Beneficial Effects of Tacrolimus on Brain-Death-Associated Right Ventricular Dysfunction in Pigs
Abstract
1. Introduction
New and Noteworthy
2. Results
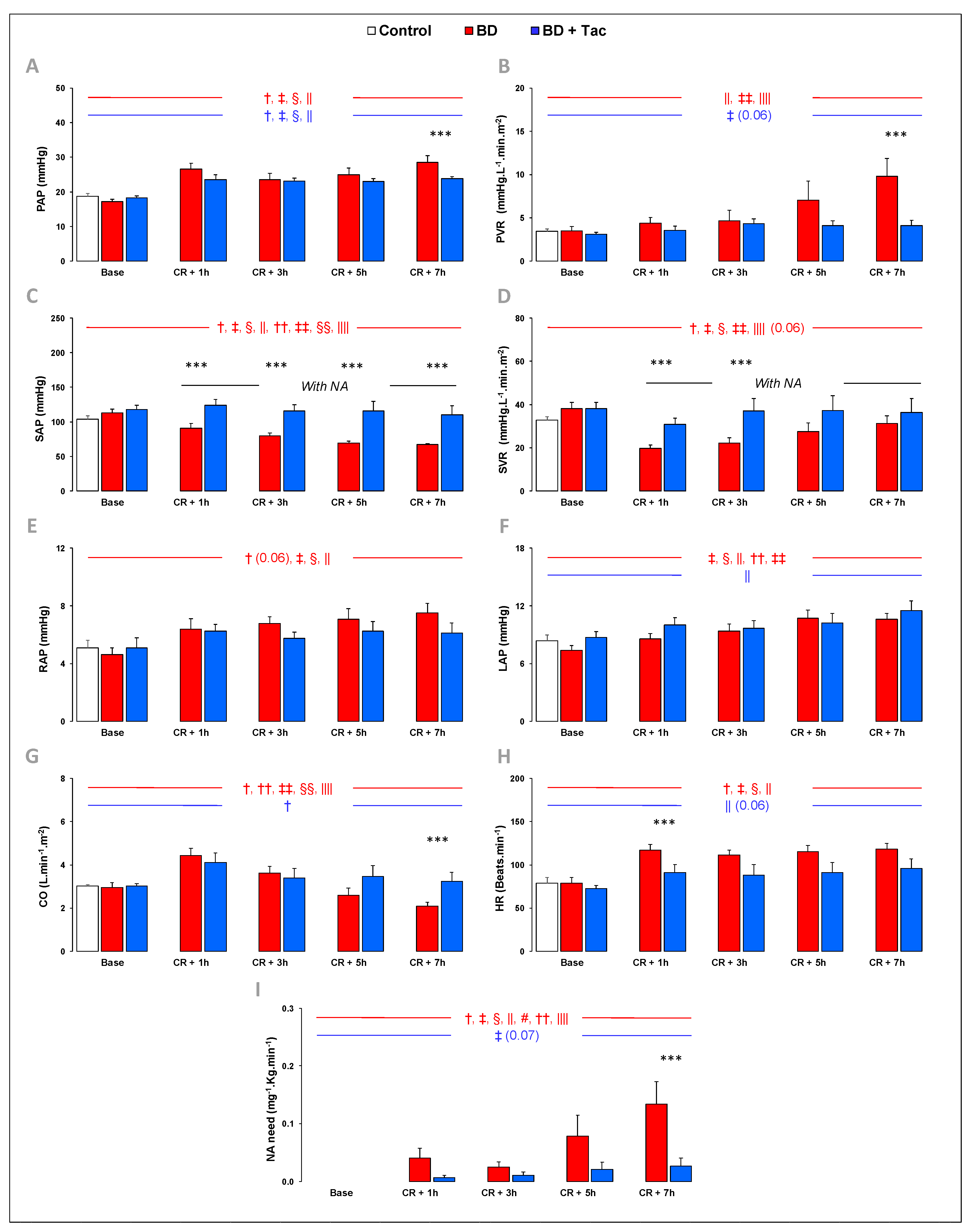
2.1. Hemodynamic Evaluation (Figure 1)
2.2. Tacrolimus Pretreatment Prevents Right Ventriculo-Arterial Uncoupling (Figure 2)
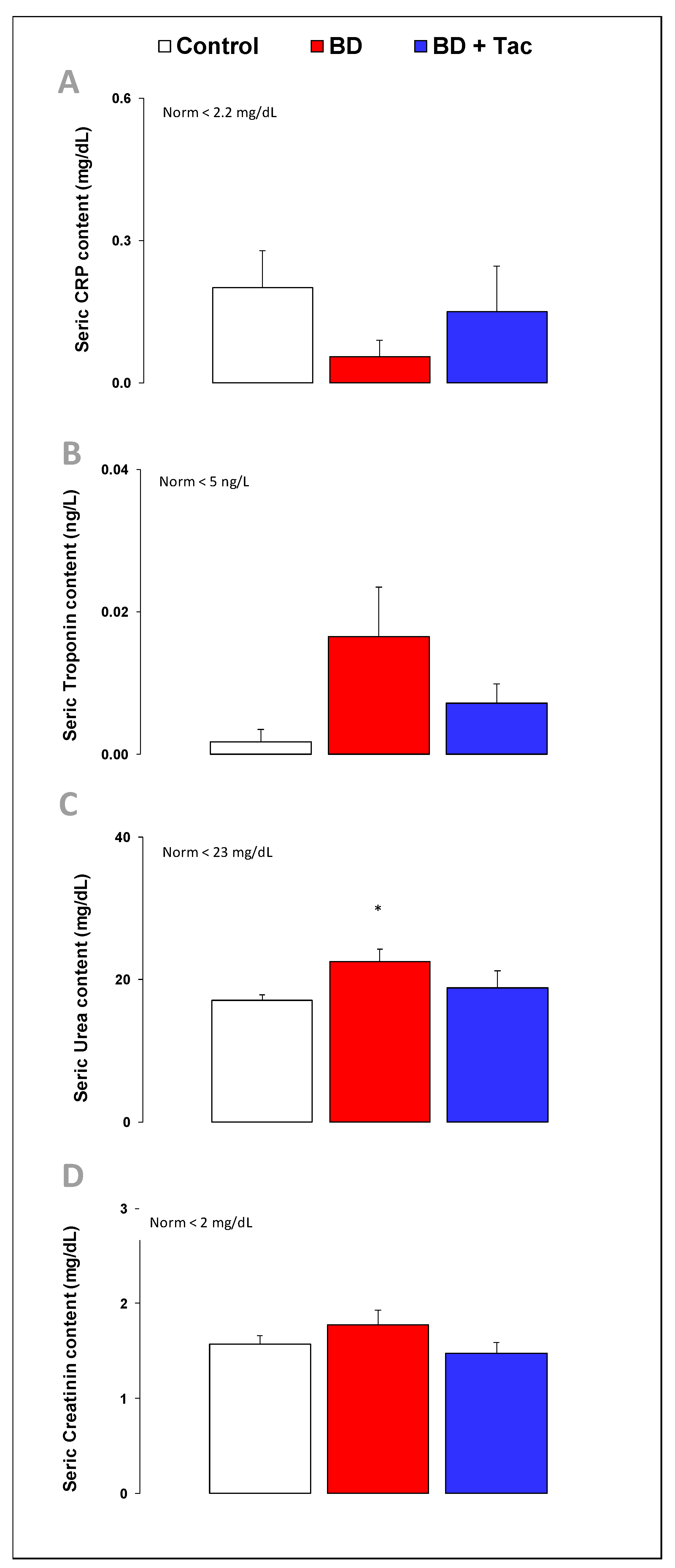
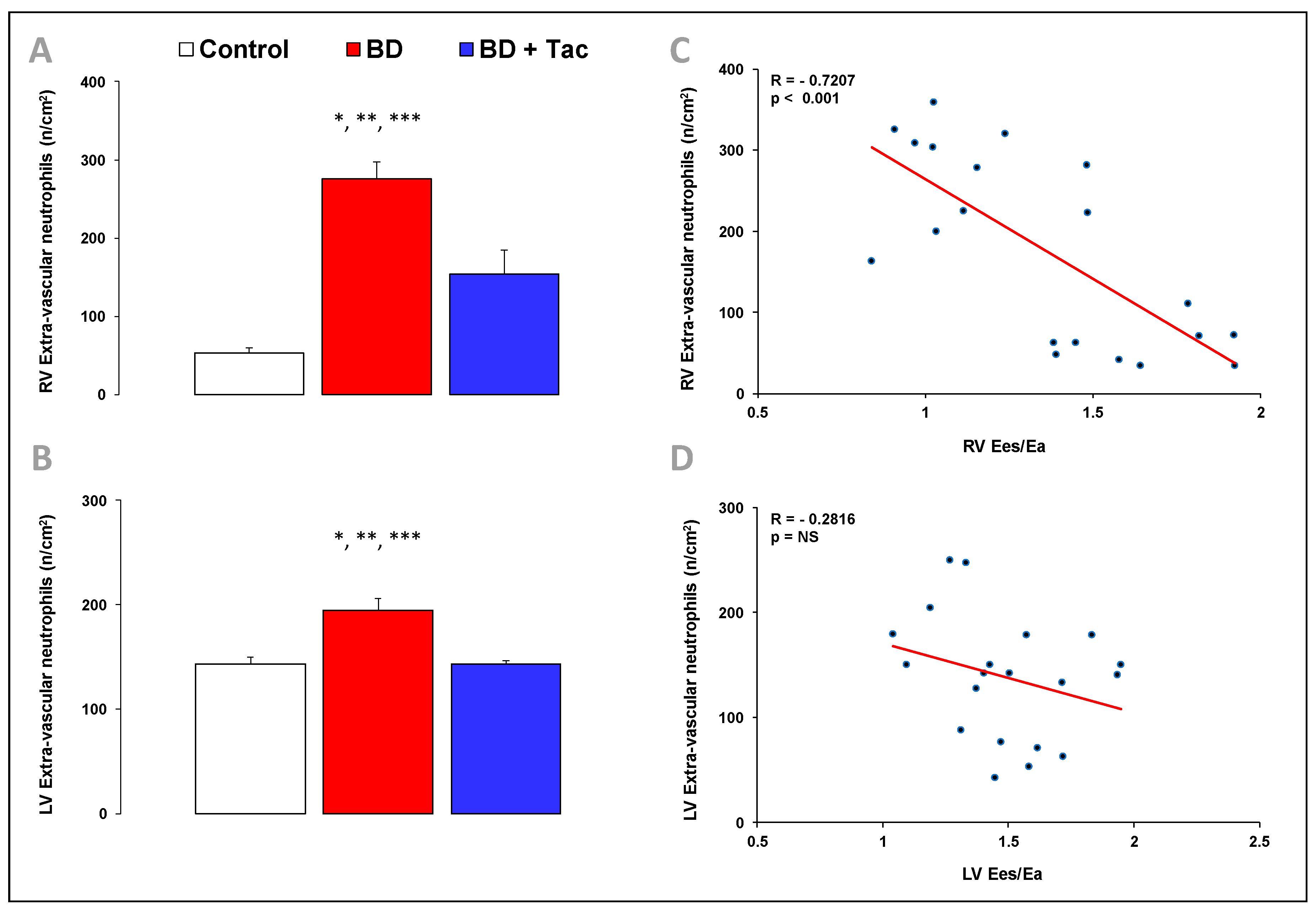
2.3. Circulating Cardiac Markers (Figure 3)
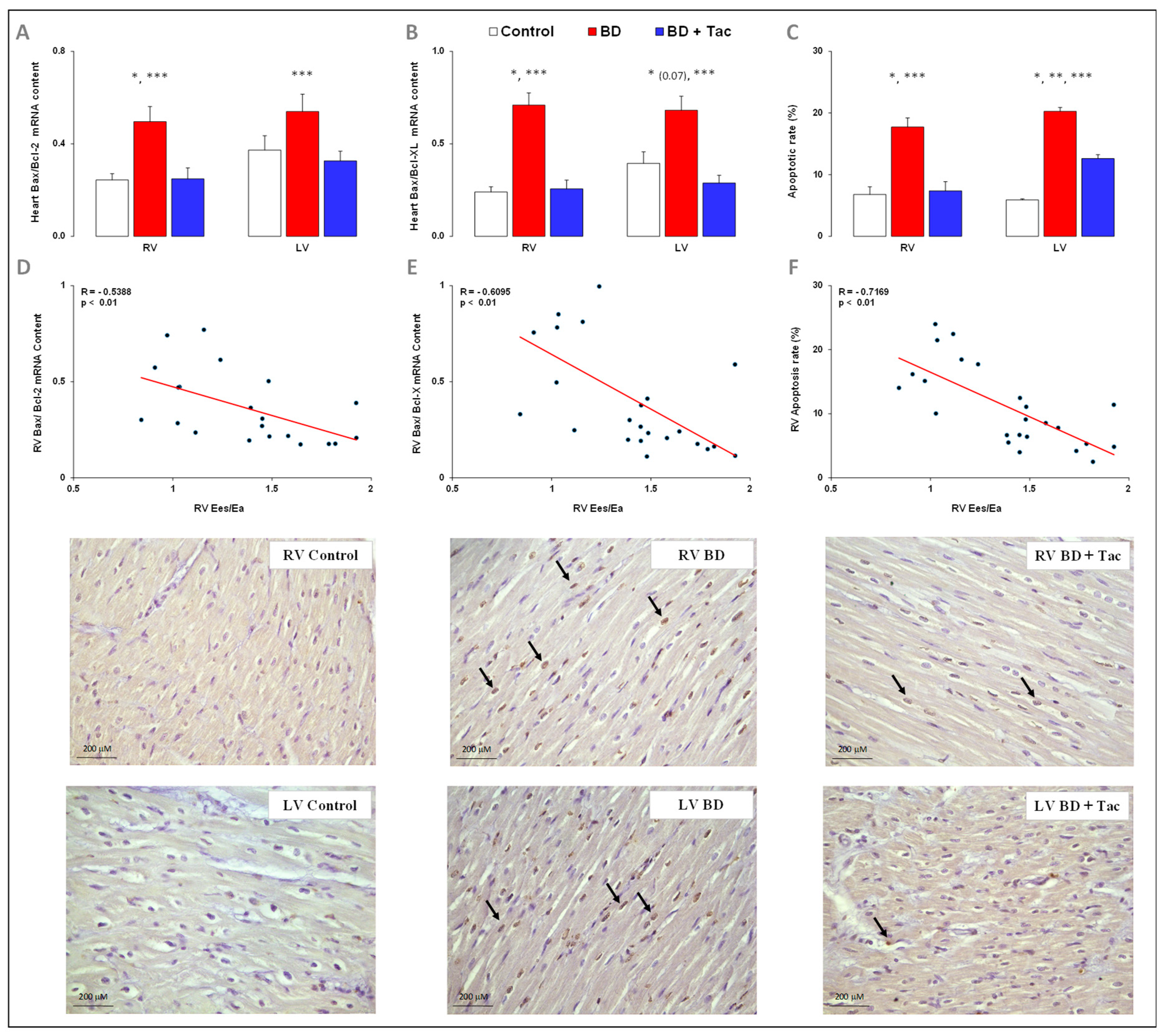
2.4. Tacrolimus Prevents RV and LV Activation of Apoptosis (Figure 4)
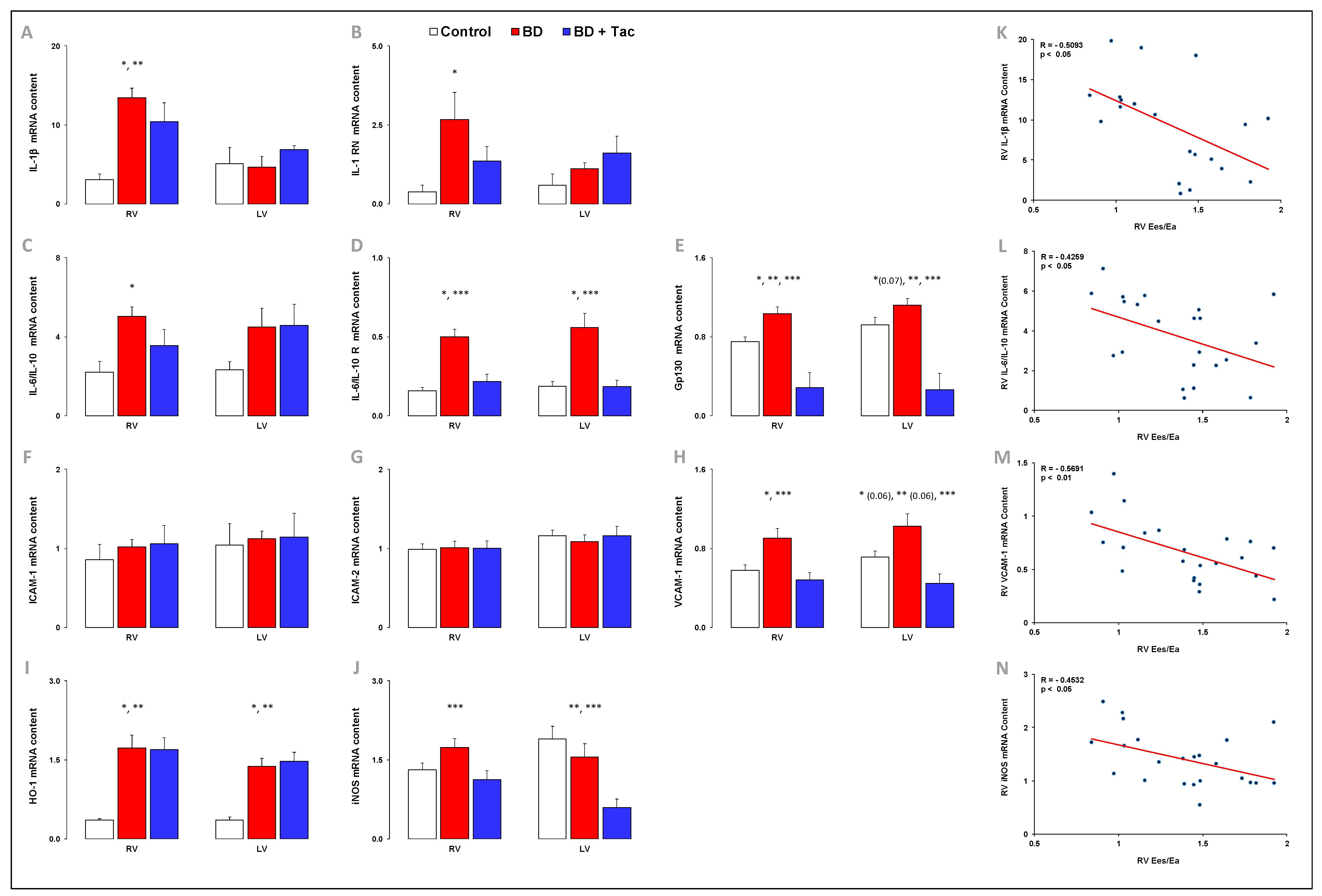
2.5. Tacrolimus Pretreatment Alters the Expression of Inflammatory Regulators and Cytokines (Figure 5 and Figure 6)
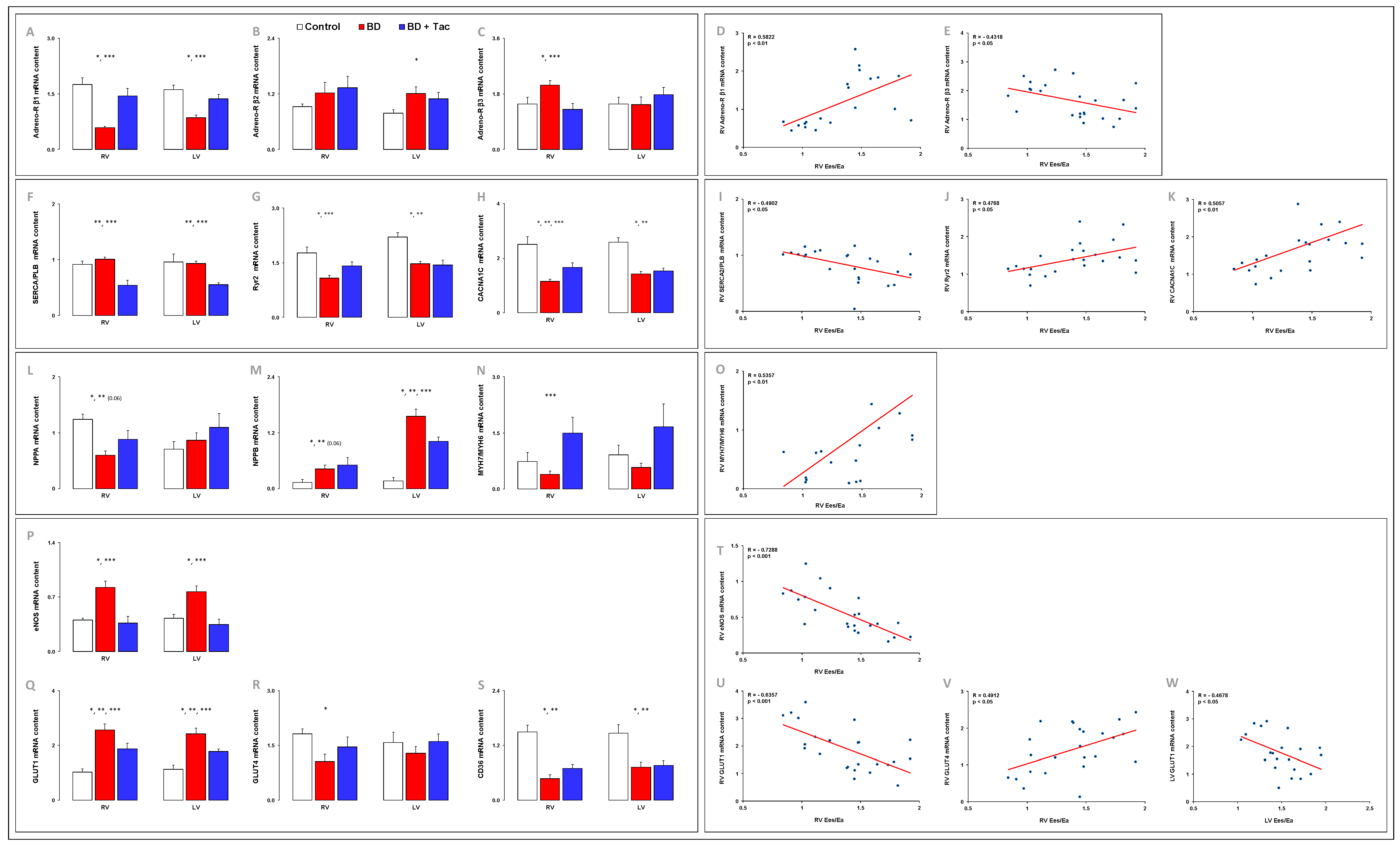
2.6. Altered Myocardial Expression of β-Adrenergic Receptors Was Prevented by Tacrolimus (Figure 7)
2.7. Tacrolimus Partly Prevented the Altered Expression of Myocardial Calcium-Handling Molecules (Figure S1)
2.8. Myocardial Expression of Cardiac Hypertrophic Markers (Figure 7)
2.9. Tacrolimus Partly Prevented the Myocardial Altered Expression of eNOS and Central Regulators of Cardiac Metabolism (Figure 7)
2.10. Myocardial Expression of Toll-Like Receptor (TLR) Signaling Pathways (Figure 8)
3. Discussion
4. Materials and Methods
4.1. Animal Preparation
4.2. Brain Death Procedure
4.3. Data Acquisition and Analysis
4.4. Biological and Histological Assessment
4.5. Immunohistochemistry: Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Staining to Assess Myocardial Apoptosis
4.6. Immunohistochemistry: Evaluation of Myeloperoxidase-Positive Inflammatory Cell Infiltrate
4.7. Plasma Levels of Inflammatory, Cardiac, and Kidney Biomarkers
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| ACTA1 | α-1 skeletal muscle actin |
| ALK1 | activin receptor-like kinase 1 |
| BMP | bone morphogenetic protein |
| BSA | bovine serum albumin |
| CACNA1C | voltage-gated Ca2+ channel subunit alpha1 C |
| CaMKIIδ | Ca2+/calmodulin-dependent protein kinase II, delta |
| CO | thermodilution cardiac output |
| CRP | C-reactive protein |
| eNOS | endothelial nitric oxide synthase |
| FiO2 | inspired oxygen fraction |
| FKBP12 | 12-kDa FK506-binding protein |
| GLUT | glucose transporter |
| HR | heart rate |
| ICAM | intercellular adhesion molecules |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LAP | left atrial pressure |
| LV | left ventricular |
| NCX1 | Na+/Ca2+ exchanger 1 |
| NLRP3 | NLR family pyrin domain containing 3 |
| NPPA | natriuretic peptide A |
| NPPB | brain natriuretic peptide |
| PAH | pulmonary arterial hypertension |
| PAP | pulmonary artery pressure |
| PBS | phosphate-buffered saline |
| PEEP | positive end-expiratory pressure |
| PLB | phospholamban |
| RAP | right atrial pressure |
| RT-qPCR | real-time quantitative polymerase chain reaction |
| RV | right ventricular |
| RyR2 | ryanodine receptor 2 |
| SaO2 | arterial oxygen saturation |
| SAP | systemic artery pressure |
