Unveiling the MUFA–Cancer Connection: Insights from Endogenous and Exogenous Perspectives
Abstract
1. Introduction
1.1. Lipid Uptake and Metabolism in Cancer
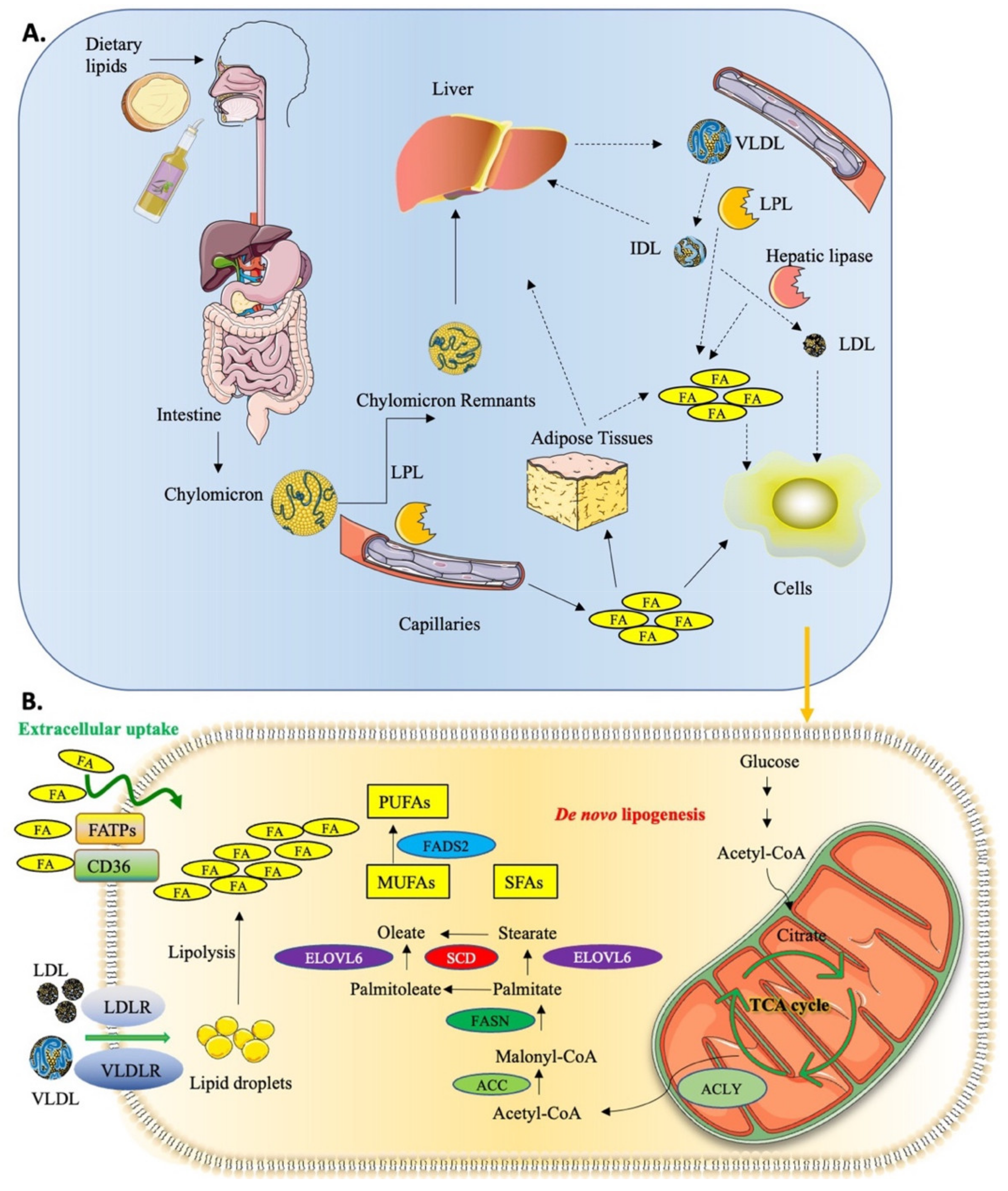
1.2. MUFA Biosynthesis
2. Exogenous MUFA and Cancer
2.1. Dietary MUFA and Cancer Risk—Evidence from Human Studies
2.2. Exogenous MUFA and Cancer Risk—Evidence from Animal Models
| Cancer Type | MUFA Source | Mouse Model | Main Outcome | Reference |
|---|---|---|---|---|
| Breast cancer | 10% olive oil diet | MMTV-neu(ndl)-YD5 transgenic mouse model | Mitigated tumor outcome (though not as efficiently as a 3% menhaden oil + 7% safflower oil mix) | [125] |
| Breast cancer | OA nanoparticles, intravenous injection and gavage | Xenograft of 4T1 cells | Inhibited tumor growth | [127] |
| Cervical cancer | High-olive-oil diet (45 kcal % fat) | Xenograft of HeLa cells | Increased tumor growth | [123] |
| (Liver metastasis) | (Subcutaneous and tail vein injection) | Increased tumor metastasis | [124] | |
| CRC | OA, injected intragastrically at a dose of 2.0 g/kg/day | Xenograft of HCT116 cells | No difference compared with controls | [128] |
| CRC | 10% extra virgin olive oil diet | Chemically induced (DSS) | Reduced incidence and multiplicity of tumors | [117] |
| CRC | Olive oil 1 g/kg through oral gavage | Chemically induced (DMH) | Inhibited tumor growth | [118] |
| CRC | Fatty acid-rich diet, 75% OA | Chemically induced (AOM/DSS) | Reduced body weight loss and number and size of tumors | [119] |
| CRC | Oral intake of OA and elaidic acid | Xenograft of HT29 cells (subcutaneous, spleen, tail vein, and peritoneum) | Increased tumor growth and metastasis | [129] |
| HNSCC/Lung metastasis | OA, tail vein injection | Xenograft of TU183 cells (tail vein injection) | Induced metastasis | [130] |
| Lung cancer | 6% OA-enriched diet | LAC1 tumor transplantation | Inhibited tumor growth but no impact on metastasis | [120] |
| Lung cancer | AIN-76A diet containing 10% OA | Chemically induced (NNK) | Reduced incidence and level of tumors | [121] |
| Pancreatic cancer | 15% olive oil diet | Xenograft of HPAF cells | Increased tumor weight | [122] |
| TSCC | OA, injected intraperitoneally at 2/4 mg/kg | Xenograft of CAL27 cells | Reduced tumor volume and weight | [126] |
2.3. Exogenous MUFA and Cancer Cell Behavior—Evidence from Cellular Models
2.3.1. Effect on Cell Proliferation
2.3.2. Effect on Cell Survival
2.3.3. Effect on Cell Migration and Invasion
2.3.4. Effect on Cancer Suppression
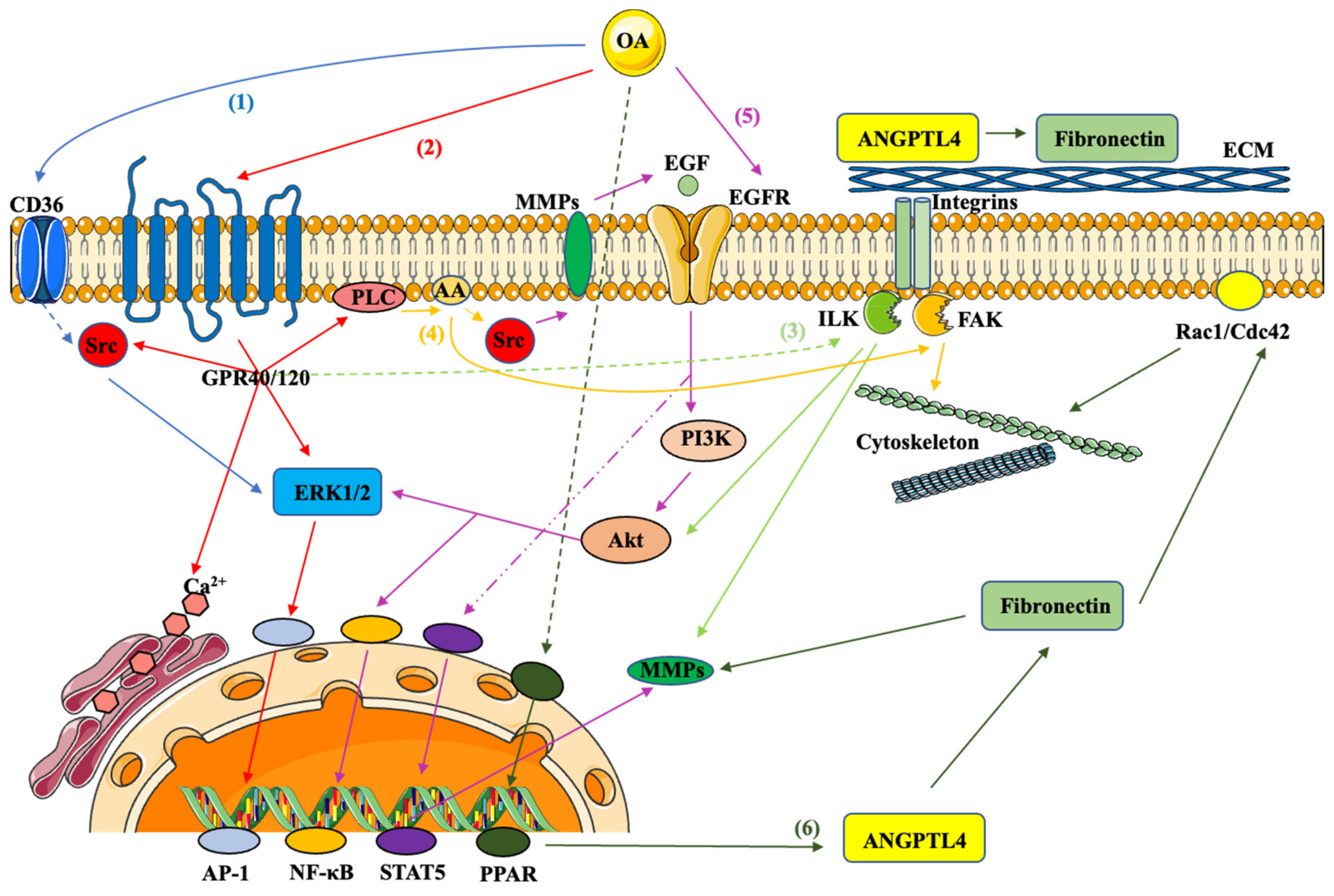
3. Role of Endogenously Synthesized MUFA in Cancer
3.1. SCD Activity and Cancer—Evidence from Human Studies
3.2. SCD Activity and Cancer—Evidence from Animal Studies
| Cancer Type | SCD Model | Mouse Model | Main Outcome | Reference |
|---|---|---|---|---|
| Bladder cancer | SCD1 knockdown, SCD1 inhibitor A37062 | Xenograft of SW780, UMUC-14 cells | Inhibited tumor growth and progression | [200] |
| Breast cancer | SCD5 overexpression | Xenograft of 4T1 cells | Reduced tumor aggressiveness | [206] |
| Colorectal cancer | SCD1 inhibitor A939572 | Xenograft of LOVO cells | Reduced tumor volume and tumor weight | [197] |
| Colorectal cancer | SCD1 knockdown | Tail vein injection xenograft of HCT116 cells | Decreased the size and number of lung metastatic tumors | [173] |
| Colorectal/Lung/Renal cancer | SCD1 inhibitor T-3764518 | Xenograft of HCT116/MSTO-211H/786-O cells | Inhibited tumor growth | [201,202] |
| Gastric cancer | SCD1 overexpression, SCD1 inhibitor A939572 | Xenograft of MKN45 cells | Overexpression of SCD1 enhanced proliferation and metastasis while inhibition reduced both tumor volume and tumor weight | [164] |
| Gastric cancer | SCD1 inhibitor A939572 | Xenograft of GA16 cells | Inhibited tumor growth | [165] |
| Liver cancer | SCD1 knockdown | Xenograft of HepG2 cells | Inhibited tumor size and metastasis | [204] |
| Liver fibrosis | SCD2 conditional knockout, SCD inhibitor A939572 | SCD2 conditional knockout | Reduced liver fibrosis, tumor formations, tumor size and tumor multiplicity | [203] |
| Lung cancer | SCD1 knockdown, SCD1 inhibitor A939572 | Xenograft of A549 cells | Reduced tumor growth, fewer tumor formations and increase in tumor latency | [32] |
| Lung cancer | SCD1 knockdown | Xenograft of H1650 cells | Reduced tumor weight and volume | [172] |
| Lung cancer | SCD1 inhibitor A939572 | Xenograft of H460 cells | Inhibited tumor growth | [196] |
| Melanoma | SCD1 inhibitor A939572 | Xenograft of B16F1, SW1 cells | Inhibited primary tumors growth but increased lung metastases | [205] |
| Melanoma | SCD5 overexpression | Xenograft of A375M, 4T1 cells | Reduced metastases | [178] |
| Ovarian cancer | SCD1 inhibitor A939572 | Xenograft of FT-t cells | Reduced tumor number and mass | [171] |
| Pancreatic cancer | SCD1 inhibitor A939572 | Xenograft of Panc02 cells | Reduced tumor size | [198] |
| Prostate cancer | SCD1 inhibitor BZ36 | Xenograft of LNCaP, C4-2 cells | Inhibited tumor volume and tumor growth rate | [169] |
| Prostate cancer | SCD1 knockdown | Xenograft of DU145 cells | Inhibited tumor growth | [167] |
| Prostate cancer | SCD1 overexpression | Xenograft of LN cells | Increased tumor formation and growth | [199] |
3.3. SCD Activity and Cancer—Evidence from Cellular Models
3.3.1. Role of SCD1 in Cell Proliferation and Cell Cycle
3.3.2. Role of SCD1 in Cell Migration and Invasion
3.3.3. Role of SCD1 in Cell Death
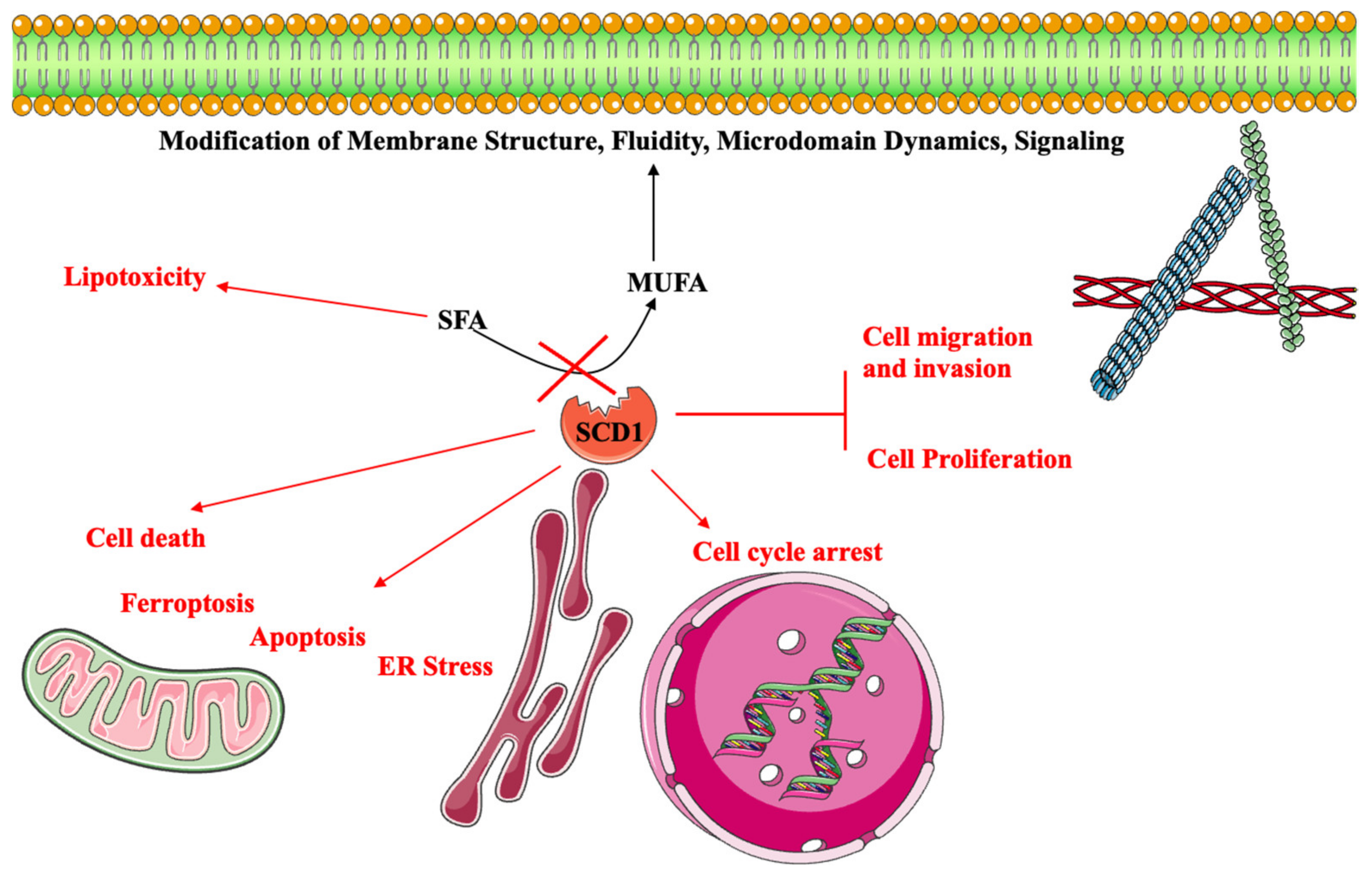
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ACC | acetyl-CoA carboxylases |
| ACLY | ATP citrate lyase |
| ANGPTL4 | angiopoietin-like 4 |
| AOM | azoxymethane |
| CRC | colorectal cancer |
| DMH | dimethylhydrazine |
| DSS | dextran sodium sulfate |
| ECMELOVL | extracellular matrix fatty acid elongases |
| EMT | epithelial to mesenchymal transition |
| EVOO | extra-virgin olive oil |
| FA | fatty acid |
| FABP | fatty acid-binding protein |
| FADS | fatty acid desaturase |
| FAK | focal adhesion kinase |
| FASN | fatty acid synthase |
| GPR | G protein-coupled receptors |
| HAMLET | human alpha-lactalbumin made lethal to tumor cell |
| HIF-1α | hypoxia-inducible factor-1 alpha |
| HNSCC | head and neck squamous cell carcinoma |
| ILDL | intermediate-density lipoprotein |
| ILK | integrin-linked kinase |
| LA | linoleic acid |
| LDL | low-density lipoprotein |
| LPL | lipoprotein lipase |
| MUFA | monounsaturated fatty acid |
| mTOR | mammalian target of rapamycin |
| NNK | N-nitrosamine: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone |
| NOX4 | NADPH oxidase 4 |
| PD-1 | programmed death-ligand 1 |
| PI3K | phosphatidylinositol-3 kinase |
| PLD2 | phospholipase D2 |
| PPAR | peroxisome proliferator-activated receptor |
| ROS | reactive oxygen species |
| SCD | stearoyl-CoA desaturases |
| SFA | saturated fatty acid |
| SFO | sunflower oil |
| SOCE | store-operated Ca2+ entry |
| SPARC | secreted protein and rich in cysteine |
| Stat5 | signal transducers and activators of transcription 5 |
| TG | triacylglycerol |
| TSCC | tongue squamous cell carcinoma |
| VLDL | very-low-density lipoprotein |
References
- Karpińska-Mirecka, A.; Bartosińska, J.; Krasowska, D. The Impact of Hypertension, Diabetes, Lipid Disorders, Overweight/Obesity and Nicotine Dependence on Health-Related Quality of Life and Psoriasis Severity in Psoriatic Patients Receiving Systemic Conventional and Biological Treatment. Int. J. Environ. Res. Public Health 2021, 18, 13167. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, G.D.; Rose, D.P. Breast Cancer and Obesity: An Update. Nutr. Cancer 2003, 45, 1–16. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. eBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Judd, S.E.; Gilchrist, S.C.; Cushman, M.; Pisu, M.; Safford, M.; Akinyemiju, T. Association between obesity and biomarkers of inflammation and metabolism with cancer mortality in a prospective cohort study. Metabolism 2019, 94, 69–76. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Rosenbloom, A.L. Obesity, diabetes and cancer: Insight into the relationship from a cohort with growth hormone receptor deficiency. Diabetologia 2015, 58, 37–42. [Google Scholar] [CrossRef]
