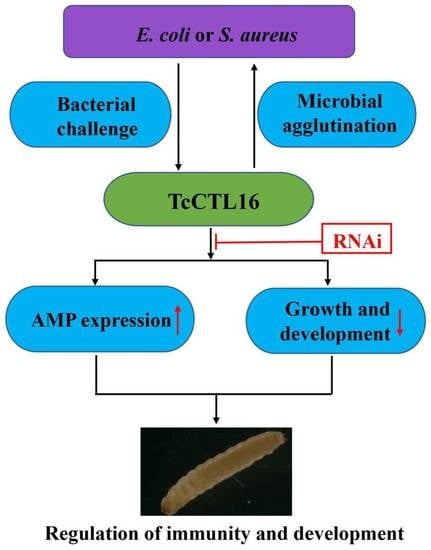
Functional Analysis of a CTL-X-Type Lectin CTL16 in Development and Innate Immunity of Tribolium castaneum
Abstract
1. Introduction
2. Results
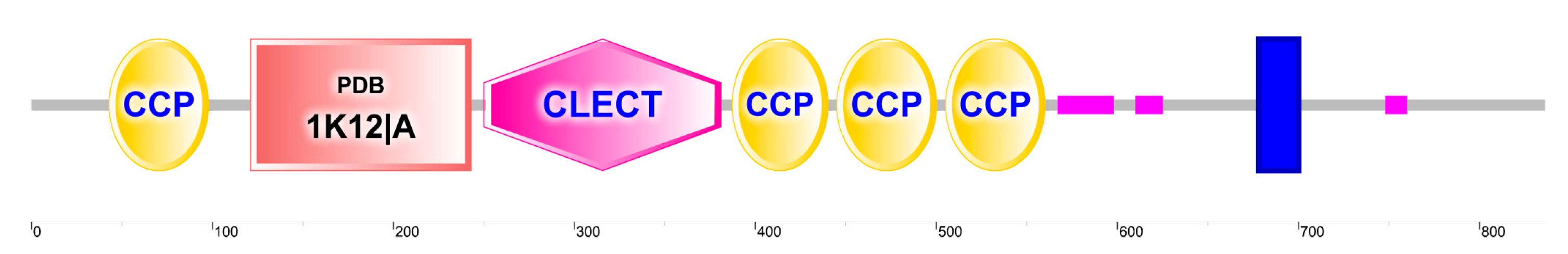
2.1. Domain Organization, Structure, and Evolutionary Relationships among CTLs
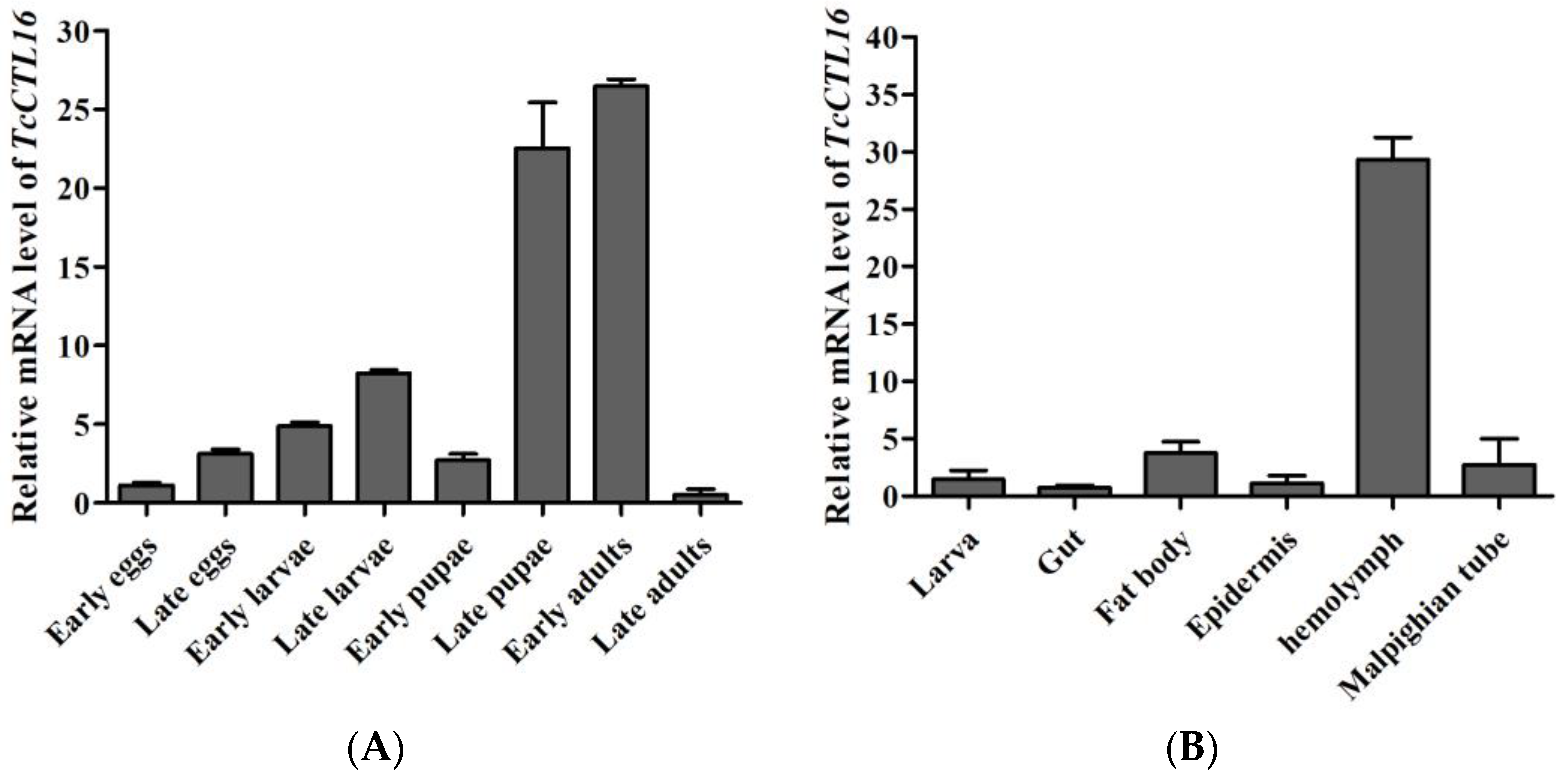
2.2. Spatiotemporal Expression of TcCTL16
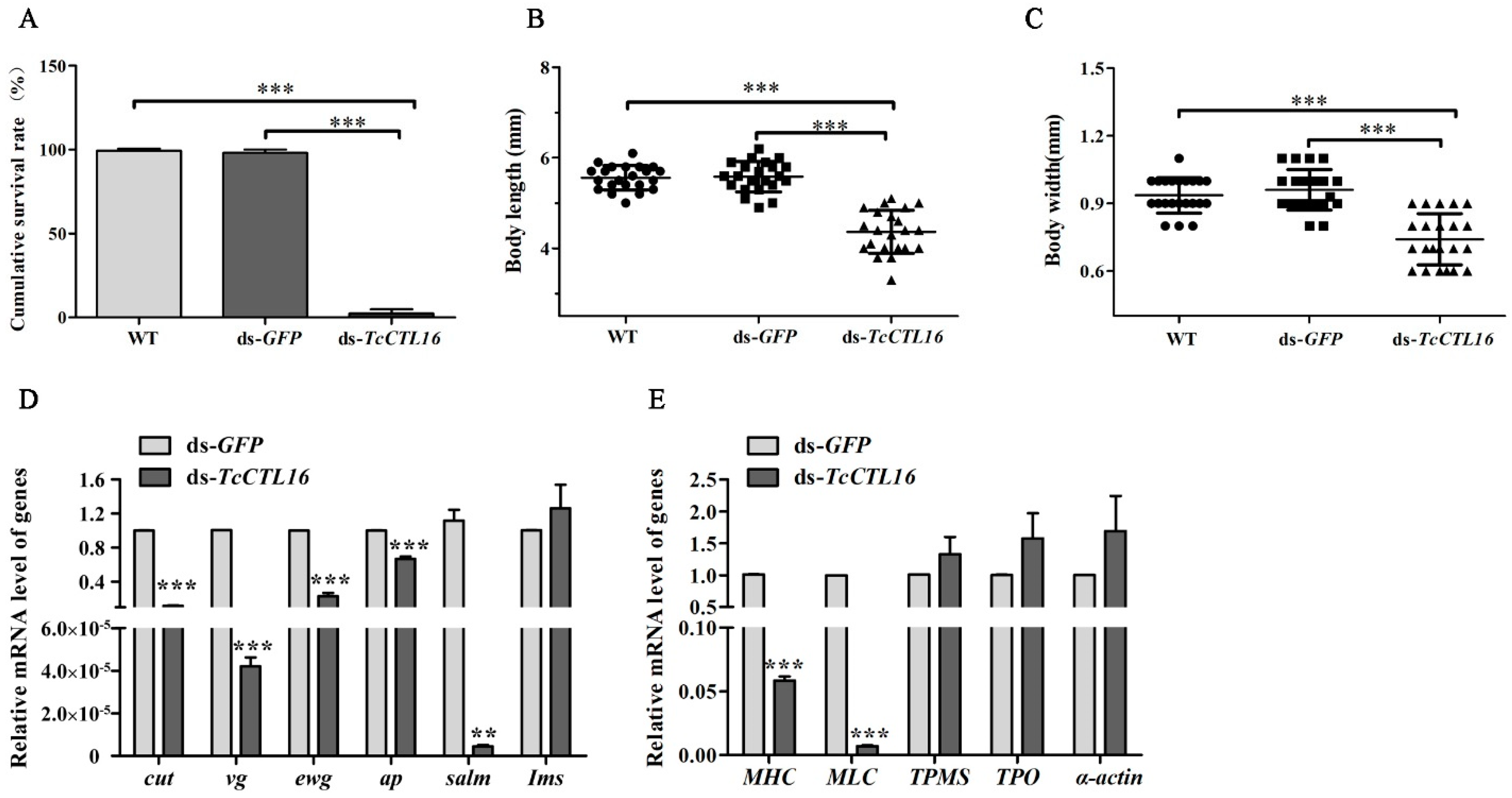
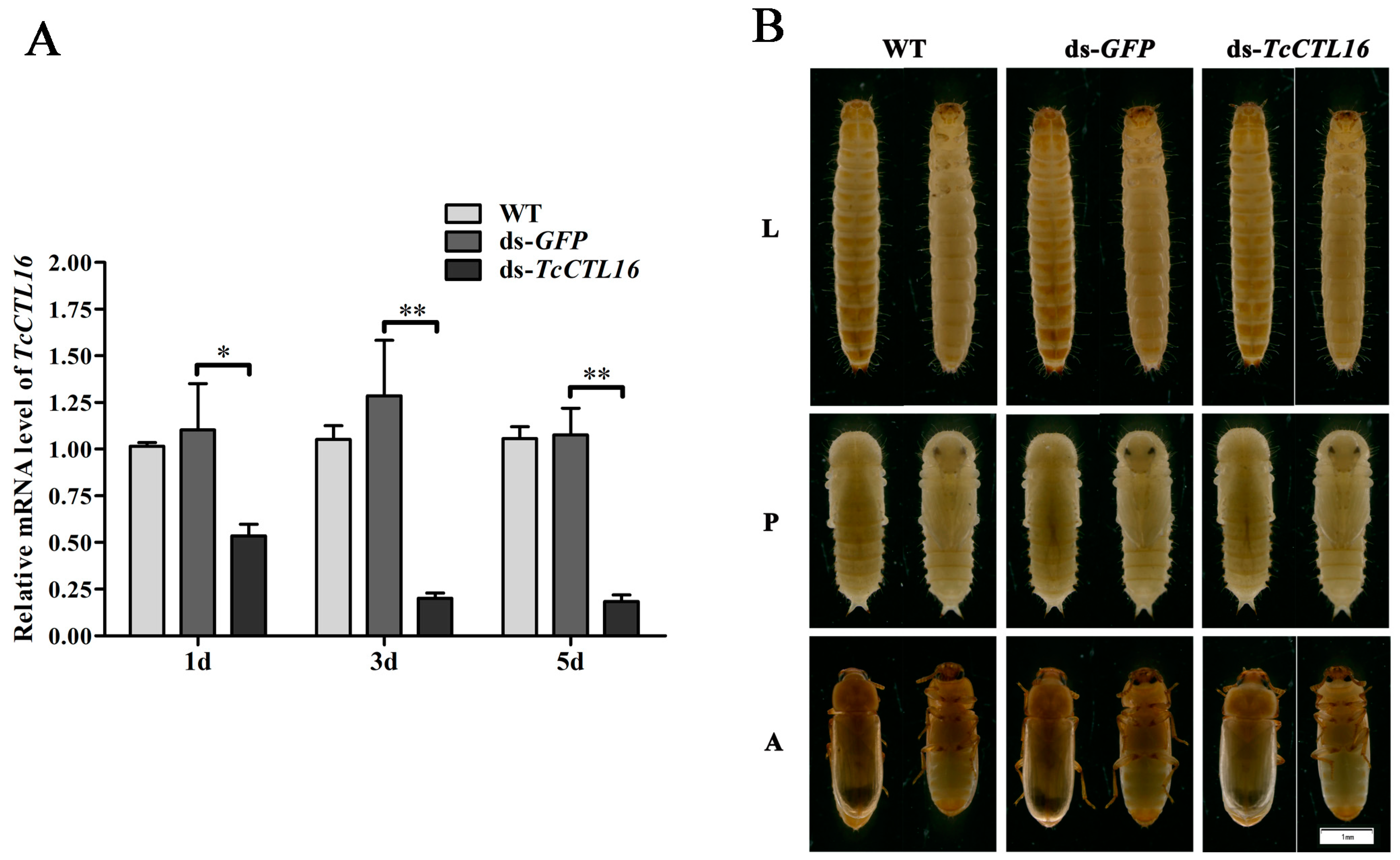
2.3. TcCTL16 Silencing in Early Larvae Influence Growth and Development
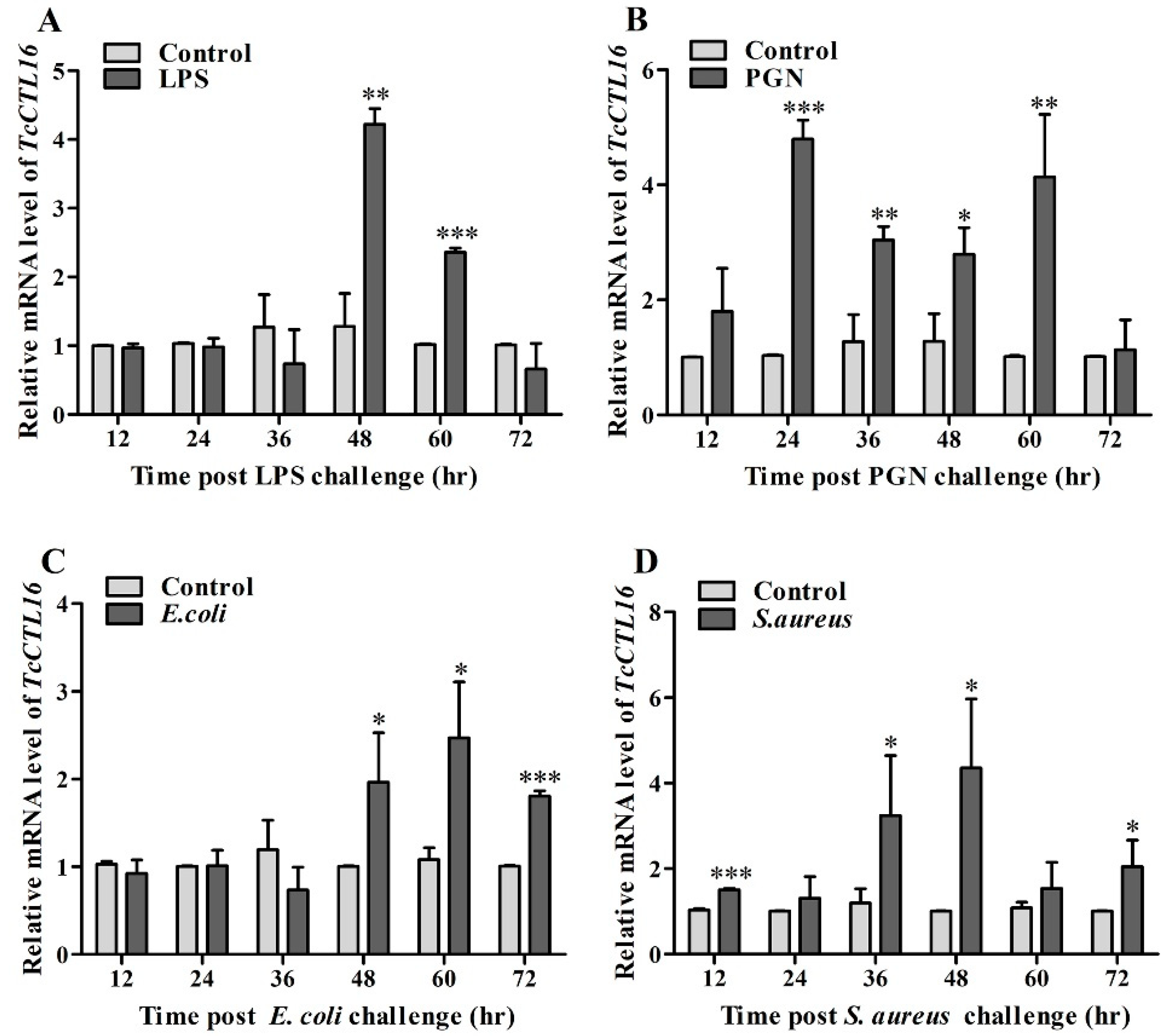
2.4. TcCTL16 Responses to Polysaccharide or Bacterial Challenge
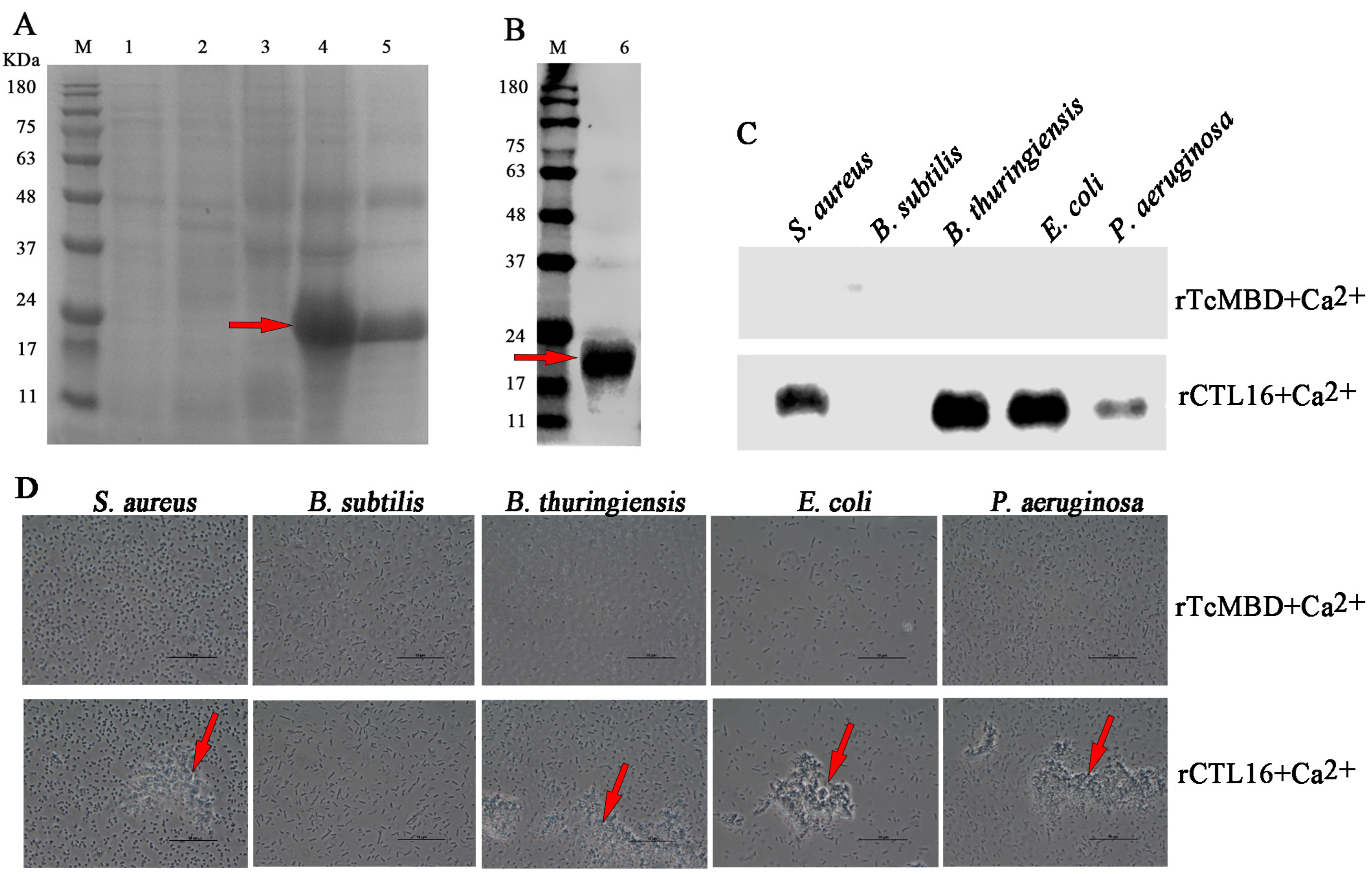
2.5. Production of rTcCTL16 CRD and Its Binding and Agglutination Capacity to Microbes
2.6. TcCTL16 Silencing on Late Larvae Does Not Affect Viability of Beetles
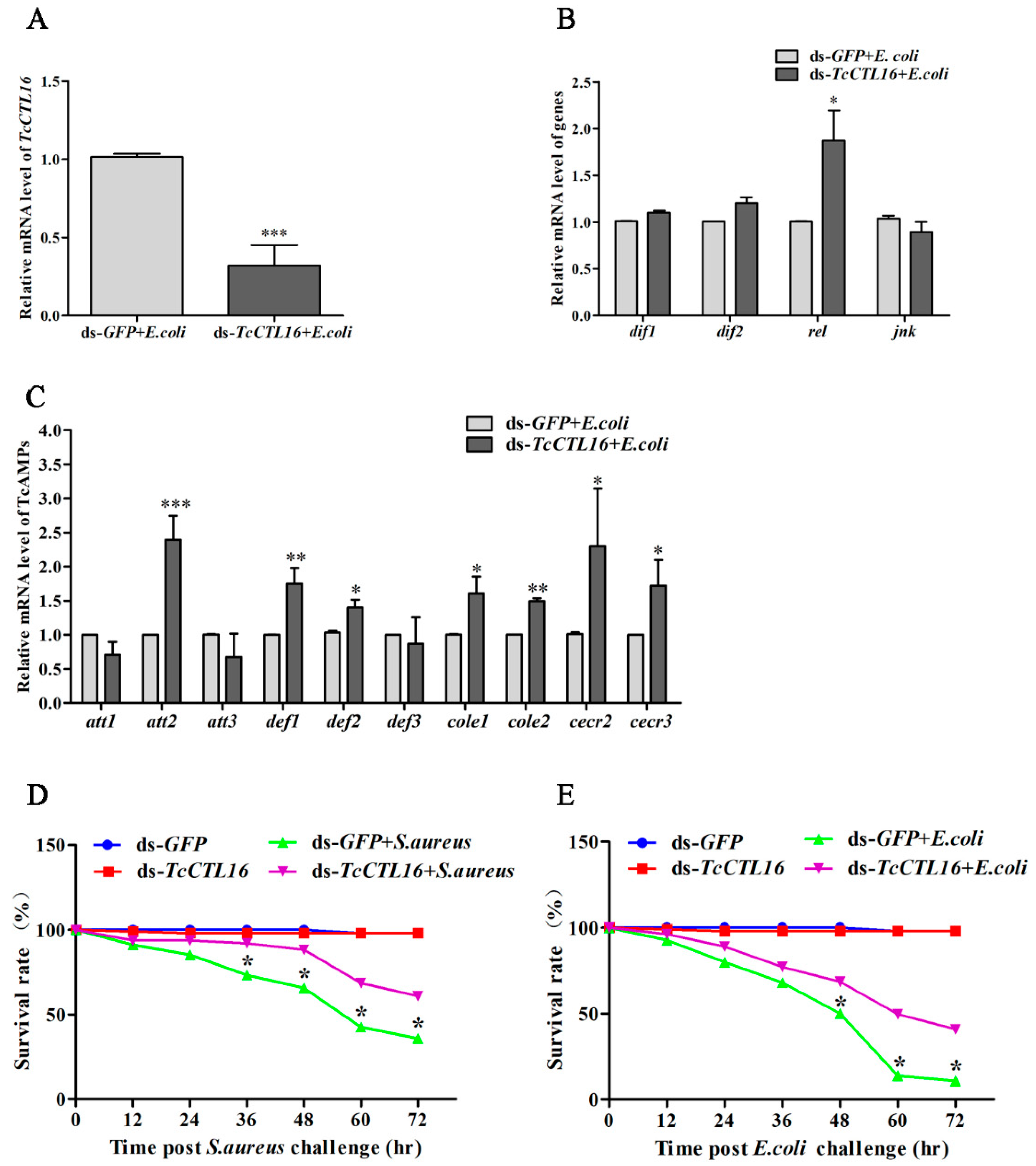
2.7. Effects of TcCTL16 Gene Knockdown on Defense against Bacterial Infection
3. Discussion
4. Materials and Methods
4.1. Animals and Chemicals
4.2. Identification and Cloning of TcCTL16 Gene
4.3. Sequence and Phylogenetic Analysis
4.4. Spatiotemporal Expression of TcCTL16
4.5. Expression Profiles of TcCTL16 after Being Challenged with Polysaccharides and Bacteria
4.6. Production of Recombinant TcCTL16 and Western Blot Analysis
4.7. Microorganism Binding and Agglutination of TcCTL16
4.8. dsRNA-Mediated RNAi Assay
4.9. Survival Assay
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Zhang, Y.; Yang, S.; Hong, Y.; Du, Y.; Hu, Z.; Tang, J.; Wang, S.; Feng, F.; Li, B. Functional analysis of TcCTL12 in innate immunity and development in Tribolium castaneum. Int. J. Biol. Macromol. 2022, 206, 422–434. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Cao, X.; Gunaratna, R.T.; Chen, Y.-R.; Blissard, G.; Kanost, M.R.; Jiang, H. Phylogenetic analysis and expression profiling of the pattern recognition receptors: Insights into molecular recognition of invading pathogens in Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 38–50. [Google Scholar] [CrossRef]