| SERCA2A | sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2a |
| TLR | toll-like receptor |
| VCAM | vascular cell adhesion molecule |
References
- Kirklin, J.; Naftel, D.; Bourge, R.; McGiffin, D.; Hill, J.; Rodeheffer, R.; Jaski, B.; Hauptman, P.; Weston, M.; White-Williams, C. Evolving trends in risk profiles and causes of death after heart transplantation: A ten-year multi-institutional study. J. Thorac. Cardiovasc. Surg. 2003, 125, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Van Trigt, P.; Bittner, H.B.; Kendall, S.W.; Milano, C.A. Mechanisms of Transplant Right Ventricular Dysfunction. Ann. Surg. 1995, 221, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Borlaug, B.A. Pulmonary hypertension due to left heart disease. Circulation 2012, 126, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Brimioulle, S.; Dewachter, L. Biomechanics of the Right Ventricle in Health and Disease (2013 Grover Conference Series). Pulm. Circ. 2014, 4, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Bittner, H.B.; Kendall, S.W.; Chen, E.P.; Davis, R.D.; Van Trigt, P. Myocardial performance after graft preservation and subsequent cardiac transplantation from brain-dead donors. Ann. Thorac. Surg. 1995, 60, 47–54. [Google Scholar] [CrossRef]
- Belhaj, A.; Dewachter, L.; Rorive, S.; Remmelink, M.; Weynand, B.; Melot, C.; Galanti, L.; Hupkens, E.; Sprockeels, T.; Dewachter, C.; et al. Roles of inflammation and apoptosis in experimental brain death–induced right ventricular failure. J. Heart Lung Transplant. 2016, 35, 1505–1518. [Google Scholar] [CrossRef]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kang, K.M.; Kim, J.H.; Bahn, J.J.; Jeon, D.; Kim, M.; Lee, S.K.; Roh, J.K. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010, 1309, 164–171. [Google Scholar] [CrossRef]
- Anyanwu, A.C.; Banner, N.R.; Radley-Smith, R.; Khaghani, A.; Yacoub, M.H. Long-term results of cardiac transplantation from live donors: The domino heart transplant. J. Heart. Lung Transplant. 2002, 21, 971–975. [Google Scholar] [CrossRef]
- Khaghani, A.; Birks, E.; Anyanwu, A.; Banner, N. Heart transplantation from live donors: “domino procedure”. Heart Lung Transplant. 2004, 23 (Suppl. S9), S257–S259. [Google Scholar] [CrossRef]
- Sousa, K.d.S.; Quiles, C.L.; Muxel, S.M.; Trevisan, I.L.; Ferreira, Z.S.; Markus, R.P. Brain Damage-linked ATP Promotes P2X7 Receptors Mediated Pineal N-acetylserotonin Release. Neuroscience 2022, 499, 12–22. [Google Scholar] [CrossRef]
- Bittner, H.B.; Kendall, S.W.; Chen, E.P.; Craig, D.; Van Trigt, P. The Effects of Brain Death on Cardiopulmonary Hemodynamics and Pulmonary Blood Flow Characteristics. Chest 1995, 108, 1358–1363. [Google Scholar] [CrossRef]
- Wells, M.A.; Hoe, L.E.S.; Molenaar, P.; Pedersen, S.; Obonyo, N.G.; McDonald, C.I.; Mo, W.; Bouquet, M.; Hyslop, K.; Passmore, M.R.; et al. Compromised right ventricular contractility in an ovine model of heart transplantation following 24 h donor brain stem death. Pharmacol. Res. 2021, 169, 105631. [Google Scholar] [CrossRef]
