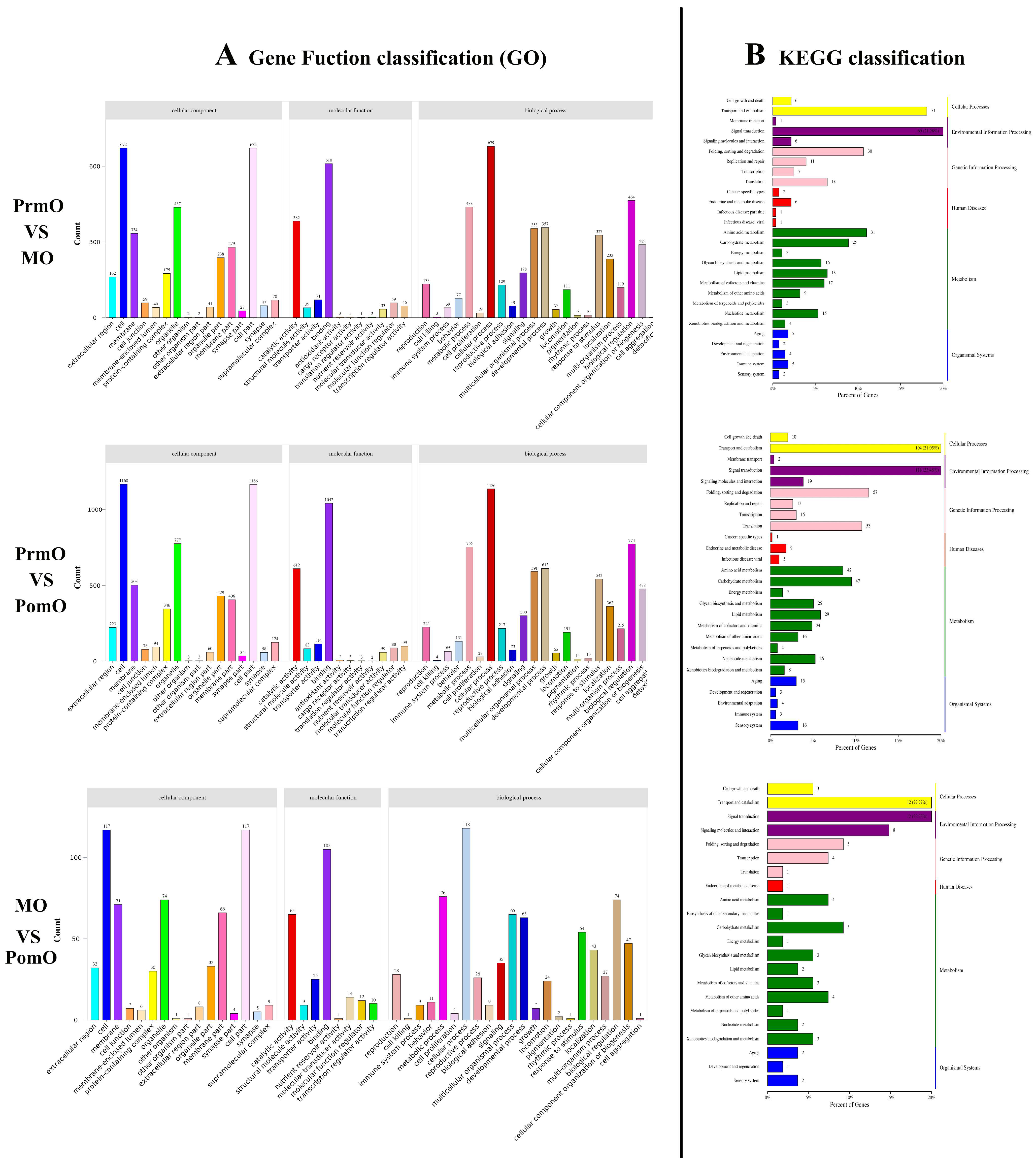
2.3. GO Enrichment and Pathway Annotation of DEGs
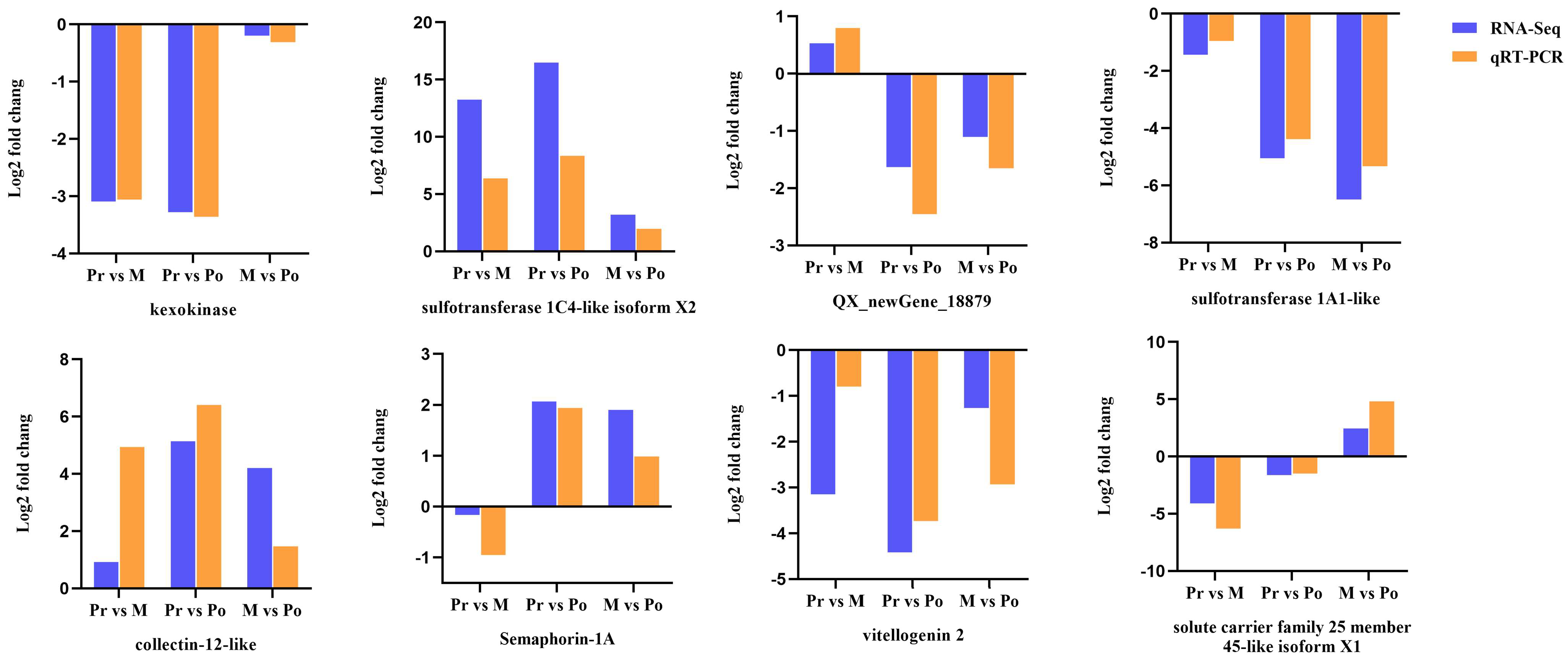
2.4. qRT-PCR Validation of DEGs
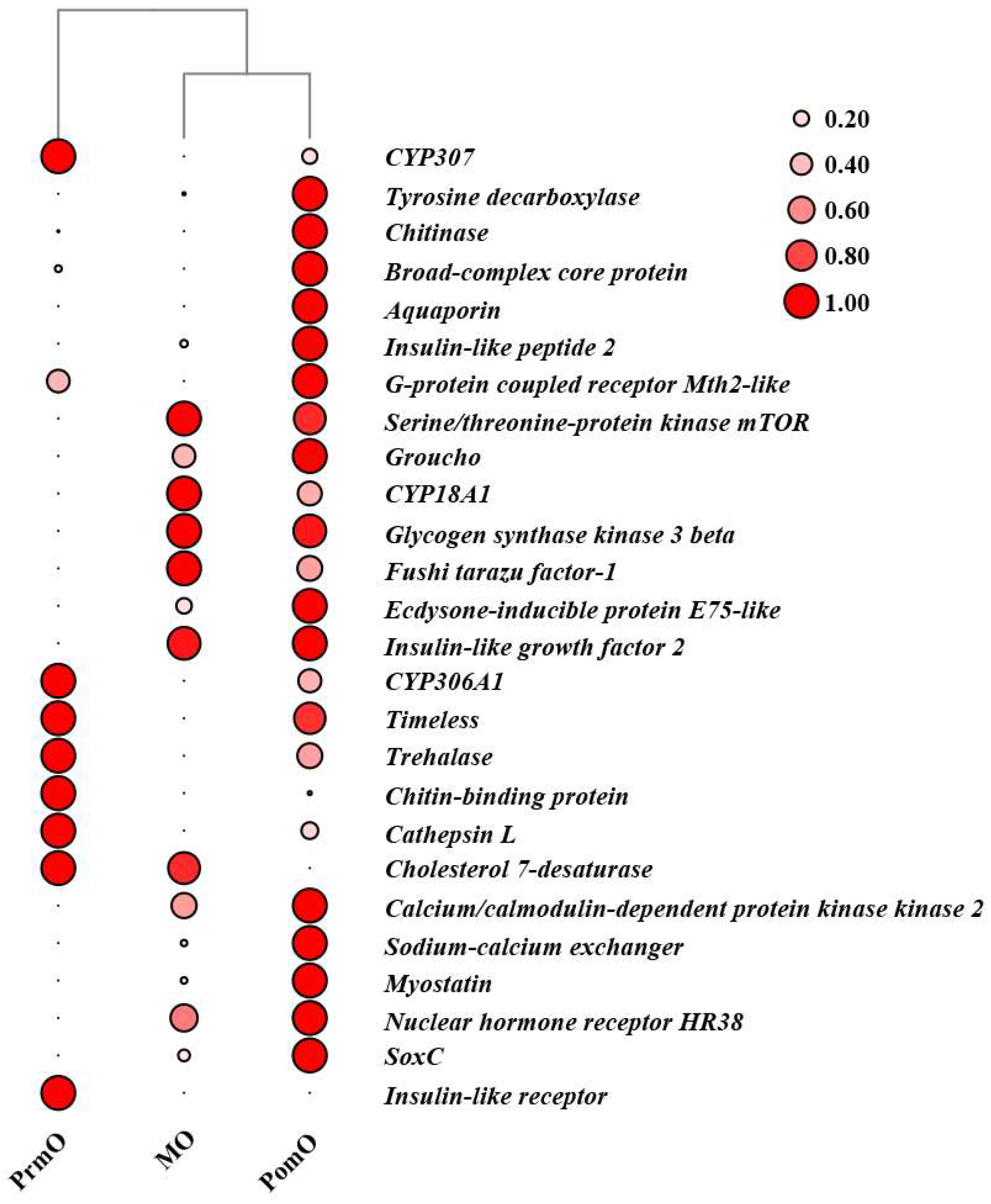
2.5. Candidate DEGs Associated with Molting and Their Expression Pattern
2.6. Analysis of DEGs in Signaling Pathways Involved in Molting Regulation
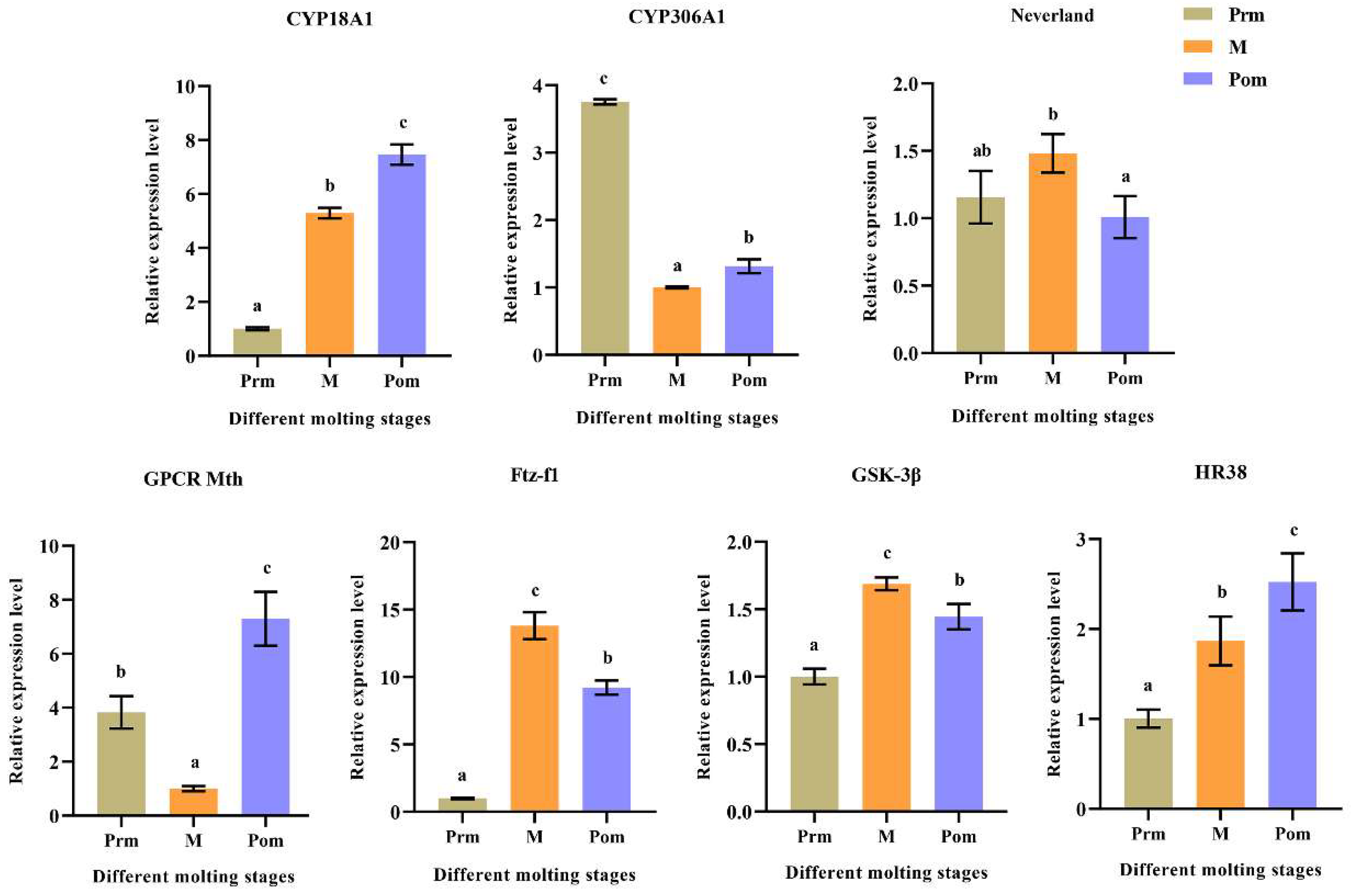
2.7. Expression of Various Molting-Related Genes at Different Molting Stages
3. Discussion
4. Materials and Methods
4.1. Sample Collection and RNA Isolation
4.2. The cDNA Library Construction and Sequencing
4.3. Assembly and Gene Annotation
4.4. Quantitative Real-Time PCR (qRT-PCR) Validation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Y.; Villeneuve, D.L.; Toyota, K.; Iguchi, T.; Tollefsen, K.E. Ecdysone receptor agonism leading to lethal molting disruption in arthropods: Review and adverse outcome pathway development. Environ. Sci. Technol. 2017, 51, 4142–4157. [Google Scholar] [CrossRef]
- Subramoniam, T. Crustacean ecdysteriods in reproduction and embryogenesis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 125, 135–156. [Google Scholar] [CrossRef]
- Waddy, S.; Aiken, D.; DeKlein, D. Control of growth and reproduction. In Biology of the Lobster Homarus americanus (Red. JR Factor); Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Wilder, M.N.; Okumura, T.; Aida, K. Accumulation of ovarian ecdysteroids in synchronization with gonadal development in the giant freshwater prawn, Macrobrachium rosenbergii. Zool. Sci. 1991, 8, 919–927. [Google Scholar]
- Okumura, T. Changes in hemolymph vitellogenin and ecdysteroid levels during the reproductive and non-reproductive molt cycles in the freshwater prawn Macrobrachium nipponense. Zool. Sci. 1992, 9, 37–45. [Google Scholar]