- Stokes, B.A.; Yadav, S.; Shokal, U.; Smith, L.C.; Eleftherianos, I. Bacterial and fungal pattern recognition receptors in homologous innate signaling pathways of insects and mammals. Front. Microbiol. 2015, 6, 19. [Google Scholar] [CrossRef]
- Chettri, D.; Boro, M.; Sarkar, L.; Verma, A.K. Lectins: Biological significance to biotechnological application. Carbohydr. Res. 2021, 506, 108367. [Google Scholar] [CrossRef]
- Rao, X.-J.; Cao, X.; He, Y.; Hu, Y.; Zhang, X.; Chen, Y.-R.; Blissard, G.; Kanost, M.R.; Yu, X.-Q.; Jiang, H. Structural features, evolutionary relationships, and transcriptional regulation of C-type lectin-domain proteins in Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 75–85. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, Z.; Wang, J.-M.; Xing, L.-S.; Xiong, G.-H.; Zou, Z. CTL14, a recognition receptor induced in late stage larvae, modulates anti-fungal immunity in cotton bollworm Helicoverpa armigera. Dev. Comp. Immunol. 2018, 84, 142–152. [Google Scholar] [CrossRef]
- Wang, X.-W.; Zhao, X.-F.; Wang, J.-X. C-type Lectin Binds to β-Integrin to Promote Hemocytic Phagocytosis in an Invertebrate. J. Biol. Chem. 2014, 289, 2405–2414. [Google Scholar] [CrossRef]
- Zhan, M.-Y.; Shahzad, T.; Yang, P.-J.; Liu, S.; Yu, X.-Q.; Rao, X.-J. A single-CRD C-type lectin is important for bacterial clearance in the silkworm. Dev. Comp. Immunol. 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Ao, J.; Ling, E.; Yu, X.-Q. Drosophila C-type lectins enhance cellular encapsulation. Mol. Immunol. 2007, 44, 2541–2548. [Google Scholar] [CrossRef]
- Arumugam, G.; Sreeramulu, B.; Paulchamy, R.; Thangavel, S.; Sundaram, J. Purification and functional characterization of lectin with phenoloxidase activity from the hemolymph of cockroach, Periplaneta americana. Arch. Insect Biochem. Physiol. 2017, 95, e21390. [Google Scholar] [CrossRef]
- Sun, J.-J.; Lan, J.-F.; Zhao, X.-F.; Vasta, G.R.; Wang, J.-X. Binding of a C-type lectin’s coiled-coil domain to the Domeless receptor directly activates the JAK/STAT pathway in the shrimp immune response to bacterial infection. PLoS Pathog. 2017, 13, e1006626. [Google Scholar] [CrossRef]