- Marasco, S.F.; Arthur, J.F.; Ou, R.; Bailey, M.; Rosenfeldt, F. Apoptotic Markers in Donor Hearts After Brain Death vs Circulatory Death. Transplant. Proc. 2020, 53, 612–619. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Lin, J.X.; Vilcek, J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol. Cell. Biol. 1990, 10, 3818–3823. [Google Scholar] [CrossRef]
- Stoica, S.C.; Satchithananda, D.K.; White, P.A.; Parameshwar, J.; Redington, A.N.; Large, S.R. Noradrenaline Use in the Human Donor and Relationship with Load-Independent Right Ventricular Contractility. Transplantation 2004, 78, 1193–1197. [Google Scholar] [CrossRef]
- Földes, G.; von Haehling, S.; Okonko, D.O.; Jankowska, E.A.; Poole-Wilson, P.A.; Anker, S.D. Fluvastatin reduces increased blood monocyte Toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int. J. Cardiol. 2008, 124, 80–85. [Google Scholar] [CrossRef]
- Mann, D.L. The emerging role of innate immunity in the heart and vascular system: For whom the cell tolls. Circ. Res. 2011, 108, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- He, R.L.; Wu, Z.J.; Liu, X.R.; Gui, L.X.; Wang, R.X.; Lin, M.J. Calcineurin/NFAT Signaling Modulates Pulmonary Artery Smooth Muscle Cell Proliferation, Migration and Apoptosis in Monocrotaline-Induced Pulmonary Arterial Hypertension Rats. Cell Physiol. Biochem. 2018, 49, 172–189. [Google Scholar] [CrossRef] [PubMed]
- de Frutos, S.; Nitta, C.H.; Caldwell, E.; Friedman, J.; Bosc, L.V.G.; Plomaritas, D.R.; Herbert, L.M.; Giermakowska, W.; Browning, C.; Jernigan, N.L.; et al. Regulation of soluble guanylyl cyclase-α1 expression in chronic hypoxia-induced pulmonary hypertension: Role of NFATc3 and HuR. Am. J. Physiol. Cell. Mol. Physiol. 2009, 297, L475–L486. [Google Scholar] [CrossRef] [PubMed]
- Na Qin, N.; Gong, Q.H.; Wei, L.W.; Wu, Q.; Huang, X.N. Total ginsenosides inhibit the right ventricular hypertrophy induced by monocrotaline in rats. Biol. Pharm. Bull. 2008, 31, 1530–1535. [Google Scholar] [CrossRef]
- Koulmann, N.; Novel-Chaté, V.; Peinnequin, A.; Chapot, R.; Serrurier, B.; Simler, N.; Richard, H.; Ventura-Clapier, R.; Bigard, X. Cyclosporin A Inhibits Hypoxia-induced Pulmonary Hypertension and Right Ventricle Hypertrophy. Am. J. Respir. Crit. Care Med. 2006, 174, 699–705. [Google Scholar] [CrossRef]
- Wang, Y.X.; Reyes-García, J.; Di Mise, A.; Zheng, Y.M. Role of ryanodine receptor 2 and FK506-binding protein 12.6 dissociation in pulmonary hypertension. J. Gen. Physiol. 2023, 155, e202213100. [Google Scholar] [CrossRef]
- Su, Z.; Sugishita, K.; Li, F.; Ritter, M.; Barry, W.H. Effects of FK506 on [Ca2+]i differ in mouse and rabbit ventricular myocytes. J. Pharmacol. Exp. Ther. 2003, 304, 334–341. [Google Scholar] [CrossRef]
- Spiekerkoetter, E.; Tian, X.; Cai, J.; Hopper, R.K.; Sudheendra, D.; Li, C.G.; El-Bizri, N.; Sawada, H.; Haghighat, R.; Chan, R.; et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J. Clin. Investig. 2013, 123, 3600–3613. [Google Scholar] [CrossRef]