- Yuan, H.; Qiao, H.; Fu, Y.; Fu, H.; Zhang, W.; Jin, S.; Gong, Y.; Jiang, S.; Xiong, Y.; Hu, Y. RNA interference shows that Spook, the precursor gene of 20-hydroxyecdysone (20E), regulates the molting of Macrobrachium nipponense. J. Steroid Biochem. Mol. Biol. 2021, 213, 105976. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, X.; Wei, J.; Sun, X.; Yuan, J.; Li, F.; Xiang, J. Whole transcriptome analysis provides insights into molecular mechanisms for molting in Litopenaeus vannamei. PLoS ONE 2015, 10, e0144350. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, W.; Jin, S.; Jiang, S.; Xiong, Y.; Chen, T.; Gong, Y.; Qiao, H.; Fu, H. Transcriptome analysis provides novel insights into the immune mechanisms of Macrobrachium nipponense during molting. Fish Shellfish Immunol. 2022, 131, 454–469. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, A.; Li, S.; Wang, G.; Ye, H. Hepatopancreas immune response during molt cycle in the mud crab, Scylla paramamosain. Sci. Rep. 2020, 10, 13102. [Google Scholar] [CrossRef]
- Das, S.; Vraspir, L.; Zhou, W.; Durica, D.S.; Mykles, D.L. Transcriptomic analysis of differentially expressed genes in the molting gland (Y-organ) of the blackback land crab, Gecarcinus lateralis, during molt-cycle stage transitions. Comp. Biochem. Physiol. D Genom. Proteom. 2018, 28, 37–53. [Google Scholar] [CrossRef]
- Shyamal, S.; Das, S.; Guruacharya, A.; Mykles, D.; Durica, D. Transcriptomic analysis of crustacean molting gland (Y-organ) regulation via the mTOR signaling pathway. Sci. Rep. 2018, 8, 7307. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Mykles, D.L.; Chang, E.S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. Gen. Comp. Endocrinol. 2020, 294, 113493. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Connacher, R.P.; O’Connor, M.B. Control of the insect metamorphic transition by ecdysteroid production and secretion. Curr. Opin. Insect Sci. 2021, 43, 11–20. [Google Scholar] [CrossRef]