- Xia, X.; You, M.; Rao, X.J.; Yu, X.Q. Insect C-type lectins in innate immunity. Dev. Comp. Immunol. 2018, 83, 70–79. [Google Scholar] [CrossRef]
- Tanji, T.; Ohashi-Kobayashi, A.; Natori, S. Participation of a galactose-specific C-type lectin in Drosophila immunity. Biochem. J. 2006, 396, 127–138. [Google Scholar] [CrossRef]
- Yu, X.Q.; Kanost, M.R. A family of C-type lectins in Manduca sexta. Adv. Exp. Med. Biol. 2001, 484, 191–194. [Google Scholar] [PubMed]
- Kubo, T.; Kawasaki, K.; Natori, S. Transient Appearance and Localization of a 26-kDa Lectin, a Novel Member of the Periplaneta Lectin Family, in Regenerating Cockroach Leg. Dev. Biol. 1993, 156, 381–390. [Google Scholar] [CrossRef]
- Wang, P.; Zhuo, X.R.; Tang, L.; Liu, X.S.; Wang, Y.F.; Wang, G.X.; Yu, X.Q.; Wang, J.L. C-type lectin interacting with β-integrin enhances hemocytic encapsulation in the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 2017, 86, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.-K.; Tian, M.-L.; Dong, Y.-P.; Ren, C.-B.; Du, Y.; Hu, J. The C-type lectin IML-10 promotes hemocytic encapsulation by enhancing aggregation of hemocytes in the Asian corn borer Ostrinia furnacalis. Insect Biochem. Mol. Biol. 2020, 118, 103314. [Google Scholar] [CrossRef]
- Rao, X.-J.; Shahzad, T.; Liu, S.; Wu, P.; He, Y.-T.; Sun, W.-J.; Fan, X.-Y.; Yang, Y.-F.; Shi, Q.; Yu, X.-Q. Identification of C-type lectin-domain proteins (CTLDPs) in silkworm Bombyx mori. Dev. Comp. Immunol. 2015, 53, 328–338. [Google Scholar] [CrossRef]
- Chin, M.-L.; Mlodzik, M. The Drosophila Selectin Furrowed Mediates Intercellular Planar Cell Polarity Interactions via Frizzled Stabilization. Dev. Cell 2013, 26, 455–468. [Google Scholar] [CrossRef]
- A Leshko-Lindsay, L.; Corces, V.G. The role of selectins in Drosophila eye and bristle development. Development 1997, 124, 169–180. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, A.M.; Paik, R.; Li, J.; Bhat, M.A. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Sarrailh, C.; Banerjee, S.; Li, J.; Hortsch, M.; Laval, M.; Bhat, M.A. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development 2004, 131, 4931–4942. [Google Scholar] [CrossRef]
