Effect of Omega-3 Rich High-Fat Diet on Markers of Tissue Lipid Metabolism in Glucocorticoid-Treated Mice
Abstract
1. Introduction
2. Results
2.1. Body Weight and Body Composition
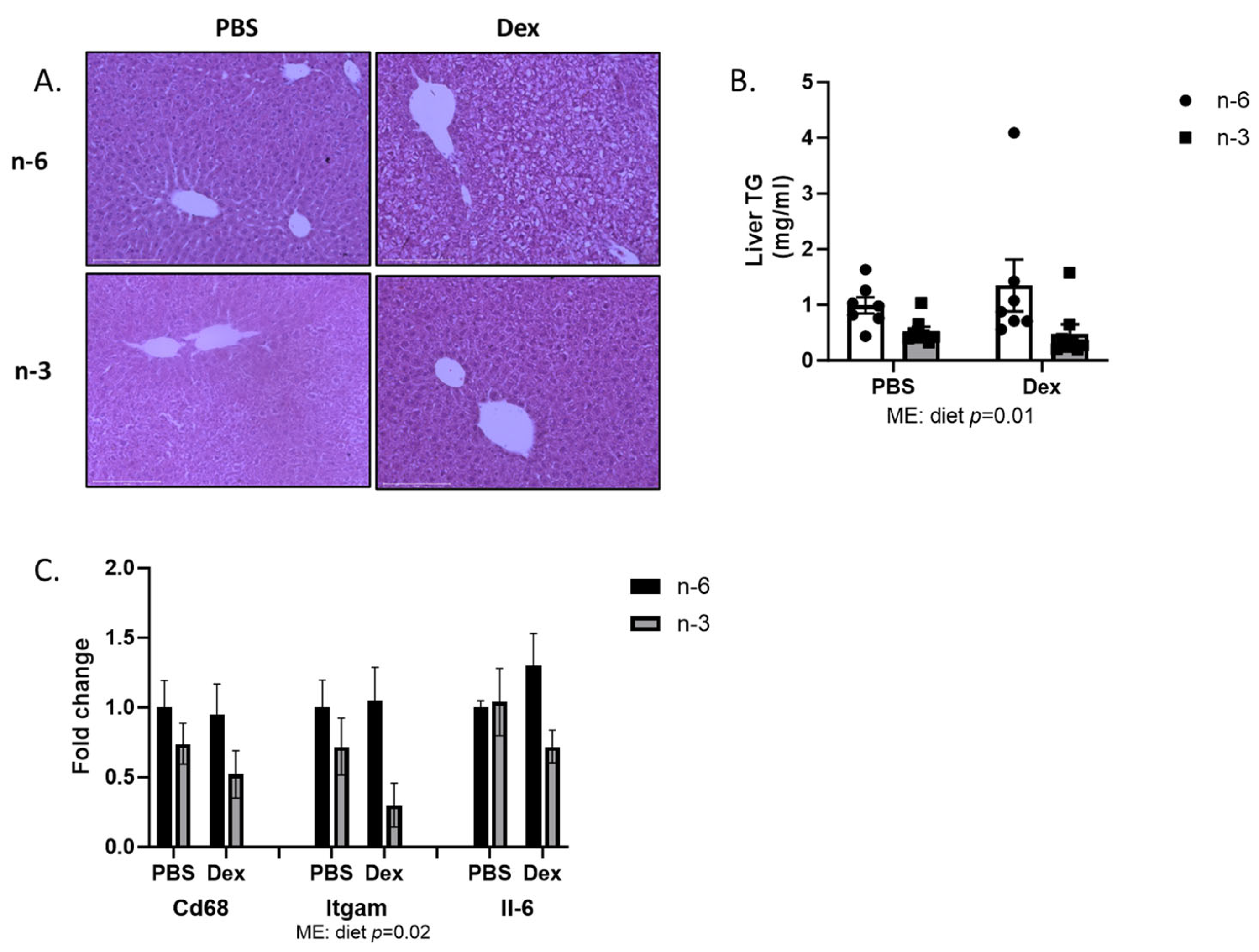
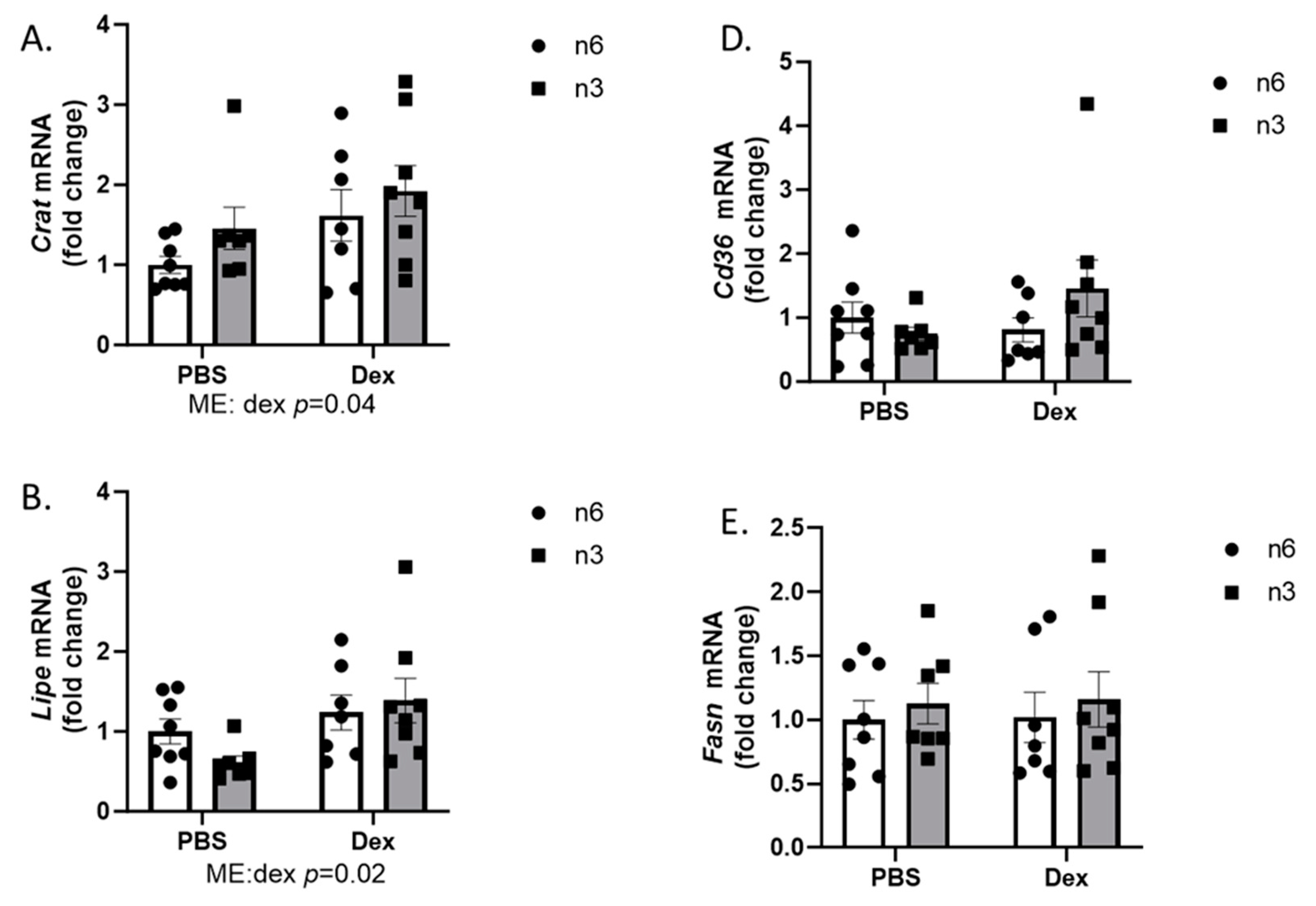
2.2. Liver Lipid Regulation
2.3. Muscle LIPID Regulation
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Non-Survival Surgery
4.3. Triglyceride Assay
4.4. RNA Isolation and qPCR
4.5. Histology
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, F. Obesity Epidemiology; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids (Commentary). J. Am. Diet. Assoc. 2002, 102, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Preuss, H.G. Phytopharmaceuticals in Cancer Chemoprevention; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Riccardi, G.; Giacco, R.; Rivellese, A.A. Dietary Fat, Insulin Sensitivity and the Metabolic Syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef]
- Braun, S.; Bitton-Worms, K.; LeRoith, D. The link between the metabolic syndrome and cancer. Int. J. Biol. Sci. 2011, 7, 1003–1015. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Trans fatty acids: Effects on metabolic syndrome, heart disease and diabetes. Nat. Rev. Endocrinol. 2009, 5, 335–344. [Google Scholar] [CrossRef]
- Bradbury, M.W. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: Possible role in steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G194–G198. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell. Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Montgomery, M.K.; De Nardo, W.; Watt, M.J. Impact of Lipotoxicity on Tissue “Cross Talk” and Metabolic Regulation. Physiology 2019, 34, 134–149. [Google Scholar] [CrossRef]
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose tissue: Between the extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef]
- Sidoroff, M.; Kolho, K.L. Glucocorticoids in pediatric inflammatory bowel disease. Scand. J. Gastroenterol. 2012, 47, 745–750. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef]