- Spiekerkoetter, E.; Sung, Y.K.; Sudheendra, D.; Scott, V.; Del Rosario, P.; Bill, M.; Haddad, F.; Long-Boyle, J.; Hedlin, H.; Zamanian, R.T. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1602449. [Google Scholar] [CrossRef]
- Hoe, L.E.S.; Wells, M.A.; Bartnikowski, N.; Obonyo, N.G.; Millar, J.E.; Khoo, A.; Ki, K.K.; Shuker, T.; Ferraioli, A.; Colombo, S.M.; et al. Heart Transplantation From Brain Dead Donors: A Systematic Review of Animal Models. Transplantation 2020, 104, 2272–2289. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Monchi, M.; Fulgencio, J.-P.; Cottias, P.; Haouache, H.; Alvarez-Gonzalvez, A.; Guerrini, P.; Cavaillon, J.-M.; Adib-Conquy, M. Immune status and apoptosis activation during brain death. Shock 2010, 33, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, A.; Dewachter, L.; Hupkens, E.; Remmelink, M.; Galanti, L.; Rorive, S.; Melot, C.; Naeije, R.; Rondelet, B. Tacrolimus Prevents Mechanical and Humoral Alterations in Brain Death-Induced Lung Injury in Pigs. Am. J. Respir. Crit. Care Med. 2022, 206, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, A.; Dewachter, L.; Rorive, S.; Remmelink, M.; Weynand, B.; Melot, C.; Hupkens, E.; Dewachter, C.; Creteur, J.; Mc Entee, K.; et al. Mechanical versus humoral determinants of brain death-induced lung injury. PLoS ONE 2017, 12, e0181899. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ge, L.; Niu, L.; Lian, X.; Ma, H.; Pang, L. The dual role of inducible nitric oxide synthase in myocardial ischemia/reperfusion injury: Friend or foe? Oxid. Med. Cell Longev. 2018, 2018, 8364848. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Feng, Z. The Role of Toll-Like Receptor Signaling in the Progression of Heart Failure. Mediat. Inflamm. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Bittner, H.B.; Chen, E.P.; Biswas, S.S.; Van Trigt, P., 3rd; Davis, R.D. Right ventricular dysfunction after cardiac transplantation: Primarily related to status of donor heart. Ann. Thorac. Cardiovasc. Surg. 1999, 68, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Sebening, C.; Hagl, C.; Szabo, G.; Tochtermann, U.; Strobel, G.; Schnabel, P.; Amann, K.; Vahl, C.; Hagl, S. Cardiocirculatory effects of acutely increased intracranial pressure and subsequent brain death. Eur. J. Cardio-Thoracic Surg. 1995, 9, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Roozekrans, N.T.; van Zwieften, P. The role of the adrenergic nervous system in the pressor response due to acute elevation of intracranial pressure (Cushing’s response). A pharmacological analysis. Arch. Int. Pharmacodyn. Ther. 1977, 228, 79–91. [Google Scholar]
- Brashear, R.E.; Ross, J.C. Hemodynamic effects of elevated cerebrospinal fluid pressure: Alterations with adrenergic blockade. J. Clin. Investig. 1970, 49, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.; Novitzky, D.; Wicomb, W.N. The pathophysiological effects of brain death on potential donor organs, with particular reference to the heart. Ind. Mark. Manag. 1989, 71, 261–266. [Google Scholar]
