Induction of the Erythroid Differentiation of K562 Cells Is Coupled with Changes in the Inter-Chromosomal Contacts of rDNA Clusters
Abstract
1. Introduction
2. Results
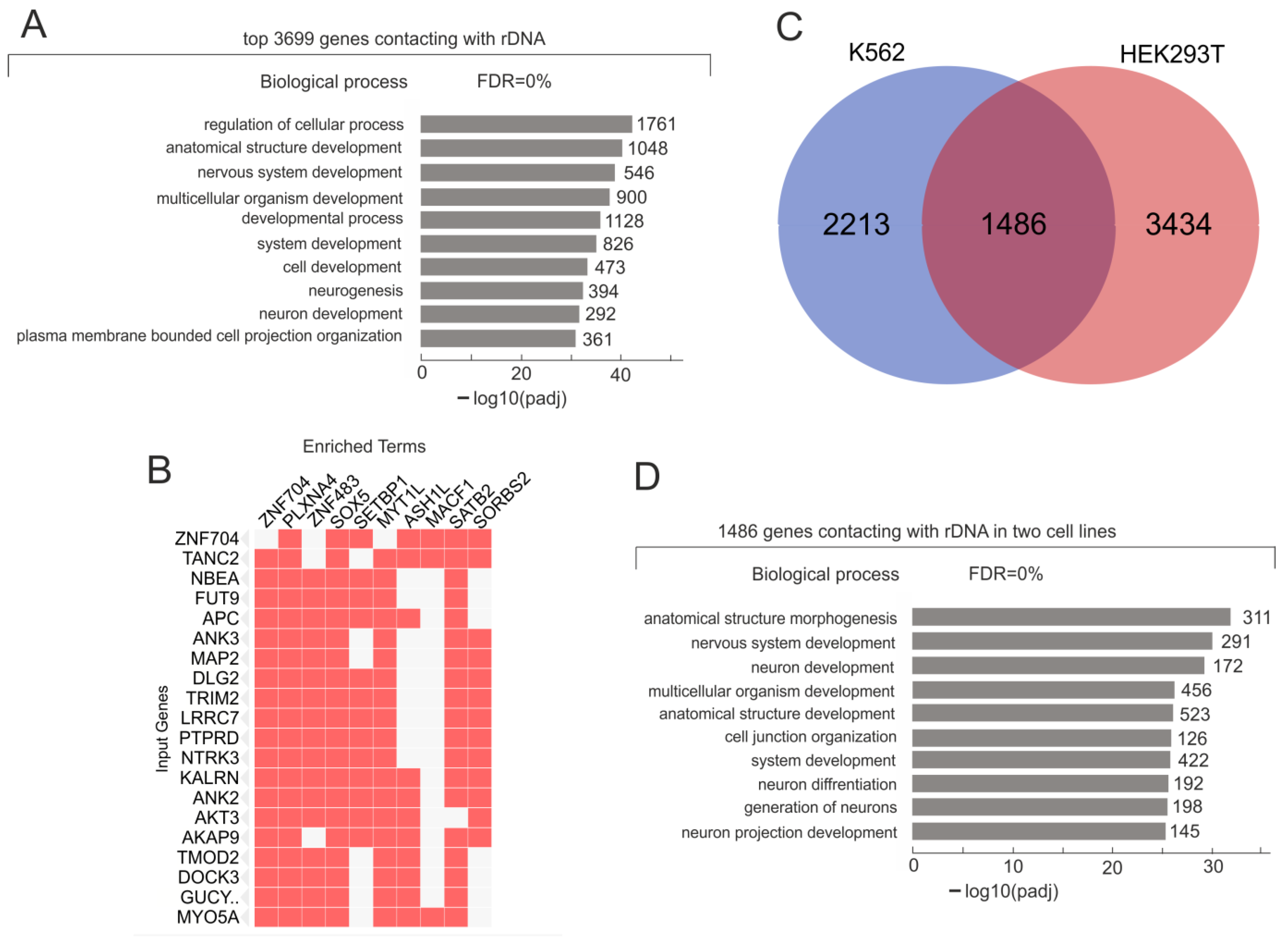
2.1. rDNA Clusters Contact with the Numerous Genes in Initial K562 Cells
2.2. Induced Differentiation of K562 Cells Leads to Essential Changes in the Pattern of rDNA Inter-Chromosomal Contacts
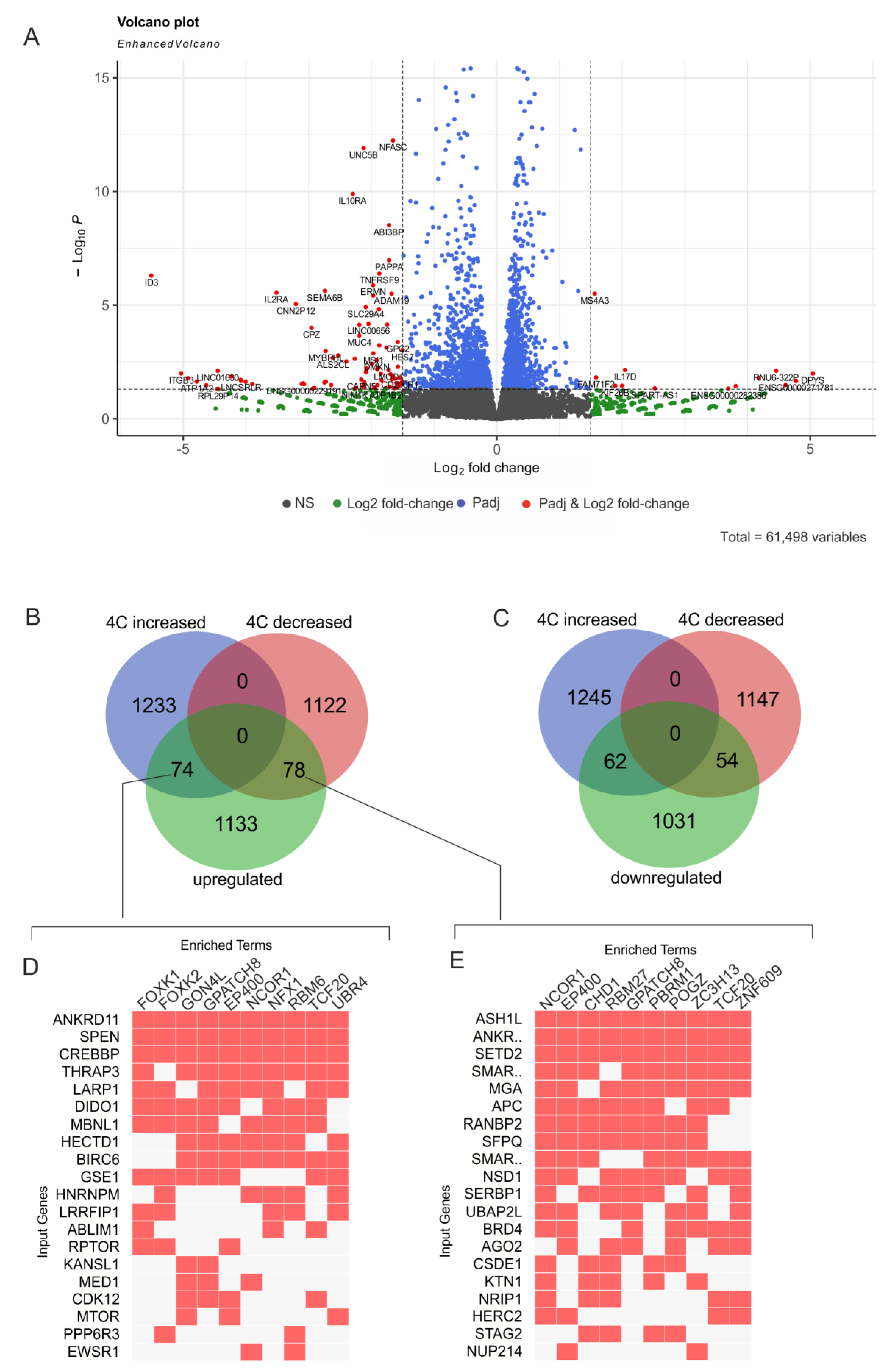
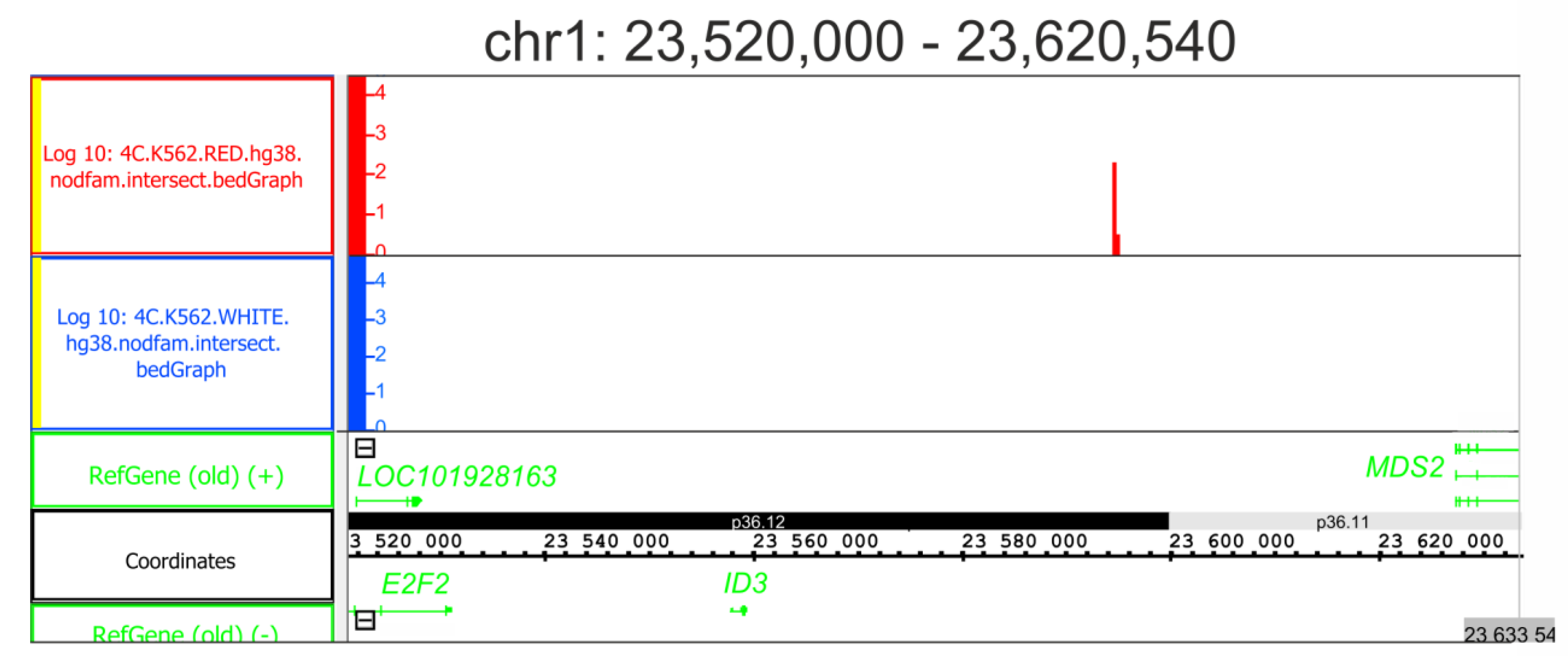
2.3. Induced Differentiation of K562 Cells Leads to Extreme Downregulation of the ID3 Gene