- Gibbens, Y.Y.; Warren, J.T.; Gilbert, L.I.; O’Connor, M.B. Neuroendocrine regulation of Drosophila metamorphosis requires TGFβ/Activin signaling. Development 2011, 138, 2693–2703. [Google Scholar] [CrossRef]
- Chang, E.S.; Mykles, D.L. Regulation of crustacean molting: A review and our perspectives. Gen. Comp. Endocrinol. 2011, 172, 323–330. [Google Scholar] [CrossRef]
- Gilbert, L.I. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Mol. Cell. Endocrinol. 2004, 215, 1–10. [Google Scholar] [CrossRef]
- Petryk, A.; Warren, J.T.; Marqués, G.; Jarcho, M.P.; Gilbert, L.I.; Kahler, J.; Parvy, J.P.; Li, Y.; Dauphin-Villemant, C.; O’Connor, M.B. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. USA 2003, 100, 13773–13778. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, G.A.; Mandal, P.K.; Mandal, A. Calcium regulation in crustaceans during the molt cycle: A review and update. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 247–257. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Ma, L.; Tian, L.; Wang, S.; Sheng, Z.; Jiang, R.J.; Bendena, W.G.; Li, S. Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the Bombyx fat body. J. Insect Physiol. 2010, 56, 1436–1444. [Google Scholar] [CrossRef]
- Hongtuo, F.; Sufei, J.; Yiwei, X. Current status and prospects of farming the giant river prawn (Macrobrachium rosenbergii) and the oriental river prawn (M acrobrachium nipponense) in China. Aquac. Res. 2012, 43, 993–998. [Google Scholar] [CrossRef]