- Lashinger, L.M.; Ford, N.A.; Hursting, S.D. Interacting Inflammatory and Growth Factor Signals Underlie the Obesity-Cancer Link. J. Nutr. 2014, 144, 109–113. [Google Scholar] [CrossRef]
- Wang, W.; Bai, L.; Li, W.; Cui, J. The Lipid Metabolic Landscape of Cancers and New Therapeutic Perspectives. Front. Oncol. 2020, 10, 605154. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. Med. Comm. 2021, 2, 27–59. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Sotgia, F.; Martinez-Outschoorn, U.E.; Lisanti, M.P. Cancer Metabolism: New Validated Targets for Drug Discovery. Oncotarget 2013, 4, 1309–1316. [Google Scholar] [CrossRef]
- Prendeville, H.; Lynch, L. Diet, lipids, and antitumor immunity. Cell. Mol. Immunol. 2022, 19, 432–444. [Google Scholar] [CrossRef]
- Karagiota, A.; Chachami, G.; Paraskeva, E. Lipid Metabolism in Cancer: The Role of Acylglycerolphosphate Acyltransferases (AGPATs). Cancers 2022, 14, 228. [Google Scholar] [CrossRef]
- Pope, E.D., 3rd; Kimbrough, E.O.; Vemireddy, L.P.; Surapaneni, P.K.; Copland, J.A., 3rd; Mody, K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin. Ther. Targets 2019, 23, 473–483. [Google Scholar] [CrossRef]
- Su, X.; Abumrad, N.A. Cellular fatty acid uptake: A pathway under construction. Trends Endocrinol. Metab. 2009, 20, 72–77. [Google Scholar] [CrossRef]
- Anderson, C.M.; Stahl, A. SLC27 fatty acid transport proteins. Mol. Asp. Med. 2013, 34, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular Fatty Acid Metabolism and Cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Denkert, C.; Lehtinen, L.; Müller, B.; Brockmöller, S.; Seppänen-Laakso, T.; Budczies, J.; Bucher, E.; Yetukuri, L.; Castillo, S.; et al. Novel Theranostic Opportunities Offered by Characterization of Altered Membrane Lipid Metabolism in Breast Cancer Progression. Cancer Res. 2011, 71, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Yu, X.; Mi, S.; Ye, J.; Lou, G. Aberrant lipid metabolism in cancer cells and tumor microenvironment: The player rather than bystander in cancer progression and metastasis. J. Cancer 2021, 12, 7498–7506. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Brusselmans, K.; Verhoeven, G. Increased lipogenesis in cancer cells: New players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 358–365. [Google Scholar] [CrossRef]
- Medes, G.; Thomas, A.; Weinhouse, S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953, 13, 27–29. [Google Scholar]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Long, J.-P.; Li, X.-N.; Zhang, F. Targeting metabolism in breast cancer: How far we can go? World J. Clin. Oncol. 2016, 7, 122–130. [Google Scholar] [CrossRef]
- Van Kappel, A.-L.; Winkvist, A.; Kaaks, R.; Lenner, P.; Riboli, E. Fatty-acid composition in serum phospholipids and risk of breast cancer: An incident case-control study in Sweden. Int. J. Cancer 1999, 83, 585–590. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M.; Dobrzyn, A. Regulation of stearoyl-CoA desaturase expression. Lipids 2004, 39, 1061–1065. [Google Scholar] [CrossRef]
- Popeijus, H.E.; Saris, W.H.M.; Mensink, R.P. Role of stearoyl-CoA desaturases in obesity and the metabolic syndrome. Int. J. Obes. 2008, 32, 1076–1082. [Google Scholar] [CrossRef]
- Flowers, M.T.; Ntambi, J.M. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2009, 1791, 85–91. [Google Scholar] [CrossRef]
- Brown, J.M.; Rudel, L.L. Stearoyl-coenzyme A desaturase 1 inhibition and the metabolic syndrome: Considerations for future drug discovery. Curr. Opin. Infect. Dis. 2010, 21, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Scaglia, N.; Igal, R.A. Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int. J. Oncol. 2008, 33, 839–850. [Google Scholar] [PubMed]
- Pala, V.; Krogh, V.; Muti, P.; Chajès, V.; Riboli, E.; Micheli, A.; Saadatian, M.; Sieri, S.; Berrino, F. Erythrocyte Membrane Fatty Acids and Subsequent Breast Cancer: A Prospective Italian Study. JNCI 2001, 93, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Y.; Zhou, D.; Li, Z. Significantly increased monounsaturated lipids relative to polyunsaturated lipids in six types of cancer microenvironment are observed by mass spectrometry imaging. Sci. Rep. 2014, 4, srep05959. [Google Scholar] [CrossRef] [PubMed]
- Zureik, M.; Ducimetiere, P.; Warnet, J.-M.; Orssaud, G. Fatty acid proportions in cholesterol esters and risk of premature death from cancer in middle aged French men. BMJ 1995, 311, 1251–1254. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Kenfield, S.A.; Stampfer, M.J.; Loda, M.; Campos, H.; Sesso, H.D.; Ma, J. Blood Levels of Saturated and Monounsaturated Fatty Acids as Markers of De Novo Lipogenesis and Risk of Prostate Cancer. Am. J. Epidemiol. 2013, 178, 1246–1255. [Google Scholar] [CrossRef]
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.-Y.; Fang, X. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef]
- Wu, X.; Daniels, G.; Lee, P.; Monaco, M.E. Lipid metabolism in prostate cancer. Am. J. Clin. Exp. Urol. 2014, 2, 111–120. [Google Scholar]
- Monaco, M.E. Fatty acid metabolism in breast cancer subtypes. Oncotarget 2017, 8, 29487–29500. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid. Res. 2004, 43, 91–104. [Google Scholar] [CrossRef]
- Castro, L.F.C.; Wilson, J.M.; Gonçalves, O.; Galante-Oliveira, S.; Rocha, E.; Cunha, I. The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates. BMC Evol. Biol. 2011, 11, 132. [Google Scholar] [CrossRef]
- Alloatti, A.; Gupta, S.; Gualdrón-López, M.; Nguewa, P.A.; Altabe, S.G.; Deumer, G.; Wallemacq, P.; Michels, P.A.; Uttaro, A.D. Stearoyl-CoA desaturase is an essential enzyme for the parasitic protist Trypanosoma brucei. Biochem. Biophys. Res. Commun. 2011, 412, 286–290. [Google Scholar] [CrossRef]
- Ntambi, J.M. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J. Lipid. Res. 1999, 40, 1549–1558. [Google Scholar] [CrossRef]
- Igal, R.A. Roles of StearoylCoA Desaturase-1 in the Regulation of Cancer Cell Growth, Survival and Tumorigenesis. Cancers 2011, 3, 2462–2477. [Google Scholar] [CrossRef]
- Horikawa, M.; Nomura, T.; Hashimoto, T.; Sakamoto, K. Elongation and Desaturation of Fatty Acids are Critical in Growth, Lipid Metabolism and Ontogeny of Caenorhabditis elegans. J. Biochem. 2008, 144, 149–158. [Google Scholar] [CrossRef]
- Wang, J.; Yu, L.; Schmidt, R.E.; Su, C.; Huang, X.; Gould, K.; Cao, G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem. Biophys. Res. Commun. 2005, 332, 735–742. [Google Scholar] [CrossRef]
- Igal, R.A. Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2016, 1861, 1865–1880. [Google Scholar] [CrossRef]
- Igal, R.A.; Sinner, D.I. Stearoyl-CoA desaturase 5 (SCD5), a Δ-9 fatty acyl desaturase in search of a function. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2020, 1866, 158840. [Google Scholar] [CrossRef]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef]