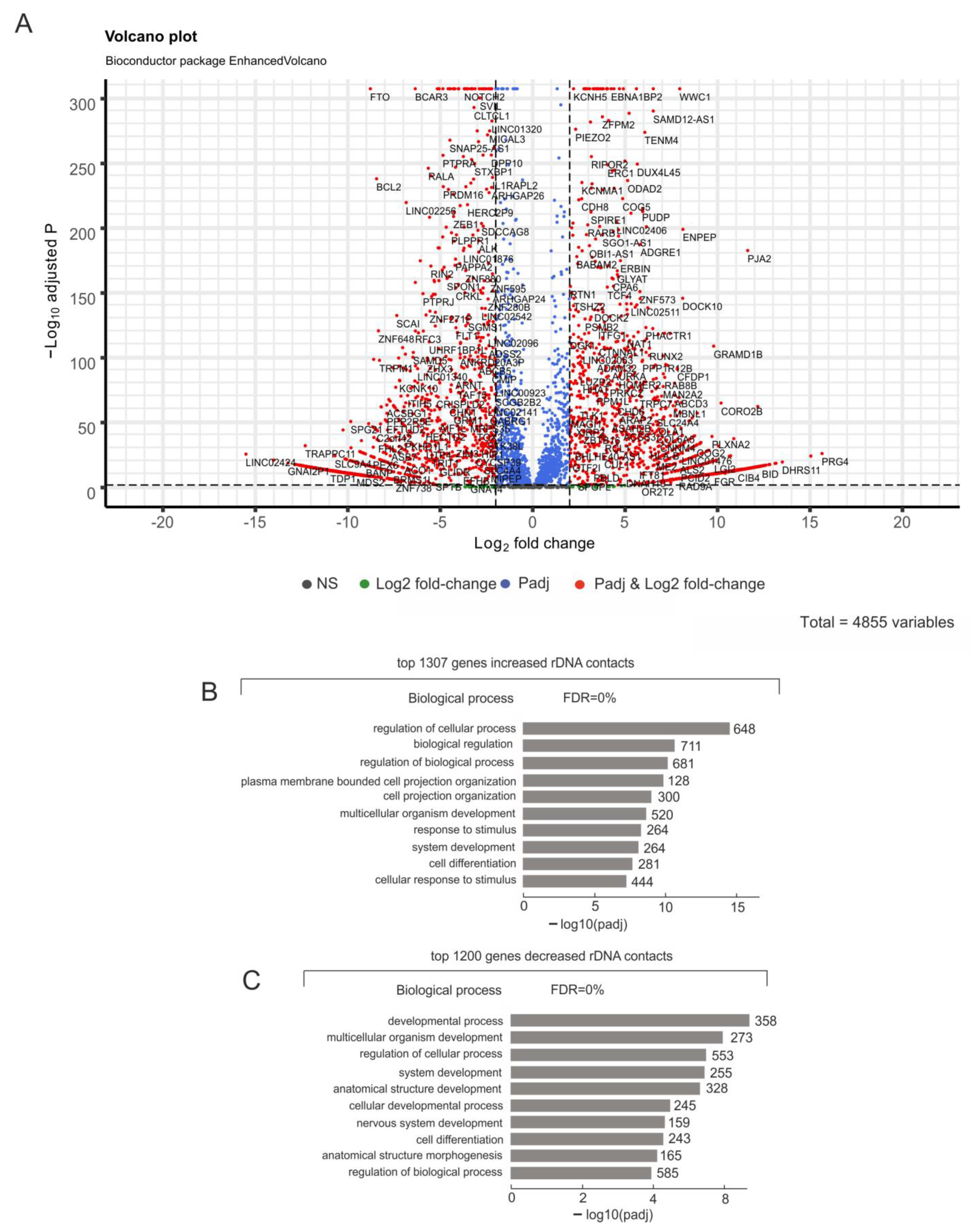
2.4. Hemin-Induced Differentiation Leads to the Up- or Downregulation of Thousands of Genes, Including More Than 200 rDNA-Contacting Genes
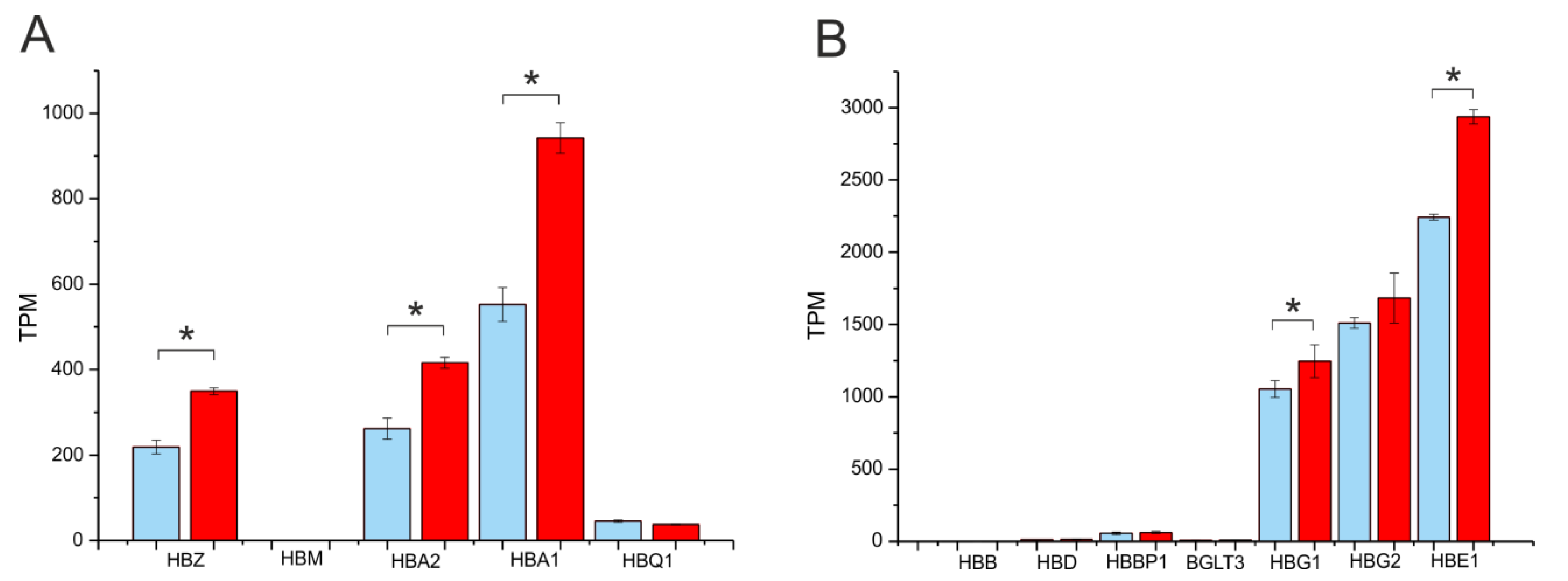
2.5. Differentiation Is Coupled with Changes in rDNA Contacts in Areas Located around Globin-Gene Clusters
2.6. rDNA Contact Appearance at the Region of the ID3 Gene Is Coupled with Its Strong Repression
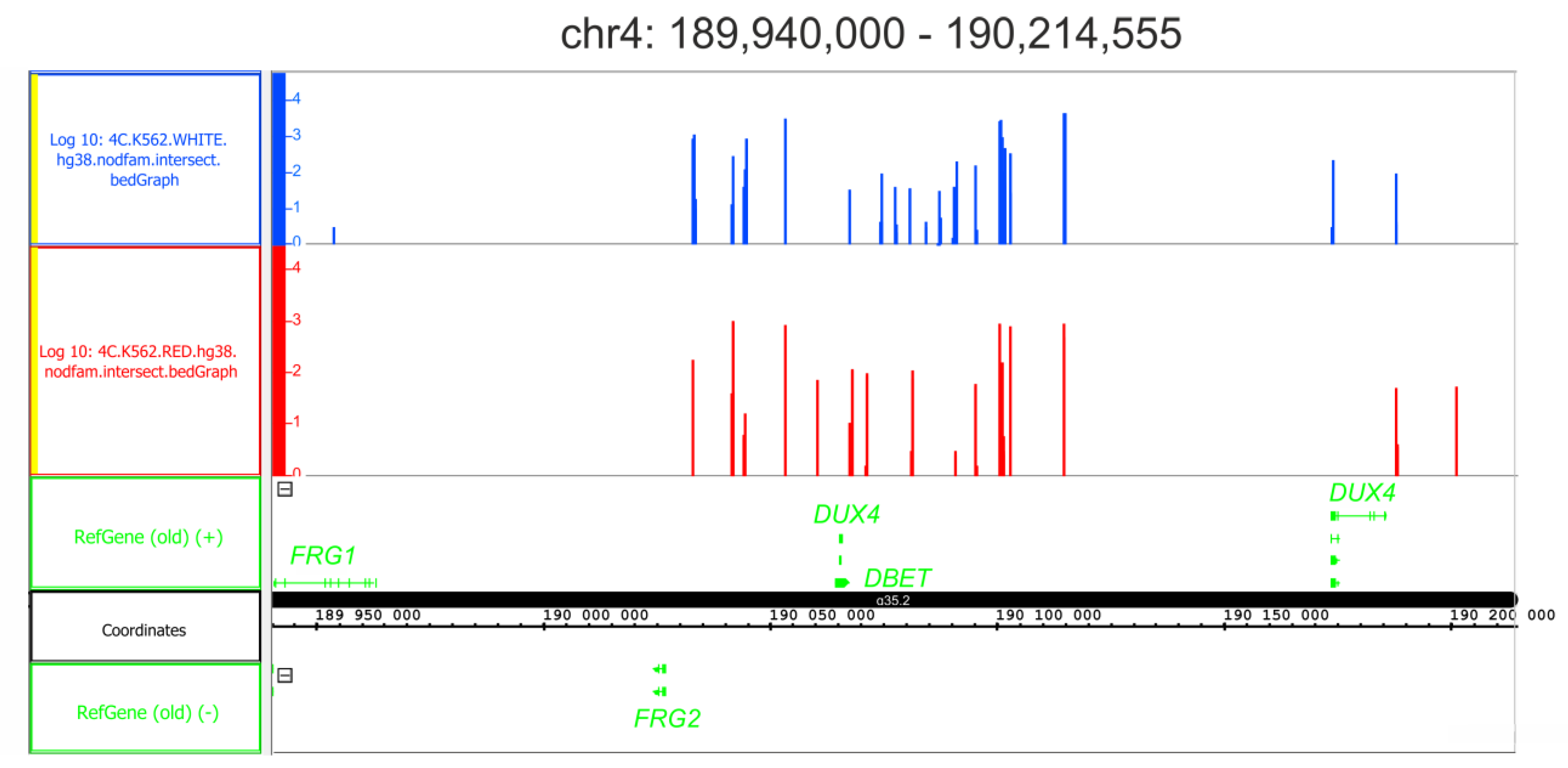