- Sumiya, E.; Ogino, Y.; Toyota, K.; Miyakawa, H.; Miyagawa, S.; Iguchi, T. Neverland regulates embryonic moltings through the regulation of ecdysteroid synthesis in the water flea Daphnia magna, and may thus act as a target for chemical disruption of molting. J. Appl. Toxicol. 2016, 36, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Sathapondecha, P.; Panyim, S.; Udomkit, A. An essential role of Rieske domain oxygenase Neverland in the molting cycle of black tiger shrimp, Penaeus monodon. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 213, 11–19. [Google Scholar] [CrossRef]
- Pan, F.; Fu, Y.; Zhang, W.; Jiang, S.; Xiong, Y.; Yan, Y.; Gong, Y.; Qiao, H.; Fu, H. Characterization, expression and functional analysis of CYP306a1 in the oriental river prawn, Macrobrachium nipponense. Aquac. Rep. 2022, 22, 101009. [Google Scholar] [CrossRef]
- Guittard, E.; Blais, C.; Maria, A.; Parvy, J.P.; Pasricha, S.; Lumb, C.; Lafont, R.; Daborn, P.J.; Dauphin-Villemant, C. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev. Biol. 2011, 349, 35–45. [Google Scholar] [CrossRef]
- Charles, J.P. The regulation of expression of insect cuticle protein genes. Insect Biochem. Mol. Biol. 2010, 40, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, W.; Fu, Y.; Jiang, S.; Xiong, Y.; Zhai, S.; Gong, Y.; Qiao, H.; Fu, H.; Wu, Y. MnFtz-f1 is required for molting and ovulation of the oriental river prawn Macrobrachium nipponense. Front. Endocrinol. 2021, 12, 1765. [Google Scholar] [CrossRef]
- Kozlova, T.; Lam, G.; Thummel, C.S. Drosophila DHR38 nuclear receptor is required for adult cuticle integrity at eclosion. Dev. Dyn. 2010, 238, 701–707. [Google Scholar] [CrossRef]