- Dentin, R.; Pégorier, J.-P.; Benhamed, F.; Foufelle, F.; Ferre, P.; Fauveau, V.; Magnuson, M.A.; Girard, J.; Postic, C. Hepatic Glucokinase Is Required for the Synergistic Action of ChREBP and SREBP-1c on Glycolytic and Lipogenic Gene Expression. J. Biol. Chem. 2004, 279, 20314–20326. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Kon, N.; Zhao, W.; Jiang, L.; Knight, C.M.; Welch, C.; Pajvani, U.; Gu, W.; Accili, D. Hepatic SirT1-Dependent Gain of Function of Stearoyl-CoA Desaturase-1 Conveys Dysmetabolic and Tumor Progression Functions. Cell Rep. 2015, 11, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Ricoult, S.J.H.; Yecies, J.L.; Ben-Sahra, I.; Manning, B.D. Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene 2016, 35, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.; Samarajiwa, S.A.; Cairns, J.M.; Menon, S.; Pérez-Mancera, P.A.; Tomimatsu, K.; Bermejo-Rodriguez, C.; Ito, Y.; Chandra, T.; Narita, M.; et al. Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53. PLoS Genet. 2015, 11, e1005053. [Google Scholar] [CrossRef] [PubMed]
- Young, R.S.; Bowman, A.P.; Williams, E.D.; Tousignant, K.D.; Bidgood, C.L.; Narreddula, V.R.; Gupta, R.; Marshall, D.L.; Poad, B.L.; Nelson, C.C.; et al. Apocryphal FADS2 activity promotes fatty acid diversification in cancer. Cell Rep. 2021, 34, 108738. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, D.; Feng, J.; Hu, Q.; Tan, A.; Xie, Z.; Chen, Q.; Huang, H.; Wei, Y.; Ouyang, Z.; et al. Metabolic Pathway of Monounsaturated Lipids Revealed by In-Depth Structural Lipidomics by Mass Spectrometry. Research 2023, 6, 87. [Google Scholar] [CrossRef]
- Vriens, K.; Christen, S.; Parik, S.; Broekaert, D.; Yoshinaga, K.; Talebi, A.; Dehairs, J.; Escalona-Noguero, C.; Schmieder, R.; Cornfield, T.; et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 2019, 566, 403–406. [Google Scholar] [CrossRef]
- Iguchi, K.; Okumura, N.; Usui, S.; Sajiki, H.; Hirota, K.; Hirano, K. Myristoleic acid, a cytotoxic component in the extract fromSerenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate 2001, 47, 59–65. [Google Scholar] [CrossRef]
- Byberg, L.; Kilander, L.; Lemming, E.W.; Michaëlsson, K.; Vessby, B. Cancer death is related to high palmitoleic acid in serum and to polymorphisms in the SCD-1 gene in healthy Swedish men. Am. J. Clin. Nutr. 2014, 99, 551–558. [Google Scholar] [CrossRef]
- Hess, D.; Chisholm, J.W.; Igal, R.A. Inhibition of StearoylCoA Desaturase Activity Blocks Cell Cycle Progression and Induces Programmed Cell Death in Lung Cancer Cells. PLoS ONE 2010, 5, e11394. [Google Scholar] [CrossRef]
- Bermúdez, M.A.; Pereira, L.; Fraile, C.; Valerio, L.; Balboa, M.A.; Balsinde, J. Roles of Palmitoleic Acid and Its Positional Isomers, Hypogeic and Sapienic Acids, in Inflammation, Metabolic Diseases and Cancer. Cells 2022, 11, 14. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Meana, C.; Bermúdez, M.A.; Pérez-Encabo, A.; Balboa, M.A.; Balsinde, J. Release of Anti-Inflammatory Palmitoleic Acid and Its Positional Isomers by Mouse Peritoneal Macrophages. Biomedicines 2020, 8, 480. [Google Scholar] [CrossRef]
- Küçüksayan, E.; Sansone, A.; Chatgilialoglu, C.; Ozben, T.; Tekeli, D.; Talibova, G.; Ferreri, C. Sapienic Acid Metabolism Influences Membrane Plasticity and Protein Signaling in Breast Cancer Cell Lines. Cells 2022, 11, 225. [Google Scholar] [CrossRef]
- Awad, A.B.; Herrmann, T.; Fink, C.S.; Horvath, P.J. 18:1 n7 fatty acids inhibit growth and decrease inositol phosphate release in HT-29 cells compared to n9 fatty acids. Cancer Lett. 1995, 91, 55–61. [Google Scholar] [CrossRef]
- Lim, J.-N.; Oh, J.-J.; Wang, T.; Lee, J.-S.; Kim, S.-H.; Kim, Y.-J.; Lee, H.-G. trans-11 18:1 Vaccenic Acid (TVA) Has a Direct Anti-Carcinogenic Effect on MCF-7 Human Mammary Adenocarcinoma Cells. Nutrients 2014, 6, 627–636. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Fan, X.; Wu, H.; Han, J.; Yang, M.; Lu, L.; Nie, G. Trans-vaccenic acid inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cells via a mitochondrial-mediated apoptosis pathway. Lipids Health Dis. 2019, 18, 46. [Google Scholar] [CrossRef]
- Avato, P.; Pesante, M.A.; Fanizzi, F.P.; Santos, C.A.D.M. Seed oil composition of Paullinia cupana var. sorbilis (Mart.) Ducke. Lipids 2003, 38, 773–780. [Google Scholar] [CrossRef]
- Ohmori, H.; Fujii, K.; Kadochi, Y.; Mori, S.; Nishiguchi, Y.; Fujiwara, R.; Kishi, S.; Sasaki, T.; Kuniyasu, H. Elaidic Acid, a Trans-Fatty Acid, Enhances the Metastasis of Colorectal Cancer Cells. Pathobiology 2017, 84, 144–151. [Google Scholar] [CrossRef]
- Delbeke, E.I.P.; Everaert, J.; Uitterhaegen, E.; Verweire, S.; Verlee, A.; Talou, T.; Soetaert, W.; Van Bogaert, I.N.A.; Stevens, C.V. Petroselinic acid purification and its use for the fermentation of new sophorolipids. AMB Express 2016, 6, 28. [Google Scholar] [CrossRef]
- Awad, N.A.; Eliraq, M.; El-Bassel, E.H.; Ismail, A.S.M.; El-Aziz, Y.S.G.A.; Gawish, M.S.; Zewail, R.M.Y.; Sami, R.; Khojah, E.; Hilary, U.; et al. Evaluation of the Effect of Elite Jojoba Lines on the Chemical Properties of their Seed Oil. Molecules 2022, 27, 3904. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, M.Z. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J. Genet. Eng. Biotechnol. 2022, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, R.Z.; Christophersen, B.; Bremer, J. Monoethylenic C20 and C22fatty acids in marine oil and rapeseed oil. studies on their oxidation and on their relative ability to inhibit palmitate oxidation in heart and liver mitochondria. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1977, 487, 28–36. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, T.; Wang, Q.; Liu, P.; Zhang, T.; Zetterström, R.; Strandvik, B. Fatty acid composition of diet, cord blood and breast milk in Chinese mothers with different dietary habits. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Altinoz, M.A.; Elmaci, I.; Hacimuftuoglu, A.; Ozpinar, A.; Hacker, E.; Ozpinar, A. PPARδ and its ligand erucic acid may act anti-tumoral, neuroprotective, and myelin protective in neuroblastoma, glioblastoma, and Parkinson’s disease. Mol. Asp. Med. 2021, 78, 100871. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Bilir, A.; Elmaci, I. Erucic acid, a component of Lorenzo’s oil and PPAR-δ ligand modifies C6 glioma growth and toxicity of doxorubicin. Experimental data and a comprehensive literature analysis. Chem. Interact. 2018, 294, 107–117. [Google Scholar] [CrossRef]
- Liu, F.; Wang, P.; Xiong, X.; Zeng, X.; Zhang, X.; Wu, G. A Review of Nervonic Acid Production in Plants: Prospects for the Genetic Engineering of High Nervonic Acid Cultivars Plants. Front. Plant Sci. 2021, 12, 626625. [Google Scholar] [CrossRef]
- Umemoto, H.; Yasugi, S.; Tsuda, S.; Yoda, M.; Ishiguro, T.; Kaba, N.; Itoh, T. Protective Effect of Nervonic Acid Against 6-Hydroxydopamine-Induced Oxidative Stress in PC-12 Cells. J. Oleo Sci. 2021, 70, 95–102. [Google Scholar] [CrossRef]
- Gunstone, F.D. Fatty Acids—Nomenclature, Structure, Isolation and Structure Determination, Biosynthesis and Chemical Synthesis. In Fatty Acid and Lipid Chemistry; Gunstone, F.D., Ed.; Springer: Boston, MA, USA, 1996; pp. 1–34. [Google Scholar]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Assy, N.; Nassar, F.; Nasser, G.; Grosovski, M. Olive oil consumption and non-alcoholic fatty liver disease. World J. Gastroenterol. 2009, 15, 1809–1815. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef]
- Binukumar, B.; Mathew, A. Dietary fat and risk of breast cancer. World J. Surg. Oncol. 2005, 3, 45. [Google Scholar] [CrossRef]
- Aglago, E.K.; Murphy, N.; Huybrechts, I.; Nicolas, G.; Casagrande, C.; Fedirko, V.; Weiderpass, E.; Rothwell, J.A.; Dahm, C.C.; Olsen, A.; et al. Dietary intake and plasma phospholipid concentrations of saturated, monounsaturated and trans fatty acids and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Int. J. Cancer 2021, 149, 865–882. [Google Scholar] [CrossRef]
- Banim, P.J.; Luben, R.; Khaw, K.-T.; Hart, A.R. Dietary oleic acid is inversely associated with pancreatic cancer—Data from food diaries in a cohort study. Pancreatology 2018, 18, 655–660. [Google Scholar] [CrossRef]
- Nkondjock, A.; The Canadian Cancer Registries Epidemiology Research Group; Krewski, D.; Johnson, K.C.; Ghadirian, P. Specific fatty acid intake and the risk of pancreatic cancer in Canada. Br. J. Cancer 2005, 92, 971–977. [Google Scholar] [CrossRef]
