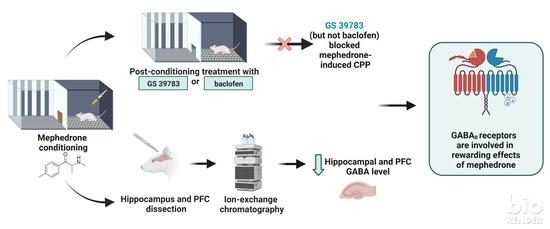
Relationship between GABA-Ergic System and the Expression of Mephedrone-Induced Reward in Rats—Behavioral, Chromatographic and In Vivo Imaging Study
Abstract
1. Introduction
2. Results
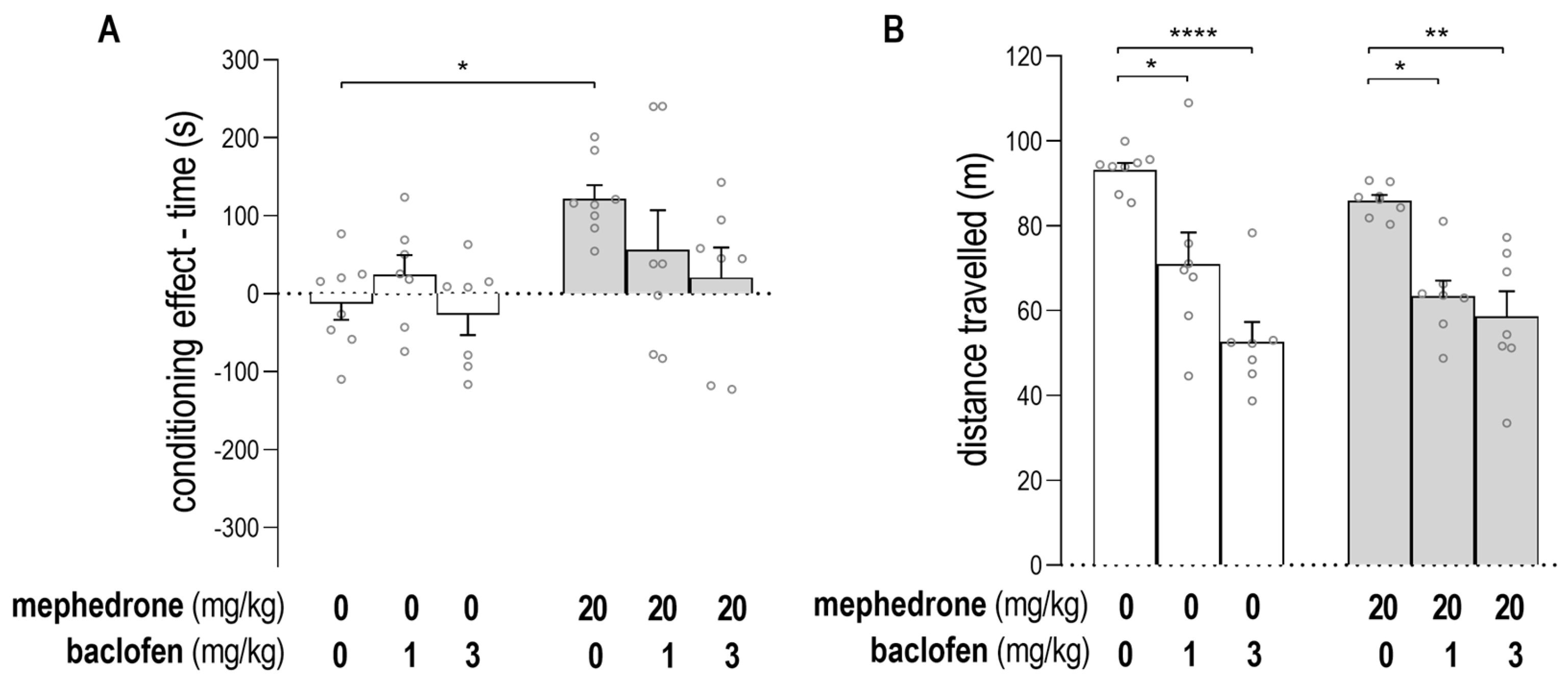
2.1. Impact of Baclofen on the Expression of Mephedrone-Induced CPP and on Animals’ Locomotor Activity
2.2. Impact of GS39783 on the Expression of Mephedrone-Induced CPP and on Animals’s Locomotor Activity
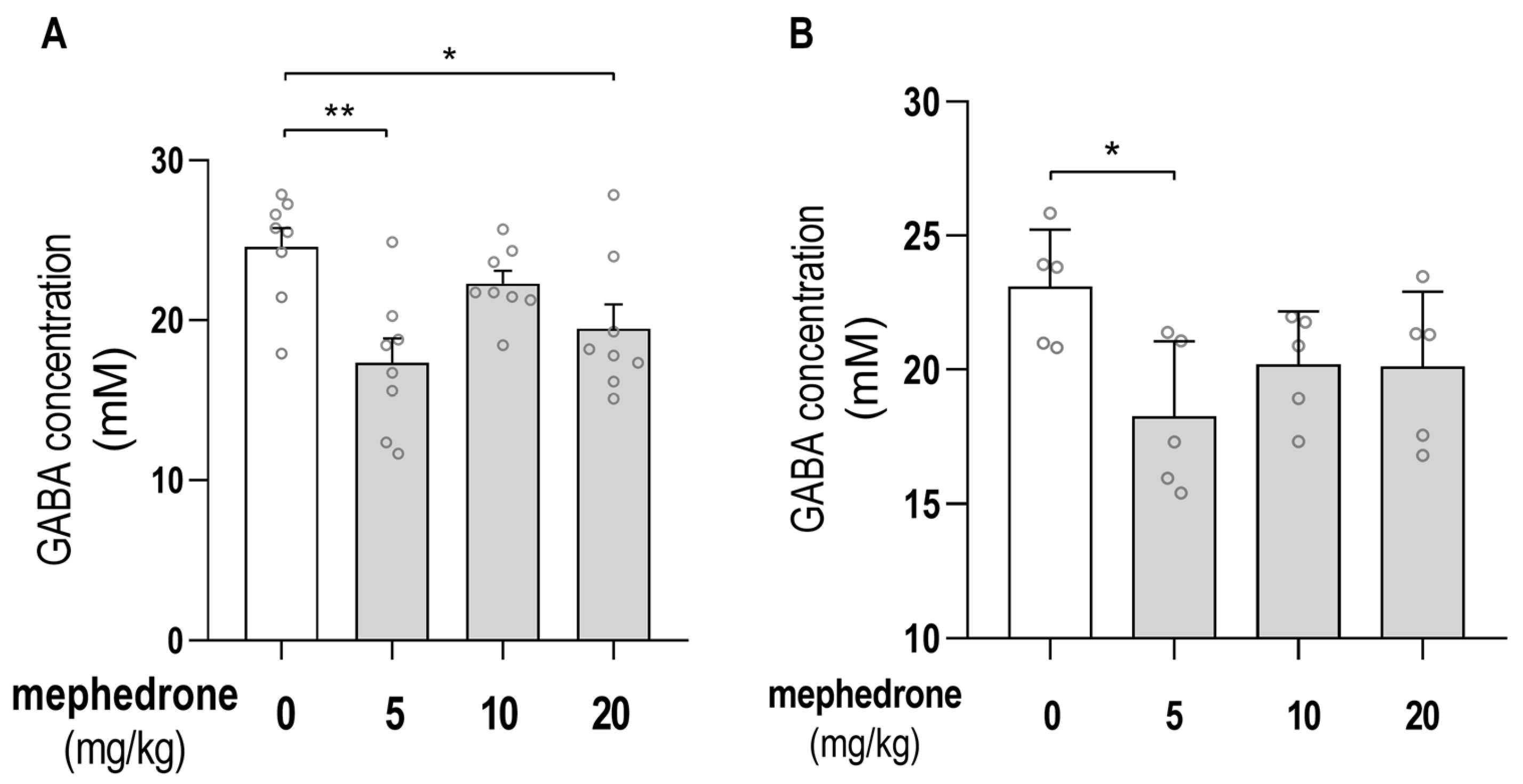
2.3. Chromatographic Determination of GABA Hippocampal/PFC Concentrations
2.4. MRI Analysis of GABA Hippocampal Level
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ethics Statement
4.3. Drugs
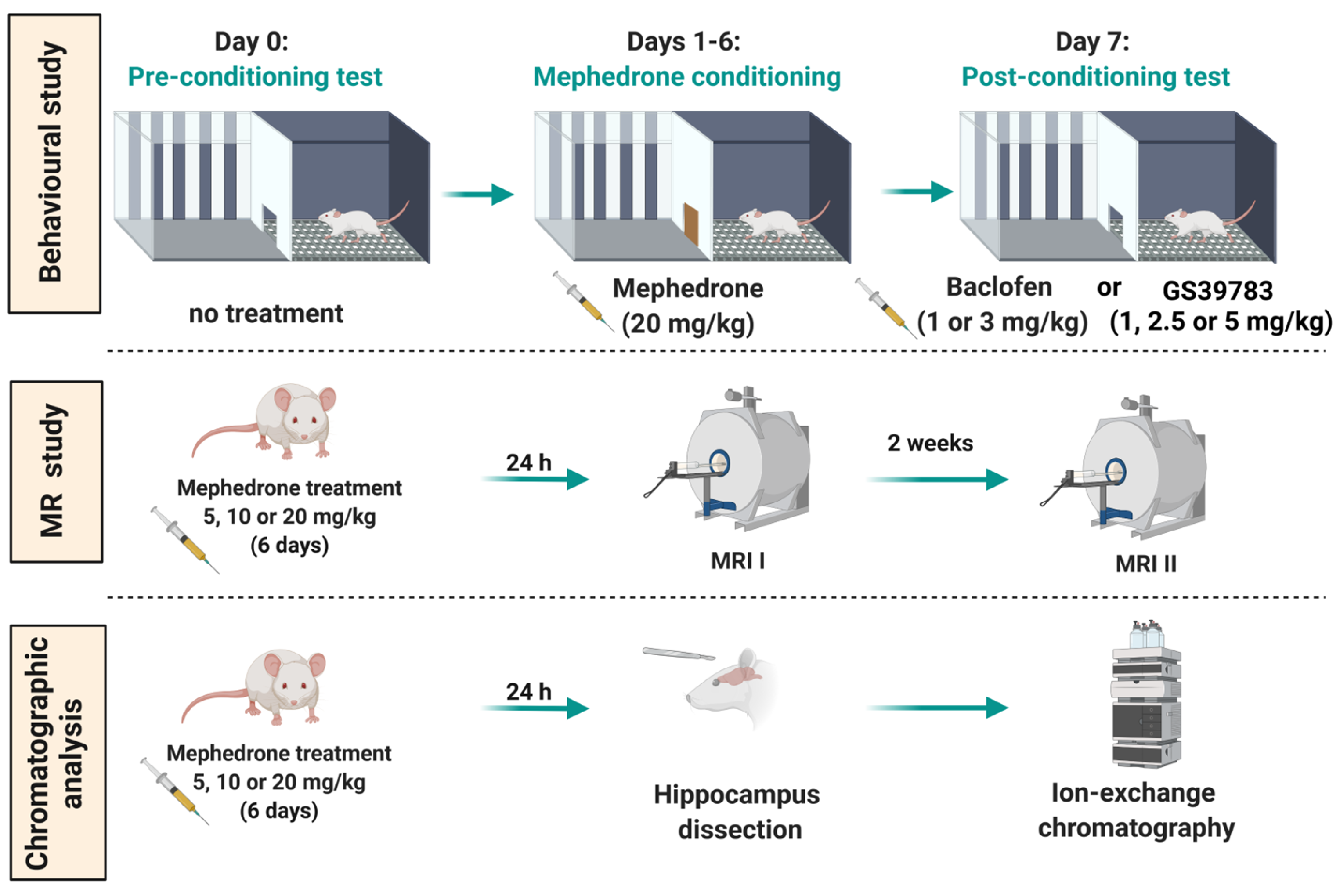
4.4. Experimental Procedure and Treatment
4.4.1. CPP Paradigm
- CPP apparatus, software and groups assignment
- CPP test
4.4.2. Locomotor Activity
4.4.3. MRS
4.4.4. Chromatographic Analysis
4.4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Monitoring Centre for Drugs and Drug Addiction and Europol. Europol–EMCDDA Joint Report on a New Psychoactive Substance: 4-Methylmethcathinone (Mephedrone); EMCDDA: Lisbon, Portugal, 2010. [Google Scholar]
- Docherty, J.R.; Alsufyani, H.A. Pharmacology of Drugs Used as Stimulants. J. Clin. Pharmacol. 2021, 61 (Suppl. S2), S53–S69. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.; Davies, S.; Puchnarewicz, M.; Button, J.; Archer, R.; Ovaska, H.; Ramsey, J.; Lee, T.; Holt, D.W.; Dargan, P.I. Recreational Use of Mephedrone (4-Methylmethcathinone, 4-MMC) with Associated Sympathomimetic Toxicity. J. Med. Toxicol. 2010, 6, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Ayestas, M.A., Jr.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology 2011, 37, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, G.C.; Webb, K.M.; McFadden, L.M.; Chu, P.W.; Ellis, J.D.; Allen, S.C.; Andrenyak, D.M.; Vieira-Brock, P.L.; German, C.L.; Conrad, K.M.; et al. 4-Methylmethcathinone (Mephedrone): Neuropharmacological Effects of a Designer Stimulant of Abuse. Experiment 2011, 339, 530–536. [Google Scholar] [CrossRef]
- López-Arnau, R.; Martínez-Clemente, J.; Pubill, D.; Escubedo, E.; Camarasa, J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: Butylone, mephedrone and methylone. Br. J. Pharmacol. 2012, 167, 407–420. [Google Scholar] [CrossRef]
- Martínez-Clemente, J.; Escubedo, E.; Pubill, D.; Camarasa, J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol. 2012, 22, 231–236. [Google Scholar] [CrossRef]