- García-Eguren, G.; Sala-Vila, A.; Giró, O.; Vega-Beyhart, A.; Hanzu, F.A. Long-term hypercortisolism induces lipogenesis promoting palmitic acid accumulation and inflammation in visceral adipose tissue compared with HFD-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E995–E1003. [Google Scholar] [CrossRef]
- D’Souza, A.M.; Beaudry, J.L.; Szigiato, A.A.; Trumble, S.J.; Snook, L.A.; Bonen, A.; Giacca, A.; Riddell, M.C. Consumption of a high-fat diet rapidly exacerbates the development of fatty liver disease that occurs with chronically elevated glucocorticoids. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G850–G863. [Google Scholar] [CrossRef]
- Robinson, L.E.; Buchholz, A.C.; Mazurak, V.C. Inflammation, obesity, and fatty acid metabolism: Influence of n-3 polyunsaturated fatty acids on factors contributing to metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 1008–1024. [Google Scholar] [CrossRef] [PubMed]
- Sakamuri, A.; Sakamuri, S.S.; Kona, S.R.; Jeyapal, S.; Ibrahim, A. Diets with low n-6:n-3 PUFA ratio protects rats from fructose-induced dyslipidemia and associated hepatic changes: Comparison between 18:3 n-3 and long-chain n-3 PUFA. Prostaglandins Leukot. Essent. Fatty Acids 2020, 155, 102082. [Google Scholar] [CrossRef]
- Hill, J.L.; Wyman, J.M.; Godwin, S.M.; Beech, L.A.; Buddington, R.K.; Sutter, T.R.; Ringwald-Smith, K.; van der Merwe, M. Dietary omega-3 fatty acids reduce adiposity and alter glucocorticoid-associated transcripts in epididymal white adipose tissue of C57BL/6 male mice raised on a high fat diet. J. Obes. Chronic Dis. 2020, 4, 13–22. [Google Scholar] [CrossRef]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef] [PubMed]