- Sellem, L.; Srour, B.; Guéraud, F.; Pierre, F.; Kesse-Guyot, E.; Fiolet, T.; Lavalette, C.; Egnell, M.; Latino-Martel, P.; Fassier, P.; et al. Saturated, mono- and polyunsaturated fatty acid intake and cancer risk: Results from the French prospective cohort NutriNet-Santé. Eur. J. Nutr. 2019, 58, 1515–1527. [Google Scholar] [CrossRef]
- Norrish, A.E.; Jackson, R.T.; Sharpe, S.J.; Skeaff, C.M. Men who consume vegetable oils rich in monounsaturated fat: Their dietary patterns and risk of prostate cancer (New Zealand). Cancer Causes Control 2000, 11, 609–615. [Google Scholar] [CrossRef]
- Jackson, M.D.; Walker, S.P.; Simpson-Smith, C.M.; Lindsay, C.M.; Smith, G.; McFarlane-Anderson, N.; Bennett, F.I.; Coard, K.C.M.; Aiken, W.D.; Tulloch, T.; et al. Associations of whole-blood fatty acids and dietary intakes with prostate cancer in Jamaica. Cancer Causes Control 2012, 23, 23–33. [Google Scholar] [CrossRef]
- Sealy, N.; Hankinson, S.E.; Houghton, S.C. Olive oil and risk of breast cancer: A systematic review and dose–response meta-analysis of observational studies. Br. J. Nutr. 2021, 125, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hou, L.; Wang, W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 2016, 138, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Lodi, M.; Kiehl, A.; Qu, F.L.; Gabriele, V.; Tomasetto, C.; Mathelin, C. Lipid Intake and Breast Cancer Risk: Is There a Link? A New Focus and Meta-Analysis. Eur. J. Breast Health 2022, 18, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, X.-Y.; Sun, S.-R.; Wang, L.-X.; Huang, T. Vegetable Oil Intake and Breast Cancer Risk: A Meta-analysis. Asian Pac. J. Cancer Prev. 2015, 16, 5125–5135. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.-W.; Kim, Y.-S.; Kwon, H.; Joh, H.-K.; Lee, J.-E.; Park, D.; Park, J.-H.; Ko, A.-R.; Kim, Y.-J. Association between dietary fat intake and colorectal adenoma in korean adults: A cross-sectional study. Medicine 2017, 96, e5759. [Google Scholar] [CrossRef]
- Yao, X.; Tian, Z. Saturated, Monounsaturated and Polyunsaturated Fatty Acids Intake and Risk of Pancreatic Cancer: Evidence from Observational Studies. PLoS ONE 2015, 10, e0130870. [Google Scholar] [CrossRef]
- Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.; Fuchs, C.S. Dietary Meat, Dairy Products, Fat, and Cholesterol and Pancreatic Cancer Risk in a Prospective Study. Am. J. Epidemiol. 2003, 157, 1115–1125. [Google Scholar] [CrossRef]
- Fan, Y.; Qiu, Y.; Wang, J.; Chen, Q.; Wang, S.; Wang, Y.; Li, Y.; Weng, Y.; Qian, J.; Chen, F.; et al. Association Between Dietary Fatty Acid Pattern and Risk of Oral Cancer. Front. Nutr. 2022, 9, 864098. [Google Scholar] [CrossRef]
- Sasanfar, B.; Toorang, F.; Zendehdel, K.; Salehi-Abargouei, A. Substitution of dietary macronutrients and their sources in association with breast cancer: Results from a large-scale case–control study. Eur. J. Nutr. 2022, 61, 2687–2695. [Google Scholar] [CrossRef]
- Thiébaut, A.C.M.; Jiao, L.; Silverman, D.T.; Cross, A.J.; Thompson, F.E.; Subar, A.F.; Hollenbeck, A.R.; Schatzkin, A.; Stolzenberg-Solomon, R.Z. Dietary Fatty Acids and Pancreatic Cancer in the NIH-AARP Diet and Health Study. JNCI 2009, 101, 1001–1011. [Google Scholar] [CrossRef]
- Gong, Z.; Holly, E.A.; Wang, F.; Chan, J.M.; Bracci, P.M. Intake of fatty acids and antioxidants and pancreatic cancer in a large population-based case-control study in the San Francisco Bay Area. Int. J. Cancer 2010, 127, 1893–1904. [Google Scholar] [CrossRef]
- Ji, X.; Wang, J.; Shen, Q.; Li, Z.; Jiang, Y.; Liu, D.; Tan, Y.; Li, H.; Xiang, Y. Dietary fat intake and liver cancer incidence: A population-based cohort study in Chinese men. Int. J. Cancer 2021, 148, 2982–2996. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, K.; Wang, L.; Yin, K.; Song, M.; Giovannucci, E.L.; Willett, W.C. Dietary fat and fatty acids in relation to risk of colorectal cancer. Eur. J. Nutr. 2022, 61, 1863–1873. [Google Scholar] [CrossRef]
- Bojková, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary Fat and Cancer—Which Is Good, Which Is Bad, and the Body of Evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Zong, G.; Willett, W.C.; Zock, P.L.; Wanders, A.J.; Hu, F.B.; Sun, Q. Associations of Monounsaturated Fatty Acids From Plant and Animal Sources With Total and Cause-Specific Mortality in Two US Prospective Cohort Studies. Circ. Res. 2019, 124, 1266–1275. [Google Scholar] [CrossRef]
- Chajès, V.; Thiébaut, A.C.M.; Rotival, M.; Gauthier, E.; Maillard, V.; Boutron-Ruault, M.-C.; Joulin, V.; Lenoir, G.M.; Clavel-Chapelon, F. Association between Serum trans-Monounsaturated Fatty Acids and Breast Cancer Risk in the E3N-EPIC Study. Am. J. Epidemiol. 2008, 167, 1312–1320. [Google Scholar] [CrossRef]
- Cruz-Lozano, M.; González-González, A.; Marchal, J.A.; Muñoz-Muela, E.; Molina, M.P.; Cara, F.E.; Brown, A.M.; García-Rivas, G.; Hernández-Brenes, C.; Lorente, J.A.; et al. Hydroxytyrosol inhibits cancer stem cells and the metastatic capacity of triple-negative breast cancer cell lines by the simultaneous targeting of epithelial-to-mesenchymal transition, Wnt/β-catenin and TGFβ signaling pathways. Eur. J. Nutr. 2019, 58, 3207–3219. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Zhang, Z.; Shen, W.-J.; Jiang, S.; Long, Y.-F.; Wu, B.; Ding, T.; Huan, F.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion of MDA-MB-231 Triple-Negative Breast Cancer Cell via Induction of Autophagy. Anti-Cancer Agents Med. Chem. 2019, 19, 1983–1990. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Xie, J.; Zhang, Z.; Zhu, J.; Jiang, S.; Shen, W.-J.; Wu, B.; Ding, T.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion via Induction of Autophagy in ER-Positive Breast Cancer Cell Lines (MCF7 and T47D). Nutr. Cancer 2021, 73, 350–360. [Google Scholar] [CrossRef]
- Chakravarti, B.; Maurya, R.; Siddiqui, J.A.; Bid, H.K.; Rajendran, S.; Yadav, P.P.; Konwar, R. In vitro anti-breast cancer activity of ethanolic extract of Wrightia tomentosa: Role of pro-apoptotic effects of oleanolic acid and urosolic acid. J. Ethnopharmacol. 2012, 142, 72–79. [Google Scholar] [CrossRef]
- Elnagar, A.Y.; Sylvester, P.W.; El Sayed, K.A. (−)-Oleocanthal as a c-Met Inhibitor for the Control of Metastatic Breast and Prostate Cancers. Planta Med. 2011, 77, 1013–1019. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Beltrán, G.; Delgado-Rodríguez, M.; Gaforio, J.J. Phytoestrogen (+)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Martínez-Hovelman, N.; Martínez-Huélamo, M.; Raventós, R.M.L.; Moreno, J.J. Extra Virgin Olive Oil Minor Compounds Modulate Mitogenic Action of Oleic Acid on Colon Cancer Cell Line. J. Agric. Food Chem. 2019, 67, 11420–11427. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Cavia, M.D.M.; Alonso-Torre, S.R. Oleic acid inhibits store-operated calcium entry in human colorectal adenocarcinoma cells. Eur. J. Nutr. 2012, 51, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Lin, J. Commentary: Anti-tumor Effect of Oleic Acid in Hepatocellular Carcinoma Cell Lines via Autophagy Reduction. Front. Cell Dev. Biol. 2021, 9, 677595. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Villegas, I.; Cárdeno, A.; Talero, E.; Sánchez-Hidalgo, M.; Motilva, V.; Alarcon de la Lastra, C. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clin. Nutr. 2010, 29, 663–673. [Google Scholar] [CrossRef]
- Nanda, N.; Mahmood, S.; Bhatia, A.; Mahmood, A.; Dhawan, D.K. Chemopreventive role of olive oil in colon carcinogenesis by targeting noncoding RNAs and methylation machinery. Int. J. Cancer 2019, 144, 1180–1194. [Google Scholar] [CrossRef]