- Pifl, C.; Reither, H.; Hornykiewicz, O. The profile of mephedrone on human monoamine transporters differs from 3,4-methylenedioxymethamphetamine primarily by lower potency at the vesicular monoamine transporter. Eur. J. Pharmacol. 2015, 755, 119–126. [Google Scholar] [CrossRef]
- Golembiowska, K.; Jurczak, A.; Kaminska, K.; Noworyta-Sokolowska, K.; Gorska, A. Effect of Some Psychoactive Drugs Used as ‘Legal Highs’ on Brain Neurotransmitters. Neurotox. Res. 2016, 29, 394–407. [Google Scholar] [CrossRef]
- Kehr, J.; Ichinose, F.; Yoshitake, S.; Goiny, M.; Sievertsson, T.; Nyberg, F. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011, 164, 1949–1958. [Google Scholar] [CrossRef]
- Marusich, J.A.; Gay, E.A.; Blough, B.E. Analysis of neurotransmitter levels in addiction-related brain regions during synthetic cathinone self-administration in male Sprague-Dawley rats. Psychopharmacology 2018, 236, 903–914. [Google Scholar] [CrossRef]
- Marusich, J.A.; Gay, E.A.; Watson, S.; Blough, B.E. Synthetic cathinone self-administration in female rats modulates neurotransmitter levels in addiction-related brain regions. Behav. Brain Res. 2019, 376, 112211. [Google Scholar] [CrossRef]
- Wronikowska, O.; Zykubek, M.; Michalak, A.; Pankowska, A.; Kozioł, P.; Boguszewska-Czubara, A.; Kurach, Ł.; Łazorczyk, A.; Kochalska, K.; Talarek, S.; et al. Insight into Glutamatergic Involvement in Rewarding Effects of Mephedrone in Rats: In Vivo and Ex Vivo Study. Mol. Neurobiol. 2021, 58, 4413–4424. [Google Scholar] [CrossRef]
- Vithlani, M.; Terunuma, M.; Moss, S.J. The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol. Rev. 2011, 91, 1009–1022. [Google Scholar] [CrossRef]
- Shaye, H.; Stauch, B.; Gati, C.; Cherezov, V. Molecular mechanisms of metabotropic GABAB receptor function. Sci. Adv. 2021, 7, eabg3362. [Google Scholar] [CrossRef]
- Terunuma, M. Diversity of structure and function of GABAB receptors: A complexity of GABAB-mediated signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 390–411. [Google Scholar] [CrossRef]
- Filip, M.; Frankowska, M.; Sadakierska-Chudy, A.; Suder, A.; Szumiec, Ł.; Mierzejewski, P.; Bienkowski, P.; Przegaliński, E.; Cryan, J.F. GABAB receptors as a therapeutic strategy in substance use disorders: Focus on positive allosteric modulators. Neuropharmacology 2015, 88, 36–47. [Google Scholar] [CrossRef]
- Li, X.; Slesinger, P.A. GABAB Receptors and Drug Addiction: Psychostimulants and Other Drugs of Abuse. Curr. Top. Behav. Neurosci. 2020, 52, 119–155. [Google Scholar] [CrossRef]
- Tyacke, R.J.; Lingford-Hughes, A.; Reed, L.J.; Nutt, D.J. GABAB Receptors in Addiction and Its Treatment. Adv. Pharmacol. 2010, 58, 373–396. [Google Scholar] [CrossRef]