- Matsuura, S.; Sakamoto, H.; Hayashida, Y.; Kuno, M. Efferent discharges of sympathetic and parasympathetic nerve fibers during increased intracranial pressure in anesthetized cats in the absence and presence of pressor response. Brain Res. 1984, 305, 291–301. [Google Scholar] [CrossRef]
- Kocsis, B.; Lenkei, Z. Coordination between cardiovascular and respiratory control systems during and after cerebral ischemia. J. Appl. Physiol. 1992, 72, 1595–1603. [Google Scholar] [CrossRef]
- Kocsis, B.; Fedina, L.; Pasztor, E. Two-phase change of sympathetic rhythms in brain ischemia, Cushing reaction, and asphyxia. Am. J. Physiol. 1989, 256, R120–R132. [Google Scholar] [CrossRef]
- Szabó, G.; Sebening, C.; Hagl, C.; Tochtermann, U.; Vahl, C.F.; Hagl, S. Right ventricular function after brain death: Response to an increased afterload1. Eur. J. Cardio-Thoracic. Surg. 1998, 13, 449–458. [Google Scholar] [CrossRef]
- Berman, M.; Ali, A.; Ashley, E.; Freed, D.; Clarke, K.; Tsui, S.; Parameshwar, J.; Large, S. Is stress cardiomyopathy the underlying cause of ventricular dysfunction associated with brain death? J. Heart Lung Transplant. 2010, 29, 957–965. [Google Scholar] [CrossRef]
- Bittner, H.B.; Chen, E.P.; Milano, C.A.; Kendall, S.W.; Jennings, R.B.; Sabiston, D.C., Jr.; Van Trigt, P. Myocardial beta-adrenergic receptor function and high-energy phosphates in brain death—Related cardiac dysfunction. Circulation 1995, 92, II472–II478. [Google Scholar] [CrossRef]
- Ducker, T.B.; Simmons, R.L. Increased intracranial pressure and pulmonary edema. 2. The hemodynamic response of dogs and monkeys to increased intracranial pressure. J. Neurosurg. 1968, 28, 118–123. [Google Scholar] [CrossRef]
- Dewachter, C.; Belhaj, A.; Rondelet, B.; Vercruyssen, M.; Schraufnagel, D.P.; Remmelink, M.; Brimioulle, S.; Kerbaul, F.; Naeije, R.; Dewachter, L. Myocardial inflammation in experimental acute right ventricular failure: Effects of prostacyclin therapy. J. Heart Lung Transplant. 2015, 34, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Mayrleitner, M.; Timerman, A.; Wiederrecht, G.; Fleischer, S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506 binding protein: Effect of FKBP-12 on single channel activity of the skeletal muscle ryanodine receptor. Cell Calcium 1994, 15, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ahern, G.P.; Junankar, P.R.; Dulhunty, A.F. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 1994, 352, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Brittain, E.L.; Trammell, A.W.; Fessel, J.P.; Austin, E.D.; Penner, N.; Maynard, K.B.; Gleaves, L.; Talati, M.; Absi, T.; et al. Evidence for Right Ventricular Lipotoxicity in Heritable Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2014, 189, 325–334. [Google Scholar] [CrossRef]
- Yano, M.; Kobayashi, S.; Kohno, M.; Doi, M.; Tokuhisa, T.; Okuda, S.; Suetsugu, M.; Hisaoka, T.; Obayashi, M.; Ohkusa, T.; et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 2003, 107, 477–484. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef]