- Ducheix, S.; Peres, C.; Härdfeldt, J.; Frau, C.; Mocciaro, G.; Piccinin, E.; Lobaccaro, J.-M.; De Santis, S.; Chieppa, M.; Bertrand-Michel, J.; et al. Deletion of Stearoyl-CoA Desaturase-1 From the Intestinal Epithelium Promotes Inflammation and Tumorigenesis, Reversed by Dietary Oleate. Gastroenterology 2018, 155, e1529. [Google Scholar] [CrossRef]
- Piegari, M.; Soria, E.A.; Eynard, A.R.; Valentich, M.A. Delay of Lung Adenocarcinoma (LAC-1) Development in Mice by Dietary Oleic Acid. Nutr. Cancer 2017, 69, 1069–1074. [Google Scholar] [CrossRef]
- Yamaki, T.; Yano, T.; Satoh, H.; Endo, T.; Matsuyama, C.; Kumagai, H.; Miyahara, M.; Sakurai, H.; Pokorny, J.; Shin, S.J.; et al. High oleic acid oil suppresses lung tumorigenesis in mice through the modulation of extracellular signal-regulated kinase cascade. Lipids 2002, 37, 783–788. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.; Duan, Y.; Zhang, D.; Li, S.; Wang, F. Four types of fatty acids exert differential impact on pancreatic cancer growth. Cancer Lett. 2015, 360, 187–194. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Luo, X.; Su, C.; Chen, Y.; Zhao, L.; Wei, L.; Zeng, H.; Varghese, Z.; Moorhead, J.F.; et al. High olive oil diets enhance cervical tumour growth in mice: Transcriptome analysis for potential candidate genes and pathways. Lipids Health Dis. 2019, 18, 76. [Google Scholar] [CrossRef]
- Yang, P.; Su, C.; Luo, X.; Zeng, H.; Zhao, L.; Wei, L.; Zhang, X.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; et al. Dietary oleic acid-induced CD36 promotes cervical cancer cell growth and metastasis via up-regulation Src/ERK pathway. Cancer Lett. 2018, 438, 76–85. [Google Scholar] [CrossRef]
- Hillyer, L.M.; Hucik, B.; Baracuhy, E.M.; Lin, Z.; Muller, W.J.; Robinson, L.E.; Ma, D.W.L. Her-2 Breast Cancer Outcomes Are Mitigated by Consuming n-3 Polyunsaturated, Saturated, and Monounsaturated Fatty Acids Compared to n-6 Polyunsaturated Fatty Acids. Nutrients 2020, 12, 3901. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci. Rep. 2017, 7, 11277. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Li, H.; Lu, L.; Han, M.; Guo, Y.; Wang, X. A simple but efficient tumor-targeted nanoparticle delivery system constructed by oleic acid. Drug Deliv. 2022, 29, 2539–2548. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Lv, B.; Hou, X.; Liu, Q.; Liao, C.; Xu, R.; Zhang, Y.; Xu, F.; Zhang, P. Oleic Acid and Insulin as Key Characteristics of T2D Promote Colorectal Cancer Deterioration in Xenograft Mice Revealed by Functional Metabolomics. Front. Oncol. 2021, 11, 685059. [Google Scholar] [CrossRef]
- Kishi, S.; Fujiwara-Tani, R.; Luo, Y.; Kawahara, I.; Goto, K.; Fujii, K.; Ohmori, H.; Nakashima, C.; Sasaki, T.; Kuniyasu, H. Pro-metastatic signaling of the trans fatty acid elaidic acid is associated with lipid rafts. Oncol. Lett. 2018, 15, 4423–4426. [Google Scholar] [CrossRef]
- Shen, C.-J.; Chan, S.-H.; Lee, C.-T.; Huang, W.-C.; Tsai, J.-P.; Chen, B.-K. Oleic acid-induced ANGPTL4 enhances head and neck squamous cell carcinoma anoikis resistance and metastasis via up-regulation of fibronectin. Cancer Lett. 2017, 386, 110–122. [Google Scholar] [CrossRef]
- Yonezawa, T.; Katoh, K.; Obara, Y. Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem. Biophys. Res. Commun. 2004, 314, 805–809. [Google Scholar] [CrossRef]
- Hardy, S.; St-Onge, G.G.; Joly, E.; Langelier, Y.; Prentki, M. Oleate Promotes the Proliferation of Breast Cancer Cells via the G Protein-coupled Receptor GPR40. J. Biol. Chem. 2005, 280, 13285–13291. [Google Scholar] [CrossRef] [PubMed]
- Marcial-Medina, C.; Ordoñez-Moreno, A.; Gonzalez-Reyes, C.; Cortes-Reynosa, P.; Perez Salazar, E. Oleic acid induces migration through a FFAR1/4, EGFR and AKT-dependent pathway in breast cancer cells. Endocr. Connect. 2019, 8, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Liotti, A.; Cosimato, V.; Mirra, P.; Calì, G.; Conza, D.; Secondo, A.; Luongo, G.; Terracciano, D.; Formisano, P.; Beguinot, F.; et al. Oleic acid promotes prostate cancer malignant phenotype via the G protein-coupled receptor FFA1/GPR40. J. Cell Physiol. 2018, 233, 7367–7378. [Google Scholar] [CrossRef] [PubMed]
- Soto-Guzman, A.; Robledo, T.; Lopez-Perez, M.; Salazar, E.P. Oleic acid induces ERK1/2 activation and AP-1 DNA binding activity through a mechanism involving Src kinase and EGFR transactivation in breast cancer cells. Mol. Cell Endocrinol. 2008, 294, 81–91. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, Y.; Yuan, Y.; Zhang, X.; Qin, C.; Xie, J.; Hao, Y.; Xu, T.; Wang, X. Effects of oleic acid on cell proliferation through an integrin-linked kinase signaling pathway in 786-O renal cell carcinoma cells. Oncol. Lett. 2013, 5, 1395–1399. [Google Scholar] [CrossRef]
- Seo, J.; Jeong, D.-W.; Park, J.-W.; Lee, K.-W.; Fukuda, J.; Chun, Y.-S. Fatty-acid-induced FABP5/HIF-1 reprograms lipid metabolism and enhances the proliferation of liver cancer cells. Commun. Biol. 2020, 3, 638. [Google Scholar] [CrossRef]
- Przybytkowski, E.; Joly, E.; Nolan, C.; Hardy, S.; Francoeur, A.-M.; Langelier, Y.; Prentki, M. Upregulation of cellular triacylglycerol—free fatty acid cycling by oleate is associated with long-term serum-free survival of human breast cancer cells. Biochem. Cell Biol. 2007, 85, 301–310. [Google Scholar] [CrossRef]
- Li, S.; Zhou, T.; Li, C.; Dai, Z.; Che, D.; Yao, Y.; Li, L.; Ma, J.; Yang, X.; Gao, G. High Metastaticgastric and Breast Cancer Cells Consume Oleic Acid in an AMPK Dependent Manner. PLoS ONE 2014, 9, e97330. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, B.; Zhao, Y.; Tao, Z.; Wang, Y.; Chen, G.; Hu, X. Mammary adipocytes protect triple-negative breast cancer cells from ferroptosis. J. Hematol. Oncol. 2022, 15, 72. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Yang, Y.; Jung, J.-H.; Kim, Y. Oleic acid from cancer-associated fibroblast promotes cancer cell stemness by stearoyl-CoA desaturase under glucose-deficient condition. Cancer Cell Int. 2022, 22, 404. [Google Scholar] [CrossRef]
- Xie, X.; Tian, L.; Zhao, Y.; Liu, F.; Dai, S.; Gu, X.; Ye, Y.; Zhou, L.; Liu, X.; Sun, Y.; et al. BACH1-induced ferroptosis drives lymphatic metastasis by repressing the biosynthesis of monounsaturated fatty acids. Cell Death Dis. 2023, 14, 48. [Google Scholar] [CrossRef]
- Soto-Guzman, A.; Navarro-Tito, N.; Castro-Sanchez, L.; Martinez-Orozco, R.; Salazar, E.P. Oleic acid promotes MMP-9 secretion and invasion in breast cancer cells. Clin. Exp. Metastasis 2010, 27, 505–515. [Google Scholar] [CrossRef]
- Soto-Guzman, A.; Villegas-Comonfort, S.; Cortes-Reynosa, P.; Salazar, E.P. Role of arachidonic acid metabolism in Stat5 activation induced by oleic acid in MDA-MB-231 breast cancer cells. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 243–249. [Google Scholar] [CrossRef]
- Navarro-Tito, N.; Soto-Guzman, A.; Castro-Sanchez, L.; Martinez-Orozco, R.; Salazar, E.P. Oleic acid promotes migration on MDA-MB-231 breast cancer cells through an arachidonic acid-dependent pathway. Int. J. Biochem. Cell Biol. 2010, 42, 306–317. [Google Scholar] [CrossRef]
- Lingrand, M.; Lalonde, S.; Jutras-Carignan, A.; Bergeron, K.-F.; Rassart, E.; Mounier, C. SCD1 activity promotes cell migration via a PLD-mTOR pathway in the MDA-MB-231 triple-negative breast cancer cell line. Breast Cancer 2020, 27, 594–606. [Google Scholar] [CrossRef]
- Shen, C.-J.; Chang, K.-Y.; Lin, B.-W.; Lin, W.-T.; Su, C.-M.; Tsai, J.-P.; Liao, Y.-H.; Hung, L.-Y.; Chang, W.-C.; Chen, B.-K. Oleic acid-induced NOX4 is dependent on ANGPTL4 expression to promote human colorectal cancer metastasis. Theranostics 2020, 10, 7083–7099. [Google Scholar] [CrossRef]
- Xiang, F.; Wu, K.; Liu, Y.; Shi, L.; Wang, D.; Li, G.; Tao, K.; Wang, G. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int. J. Biochem. Cell Biol. 2017, 84, 14–21. [Google Scholar] [CrossRef]