- Siivonen, M.S.; de Miguel, E.; Aaltio, J.; Manner, A.K.; Vahermo, M.; Yli-Kauhaluoma, J.; Linden, A.-M.; Aitta-Aho, T.; Korpi, E.R. Conditioned Reward of Opioids, but not Psychostimulants, is Impaired in GABA-A Receptor δ Subunit Knockout Mice. Basic Clin. Pharmacol. Toxicol. 2018, 123, 558–566. [Google Scholar] [CrossRef]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Floresco, S.B.; West, A.R.; Ash, B.; Moore, H.; Grace, A.A. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 2003, 6, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M. The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, A.; Calza, A.; Panzanelli, P.; Concas, A.; Giustetto, M.; Sassoè-Pognetto, M. Organization of GABAergic Synaptic Circuits in the Rat Ventral Tegmental Area. PLoS ONE 2012, 7, e46250. [Google Scholar] [CrossRef] [PubMed]
- Soden, M.E.; Chung, A.S.; Cuevas, B.; Resnick, J.M.; Awatramani, R.; Zweifel, L.S. Anatomic resolution of neurotransmitter-specific projections to the VTA reveals diversity of GABAergic inputs. Nat. Neurosci. 2020, 23, 968–980. [Google Scholar] [CrossRef]
- Ilari, A.; Curti, L.; Petrella, M.; Cannella, N.; La Rocca, A.; Ranieri, G.; Gerace, E.; Iezzi, D.; Silvestri, L.; Mannaioni, G.; et al. Moderate ethanol drinking is sufficient to alter Ventral Tegmental Area dopamine neurons activity via functional and structural remodeling of GABAergic transmission. Neuropharmacology 2021, 203, 108883. [Google Scholar] [CrossRef]
- Marcos, A.; Ballesteros-Yáñez, I.; Castillo-Sarmiento, C.A.; Pardo, F.; Roura-Martínez, D.; Muñoz-Rodríguez, J.R.; Higuera-Matas, A.; Ambrosio, E. The interactions of alcohol and cocaine regulate the expression of genes involved in the GABAergic, glutamatergic and endocannabinoid systems of male and female rats. Neuropharmacology 2021, 206, 108937. [Google Scholar] [CrossRef]
- Matsui, A.; Jarvie, B.C.; Robinson, B.G.; Hentges, S.T.; Williams, J.T. Separate GABA Afferents to Dopamine Neurons Mediate Acute Action of Opioids, Development of Tolerance, and Expression of Withdrawal. Neuron 2014, 82, 1346–1356. [Google Scholar] [CrossRef]
- Janhsen, K.; Roser, P.; Hoffmann, K. The Problems of Long-Term Treatment with Benzodiazepines and Related Substances. Dtsch. Ärzteblatt Int. 2015, 112, 1–7. [Google Scholar] [CrossRef]
- Cousins, M.S.; Roberts, D.C.; de Wit, H. GABAB receptor agonists for the treatment of drug addiction: A review of recent findings. Drug Alcohol Depend. 2002, 65, 209–220. [Google Scholar] [CrossRef]