- Rowan, R.A.; Billingham, M.E. Pathologic changes in the long-term transplanted heart: A morphometric study of myocardial hypertrophy, vascularity, and fibrosis. Hum. Pathol. 1990, 21, 767–772. [Google Scholar] [CrossRef]
- Luedde, M.; Katus, H.; Frey, N. Novel Molecular Targets in the Treatment of Cardiac Hypertrophy. Recent Patents Cardiovasc. Drug Discov. 2006, 1, 1–20. [Google Scholar] [CrossRef]
- Harvey, P.A.; Leinwand, L.A. The cell biology of disease: Cellular mechanisms of cardiomyopathy. J. Cell Biol. 2011, 194, 355–365. [Google Scholar] [CrossRef]
- Rosano, G.M.; Vitale, C. Metabolic modulation of cardiac metabolism in heart failure. Card Fail Rev. 2018, 4, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Rondelet, B.; Dewachter, C.; Kerbaul, F.; Kang, X.; Fesler, P.; Brimioulle, S.; Naeije, R.; Dewachter, L. Prolonged overcirculation-induced pulmonary arterial hypertension as a cause of right ventricular failure. Eur. Heart J. 2011, 33, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Rondelet, B.; Kerbaul, F.; Motte, S.; van Beneden, R.; Remmelink, M.; Brimioulle, S.; Mc Entee, K.; Wauthy, P.; Salmon, I.; Ketelslegers, J.-M.; et al. Bosentan for the Prevention of Overcirculation-Induced Experimental Pulmonary Arterial Hypertension. Circulation 2003, 107, 1329–1335. [Google Scholar] [CrossRef]
- Van Raemdonck, D.; Neyrinck, A.; Verleden, G.M.; Dupont, L.; Coosemans, W.; Decaluwe, H.; Decker, G.; De Leyn, P.; Nafteux, P.; Lerut, T. Lung Donor Selection and Management. Proc. Am. Thorac. Soc. 2009, 6, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.R.; Marshall, S.G. Apnea Testing: The Effects of Insufflation Catheter Size and Flow on Pressure and Volume in a Test Lung. Respir. Care 2013, 59, 406–410. [Google Scholar] [CrossRef]
- Morenski, J.D.; Oro, J.J.; Tobias, J.D.; Singh, A. Determination of Death by Neurological Criteria. J. Intensiv. Care Med. 2003, 18, 211–221. [Google Scholar] [CrossRef]
- Sagawa, K. The end-systolic pressure-volume relation of the ventricle: Definition, modifications and clinical use. Circulation 1981, 63, 1223–1227. [Google Scholar] [CrossRef]
- Belhaj, A.; Dewachter, L.; Kerbaul, F.; Brimioulle, S.; Dewachter, C.; Naeije, R.; Rondelet, B. Heme Oxygenase-1 and Inflammation in Experimental Right Ventricular Failure on Prolonged Overcirculation-Induced Pulmonary Hypertension. PLoS ONE 2013, 8, e69470. [Google Scholar] [CrossRef]
- Winer, J.; Jung, C.K.; Shackel, I.; Williams, P.M. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal. Biochem. 1999, 270, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, C.; Dewachter, L.; Rondelet, B.; Fesler, P.; Brimioulle, S.; Kerbaul, F.; Naeije, R. Activation of apoptotic pathways in experimental acute afterload-induced right ventricular failure*. Crit. Care Med. 2010, 38, 1405–1413. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequences |
|---|---|
| Bcl-2 associated X apoptosis regulator (Bax) | |