- Stork, T.; Engelen, D.; Krudewig, A.; Silies, M.; Bainton, R.J.; Klämbt, C. Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 587–597. [Google Scholar] [CrossRef]
- Tonk, M.; Knorr, E.; Cabezas-Cruz, A.; Valdés, J.J.; Kollewe, C.; Vilcinskas, A. Tribolium castaneum defensins are primarily active against Gram-positive bacteria. J. Invertebr. Pathol. 2015, 132, 208–215. [Google Scholar] [CrossRef]
- Consortium, T.G.S. The first genome sequence of a beetle, Tribolium castaneum, a model for insect development and pest biology. Nature 2008, 452, 949–955. [Google Scholar]
- Zou, Z.; Evans, J.D.; Lu, Z.; Zhao, P.; Williams, M.; Sumathipala, N.; Hetru, C.; Hultmark, D.; Jiang, H. Comparative genomic analysis of the Tribolium immune system. Genome Biol. 2007, 8, R177. [Google Scholar] [CrossRef]
- Bi, J.; Ning, M.; Li, J.; Zhang, P.; Wang, L.; Xu, S.; Zhong, Y.; Wang, Z.; Song, Q.; Li, B. A C-type lectin with dual-CRD from Tribolium castaneum is induced in response to bacterial challenge. Pest Manag. Sci. 2020, 76, 3965–3974. [Google Scholar] [CrossRef]
- Li, J.; Bi, J.; Zhang, P.; Wang, Z.; Zhong, Y.; Xu, S.; Wang, L.; Li, B. Functions of a C-type lectin with a single carbohydrate-recognition domain in the innate immunity and movement of the red flour beetle, Tribolium castaneum. Insect Mol. Biol. 2021, 30, 90–101. [Google Scholar] [CrossRef]
- Cheng, G.; Cox, J.; Wang, P.; Krishnan, M.N.; Dai, J.; Qian, F.; Anderson, J.F.; Fikrig, E. A C-Type Lectin Collaborates with a CD45 Phosphatase Homolog to Facilitate West Nile Virus Infection of Mosquitoes. Cell 2010, 142, 714–725. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.; Liu, J.; Xiao, X.; Zhang, S.; Qin, C.; Xiang, Y.; Wang, P.; Cheng, G. Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention. PLoS Pathog. 2014, 10, e1003931. [Google Scholar] [CrossRef]