- Ragheb, R.; Shanab, G.M.; Medhat, A.M.; Seoudi, D.M.; Adeli, K.; Fantus, I. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: Evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem. Biophys. Res. Commun. 2009, 389, 211–216. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The effect of insulin on the disposal of intravenous glucose: Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cancer cachexia: Metabolic alterations and clinical manifestations. Nutrition 1997, 13, 1–7. [Google Scholar] [CrossRef]
- Perseghin, G. Muscle lipid metabolism in the metabolic syndrome. Curr. Opin. Lipidol. 2005, 16, 416–420. [Google Scholar] [CrossRef]
- Turcotte, L.P.; Fisher, J.S. Skeletal muscle insulin resistance: Roles of fatty acid metabolism and exercise. Phys. Ther. 2008, 88, 1279–1296. [Google Scholar] [CrossRef] [PubMed]
- da Cunha de Sá, R.D.C.; Cruz, M.M.; de Farias, T.M.; da Silva, V.S.; de Jesus Simão, J.; Telles, M.M.; Alonso-Vale, M.I.C. Fish oil reverses metabolic syndrome, adipocyte dysfunction, and altered adipokines secretion triggered by high-fat diet-induced obesity. Physiol. Rep. 2020, 8, e14380. [Google Scholar] [CrossRef]
- Han, H.; Qiu, F.; Zhao, H.; Tang, H.; Li, X.; Shi, D. Dietary Flaxseed Oil Prevents Western-Type Diet-Induced Nonalcoholic Fatty Liver Disease in Apolipoprotein-E Knockout Mice. Oxid. Med. Cell. Longev. 2017, 2017, 3256241. [Google Scholar] [CrossRef]
- Adamcova, K.; Horakova, O.; Bardova, K.; Janovska, P.; Brezinova, M.; Kuda, O.; Rossmeisl, M.; Kopecky, J. Reduced Number of Adipose Lineage and Endothelial Cells in Epididymal fat in Response to Omega-3 PUFA in Mice Fed High-Fat Diet. Mar. Drugs. 2018, 16, 515. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, I.J.; Wei, X.; Semenkovich, C.F. Lipoexpediency: De novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol. Metab. 2011, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Tandon, N.N.; Woldegiorgis, G.; Luiken, J.J.; Glatz, J.F.; Bonen, A. A novel function for fatty acid translocase (FAT)/CD36: Involvement in long chain fatty acid transfer into the mitochondria. J. Biol. Chem. 2004, 279, 36235–36241. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs—Mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015, 11, 592–605. [Google Scholar] [CrossRef]