- Gawlińska, K.; Jastrzębska, J.; Gamberini, S.; Gawliński, D.; Pieniążek, R.; Suder, A.; Wydra, K.; Frankowska, M. The impact of GABAB receptors and their pharmacological stimulation on cocaine reinforcement and drug-seeking behaviors in a rat model of depression. Eur. J. Pharmacol. 2020, 883, 173324. [Google Scholar] [CrossRef]
- Slattery, D.A.; Markou, A.; Froestl, W.; Cryan, J.F. The GABAB Receptor-Positive Modulator GS39783 and the GABAB Receptor Agonist Baclofen Attenuate the Reward-Facilitating Effects of Cocaine: Intracranial Self-Stimulation Studies in the Rat. Neuropsychopharmacology 2005, 30, 2065–2072. [Google Scholar] [CrossRef]
- Fattore, L.; Cossu, G.; Martellotta, M.C.; Fratta, W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002, 37, 495–498. [Google Scholar] [CrossRef]
- Varani, A.P.; Aso, E.; Moutinho, L.M.; Maldonado, R.; Balerio, G.N. Attenuation by baclofen of nicotine rewarding properties and nicotine withdrawal manifestations. Psychopharmacology 2014, 231, 3031–3040. [Google Scholar] [CrossRef]
- Di Ciano, P.; Everitt, B.J. The GABAB receptor agonist baclofen attenuates cocaine- and heroin-seeking behavior by rats. Neuropsychopharmacology 2003, 28, 510–518. [Google Scholar] [CrossRef]
- Spano, M.S.; Fattore, L.; Fratta, W.; Fadda, P. The GABAB receptor agonist baclofen prevents heroin-induced reinstatement of heroin-seeking behavior in rats. Neuropharmacology 2007, 52, 1555–1562. [Google Scholar] [CrossRef]
- Brebner, K.; Ahn, S.; Phillips, A.G. Attenuation of d-amphetamine self-administration by baclofen in the rat: Behavioral and neurochemical correlates. Psychopharmacology 2004, 177, 409–417. [Google Scholar] [CrossRef]
- Marti-Prats, L.; Belin-Rauscent, A.; Fouyssac, M.; Puaud, M.; Cocker, P.J.; Everitt, B.J.; Belin, D. Baclofen decreases compulsive alcohol drinking in rats characterized by reduced levels of GAT-3 in the central amygdala. Addict. Biol. 2021, 26, e13011. [Google Scholar] [CrossRef]
- Minnaard, A.M.; Ramakers, G.M.; Vanderschuren, L.J.; Lesscher, H.M. Baclofen and naltrexone, but not N-acetylcysteine, affect voluntary alcohol drinking in rats regardless of individual levels of alcohol intake. Behav. Pharmacol. 2020, 32, 251–257. [Google Scholar] [CrossRef]
- Fadda, P.; Scherma, M.; Fresu, A.; Collu, M.; Fratta, W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse 2003, 50, 1–6. [Google Scholar] [CrossRef]
- Palpacuer, C.; Duprez, R.; Huneau, A.; Locher, C.; Boussageon, R.; Laviolle, B.; Naudet, F. Pharmacologically controlled drinking in the treatment of alcohol dependence or alcohol use disorders: A systematic review with direct and network meta-analyses on nalmefene, naltrexone, acamprosate, baclofen and topiramate. Addiction 2017, 113, 220–237. [Google Scholar] [CrossRef]