- Kimura, Y. Carp Oil or Oleic Acid, but Not Linoleic Acid or Linolenic Acid, Inhibits Tumor Growth and Metastasis in Lewis Lung Carcinoma–Bearing Mice. J. Nutr. 2002, 132, 2069–2075. [Google Scholar] [CrossRef]
- Moon, H.-S.; Batirel, S.; Mantzoros, C.S. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism 2014, 63, 1447–1454. [Google Scholar] [CrossRef]
- Giulitti, F.; Petrungaro, S.; Mandatori, S.; Tomaipitinca, L.; de Franchis, V.; D’Amore, A.; Filippini, A.; Gaudio, E.; Ziparo, E.; Giampietri, C. Anti-tumor Effect of Oleic Acid in Hepatocellular Carcinoma Cell Lines via Autophagy Reduction. Front. Cell Dev. Biol. 2021, 9, 629182. [Google Scholar] [CrossRef]
- Mizushina, Y.; Takeuchi, T.; Sugawara, F.; Yoshida, H. Anti-Cancer Targeting Telomerase Inhibitors: β-Rubromycin and Oleic Acid. Mini-Reviews Med. Chem. 2012, 12, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Uzu, E.; Yoshigai, Y.; Kato, C.; Tagami, M. Oleic acid and oleoylethanolamide decrease interferon-γ-induced expression of PD-L1 and induce apoptosis in human lung carcinoma cells. Eur. J. Pharmacol. 2021, 903, 174116. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin™) in breast cancer cells with Her-2/neu oncogene amplification. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R. Oleic acid suppresses overexpression of ERBB2 oncogene. Lancet Oncol. 2005, 6, 69. [Google Scholar] [CrossRef]
- Rinaldi, F.; Forte, J.; Pontecorvi, G.; Hanieh, P.N.; Carè, A.; Bellenghi, M.; Tirelli, V.; Ammendolia, M.G.; Mattia, G.; Marianecci, C.; et al. pH-responsive oleic acid based nanocarriers: Melanoma treatment strategies. Int. J. Pharm. 2022, 613, 121391. [Google Scholar] [CrossRef]
- Muhammad, N.; Ruiz, F.; Stanley, J.; Rashmi, R.; Cho, K.; Jayachandran, K.; Zahner, M.C.; Huang, Y.; Zhang, J.; Markovina, S.; et al. Monounsaturated and Diunsaturated Fatty Acids Sensitize Cervical Cancer to Radiation Therapy. Cancer Res. 2022, 82, 4515–4527. [Google Scholar] [CrossRef]
- Jung, S.; Lee, S.; Lee, H.; Yoon, J.; Lee, E. Oleic acid-embedded nanoliposome as a selective tumoricidal agent. Colloids Surf. B Biointerfaces 2016, 146, 585–589. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Prasanna, X.; Agiru, P.; Chakraborty, H.; Rydström, A.; Ho, J.C.S.; Svanborg, C.; Sengupta, D.; Chattopadhyay, A. Protein-dependent Membrane Interaction of A Partially Disordered Protein Complex with Oleic Acid: Implications for Cancer Lipidomics. Sci. Rep. 2016, 6, 35015. [Google Scholar] [CrossRef]
- Brinkmann, C.R.; Heegaard, C.W.; Petersen, T.E.; Jensenius, J.C.; Thiel, S. The toxicity of bovine α-lactalbumin made lethal to tumor cells is highly dependent on oleic acid and induces killing in cancer cell lines and noncancer-derived primary cells. FEBS J. 2011, 278, 1955–1967. [Google Scholar] [CrossRef]
- Ruggiero, M.; Ward, E.; Smith, R.; Branca, J.J.V.; Noakes, D.; Morucci, G.; Taubmann, M.; Thyer, L.; Pacini, S. Oleic Acid, deglycosylated vitamin D-binding protein, nitric oxide: A molecular triad made lethal to cancer. Anticancer Res. 2014, 34, 3569–3578. [Google Scholar]
- Houshaymi, B.; Nasreddine, N.; Kedees, M.; Soayfane, Z. Oleic acid increases uptake and decreases the P-gp-mediated efflux of the veterinary anthelmintic Ivermectin. Drug Res. 2019, 69, 173–180. [Google Scholar] [CrossRef]
- Aspenström-Fagerlund, B.; Tallkvist, J.; Ilbäck, N.-G.; Glynn, A.W. Oleic acid decreases BCRP mediated efflux of mitoxantrone in Caco-2 cell monolayers. Food Chem. Toxicol. 2012, 50, 3635–3645. [Google Scholar] [CrossRef]
- Wang, C.; Shi, M.; Ji, J.; Cai, Q.; Zhao, Q.; Jiang, J.; Liu, J.; Zhang, H.; Zhu, Z.; Zhang, J. Stearoyl-CoA desaturase 1 (SCD1) facilitates the growth and anti-ferroptosis of gastric cancer cells and predicts poor prognosis of gastric cancer. Aging 2020, 12, 15374–15391. [Google Scholar] [CrossRef]
- Roongta, U.V.; Pabalan, J.G.; Wang, X.; Ryseck, R.-P.; Fargnoli, J.; Henley, B.J.; Yang, W.-P.; Zhu, J.; Madireddi, M.T.; Lawrence, R.M.; et al. Cancer Cell Dependence on Unsaturated Fatty Acids Implicates Stearoyl-CoA Desaturase as a Target for Cancer Therapy. Mol. Cancer Res. 2011, 9, 1551–1561. [Google Scholar] [CrossRef]
- Holder, A.M.; Gonzalez-Angulo, A.M.; Chen, H.; Akcakanat, A.; Do, K.-A.; Symmans, W.F.; Pusztai, L.; Hortobagyi, G.N.; Mills, G.B.; Meric-Bernstam, F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res. Treat. 2013, 137, 319–327. [Google Scholar] [CrossRef]
- Peck, B.; Schug, Z.T.; Zhang, Q.; Dankworth, B.; Jones, D.T.; Smethurst, E.; Patel, R.; Mason, S.; Jiang, M.; Saunders, R.; et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016, 4, 6. [Google Scholar] [CrossRef]
- Presler, M.; Wojtczyk-Miaskowska, A.; Schlichtholz, B.; Kaluzny, A.; Matuszewski, M.; Mika, A.; Sledzinski, T.; Swierczynski, J. Increased expression of the gene encoding stearoyl-CoA desaturase 1 in human bladder cancer. Mol. Cell Biochem. 2018, 447, 217–224. [Google Scholar] [CrossRef]
- Fritz, V.; Benfodda, Z.; Rodier, G.; Henriquet, C.; Iborra, F.; Avancès, C.; Allory, Y.; de la Taille, A.; Culine, S.; Blancou, H.; et al. Abrogation of De novo Lipogenesis by Stearoyl-CoA Desaturase 1 Inhibition Interferes with Oncogenic Signaling and Blocks Prostate Cancer Progression in Mice. Mol. Cancer Ther. 2010, 9, 1740–1754. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Z.; Liu, Y.; Zhang, Z.; Wang, M.; Gong, A.; Xia, L.; Liao, X.; Wang, D.; Zhu, H. Stearoyl-CoA Desaturase 1 Potentiates Hypoxic plus Nutrient-Deprived Pancreatic Cancer Cell Ferroptosis Resistance. Oxidative Med. Cell Longev. 2021, 2021, 662980. [Google Scholar] [CrossRef]
- Tesfay, L.; Paul, B.T.; Konstorum, A.; Deng, Z.; Cox, A.O.; Lee, J.; Furdui, C.M.; Hegde, P.; Torti, F.M.; Torti, S.V. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res 2019, 79, 5355–5366. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.-X.; He, J.; Pan, H.; Li, R.Z.; Huang, L.; Jiang, Z.; Yao, X.-J.; Liu, L.; Leung, E.L.; et al. SCD1 is associated with tumor promotion, late stage and poor survival in lung adenocarcinoma. Oncotarget 2016, 7, 39970–39979. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.; Zhu, Y.; Deng, R.; Zhang, Q.; Liu, X.; Feng, M.; Zhong, J.; Lin, S.; Tong, X.; Su, Q. Stearoyl-CoA desaturase-1 promotes colorectal cancer metastasis in response to glucose by suppressing PTEN. J. Exp. Clin. Cancer Res. 2018, 37, 54. [Google Scholar] [CrossRef] [PubMed]
- Von Roemeling, C.A.; Marlow, L.A.; Wei, J.J.; Cooper, S.J.; Caulfield, T.R.; Wu, K.; Tan, W.W.; Tun, H.W.; Copland, J.A. Stearoyl-CoA Desaturase 1 Is a Novel Molecular Therapeutic Target for Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2013, 19, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Zhu, L.; Zou, Y.; Kong, W.; Dong, B.; Huang, J.; Chen, Y.; Xue, W.; Huang, Y.; et al. High Expression of Stearoyl-CoA Desaturase 1 Predicts Poor Prognosis in Patients with Clear-Cell Renal Cell Carcinoma. PLoS ONE 2016, 11, e0166231. [Google Scholar] [CrossRef]
- Wang, L.; Ye, G.; Wang, Y.; Wang, C. Stearoyl-CoA desaturase 1 regulates malignant progression of cervical cancer cells. Bioengineered 2022, 13, 12941–12954. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, L.; Li, X.; Wang, J.; Zhu, Y.; Jia, Y.; Tong, Z. SCD5 expression correlates with prognosis and response to neoadjuvant chemotherapy in breast cancer. Sci. Rep. 2021, 11, 8976. [Google Scholar] [CrossRef]
- Bellenghi, M.; Puglisi, R.; Pedini, F.; De Feo, A.; Felicetti, F.; Bottero, L.; Sangaletti, S.; Errico, M.C.; Petrini, M.; Gesumundo, C.; et al. SCD5-induced oleic acid production reduces melanoma malignancy by intracellular retention of SPARC and cathepsin B. J. Pathol. 2015, 236, 315–325. [Google Scholar] [CrossRef]
- Ruggieri, S.; Roblin, R.; Black, P.H. Lipids of whole cells and plasma membrane fractions from Balb/c3T3, SV3T3, and concanavalin A-selected revertant cells. J. Lipid. Res. 1979, 20, 760–771. [Google Scholar] [CrossRef]