2.7. Differentiation Does Not Affect Either the Frequency of rDNA Contacts or the Expression of DUX4 Genes
3. Discussion
3.1. How Does Hemin Affect rDNA Inter-Chromosomal Contacts?
3.2. rDNA Inter-Chromosomal Contacts and Mechanisms of Gene Expression Regulation
4. Materials and Methods
4.1. Cell Culture Growth and the Induction of Differentiation by Hemin
4.2. 4C-rDNA Procedure
4.3. Mapping and Processing of 4C-rDNA 4C Data
- 4C full-length adapters (both direct and reverse complement (RC)):
- A1D
- TTCACTTCTGACATCCCAGATTTGATCTCCCTACAGAATGCTGTACAGAACTGGCGAGTTGATTTCTGGACTT,
- A1RC
- AAGTCCAGAAATCAACTCGCCAGTTCTGTACAGCATTCTGTAGGGAGATCAAATCTGGGATGTCAGAAGTGAA,
- A2D
- TCTTTGAAAAAAATCCCAGAAGTGGTTTTGGCTTTTTGGCTAGGAGGCCTAAGCCTGCTGAGAACTTTCCTGCCCAGGATCCT,
- A2RC
- AGGATCCTGGGCAGGAAAGTTCTCAGCAGGCTTAGGCCTCCTAGCCAAAAAGCCAAAACCACTTCTGGGATTTTTTTCAAAGA
were removed at 5′ ends by the options -O 10 (minimal overlap adapter with the read) --trim-n (omit N’s at the ends of reads) --times = 4 (search for the adapter up to 4 times in the read consequently) --minimum-length = 20 (minimum acceptable read length after trimming) -q 24 (minimal acceptable quality). All untrimmed reads were collected in a separate file. - Illumina 3′ adapter arrays from AGATCGGAAGAGC to AGATCGGAA-GAGCNNNNNNNNNN and from GATCGGAAGAGC to GATCGGAA-GAGCNNNNNNNNNN anchored to 3′ ends of reads were removed by cutadapt with the following options: -O 10 (minimal overlap adapter with the read) --times = 4 (search for the adapter up to 4 times in the read consequently) --minimum-length = 20 (minimum acceptable read length after trimming) -q 24 (minimal acceptable quality).