- Ertzgaard, P.; Campo, C.; Calabrese, A. Efficacy and safety of oral baclofen in the management of spasticity: A rationale for intrathecal baclofen. J. Rehabil. Med. 2017, 49, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Rolland, B.; Labreuche, J.; Duhamel, A.; Deheul, S.; Gautier, S.; Auffret, M.; Pignon, B.; Valin, T.; Bordet, R.; Cottencin, O. Baclofen for alcohol dependence: Relationships between baclofen and alcohol dosing and the occurrence of major sedation. Eur. Neuropsychopharmacol. 2015, 25, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Kruse, L.C.; Linsenbardt, D.N.; Boehm, S.L., 2nd. Positive allosteric modulation of the GABAB receptor by GS39783 attenuates the locomotor stimulant actions of ethanol and potentiates the induction of locomotor sensitization. Alcohol 2012, 46, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Lorrai, I.; Contini, A.; Gessa, G.L.; Mugnaini, C.; Corelli, F.; Colombo, G.; Maccioni, P. Operant, oral alcohol self-administration: Sex differences in Sardinian alcohol-preferring rats. Alcohol 2019, 79, 147–162. [Google Scholar] [CrossRef]
- Maccioni, P.; Fantini, N.; Froestl, W.; Carai, M.A.M.; Gessa, G.L.; Colombo, G. Specific reduction of alcohol’s motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783-comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin. Exp. Res. 2008, 32, 1558–1564. [Google Scholar] [CrossRef]
- Maccioni, P.; Flore, P.; Carai, M.A.M.; Mugnaini, C.; Pasquini, S.; Corelli, F.; Gessa, G.L.; Colombo, G. Reduction by the Positive Allosteric Modulator of the GABAB Receptor, GS39783, of Alcohol Self-Administration in Sardinian Alcohol-Preferring Rats Exposed to the “Sipper” Procedure. Front. Psychiatry 2010, 1, 20. [Google Scholar] [CrossRef]
- Orrù, A.; Lai, P.; Lobina, C.; Maccioni, P.; Piras, P.; Scanu, L.; Froestl, W.; Gessa, G.L.; Carai, M.A.M.; Colombo, G. Reducing effect of the positive allosteric modulators of the GABAB receptor, CGP7930 and GS39783, on alcohol intake in alcohol-preferring rats. Eur. J. Pharmacol. 2005, 525, 105–111. [Google Scholar] [CrossRef]
- Lhuillier, L.; Mombereau, C.; Cryan, J.F.; Kaupmann, K. GABAB receptor-positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology 2007, 32, 388–398. [Google Scholar] [CrossRef]
- Halbout, B.; Quarta, D.; Valerio, E.; Heidbreder, C.A.; Hutcheson, D.M. The GABA-B positive modulator GS39783 decreases psychostimulant conditioned-reinforcement and conditioned-reward. Addict. Biol. 2011, 16, 416–427. [Google Scholar] [CrossRef]