- Guha, S.; Cullen, J.P.; Morrow, D.; Colombo, A.; Lally, C.; Walls, D.; Redmond, E.M.; Cahill, P.A. Glycogen synthase kinase 3 beta positively regulates Notch signaling in vascular smooth muscle cells: Role in cell proliferation and survival. Basic Res. Cardiol. 2011, 106, 773–785. [Google Scholar] [CrossRef]
- Shin, S.; Wolgamott, L.; Yu, Y.; Blenis, J.; Yoon, S.O. Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc. Natl. Acad. Sci. USA 2011, 108, E1204–E1213. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, X.; Yuan, J.; Zhang, X.; Su, M.; Li, F. Glycogen Synthase Kinase 3 Gene Is Important in Growth and Molting of the Pacific White Shrimp Litopenaeus vannamei. Front. Mar. Sci. 2021, 8, 681966. [Google Scholar] [CrossRef]
- Zhao, X.F. G protein-coupled receptors function as cell membrane receptors for the steroid hormone 20-hydroxyecdysone. Cell Commun. Signal. 2020, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.M.; Mykles, D.L.; Elizur, A.; Ventura, T. Characterization of G-protein coupled receptors from the blackback land crab Gecarcinus lateralis Y organ transcriptome over the molt cycle. BMC Genom. 2019, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.J.; Fitzgibbon, Q.P.; Smith, G.G.; Ventura, T. In silico prediction of the G-protein coupled receptors expressed during the metamorphic molt of Sagmariasus verreauxi (Crustacea: Decapoda) by mining transcriptomic data: RNA-seq to repertoire. Gen. Comp. Endocrinol. 2016, 228, 111–127. [Google Scholar] [CrossRef]
- HuangFu, N.; Zhu, X.; Chang, G.; Wang, L.; Li, D.; Zhang, K.; Gao, X.; Ji, J.; Luo, J.; Cui, J. Dynamic transcriptome analysis and Methoprene-tolerant gene knockdown reveal that juvenile hormone regulates oogenesis and vitellogenin synthesis in Propylea Japonica. Genom. 2021, 113, 2877–2889. [Google Scholar] [CrossRef]