- Bougnoux, P.; Verrelle, P.; Chassagne, J. Intratumoral biological markers in breast cancers. Rev. Prat. 1992, 42, 1935–1942. [Google Scholar]
- Williams, C.M.; Maunder, K. Fatty acid compositions of inositol and choline phospholipids of breast tumours and normal breast tissue. Eur. J. Clin. Nutr. 1993, 47, 260–267. [Google Scholar]
- Amézaga, J.; Arranz, S.; Urruticoechea, A.; Ugartemendia, G.; Larraioz, A.; Louka, M.; Uriarte, M.; Ferreri, C.; Tueros, I. Altered Red Blood Cell Membrane Fatty Acid Profile in Cancer Patients. Nutrients 2018, 10, 1853. [Google Scholar] [CrossRef]
- Shetty, V.; Preethika, A.; Kumari, S.; Shetty, J. Plasma fatty acids composition and estimated delta desaturases activity in women with breast cancer. J. Cancer Res. Ther. 2020, 16, 1382–1386. [Google Scholar] [CrossRef]
- Da Conceição, L.L.; Dias, M.D.M.; Pessoa, M.C.; Pena, G.D.G.; Mendes, M.C.S.; Neves, C.V.B.; Hermsdorff, H.H.M.; Freitas, R.N.; Peluzio, M.D.C.G. Difference in fatty acids composition of breast adipose tissue in women with breast cancer and benign breast disease. Nutr. Hosp. 2016, 33, 1354–1360. [Google Scholar] [CrossRef]
- Budhu, A.; Roessler, S.; Zhao, X.; Yu, Z.; Forgues, M.; Ji, J.; Karoly, E.; Qin, L.; Ye, Q.; Jia, H.; et al. Integrated Metabolite and Gene Expression Profiles Identify Lipid Biomarkers Associated With Progression of Hepatocellular Carcinoma and Patient Outcomes. Gastroenterology 2013, 144, 1066–1075.e1. [Google Scholar] [CrossRef]
- Park, S.-Y.; Wilkens, L.R.; Henning, S.M.; Le Marchand, L.; Gao, K.; Goodman, M.T.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N. Circulating fatty acids and prostate cancer risk in a nested case–control study: The Multiethnic Cohort. Cancer Causes Control 2009, 20, 211–223. [Google Scholar] [CrossRef]
- Butler, L.M.; Yuan, J.-M.; Huang, J.Y.; Su, J.; Wang, R.; Koh, W.-P.; Ong, C.-N. Plasma fatty acids and risk of colon and rectal cancers in the Singapore Chinese Health Study. NPJ Precis. Oncol. 2017, 1, 38. [Google Scholar] [CrossRef]
- May-Wilson, S.; Sud, A.; Law, P.J.; Palin, K.; Tuupanen, S.; Gylfe, A.; Hänninen, U.A.; Cajuso, T.; Tanskanen, T.; Kondelin, J.; et al. Pro-inflammatory fatty acid profile and colorectal cancer risk: A Mendelian randomisation analysis. Eur. J. Cancer 2017, 84, 228–238. [Google Scholar] [CrossRef]
- Shishavan, N.G.; Mohamadkhani, A.; Sepanlou, S.G.; Masoudi, S.; Sharafkhah, M.; Poustchi, H.; Hekmatdoost, A.; Pourshams, A. Circulating plasma fatty acids and risk of pancreatic cancer: Results from the Golestan Cohort Study. Clin. Nutr. 2021, 40, 1897–1904. [Google Scholar] [CrossRef]
- Chiu, Y.; Bertrand, K.A.; Zhang, S.; Laden, F.; Epstein, M.M.; Rosner, B.A.; Chiuve, S.; Campos, H.; Giovannucci, E.L.; Chavarro, J.E.; et al. A prospective analysis of circulating saturated and monounsaturated fatty acids and risk of non-Hodgkin lymphoma. Int. J. Cancer 2018, 143, 1914–1922. [Google Scholar] [CrossRef]
- Cheung, S.M.; Husain, E.; Mallikourti, V.; Masannat, Y.; Heys, S.; He, J. Intra-tumoural lipid composition and lymphovascular invasion in breast cancer via non-invasive magnetic resonance spectroscopy. Eur. Radiol. 2021, 31, 3703–3711. [Google Scholar] [CrossRef]
- Chajès, V.; Joulin, V.; Clavel-Chapelon, F. The fatty acid desaturation index of blood lipids, as a biomarker of hepatic stearoyl-CoA desaturase expression, is a predictive factor of breast cancer risk. Curr. Opin. Infect. Dis. 2011, 22, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Man, W.C.; Miyazaki, M.; Chu, K.; Ntambi, J.M. Membrane Topology of Mouse Stearoyl-CoA Desaturase 1. J. Biol. Chem. 2006, 281, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Flowers, M.T.; Liu, X.; Paton, C.M.; Sullivan, R.; Chu, K.; Zhao, M.; Ntambi, J.M. Skin-specific Deletion of Stearoyl-CoA Desaturase-1 Alters Skin Lipid Composition and Protects Mice from High Fat Diet-induced Obesity. J. Biol. Chem. 2009, 284, 19961–19973. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl–CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef]
- Hu, X.; Xiang, J.; Li, Y.; Xia, Y.; Xu, S.; Gao, X.; Qiao, S. Inhibition of Stearoyl-CoA Desaturase 1 Potentiates Anti-tumor Activity of Amodiaquine in Non-small Cell Lung Cancer. Biol. Pharm. Bull. 2022, 45, 438–445. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.; Yang, L.; Li, Y.; Fu, J.; Li, Y.; Tian, Y.; Qiu, F.; Liu, Z.; Qiu, Y. Stearoyl-CoA desaturase-1 mediated cell apoptosis in colorectal cancer by promoting ceramide synthesis. Sci. Rep. 2016, 6, 19665. [Google Scholar] [CrossRef]
- Hackney, A.B.; Chung, W.Y.; Isherwood, J.; Dennison, A.R.; Martin, N. Stearoyl-CoA desaturase 1 inhibitor supplemented with gemcitabine treatment reduces the viability and fatty acid content of pancreatic cancer cells in vitro. J. Pancreatol. 2021, 4, 170–177. [Google Scholar] [CrossRef]
- Kim, S.-J.; Choi, H.; Park, S.-S.; Chang, C.; Kim, E. Stearoyl CoA desaturase (SCD) facilitates proliferation of prostate cancer cells through enhancement of androgen receptor transactivation. Mol. Cells 2011, 31, 371–377. [Google Scholar] [CrossRef]
- Du, X.; Wang, Q.-R.; Chan, E.; Merchant, M.; Liu, J.; French, D.; Ashkenazi, A.; Qing, J. FGFR3 Stimulates Stearoyl CoA Desaturase 1 Activity to Promote Bladder Tumor Growth. Cancer Res 2012, 72, 5843–5855. [Google Scholar] [CrossRef]
- Imamura, K.; Tomita, N.; Kawakita, Y.; Ito, Y.; Ono, K.; Nii, N.; Miyazaki, T.; Yonemori, K.; Tawada, M.; Sumi, H.; et al. Discovery of Novel and Potent Stearoyl Coenzyme A Desaturase 1 (SCD1) Inhibitors as Anticancer Agents. Bioorg. Med. Chem. 2017, 25, 3768–3779. [Google Scholar] [CrossRef]
- Nishizawa, S.; Sumi, H.; Satoh, Y.; Yamamoto, Y.; Kitazawa, S.; Honda, K.; Araki, H.; Kakoi, K.; Imamura, K.; Sasaki, M.; et al. In vitro and in vivo antitumor activities of T-3764518, a novel and orally available small molecule stearoyl-CoA desaturase 1 inhibitor. Eur. J. Pharmacol. 2017, 807, 21–31. [Google Scholar] [CrossRef]
- Lai, K.K.; Kweon, S.-M.; Chi, F.; Hwang, E.; Kabe, Y.; Higashiyama, R.; Qin, L.; Yan, R.; Wu, R.P.; Lai, K.; et al. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology 2017, 152, 1477–1491. [Google Scholar] [CrossRef]
- Liu, H.-H.; Xu, Y.; Li, C.-J.; Hsu, S.-J.; Lin, X.-H.; Zhang, R.; Chen, J.; Chen, J.; Gao, D.-M.; Cui, J.-F.; et al. An SCD1-dependent mechanoresponsive pathway promotes HCC invasion and metastasis through lipid metabolic reprogramming. Mol. Ther. 2022, 30, 2554–2567. [Google Scholar] [CrossRef]
- Vivas-García, Y.; Falletta, P.; Liebing, J.; Louphrasitthiphol, P.; Feng, Y.; Chauhan, J.; Scott, D.; Glodde, N.; Calvo, A.C.; Bonham, S.; et al. Lineage-Restricted Regulation of SCD and Fatty Acid Saturation by MITF Controls Melanoma Phenotypic Plasticity. Mol. Cell 2020, 77, 120–137.e9. [Google Scholar] [CrossRef]
- Bellenghi, M.; Talarico, G.; Botti, L.; Puglisi, R.; Tabolacci, C.; Portararo, P.; Piva, A.; Pontecorvi, G.; Carè, A.; Colombo, M.P.; et al. SCD5-dependent inhibition of SPARC secretion hampers metastatic spreading and favors host immunity in a TNBC murine model. Oncogene 2022, 41, 4055–4065. [Google Scholar] [CrossRef]
- Ferreri, C.; Sansone, A.; Buratta, S.; Urbanelli, L.; Costanzi, E.; Emiliani, C.; Chatgilialoglu, C. The n-10 Fatty Acids Family in the Lipidome of Human Prostatic Adenocarcinoma Cell Membranes and Extracellular Vesicles. Cancers 2020, 12, 900. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, Y.J.; Kim, H.; Lee, H.; Chung, H.; Nam, M.-H.; Moon, J.-Y.; Lee, H.S.; Yoon, S.; Kim, W.-Y. Clinical and biochemical relevance of monounsaturated fatty acid metabolism targeting strategy for cancer stem cell elimination in colon cancer. Biochem. Biophys. Res. Commun. 2019, 519, 100–105. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kenny, H.A.; Lengyel, E. Unsaturated Fatty Acids Maintain Cancer Cell Stemness. Cell Stem Cell 2017, 20, 291–292. [Google Scholar] [CrossRef]