- Miyata, Y.; Shimomura, I. Metabolic flexibility and carnitine flux: The role of carnitine acyltransferase in glucose homeostasis. J. Diabetes Investig. 2013, 4, 247–249. [Google Scholar] [CrossRef]
- Morgan, S.A.; Gathercole, L.L.; Simonet, C.; Hassan-Smith, Z.K.; Bujalska, I.; Guest, P.; Abrahams, L.; Smith, D.M.; Stewart, P.M.; Lavery, G.G.; et al. Regulation of lipid metabolism by glucocorticoids and 11beta-HSD1 in skeletal muscle. Endocrinology 2013, 154, 2374–2384. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.A.; Sherlock, M.; Gathercole, L.L.; Lavery, G.G.; Lenaghan, C.; Bujalska, I.J.; Laber, D.; Yu, A.; Convey, G.; Mayers, R.; et al. 11beta-hydroxysteroid dehydrogenase type 1 regulates glucocorticoid-induced insulin resistance in skeletal muscle. Diabetes 2009, 58, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Persinger, A.; Butawan, M.; Faietti, M.; Pryke, A.; Rose, K.; van der Merwe, M.; Bloomer, R.J.; Puppa, M.J. Effects of Feeding Time on Markers of Muscle Metabolic Flexibility Following Acute Aerobic Exercise in Trained Mice Undergoing Time Restricted Feeding. Nutrients 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]





| Ingredient | n-6 | n-3 |
|---|---|---|
| g | g | |
| Lard | 177.5 | |
| Menhaden Oil, ARBP-F | 177.5 | |
| Soybean Oil | 25 | 25 |
| Total | 202.5 | 202.5 |
| Saturated (%) | 31.6 | 31.5 |
| Monounsaturated (%) | 35.5 | 21.8 |
| Polyunsaturated (%) | 32.9 | 46.7 |
| n-6 (g) | 57.0 | 17.9 |
| n-3 (g) | 4.4 | 66.6 |
| n6/n3 ratio | 13.1 | 0.3 |
| Gene | Primers | |
|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | |
| Crat | CTGTGGGATGGTGTATGAGC | CTGAGGTTCTGTTTGGCTTTC |
| Lipe | CACAGACCTCTAAATCCCACG | ATATCCGCTCTCCAGTTGAAC |
| Fabp3 | GCTGGGAATAGAGTTCGACG | CTTCTCATAAGTCCGAGTGCTC |
| Cd36 | GATGTGCAAAACCCAGATGAC | ACAGTGAAGGCTCAAAGATGG |