- Mykles, D.L. Signaling pathways that regulate the crustacean molting gland. Front. Endocrinol. 2021, 12, 674711. [Google Scholar] [CrossRef]
- Layalle, S.; Arquier, N.; Léopold, P. The TOR pathway couples nutrition and developmental timing in Drosophila. Dev. Cell 2008, 15, 568–577. [Google Scholar] [CrossRef]
- Abuhagr, A.M.; MacLea, K.S.; Mudron, M.R.; Chang, S.A.; Chang, E.S.; Mykles, D.L. Roles of mechanistic target of rapamycin and transforming growth factor-β signaling in the molting gland (Y-organ) of the blackback land crab, Gecarcinus lateralis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 198, 15–21. [Google Scholar] [CrossRef]
- Baretić, D.; Williams, R.L. The structural basis for mTOR function. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 36, pp. 91–101. [Google Scholar]
- Abuhagr, A.M.; MacLea, K.S.; Chang, E.S.; Mykles, D.L. Mechanistic target of rapamycin (mTOR) signaling genes in decapod crustaceans: Cloning and tissue expression of mTOR, Akt, Rheb, and p70 S6 kinase in the green crab, Carcinus maenas, and blackback land crab, Gecarcinus lateralis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 168, 25–39. [Google Scholar] [CrossRef]
- Bellés, X.; Martín, D.; Piulachs, M.D. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu. Rev. Entomol. 2005, 50, 181–199. [Google Scholar] [CrossRef]
- Nusse, R. Wnt signaling and stem cell control. Cell Res. 2008, 18, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Archbold, H.C.; Broussard, C.; Chang, M.V.; Cadigan, K.M. Bipartite recognition of DNA by TCF/Pangolin is remarkably flexible and contributes to transcriptional responsiveness and tissue specificity of wingless signaling. PLoS Genet. 2014, 10, e1004591. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, X.; Yuan, J.; Zhang, X.; Li, F.; Xiang, J. Wnt gene family members and their expression profiling in Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 77, 233–243. [Google Scholar] [CrossRef]