- Incomplete from 5′ 4C full-length adapter arrays: from TTCACTTCTGACATCCCAGATTTGATCTCCCTACAGAATGCTGTACAGAACTGGCGAGTTGATTTCTGGACTT to TTCACTTCTGACATCCCAGA (minimal length = 20) and from TCTTTGAAAAAAATCCCAGAAGTGGTTTTGGCTTTTTGGCTAGGAGGCCTAAGCCTGCTGAGAACTTTCCTGCCCAGGATCCT to TCTTTGAAAAAAATCCCAGA (minimal length = 20) both direct and reverse complement reads, anchored to 5′ ends of reads were removed by cutadapt with the same options as mentioned above.
- Illumina adapter GATCGGAAGAGC and IlluminaPE adapter AGATCGGAA-GAGC were removed by cutadapt from 3′ ends of reads with the following options: -O 5 (minimal overlap adapter with the read) --times = 4 --minimum-length = 20 (minimum acceptable read length after trimming) -q 24 (minimal acceptable quality)
- Incomplete from 3′ 4C full-length adapter arrays: from TTCACTTCTGACATCCCAGATTTGATCTCCCTACAGAATGCTGTACAGAACTGGCGAGTTGATTTCTGGACTT to GCGAGTTGATTTCTGGACTT (minimal length = 20) and from TCTTTGAAAAAAATCCCAGAAGTGGTTTTGGCTTTTTGGCTAGGAGGCCTAAGCCTGCTGAGAACTTTCCTGCCCAGGATCCT to ACTTTCCTGCCCAGGATCCT (minimal length = 20) both direct and reverse complement reads were removed by cutadapt from the 3′ ends of reads with the same options as in points 2 and 3 of this protocol.
- All untrimmed reads collected during step 1 of the described procedure (i.e., the reads without at least a 10-nucleotides overlap of the adapter and read) were trimmed again by the previously described procedure (points 1–5) with the only difference: in the first step, the changed set of cudatapt options was applied: -O 15 (instead of -O 10, thus requiring additional adapter nucleotides to overlap with the read); -e 0.2 (this option fixed the error rate at 0.2, thus enabling us to find adapters that were read with a reduced quality).
4.4. 4C-rDNA-Associated Gene Quantifications and Differential 4C Analysis
4.5. RNA-Seq Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.; Santoro, R. Regulation and Roles of the Nucleolus in Embryonic Stem Cells: From Ribosome Biogenesis to Genome Organization. Stem Cell Rep. 2020, 15, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Salmina, K.; Huna, A.; Inashkina, I.; Belyayev, A.; Krigerts, J.; Pastova, L.; Vazquez-Martin, A.; Erenpreisa, J. Nucleolar aggresomes mediate release of pericentric heterochromatin and nuclear destruction of genotoxically treated cancer cells. Nucleus 2017, 8, 205–221. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, M.; van Vuuren, C.; Mangan, H.; McStay, B. NORs on human acrocentric chromosome p-arms are active by default and can associate with nucleoli independently of rDNA. Proc. Natl. Acad. Sci. USA 2020, 117, 10368–10377. [Google Scholar] [CrossRef]
- Yang, K.; Yang, J.; Yi, J. Nucleolar Stress: Hallmarks, sensing mechanism and diseases. Cell Stress 2018, 2, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lemos, B. Ribosomal DNA harbors an evolutionarily conserved clock of biological aging. Genome Res. 2019, 29, 325–333. [Google Scholar] [CrossRef]
- Feinberg, A.P. The Nucleolus Gets the Silent Treatment. Cell Stem Cell 2014, 15, 675–676. [Google Scholar] [CrossRef]
- Savic, N.; Bär, D.; Leone, S.; Frommel, S.C.; Weber, F.A.; Vollenweider, E.; Ferrari, E.; Ziegler, U.; Kaech, A.; Shakhova, O.; et al. lncRNA maturation to initiate heterochromatin formation in the nucleolus is required for exit from pluripotency in ESCs. Cell Stem Cell 2014, 15, 720–734. [Google Scholar] [CrossRef]
- Ananiev, E.V.; Barsky, V.E.; Ilyin, Y.V.; Churikov, N.A. Localization of nucleoli in Drosophila melanogaster polytene chromosomes. Chromosoma 1981, 81, 619–628. [Google Scholar] [CrossRef]
- Tchurikov, N.A.; Fedoseeva, D.M.; Klushevskaya, E.S.; Slovohotov, I.Y.; Chechetkin, V.R.; Kravatsky, Y.V.; Kretova, O.V. rDNA Clusters Make Contact with Genes that Are Involved in Differentiation and Cancer and Change Contacts after Heat Shock Treatment. Cells 2019, 8, 1393. [Google Scholar] [CrossRef]