- Voigt, R.M.; Herrold, A.A.; Riddle, J.L.; Napier, T.C. Administration of GABAB receptor positive allosteric modulators inhibit the expression of previously established methamphetamine-induced conditioned place preference. Behav. Brain Res. 2011, 216, 419–423. [Google Scholar] [CrossRef]
- Paterson, N.E.; Vlachou, S.; Guery, S.; Kaupmann, K.; Froestl, W.; Markou, A. Positive modulation of GABAB receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J. Pharmacol. Exp. Ther. 2008, 326, 306–314. [Google Scholar] [CrossRef]
- Mombereau, C.; Lhuillier, L.; Kaupmann, K.; Cryan, J.F. GABAB receptor-positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens ΔFosB accumulation. J. Pharmacol. Exp. Ther. 2007, 321, 172–177. [Google Scholar] [CrossRef]
- Bouarab, C.; Thompson, B.; Polter, A.M. VTA GABA Neurons at the Interface of Stress and Reward. Front. Neural Circuits 2019, 13, 78. [Google Scholar] [CrossRef]
- Weitz, M.; Khayat, A.; Yaka, R. GABAergic projections to the ventral tegmental area govern cocaine-conditioned reward. Addict. Biol. 2021, 26, e13026. [Google Scholar] [CrossRef]
- Martínez-Clemente, J.; López-Arnau, R.; Carbó, M.; Pubill, D.; Camarasa, J.; Escubedo, E. Mephedrone pharmacokinetics after intravenous and oral administration in rats: Relation to pharmacodynamics. Psychopharmacology 2013, 229, 295–306. [Google Scholar] [CrossRef]
- Wronikowska, O.; Zykubek, M.; Kurach, Ł.; Michalak, A.; Boguszewska-Czubara, A.; Budzyńska, B. Vulnerability factors for mephedrone-induced conditioned place preference in rats-the impact of sex differences, social-conditioning and stress. Psychopharmacology 2021, 238, 2947–2961. [Google Scholar] [CrossRef]
- Ciudad-Roberts, A.; Camarasa, J.; Ciudad, C.J.; Pubill, D.; Escubedo, E. Alcohol enhances the psychostimulant and conditioning effects of mephedrone in adolescent mice; postulation of unique roles of D3 receptors and BDNF in place preference acquisition. Br. J. Pharmacol. 2015, 172, 4970–4984. [Google Scholar] [CrossRef]
- Miller, M.; Creehan, K.; Angrish, D.; Barlow, D.; Houseknecht, K.; Dickerson, T.; Taffe, M. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone). Drug Alcohol Depend. 2012, 127, 248–253. [Google Scholar] [CrossRef]
- Šíchová, K.; Pinterová, N.; Židková, M.; Horsley, R.R.; Lhotková, E.; Štefková, K.; Vejmola, Č.; Uttl, L.; Balíková, M.; Kuchař, M.; et al. Mephedrone (4-Methylmethcathinone): Acute Behavioral Effects, Hyperthermic, and Pharmacokinetic Profile in Rats. Front. Psychiatry 2018, 8, 306. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Kane, M.J.; Francescutti, D.M.; Sykes, K.E.; Shah, M.M.; Mohammed, A.M.; Thomas, D.M.; Kuhn, D.M. Mephedrone, an abused psychoactive component of “bath salts” and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J. Neurochem. 2012, 120, 1097–1107. [Google Scholar] [CrossRef]