- Dodd, R.; Drickamer, K. Lectin-like proteins in model organisms: Implications for evolution of carbohydrate-binding activity. Glycobiology 2001, 11, 71R–79R. [Google Scholar] [CrossRef]
- Zelensky, A.N.; E Gready, J. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef]
- Wang, X.-W.; Wang, J.-X. Diversity and multiple functions of lectins in shrimp immunity. Dev. Comp. Immunol. 2012, 39, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Pees, B.; Yang, W.; Zárate-Potes, A.; Schulenburg, H.; Dierking, K. High Innate Immune Specificity through Diversified C-Type Lectin-Like Domain Proteins in Invertebrates. J. Innate Immun. 2016, 8, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Anam, K.; Ahmed, H. Development of Galectin-3 Targeting Drugs for Therapeutic Applications in Various Diseases. Int. J. Mol. Sci. 2023, 24, 8116. [Google Scholar] [CrossRef]
- Hung, C.-H.; Huang, H.-L.; Hsu, K.-T.; Ho, S.-J.; Ho, S.-Y. Prediction of non-classical secreted proteins using informative physicochemical properties. Interdiscip. Sci. Comput. Life Sci. 2010, 2, 263–270. [Google Scholar] [CrossRef]
- Zhang, L.; Ward, R.E. uninflatable encodes a novel ectodermal apical surface protein required for tracheal inflation in Drosophila. Dev. Biol. 2009, 336, 201–212. [Google Scholar] [CrossRef]
- Rai, M.; Nongthomba, U.; Grounds, M.D. Chapter nine—Skeletal muscle degeneration and regeneration in mice and flies. Curr. Top. Dev. Biol. 2014, 108, 247–281. [Google Scholar]
- Abmayr, S.M.; Pavlath, G.K. Myoblast fusion: Lessons from flies and mice. Development 2012, 139, 641–656. [Google Scholar] [CrossRef]
- Schweitzer, R.; Zelzer, E.; Volk, T. Connecting muscles to tendons: Tendons and musculoskeletal development in flies and vertebrates. Development 2010, 137, 2807–2817. [Google Scholar] [CrossRef]
- Laurichesse, Q.; Soler, C. Muscle development: A view from adult myogenesis in Drosophila. Semin. Cell Dev. Biol. 2020, 104, 39–50. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Prabhakar, S.; Aggarwal, A.; Atreya, K.B.; VijayRaghavan, K. Adult Drosophila muscle morphometry through microCT reveals dynamics during ageing. Open Biol. 2019, 9, 190087. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Feng, F.; Li, J.; Mao, J.; Ning, M.; Song, X.; Xie, J.; Tang, J.; Li, B. A C-type lectin with a single carbohydrate-recognition domain involved in the innate immune response of Tribolium castaneum. Insect Mol. Biol. 2019, 28, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Imamura, M.; Kadotani, T.; Yaoi, K.; Iwahana, H.; Sato, R. The lipopolysaccharide-binding protein participating in hemocyte nodule formation in the silkworm Bombyx mori is a novel member of the C-type lectin superfamily with two different tandem carbohydrate-recognition domains. FEBS Lett 1999, 443, 139–143. [Google Scholar] [CrossRef]
- Shi, X.-Z.; Kang, C.-J.; Wang, S.-J.; Zhong, X.; Beerntsen, B.T.; Yu, X.-Q. Functions of Armigeres subalbatus C-type lectins in innate immunity. Insect Biochem. Mol. Biol. 2014, 52, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Watanabe, A.; Yoshizawa, Y.; Kitami, M.; Sato, R. Identification and comparative analysis of three novel C-type lectins from the silkworm with functional implications in pathogen recognition. Dev. Comp. Immunol. 2009, 33, 789–800. [Google Scholar] [CrossRef]
- Watanabe, A.; Miyazawa, S.; Kitami, M.; Tabunoki, H.; Ueda, K.; Sato, R. Characterization of a novel C-type lectin, Bombyx mori multibinding protein, from the B. mori hemolymph: Mechanism of wide-range microorganism recognition and role in immunity. J. Immunol. 2006, 177, 4594–4604. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Rimphanitchayakit, V.; Visetnan, S.; Amparyup, P.; Somboonwiwat, K.; Charoensapsri, W.; Tang, S. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization. Dev. Comp. Immunol. 2018, 80, 81–93. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, D.; Li, F.; Wang, M.; Huang, M.; Zhang, H.; Song, L. A novel C-type lectin from crab Eriocheir sinensis functions as pattern recognition receptor enhancing cellular encapsulation. Fish Shellfish. Immunol. 2013, 34, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Kanost, M.R. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J. Biol. Chem. 2000, 275, 37373–37381. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Zhang, A.; Cui, Z. A C-Type Lectin Highly Expressed in Portunus trituberculatus Intestine Functions in AMP Regulation and Prophenoloxidase Activation. Antibiotics 2021, 10, 541. [Google Scholar] [CrossRef]
- Geng, T.; Lv, D.-D.; Huang, Y.-X.; Hou, C.-X.; Qin, G.-X.; Guo, X.-J. JAK/STAT signaling pathway-mediated immune response in silkworm (Bombyx mori) challenged by Beauveria bassiana. Gene 2016, 595, 69–76. [Google Scholar] [CrossRef]
- Yu, X.-Q.; Ma, Y. Calcium is not required for immulectin-2 binding, but protects the protein from proteinase digestion. Insect Biochem. Mol. Biol. 2006, 36, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Ning, M.; Xie, X.; Fan, W.; Huang, Y.; Gu, W.; Wang, W.; Wang, L.; Meng, Q. A typical C-type lectin, perlucin-like protein, is involved in the innate immune defense of whiteleg shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2020, 103, 293–301. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, N.N.; Zheng, Y.; Liu, K.Y.; Mao, B.; Na Kong, L.; Chen, Y.; Ai, H. Identification and characterization of two novel C-type lectins from the larvae of housefly, Musca domestica L. Arch. Insect Biochem. Physiol. 2018, 98, e21467. [Google Scholar] [CrossRef]
- Yokoi, K.; Koyama, H.; Ito, W.; Minakuchi, C.; Tanaka, T.; Miura, K. Involvement of NF-κB transcription factors in antimicrobial peptide gene induction in the red flour beetle, Tribolium castaneum. Dev. Comp. Immunol. 2012, 38, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Gao, S.; Mao, J.; Wei, L.; Xie, J.; Liu, J.; Bi, J.; Song, X.; Li, B. CYP4BN6 and CYP6BQ11 mediate insecticide susceptibility and their expression is regulated by Latrophilin in Tribolium castaneum. Pest Manag. Sci. 2019, 75, 2744–2755. [Google Scholar] [CrossRef]