- Alexandratos, A.; Moulos, P.; Nellas, I.; Mavridis, K.; Dedos, S.G. Reassessing ecdysteroidogenic cells from the cell membrane receptors’ perspective. Sci. Rep. 2016, 6, 20229. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Cesar, J.R.; Zhao, B.; Malecha, S.; Ako, H.; Yang, J. Morphological and biochemical changes in the muscle of the marine shrimp Litopenaeus vannamei during the molt cycle. Aquaculture 2006, 261, 688–694. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, H.; Qiao, H.; Sun, S.; Zhang, W.; Jin, S.; Jiang, S.; Gong, Y.; Xiong, Y.; Wu, Y. Validation and evaluation of reference genes for quantitative real-time PCR in Macrobrachium nipponense. Int. J. Mol. Sci. 2018, 19, 2258. [Google Scholar] [CrossRef] [PubMed]


| Sample | ReadSum | BaseSum | GC (%) | Q30 (%) | Reads Aligned (%) |
|---|---|---|---|---|---|
| PrmO1 | 22,839,573 | 6,851,871,900 | 39.49 | 92.23 | 87.57% |
| PrmO2 | 21,278,239 | 6,383,471,700 | 39.01 | 92.33 | 88.12% |
| PrmO3 | 21,613,754 | 6,484,126,200 | 39.11 | 92.77 | 88.35% |
| MO1 | 25,537,213 | 7,661,163,900 | 39.36 | 92.31 | 88.86% |
| MO2 | 20,883,459 | 6,265,037,700 | 40.07 | 91.15 | 88.30% |
| MO3 | 22,137,657 | 6,641,297,100 | 39.61 | 91.49 | 88.35% |
| PomO1 | 26,777,746 | 8,033,323,800 | 39.58 | 92.04 | 88.63% |
| PomO2 | 30,473,592 | 9,142,077,600 | 39.33 | 92.17 | 89.15% |
| PomO3 | 30,353,545 | 9,106,063,500 | 39.63 | 92.57 | 88.01% |
| DEG_Set | Total | Swiss-Prot | GO | KEGG | COG | KOG | Pfam | NR |
|---|---|---|---|---|---|---|---|---|