| Sense | 5′-CGCATTGGAGATGAACTGG-3′ |
| Antisense | 5′-CGCCACTCGGAAAAAGACT-3′ |
| B-CELL LYMPHOMA-2 (Bcl2) | |
| Sense | 5′-GACTTTGCCGAGATGTCCAG-3′ |
| Antisense | 5′-ACAATCCTCCCCCAGTTCA-3′ |
| Bcl-2-like 1 (BclXL) | |
| Sense | 5′-TTGTGGCCTTTTTCTCCTTC-3′ |
| Antisense | 5′-CGATCCGACTCACCAATACC-3′ |
| Interleukin-1beta (IL-1β) | |
| Sense | 5′-CACCCAAAACCTGGACCTT-3′ |
| Antisense | 5′-TGCCTGATGCTCTTGTTC-3′ |
| IL-1 receptor antagonist (IL1RN1) | |
| Sense | 5′-GACTTTGCCGAGATGTCCAG-3′ |
| Antisense | 5′-ACAATCCTCCCCCAGTTCA-3′ |
| Interleukin-6 (IL-6) | |
| Sense | 5’-CCACCAGGAACGAAAGAGAG-3’ |
| Antisense | 5’-AGTAGCCATCACCAGAAGCAG-3’ |
| IL-6 receptor (IL-6R) | |
| Sense | 5′-CCGGAGGGAGACAACTCTTT-3′ |
| Antisense | 5′-GGCTGCAAGATTCCATAACC-3′ |
| IL-6 signal transducer (Gp130) | |
| Sense | 5’-ATGGCAGCGTACACAGATGA-3’ |
| Antisense | 5’-GCTAAACACACAGGCACGAC-3’ |
| Interleukin-10 (IL-10) | |
| Sense | 5’-TCATCAATTTCTGCCCTGTG-3’ |
| Antisense | 5’-TGTAGACACCCCTCTCTTGGA-3’ |
| IL-10 receptor (IL-10R) | |
| Sense | 5′-TTCAAGTCCGAGCGTTTCTT-3′ |
| Antisense | 5′-GGTTTCGTCATTGGTCGTCT-3′ |
| Tumor necrosis factor-alpha (TNF-α) | |
| Sense | 5′-TCTGGACTTTGCTGAATCTGG-3′ |
| Antisense | 5′-TGAGGGGGTCTGAAGGAGTAA-3′ |
| Heme oxygenase-1 (HO-1) | |
| Sense | 5’-CAGCATGCCCCAGGATTTGT-3 |
| Antisense | 5’-GACCTCGCCCTTCTGAAAGT-3’ |
| NITRIC OXIDE-SYNTHASE 2 (NOS2 OR iNOS) | |
| Sense | 5’-CTGCATGGATAAGTACAGGCTGACC-3 |
| Antisense | 5’-AGCTTCTGATCAATGTCATGAGCAA-3’ |
| Intercellular adhesion molecule 1 (ICAM1) | |
| Sense | 5′-ATTGTGAGGGGTGTCGAAGT-3′ |
| Antisense | 5′-TTCCCAGTTGTGTGTTTCCA-3′ |
| Intercellular adhesion molecule 2 (ICAM2) | |
| Sense | 5′-GGGCTCAGTGGAAGCTGTAT-3′ |
| Antisense | 5′-GGGAGAACACGCTGATGTTG-3′ |
| Vascular cell adhesion molecule 1 (VCAM1) | |
| Sense | 5′-GGAATTTACGTGTGCGAGGG-3′ |
| Antisense | 5′-TCCCTGGGAGCAACTTGAAC-3′ |
| Adrenoceptor beta 1 (Adreno-R b1) | |
| Sense | 5′-ACCCCAAGTGCTGCGATTT-3′ |
| Antisense | 5′-ATGCACAAGGGCACGTAGAA-3′ |
| Adrenoceptor beta 2 (Adreno-R β2) | |
| Sense | 5′-GATTCACAGGGGAGGAACTGTAG-3′ |
| Antisense | 5′-TTGTTTAGTGTTTGGCTGGGAG-3′ |
| Adrenoceptor beta 3 (Adreno-R b3) | |
| Sense | 5′-CAGAATGAGCCCTGTGGAGAT-3′ |
| Antisense | 5′-AGGTTGGTGAAAAGCCACTTG-3′ |
| ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 (SERCA2A) | |
| Sense | 5′-GGGAAAACCTTGCTGGAACT-3′ |
| Antisense | 5′-CTTCGCCTTCTTCAAACCAA-3′ |
| Phospholamban (PLB) | |
| Sense | 5′-AAACAGCCAAGGCTGCCTAAA-3′ |
| Antisense | 5′-GATACCAGGAAGGCAGGAAGC-3′ |
| Ryanodine receptor 2 (Ryr2) | |
| Sense | 5′-GTGAAGCAGCCCAAGGGTAT-3′ |
| Antisense | 5′-AAGGGACAGTGAGGCATTCG-3′ |
| Ca2+ voltage-gated channel subunit alpha1 C (CACNA1C) | |
| Sense | 5′-TTGACGCCTTGATTGTTGTG-3′ |
| Antisense | 5′-ATGGAGATGCGGGAGTTCT-3′ |
| Sodium/calcium exchanger protein (NCX1) | |
| Sense | 5′-ACTCTGGAATGCGGTTGGAG-3′ |
| Antisense | 5′-CCATGTAATGGAACATGTGTCTTCT-3′ |
| Ca2+/calmodulin-dependent protein kinase II delta (CAMKIId) | |
| Sense | 5′-GTGAAGCAGCCCAAGGGTAT-3′ |
| Antisense | 5′-AAGGGACAGTGAGGCATTCG-3′ |