- Tchurikov, N.A.; Klushevskaya, E.S.; Fedoseeva, D.M.; Alembekov, I.R.; Kravatskaya, G.I.; Chechetkin, V.R.; Kravatsky, Y.V.; Kretova, O.V. Dynamics of Whole-Genome Contacts of Nucleoli in Drosophila Cells Suggests a Role for rDNA Genes in Global Epigenetic Regulation. Cells 2020, 9, 2587. [Google Scholar] [CrossRef]
- Diesch, J.; Bywater, M.J.; Sanij, E.; Cameron, D.P.; Schierding, W.; Brajanovski, N.; Poortinga, G. Changes in long-range rDNA-genomic interactions associate with altered RNA polymerase II gene programs during malignant transformation. Commun. Biol. 2019, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Tchurikov, N.A.; Fedoseeva, D.M.; Sosin, D.V.; Snezhkina, A.V.; Melnikova, N.V.; Kudryavtseva, A.V.; Kravatsky, Y.V.; Kretova, O.V. Hot spots of DNA double-strand breaks and genomic contacts of human rDNA units are involved in epigenetic regulation. J. Mol. Cell Biol. 2015, 7, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Abraham, B.J.; Lee, T.I.; Lau, A.; Saint-André, V.; Sigova, A.A.; Hoke, H.A.; Young, R.A. Super-enhancers in the control of cell identity and disease. Cell 2013, 155, 934–947. [Google Scholar] [CrossRef]
- Kretova, O.V.; Fedoseeva, D.M.; Kravatsky, Y.V.; Klushevskaya, E.S.; Alembekov, I.R.; Slovohotov, I.Y.; Tchurikov, N.A. Contact Sites of rDNA Clusters with FANK1 Gene Correspond to Repressed Chromatin. Mol. Biol. 2020, 54, 262–266. [Google Scholar] [CrossRef]
- Yu, S.; Lemos, B. The long-range interaction map of ribosomal DNA arrays. PLoS Genet. 2018, 14, e1007258. [Google Scholar] [CrossRef]
- Tchurikov, N.A.; Alembekov, I.R.; Klushevskaya, E.S.; Kretova, A.N.; Keremet, A.M.; Sidorova, A.E.; Meilakh, P.B.; Chechetkin, V.R.; Kravatskaya, G.I.; Kravatsky, Y.V. Genes Possessing the Most Frequent DNA DSBs Are Highly Associated with Development and Cancers, and Essentially Overlap with the rDNA-Contacting Genes. Int. J. Mol. Sci. 2022, 23, 7201. [Google Scholar] [CrossRef]
- Tchurikov, N.A.; Kravatsky, Y.V. The Role of rDNA Clusters in Global Epigenetic Gene Regulation. Front. Genet. 2021, 12, 730633. [Google Scholar] [CrossRef]
- Picart-Picolo, A.; Picault, N.; Pontvianne, F. Ribosomal RNA genes shape chromatin domains associating with the nucleolus. Nucleus 2019, 10, 67–72. [Google Scholar] [CrossRef]
- Bu, S.; Lv, Y.; Liu, Y.; Qiao, S.; Wang, H. Zinc Finger Proteins in Neuro-Related Diseases Progression. Front. Neurosci. 2021, 15, 760567. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Lozzio, C.B.; Lozzio, B.B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 1975, 45, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Okabe-Kado, J.; Honma, Y.; Hayashi, M.; Hozumi, M.; Sampi, K.; Sakurai, M.; Hino, K.; Tsuruoka, N. Induction of differentiation of mouse myeloid leukemia M1 cells by serum of patients with chronic myeloid leukemia. Jpn. J. Cancer Res. 1988, 79, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, A.; Torre, D.; Keenan, A.B.; Jagodnik, K.M.; Lee, H.J.; Wang, L.; Silverstein, M.C.; Ma’ayan, A. Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002, 16, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Filiano, A.J.; Wiltbank, A.T.; Yogev, N.; Kipnis, J. Myeloid Cells in the Central Nervous System. Immunity 2017, 46, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Jögi, A.; Vaapil, M.; Johansson, M.; Påhlman, S. Cancer cell differentiation heterogeneity and aggressive behavior in solid tumors. Upsala J. Med. Sci. 2012, 117, 217–224. [Google Scholar] [CrossRef]
- Peddada, S.; Yasuil, D.H.; LaSalle, J.M. Inhibitors of differentiation (ID1, ID2, ID3 and ID4) genes are neuronal targets of MeCP2 that are elevated in Rett syndrome. Hum. Mol. Genet. 2006, 15, 2003–2014. [Google Scholar] [CrossRef]
- Tchurikov, N.A.; Klushevskaya, E.S.; Kravatsky, Y.V.; Kravatskaya, G.I.; Fedoseeva, D.M.; Kretova, O.V. Interchromosomal Contacts of rDNA Clusters with DUX Genes in Human Chromosome 4 Are Very Sensitive to Heat Shock Treatment. Dokl. Biochem. Biophys. 2020, 490, 50–53. [Google Scholar] [CrossRef]
- Yachie, A. Heme Oxygenase-1 Deficiency and Oxidative Stress: A Review of 9 Independent Human Cases and Animal Models by Division of Medical Safety, Kanazawa University Hospital, Kanazawa 920-8641, Japan. Int. J. Mol. Sci. 2021, 22, 1514. [Google Scholar] [CrossRef]