- Mayer, F.P.; Wimmer, L.; Dillon-Carter, O.; Partilla, J.S.; Burchardt, N.V.; Mihovilovic, M.D.; Baumann, M.H.; Sitte, H.H. Phase I metabolites of mephedrone display biological activity as substrates at monoamine transporters. Br. J. Pharmacol. 2016, 173, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- Shortall, S.E.; Spicer, C.H.; Ebling, F.J.P.; Green, A.R.; Fone, K.C.F.; King, M.V. Contribution of serotonin and dopamine to changes in core body temperature and locomotor activity in rats following repeated administration of mephedrone. Addict. Biol. 2015, 21, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Gatch, M.B.; Taylor, C.M.; Forster, M.J. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav. Pharmacol. 2013, 24, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Enoch, M.A.; Zhou, Z.; Kimura, M.; Mash, D.C.; Yuan, Q.; Goldman, D. GABAergic gene expression in postmortem hippocampus from alcoholics and cocaine addicts; corresponding findings in alcohol-naïve P and NP rats. PLoS ONE 2012, 7, e29369. [Google Scholar] [CrossRef] [PubMed]
- Chau, B.K.H.; Jarvis, H.; Law, C.-K.; Chong, T. Dopamine and reward: A view from the prefrontal cortex. Behav. Pharmacol. 2018, 29, 569–583. [Google Scholar] [CrossRef]
- Ceceli, A.O.; Bradberry, C.W.; Goldstein, R.Z. The neurobiology of drug addiction: Cross-species insights into the dysfunction and recovery of the prefrontal cortex. Neuropsychopharmacology 2022, 47, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic inhibitory interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Rombo, D.M.; Ribeiro, J.A.; Sebastião, A.M. Hippocampal GABAergic transmission: A new target for adenosine control of excitability. J. Neurochem. 2016, 139, 1056–1070. [Google Scholar] [CrossRef]
- Henry, M.E.; Lauriat, T.L.; Shanahan, M.; Renshaw, P.F.; Jensen, J. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: A phantom study at 4 Tesla. J. Magn. Reson. 2011, 208, 210–218. [Google Scholar] [CrossRef]
- Hsu, C.; Lin, S.; Ho, A.; Johnson, T.D.; Wang, P.C.; Scafidi, J.; Tu, T. Comparison of in vivo and in situ detection of hippocampal metabolites in mouse brain using 1H-MRS. NMR Biomed. 2020, 34, e4451. [Google Scholar] [CrossRef]
- Puts, N.A.; Edden, R.A. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 29–41. [Google Scholar] [CrossRef]
- Gruetter, R.; Weisdorf, S.A.; Rajanayagana, V.; Terpstraa, M.; Merklea, H.; Truwit, C.L.; Garwooda, M.; Nyberg, S.L.; Ugurbil, K. Resolution Improvements in In Vivo 1H NMR Spectra with Increased Magnetic Field Strength. J. Magn. Reson. 1998, 135, 260–264. [Google Scholar] [CrossRef]