| Actin alpha 1 (ACTA1) | |
| Sense | 5′-TCTATCGTCCACCGCAAAT-3′ |
| Antisense | 5′-CACTTGAGCAGATTCGTCGTC-3′ |
| Myosin heavy chain 6 (MYH6) | |
| Sense | 5′-CATCGGCGCCAAGCAAAAA-3′ |
| Antisense | 5′-AGAGTCTGGCGCTCATGTTT-3′ |
| Myosin heavy chain 7 (MYH7) | |
| Sense | 5′-CAAGGGCTTGAACGAGGAGTA-3′ |
| Antisense | 5′-TCCAGGACTGGGAGCTTTGT-3′ |
| Natriuretic peptide A (NPPA) | |
| Sense | 5′-TGTCCAATGCAGACCTGATG-3′ |
| Antisense | 5′-GGGGCATAGCCTCATCTTCT-3′ |
| Natriuretic peptide B (NPPB) | |
| Sense | 5′-AAGACGATGCGTGACTCTGG-3′ |
| Antisense | 5′-TACCTCCTGAGCACATTGCAG-3′ |
| Nitric oxide synthase (NOS3 or eNOS) | |
| Sense | 5’-CTTTCCTGTTGGCCTGACCA-3’ |
| Antisense | 5’-CCGGTTACTCAGACCCAAGG-3’ |
| Solute carrier family 2 member 1 (SLC2A1 or GLUT1) | |
| Sense | 5′-CCATTCAGACAAGCAACAGG-3′ |
| Antisense | 5′-TGGGATGTGGGTAAAGGAGA-3′ |
| Solute carrier family 2 member 4 (SLC2A4 or GLUT4) | |
| Sense | 5′-TTTCCAGTATGTTGCGGATG-3′ |
| Antisense | 5′-CGGGTTTCAGGCACTTTTAG-3′ |
| CD36 molecule (CD36) | |
| Sense | 5′-CAGCCATTTGTGGATACTTGG-3′ |
| Antisense | 5′-TGCTGGTTGGAATACAGTGG-3′ |
| Toll-like receptor 2 (TLR2) | |
| Sense | 5′-CTCTCGTTGCGGCTTCCAA-3′ |
| Antisense | 5′-TCCAGAGAGTTGACCTTGCAG-3′ |
| Toll-like receptor 4 (TLR4) | |
| Sense | 5′-CGTGCAGGTGGTTCCTAACAT-3′ |
| Antisense | 5′-TGACTGATGTGGGGATGTTGT-3′ |
| NLR family pyrin domain containing 3 (NLRP3) | |
| Sense | 5’-CAGGCTTCTGGGACACCTTT-3’ |
| Antisense | 5’-CAGGCTTCTGGGACACCTTT-3’ |
| RIBOSOMAL PROTEIN-L4 (RPL4) | |
| Sense | 5′-AAACCAAGGAGGCTGTTCTG-3′ |
| Antisense | 5′-CATTCGCTGAGAGGCATAAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belhaj, A.; Dewachter, L.; Monier, A.; Vegh, G.; Rorive, S.; Remmelink, M.; Closset, M.; Melot, C.; Creteur, J.; Salmon, I.; et al. Beneficial Effects of Tacrolimus on Brain-Death-Associated Right Ventricular Dysfunction in Pigs. Int. J. Mol. Sci. 2023, 24, 10439. https://doi.org/10.3390/ijms241310439
Belhaj A, Dewachter L, Monier A, Vegh G, Rorive S, Remmelink M, Closset M, Melot C, Creteur J, Salmon I, et al. Beneficial Effects of Tacrolimus on Brain-Death-Associated Right Ventricular Dysfunction in Pigs. International Journal of Molecular Sciences. 2023; 24(13):10439. https://doi.org/10.3390/ijms241310439
Chicago/Turabian StyleBelhaj, Asmae, Laurence Dewachter, Astrid Monier, Gregory Vegh, Sandrine Rorive, Myriam Remmelink, Mélanie Closset, Christian Melot, Jacques Creteur, Isabelle Salmon, and et al. 2023. "Beneficial Effects of Tacrolimus on Brain-Death-Associated Right Ventricular Dysfunction in Pigs" International Journal of Molecular Sciences 24, no. 13: 10439. https://doi.org/10.3390/ijms241310439
APA StyleBelhaj, A., Dewachter, L., Monier, A., Vegh, G., Rorive, S., Remmelink, M., Closset, M., Melot, C., Creteur, J., Salmon, I., & Rondelet, B. (2023). Beneficial Effects of Tacrolimus on Brain-Death-Associated Right Ventricular Dysfunction in Pigs. International Journal of Molecular Sciences, 24(13), 10439. https://doi.org/10.3390/ijms241310439