| Fasn | GATGACAGGAGATGGAAGGC | GAGTGAGGCTGGGTTGATAC |
| Fabp1 | AGTCGTCAAGCTGGAAGGTGACAA | GACAATGTCGCCCAATGTCATGGT |
| Fabp2 | AAAGGAGCTGATTGCTGTCCGAGA | TCGCTTGGCCTCAACTCCTTCATA |
| Il-6 | GACAACTTTGGCATTGTGG | ATGCAGGGATGATGTTCTG |
| Itgam | TCCTGTACCACTCATTGTGG | GGGCAGCTTCATTCATCATG |
| CD68 | CTGCTGTGGAAATGCAAGCA | TGGTCACGGTTGCAAGAGAA |
| Lxr | CCGACAGAGCTTCGTCC | CCCACAGACACTGCACAG |
| Cpt1a | GCTGGAGGTGGCTTTGGT | GCTTGGCGGATGTGGTTC |
| Slc27a2 | ACACACCGCAGAAACCAAATGACC | TGCCTTCAGTGGATGCGTAGAACT |
| Slc27a5 | TGTAACGTCCCTGAGCAACCAGAA | ATTCCCAGATCCGAATGGGACCAA |
| Rplp0 | GCTTCATTGTGGGAGCAGACA | CATGGTGTTCTTGCCCATCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, W.; Brown, K.; Persinger, A.; Pryke, A.; Lin, J.; Powell, Z.; Wallace, N.; van der Merwe, M.; Puppa, M. Effect of Omega-3 Rich High-Fat Diet on Markers of Tissue Lipid Metabolism in Glucocorticoid-Treated Mice. Int. J. Mol. Sci. 2023, 24, 11492. https://doi.org/10.3390/ijms241411492
Son W, Brown K, Persinger A, Pryke A, Lin J, Powell Z, Wallace N, van der Merwe M, Puppa M. Effect of Omega-3 Rich High-Fat Diet on Markers of Tissue Lipid Metabolism in Glucocorticoid-Treated Mice. International Journal of Molecular Sciences. 2023; 24(14):11492. https://doi.org/10.3390/ijms241411492
Chicago/Turabian StyleSon, Wangkuk, Katie Brown, Aaron Persinger, Ashley Pryke, Jason Lin, Zereque Powell, Noah Wallace, Marie van der Merwe, and Melissa Puppa. 2023. "Effect of Omega-3 Rich High-Fat Diet on Markers of Tissue Lipid Metabolism in Glucocorticoid-Treated Mice" International Journal of Molecular Sciences 24, no. 14: 11492. https://doi.org/10.3390/ijms241411492
APA StyleSon, W., Brown, K., Persinger, A., Pryke, A., Lin, J., Powell, Z., Wallace, N., van der Merwe, M., & Puppa, M. (2023). Effect of Omega-3 Rich High-Fat Diet on Markers of Tissue Lipid Metabolism in Glucocorticoid-Treated Mice. International Journal of Molecular Sciences, 24(14), 11492. https://doi.org/10.3390/ijms241411492