- Bartha, R.; Drost, D.J.; Menon, R.S.; Williamson, P.C. Comparison of the quantification precision of human short echo time 1H spectroscopy at 1.5 and 4.0 Tesla. Magn. Reson. Med. 2000, 44, 185–192. [Google Scholar] [CrossRef]
- Harris, A.D.; Saleh, M.G.; Edden, R.A. Edited 1H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magn. Reson. Med. 2017, 77, 1377–1389. [Google Scholar] [CrossRef]
- Ryner, L.; Sorenson, J.; Thomas, M. 3D Localized 2D NMR Spectroscopy on an MRI Scanner. J. Magn. Reson. Ser. B 1995, 107, 126–137. [Google Scholar] [CrossRef]
- Smith, M.A.; Yancey, D.L.; Morgan, D.; Liu, Y.; Froestl, W.; Roberts, D.C.S. Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacology 2004, 173, 105–111. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wronikowska-Denysiuk, O.; Michalak, A.; Pankowska, A.; Kurach, Ł.; Kozioł, P.; Łazorczyk, A.; Kochalska, K.; Targowska-Duda, K.; Boguszewska-Czubara, A.; Budzyńska, B. Relationship between GABA-Ergic System and the Expression of Mephedrone-Induced Reward in Rats—Behavioral, Chromatographic and In Vivo Imaging Study. Int. J. Mol. Sci. 2023, 24, 9958. https://doi.org/10.3390/ijms24129958
Wronikowska-Denysiuk O, Michalak A, Pankowska A, Kurach Ł, Kozioł P, Łazorczyk A, Kochalska K, Targowska-Duda K, Boguszewska-Czubara A, Budzyńska B. Relationship between GABA-Ergic System and the Expression of Mephedrone-Induced Reward in Rats—Behavioral, Chromatographic and In Vivo Imaging Study. International Journal of Molecular Sciences. 2023; 24(12):9958. https://doi.org/10.3390/ijms24129958
Chicago/Turabian StyleWronikowska-Denysiuk, Olga, Agnieszka Michalak, Anna Pankowska, Łukasz Kurach, Paulina Kozioł, Artur Łazorczyk, Katarzyna Kochalska, Katarzyna Targowska-Duda, Anna Boguszewska-Czubara, and Barbara Budzyńska. 2023. "Relationship between GABA-Ergic System and the Expression of Mephedrone-Induced Reward in Rats—Behavioral, Chromatographic and In Vivo Imaging Study" International Journal of Molecular Sciences 24, no. 12: 9958. https://doi.org/10.3390/ijms24129958
APA StyleWronikowska-Denysiuk, O., Michalak, A., Pankowska, A., Kurach, Ł., Kozioł, P., Łazorczyk, A., Kochalska, K., Targowska-Duda, K., Boguszewska-Czubara, A., & Budzyńska, B. (2023). Relationship between GABA-Ergic System and the Expression of Mephedrone-Induced Reward in Rats—Behavioral, Chromatographic and In Vivo Imaging Study. International Journal of Molecular Sciences, 24(12), 9958. https://doi.org/10.3390/ijms24129958