- Lord, J.C.; Hartzer, K.; Toutges, M.; Oppert, B. Evaluation of quantitative PCR reference genes for gene expression studies in Tribolium castaneum after fungal challenge. J. Microbiol. Methods 2010, 80, 219–221. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Huang, M.; Yi, Q.; Guo, Y.; Gai, Y.; Wang, H.; Zhang, H.; Song, L. A galectin from Eriocheir sinensis functions as pattern recognition receptor enhancing microbe agglutination and haemocytes encapsulation. Fish Shellfish. Immunol. 2016, 55, 10–20. [Google Scholar] [CrossRef]
| Name | Sequence (5′-3′) |
|---|---|
| PCR: | |
| TcCTL16-F | ATGGTCGTGTGTCAGTGCAAGG |
| TcCTL16-R | ATATCTCTTCTGGTCGCGGTGC |
| TcCTL16-F | atgggtcgcggatccgaattcATGGTCGTGTGTCAGTGCAAGG |
| TcCTL16-R | gtggtggtggtggtgctcgagATATCTCTTCTGGTCGCGGTGC |
| RNAi: | |
| ds-TcCTL16-F | taatacgactcactatagggATGGTCGTGTGTCAGTGCAA |
| ds-TcCTL16-R | taatacgactcactatagggATTGTGGCAGGTTTGTTCGC |
| qRT-PCR: | |
| TcCTL16-qF | TGTGAACCTGTCCAGTGCG |
| TcCTL16-qR | CATCATACTGTCCATTCGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, J.; Wang, Y.; Gao, R.; Liu, P.; Jiang, Y.; Gao, L.; Li, B.; Song, Q.; Ning, M. Functional Analysis of a CTL-X-Type Lectin CTL16 in Development and Innate Immunity of Tribolium castaneum. Int. J. Mol. Sci. 2023, 24, 10700. https://doi.org/10.3390/ijms241310700
Bi J, Wang Y, Gao R, Liu P, Jiang Y, Gao L, Li B, Song Q, Ning M. Functional Analysis of a CTL-X-Type Lectin CTL16 in Development and Innate Immunity of Tribolium castaneum. International Journal of Molecular Sciences. 2023; 24(13):10700. https://doi.org/10.3390/ijms241310700
Chicago/Turabian StyleBi, Jingxiu, Yutao Wang, Rui Gao, Pingxiang Liu, Yuying Jiang, Lei Gao, Bin Li, Qisheng Song, and Mingxiao Ning. 2023. "Functional Analysis of a CTL-X-Type Lectin CTL16 in Development and Innate Immunity of Tribolium castaneum" International Journal of Molecular Sciences 24, no. 13: 10700. https://doi.org/10.3390/ijms241310700
APA StyleBi, J., Wang, Y., Gao, R., Liu, P., Jiang, Y., Gao, L., Li, B., Song, Q., & Ning, M. (2023). Functional Analysis of a CTL-X-Type Lectin CTL16 in Development and Innate Immunity of Tribolium castaneum. International Journal of Molecular Sciences, 24(13), 10700. https://doi.org/10.3390/ijms241310700