- Jang, H.Y.; Hong, O.Y.; Chung, E.Y.; Park, K.H.; Kim, J.S. Roles of JNK/Nrf2 Pathway on Hemin-Induced Heme Oxygenase-1 Activation in MCF-7 Human Breast Cancer Cells. Medicina 2020, 56, 268. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, M.; McStay, B. Nucleolar DNA double-strand break responses underpinning rDNA genomic stability. Trends Genet. 2019, 35, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Jones, S. An overview of the basic helix-loop-helix proteins. Genome Biol. 2004, 5, 226. [Google Scholar] [CrossRef] [PubMed]
- Tchurikov, N.A.; Kretova, O.V.; Fedoseeva, D.M.; Sosin, D.V.; Grachev, S.A.; Serebraykova, M.V.; Romanenko, S.A.; Vorobieva, N.V.; Kravatsky, Y.V. DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes. PLoS Genet. 2013, 9, e1003429. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175, 1842–1855. [Google Scholar] [CrossRef]
- Bystricky, K.; Heun, P.; Gehlen, L.; Langowski, J.; Gasser, S.M. Long-range compaction and flexibility of interphase chromatin in budding yeast analyzed by high-resolution imaging techniques. Proc. Natl. Acad. Sci. USA 2004, 101, 16495–16500. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator Condensation at Super-Enhancers Links Phase Separation and Gene Control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Wang, D.; Si, S.; Wang, Q.; Luo, G.; Du, Q.; Liang, Q.; Guo, X.; Zhang, G.; Feng, J.; Leng, Z. MiR-27a Promotes Hemin-Induced Erythroid Differentiation of K562 Cells by Targeting CDC25B. Cell. Physiol. Biochem. 2018, 46, 365–374. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bushnell, B. BBTools: A Suite of Fast, Multithreaded Bioinformatics Tools Designed for Analysis of DNA and RNA Sequence Data. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 4 June 2023).
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.; Ryan, D.P.; Gruning, B.; Bhardwaj, V.; Kilpert, F.; Richter, A.S.; Heyne, S.; Dundar, F.; Manke, T. deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016, 44, W160–W165. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Neph, S.; Kuehn, M.S.; Reynolds, A.P.; Haugen, E.; Thurman, R.E.; Johnson, A.K.; Rynes, E.; Maurano, M.T.; Vierstra, J.; Thomas, S.; et al. BEDOPS: High-performance genomic feature operations. Bioinformatics 2012, 28, 1919–1920. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Blighe, K.; Lewis, M.; Rana, S. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 4 June 2023).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchurikov, N.A.; Klushevskaya, E.S.; Alembekov, I.R.; Kretova, A.N.; Chechetkin, V.R.; Kravatskaya, G.I.; Kravatsky, Y.V. Induction of the Erythroid Differentiation of K562 Cells Is Coupled with Changes in the Inter-Chromosomal Contacts of rDNA Clusters. Int. J. Mol. Sci. 2023, 24, 9842. https://doi.org/10.3390/ijms24129842
Tchurikov NA, Klushevskaya ES, Alembekov IR, Kretova AN, Chechetkin VR, Kravatskaya GI, Kravatsky YV. Induction of the Erythroid Differentiation of K562 Cells Is Coupled with Changes in the Inter-Chromosomal Contacts of rDNA Clusters. International Journal of Molecular Sciences. 2023; 24(12):9842. https://doi.org/10.3390/ijms24129842
Chicago/Turabian StyleTchurikov, Nickolai A., Elena S. Klushevskaya, Ildar R. Alembekov, Antonina N. Kretova, Vladimir R. Chechetkin, Galina I. Kravatskaya, and Yuri V. Kravatsky. 2023. "Induction of the Erythroid Differentiation of K562 Cells Is Coupled with Changes in the Inter-Chromosomal Contacts of rDNA Clusters" International Journal of Molecular Sciences 24, no. 12: 9842. https://doi.org/10.3390/ijms24129842
APA StyleTchurikov, N. A., Klushevskaya, E. S., Alembekov, I. R., Kretova, A. N., Chechetkin, V. R., Kravatskaya, G. I., & Kravatsky, Y. V. (2023). Induction of the Erythroid Differentiation of K562 Cells Is Coupled with Changes in the Inter-Chromosomal Contacts of rDNA Clusters. International Journal of Molecular Sciences, 24(12), 9842. https://doi.org/10.3390/ijms24129842