- Scaglia, N.; Chisholm, J.W.; Igal, R.A. Inhibition of StearoylCoA Desaturase-1 Inactivates Acetyl-CoA Carboxylase and Impairs Proliferation in Cancer Cells: Role of AMPK. PLoS ONE 2009, 4, e6812. [Google Scholar] [CrossRef]
- Zhang, J.; Song, F.; Zhao, X.; Jiang, H.; Wu, X.; Wang, B.; Zhou, M.; Tian, M.; Shi, B.; Wang, H.; et al. EGFR modulates monounsaturated fatty acid synthesis through phosphorylation of SCD1 in lung cancer. Mol. Cancer 2017, 16, 127. [Google Scholar] [CrossRef]
- Angelucci, C.; Maulucci, G.; Colabianchi, A.; Iacopino, F.; D’Alessio, A.; Maiorana, A.; Palmieri, V.; Papi, M.; De Spirito, M.; Di Leone, A.; et al. Stearoyl-CoA desaturase 1 and paracrine diffusible signals have a major role in the promotion of breast cancer cell migration induced by cancer-associated fibroblasts. Br. J. Cancer 2015, 112, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, C.; D’alessio, A.; Iacopino, F.; Proietti, G.; Di Leone, A.; Masetti, R.; Sica, G. Pivotal role of human stearoyl-CoA desaturases (SCD1 and 5) in breast cancer progression: Oleic acid-based effect of SCD1 on cell migration and a novel pro-cell survival role for SCD5. Oncotarget 2018, 9, 24364–24380. [Google Scholar] [CrossRef] [PubMed]
- Mauvoisin, D.; Charfi, C.; Lounis, A.M.; Rassart, E.; Mounier, C. Decreasing stearoyl-CoA desaturase-1 expression inhibits β-catenin signaling in breast cancer cells. Cancer Sci. 2013, 104, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Zhang, J.; Lv, J.; Huang, Y. Positive feedback loop and synergistic effects between hypoxia-inducible factor-2α and stearoyl-CoA desaturase-1 promote tumorigenesis in clear cell renal cell carcinoma. Cancer Sci. 2013, 104, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.; Bellenghi, M.; Pontecorvi, G.; Gulino, A.; Petrini, M.; Felicetti, F.; Bottero, L.; Mattia, G.; Carè, A. SCD5 restored expression favors differentiation and epithelial-mesenchymal reversion in advanced melanoma. Oncotarget 2018, 9, 7567–7581. [Google Scholar] [CrossRef]
- Minville-Walz, M.; Pierre, A.-S.; Pichon, L.; Bellenger, S.; Fèvre, C.; Bellenger, J.; Tessier, C.; Narce, M.; Rialland, M. Inhibition of Stearoyl-CoA Desaturase 1 Expression Induces CHOP-Dependent Cell Death in Human Cancer Cells. PLoS ONE 2010, 5, e14363. [Google Scholar] [CrossRef]
- Zhao, G.; Tan, Y.; Cardenas, H.; Vayngart, D.; Wang, Y.; Huang, H.; Keathley, R.; Wei, J.-J.; Ferreira, C.R.; Orsulic, S.; et al. Ovarian cancer cell fate regulation by the dynamics between saturated and unsaturated fatty acids. Proc. Natl. Acad. Sci. USA 2022, 119, e2203480119. [Google Scholar] [CrossRef]
- Escrich, E.; Moral, R.; Grau, L.; Costa, I.; Solanas, M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol. Nutr. Food Res. 2007, 51, 1279–1292. [Google Scholar] [CrossRef]
- Marques, S.D.O.; Muller, A.P.; Luciano, T.F.; Tramontin, N.D.S.; Caetano, M.D.S.; Pieri, B.L.d.S.; Amorim, T.L.; de Oliveira, M.A.L.; de Souza, C.T. Effects of Avocado Oil Supplementation on Insulin Sensitivity, Cognition, and Inflammatory and Oxidative Stress Markers in Different Tissues of Diet-Induced Obese Mice. Nutrients 2022, 14, 2906. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Nolasco-Hipolito, C.; Aguilar-Uscanga, M.G.; Santiesteban, G.M.; Hayward-Jones, P.M.; Barradas-Dermitz, D.M. Effect of Dietary Intake of Avocado Oil and Olive Oil on Biochemical Markers of Liver Function in Sucrose-Fed Rats. BioMed Res. Int. 2014, 2014, 595479. [Google Scholar] [CrossRef]
- Ascenzi, F.; De Vitis, C.; Maugeri-Saccà, M.; Napoli, C.; Ciliberto, G.; Mancini, R. SCD1, autophagy and cancer: Implications for therapy. J. Exp. Clin. Cancer Res. 2021, 40, 265. [Google Scholar] [CrossRef]
- Gan, A.-M.; Tracz-Gaszewska, Z.; Ellert-Miklaszewska, A.; Navrulin, V.O.; Ntambi, J.M.; Dobrzyn, P. Stearoyl-CoA Desaturase Regulates Angiogenesis and Energy Metabolism in Ischemic Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 10459. [Google Scholar] [CrossRef]
- Oatman, N.; Dasgupta, N.; Arora, P.; Choi, K.; Gawali, M.V.; Gupta, N.; Parameswaran, S.; Salomone, J.; Reisz, J.A.; Lawler, S.; et al. Mechanisms of stearoyl CoA desaturase inhibitor sensitivity and acquired resistance in cancer. Sci. Adv. 2021, 7, eabd7459. [Google Scholar] [CrossRef]
- She, K.; Fang, S.; Du, W.; Fan, X.; He, J.; Pan, H.; Huang, L.; He, P.; Huang, J. SCD1 is required for EGFR-targeting cancer therapy of lung cancer via re-activation of EGFR/PI3K/AKT signals. Cancer Cell Int. 2019, 19, 103. [Google Scholar] [CrossRef]
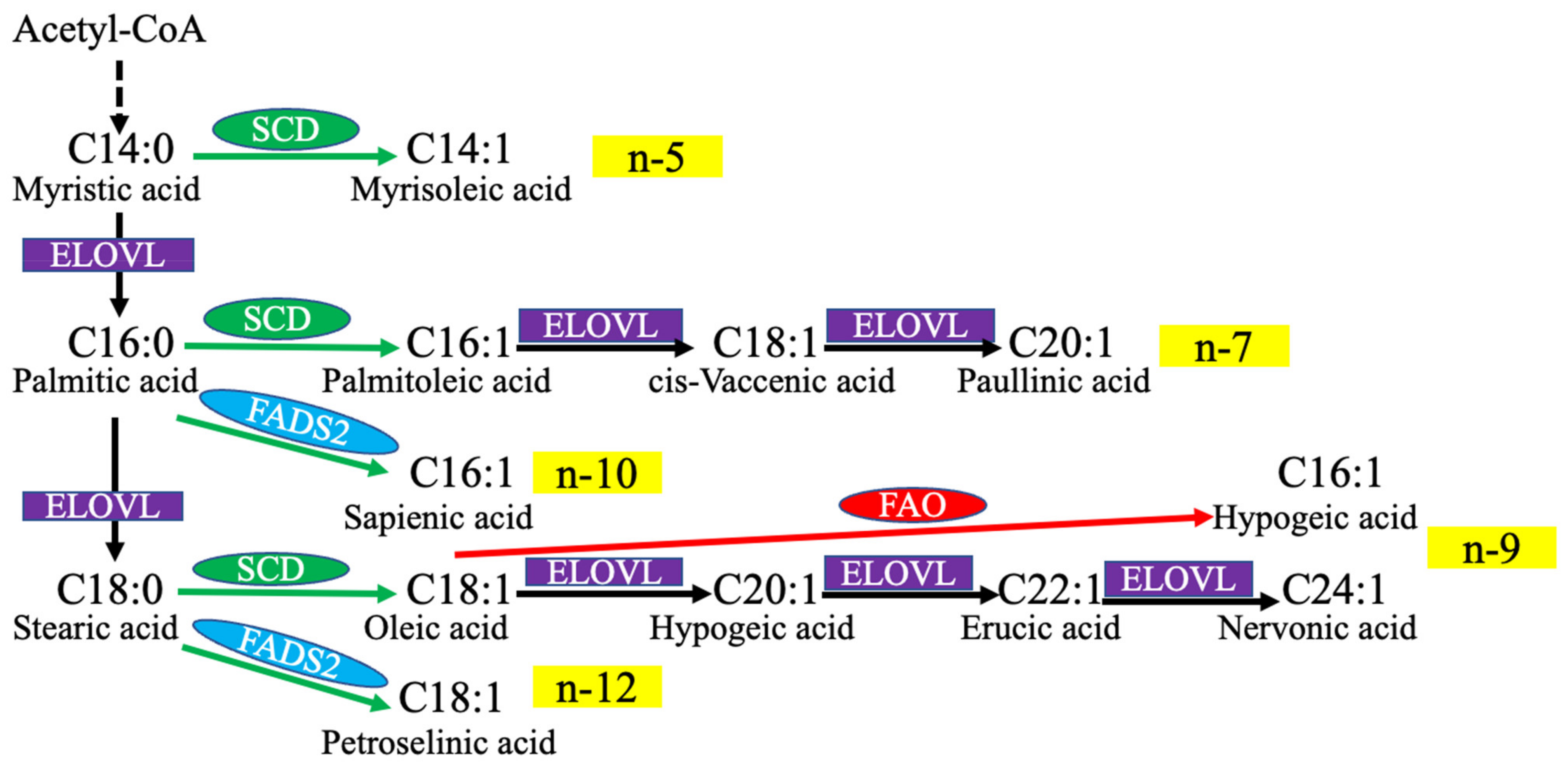
| MUFA | Source | Main Outcome | Reference | |
|---|---|---|---|---|
| Myristoleic acid | 14:1 (n-5), cis | Small amounts in nutmeg and nutmeg butter | Anti-cancer effects in prostate cancer cells | [58] |
| Palmitoleic acid | 16:1 (n-7), cis | Nuts, meats, animal fats | Related to cancer death and rescued SCD1 blockade anti-cancer effects | [32,59,60,61] |
| Hypogeic acid | 16:1 (n-9), cis | Human milk | Limited studies | [61,62] |
| Sapienic acid | 16:1 (n-10), cis | Human sebum | Increased in lung and liver carcinomas and contributed to SCD inhibition resistance | [57,61,63] |
| cis-Vaccenic acid | 18:1 (n-7), cis | Sea buckthorn oil | Inhibited colon cancer cell growth | [64] |
| Vaccenic acid | 18:1 (n-7), trans | Human milk, dairy products | Inhibited cancer cell growth and proliferation and induced apoptosis | [65,66] |
| Paullinic acid | 20:1 (n-7), cis | The seed oil of the plant Pangium edule | Limited studies | [67] |
| Oleic acid | 18:1 (n-9), cis | Vegetable oils, such as olive oil, rapeseed oil and sesame oil | Both cancer-promoting and anti-cancer effects | See Section 2.3 |
| Elaidic acid | 18:1 (n-9), trans | Small amounts in caprine, bovine milk and some meats | Promoted survival, growth, and invasion of the colorectal cancer cell lines | [65,66,68] |
| Petroselinic acid | 18:1 (n-12), cis | Several animal and vegetable fats and oils | Limited studies | [69] |
| Gondoic acid | 20:1 (n-9), cis | Plant oils and nuts, such as jojoba oil | Limited studies | [70,71] |
| Gadoleic acid | 20:1 (n-11), cis | Some fish oils, such as cod liver oil | Limited studies | [71,72] |
| Erucic acid | 22:1 (n-9), cis | Brassica seeds, Indian mustard, rapeseed | Anti-cancer activity in brain cancer and glioblastoma | [73,74,75] |
| Brassidic acid | 22:1 (n-9), trans | Seeds of certain brassica crops, such as mustard, rapeseed and kale | Limited studies | [72] |
| Nervonic acid | 24:1 (n-9), cis | Animal brain, plant seed oil | Limited studies | [71,76,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Bergeron, K.-F.; Lingrand, M.; Mounier, C. Unveiling the MUFA–Cancer Connection: Insights from Endogenous and Exogenous Perspectives. Int. J. Mol. Sci. 2023, 24, 9921. https://doi.org/10.3390/ijms24129921
Guo Z, Bergeron K-F, Lingrand M, Mounier C. Unveiling the MUFA–Cancer Connection: Insights from Endogenous and Exogenous Perspectives. International Journal of Molecular Sciences. 2023; 24(12):9921. https://doi.org/10.3390/ijms24129921
Chicago/Turabian StyleGuo, Zhiqiang, Karl-Frédérik Bergeron, Marine Lingrand, and Catherine Mounier. 2023. "Unveiling the MUFA–Cancer Connection: Insights from Endogenous and Exogenous Perspectives" International Journal of Molecular Sciences 24, no. 12: 9921. https://doi.org/10.3390/ijms24129921
APA StyleGuo, Z., Bergeron, K.-F., Lingrand, M., & Mounier, C. (2023). Unveiling the MUFA–Cancer Connection: Insights from Endogenous and Exogenous Perspectives. International Journal of Molecular Sciences, 24(12), 9921. https://doi.org/10.3390/ijms24129921