| PrmO vs. MO | 1388 | 1046 | 975 | 662 | 319 | 826 | 977 | 822 |
| PrmO vs. PomO | 2236 | 1720 | 1609 | 1139 | 521 | 1358 | 1558 | 1315 |
| MO vs. PomO | 269 | 204 | 189 | 113 | 62 | 157 | 188 | 164 |
| Total | 3893 | 2970 | 2773 | 1914 | 902 | 2341 | 2723 | 2301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Gao, Z.; Cai, P.; Zhang, W.; Jin, S.; Jiang, S.; Xiong, Y.; Gong, Y.; Qiao, H.; Fu, H. Deciphering Molecular Mechanisms Governing the Reproductive Molt of Macrobrachium nipponense: A Transcriptome Analysis of Ovaries across Various Molting Stages. Int. J. Mol. Sci. 2023, 24, 11056. https://doi.org/10.3390/ijms241311056
Yuan H, Gao Z, Cai P, Zhang W, Jin S, Jiang S, Xiong Y, Gong Y, Qiao H, Fu H. Deciphering Molecular Mechanisms Governing the Reproductive Molt of Macrobrachium nipponense: A Transcriptome Analysis of Ovaries across Various Molting Stages. International Journal of Molecular Sciences. 2023; 24(13):11056. https://doi.org/10.3390/ijms241311056
Chicago/Turabian StyleYuan, Huwei, Zijian Gao, Pengfei Cai, Wenyi Zhang, Shubo Jin, Sufei Jiang, Yiwei Xiong, Yongsheng Gong, Hui Qiao, and Hongtuo Fu. 2023. "Deciphering Molecular Mechanisms Governing the Reproductive Molt of Macrobrachium nipponense: A Transcriptome Analysis of Ovaries across Various Molting Stages" International Journal of Molecular Sciences 24, no. 13: 11056. https://doi.org/10.3390/ijms241311056
APA StyleYuan, H., Gao, Z., Cai, P., Zhang, W., Jin, S., Jiang, S., Xiong, Y., Gong, Y., Qiao, H., & Fu, H. (2023). Deciphering Molecular Mechanisms Governing the Reproductive Molt of Macrobrachium nipponense: A Transcriptome Analysis of Ovaries across Various Molting Stages. International Journal of Molecular Sciences, 24(13), 11056. https://doi.org/10.3390/ijms241311056








