Identification and Functional Characterization of ZmSCYL2 Involved in Phytosterol Accumulation in Plants
Abstract
1. Introduction
2. Results
2.1. Variation in Phytosterol Content among the Maize Inbred Lines
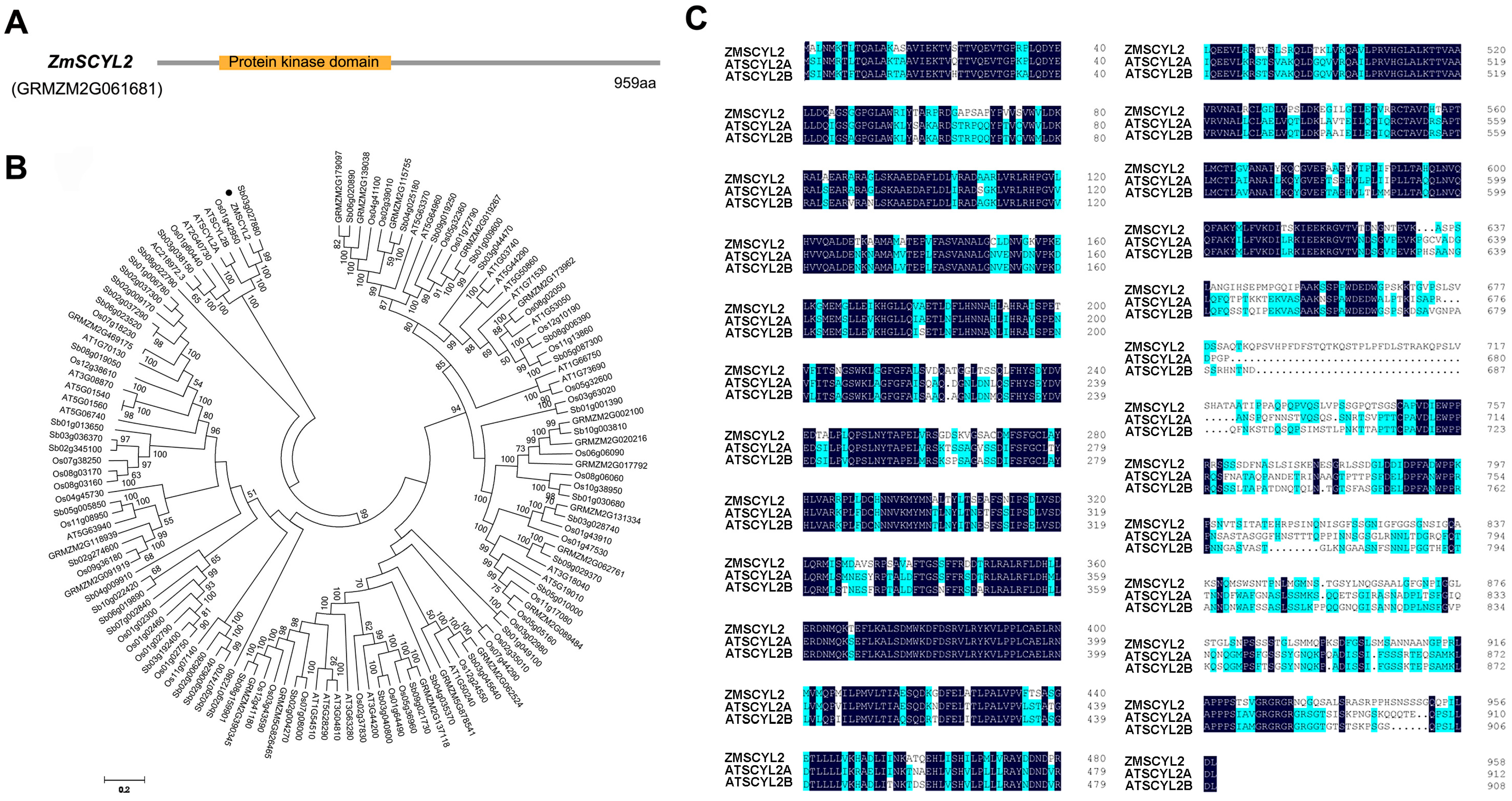
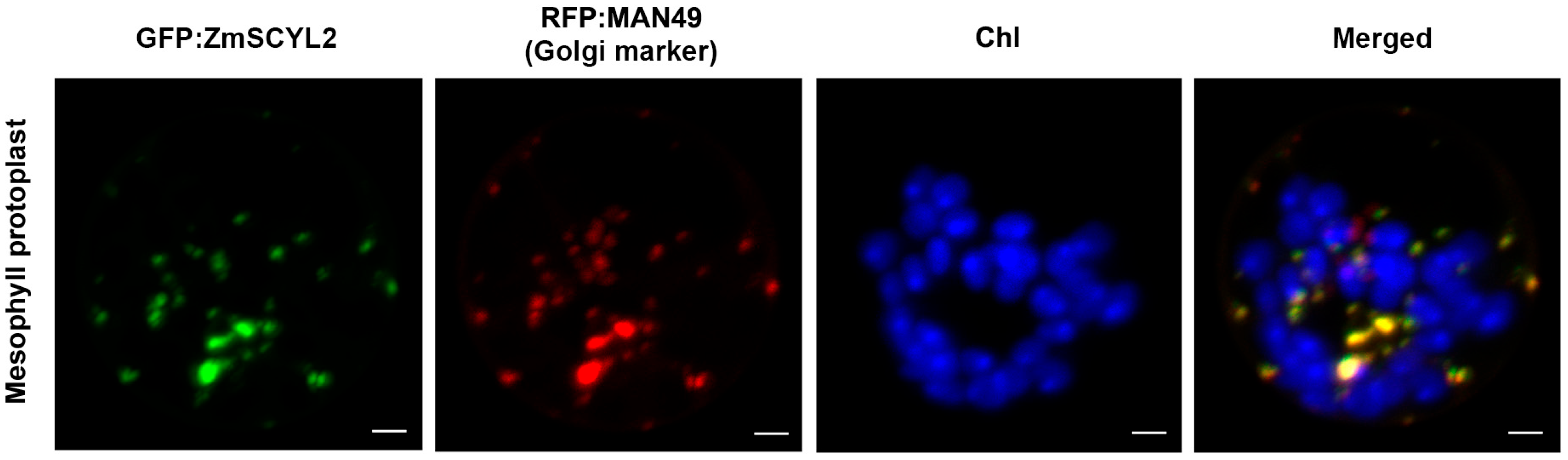
2.2. Gene Cloning and Bioinformatics Analysis of ZmSCYL2
2.3. Gene Cloning and Bioinformatics Analysis of ZmSCYL2
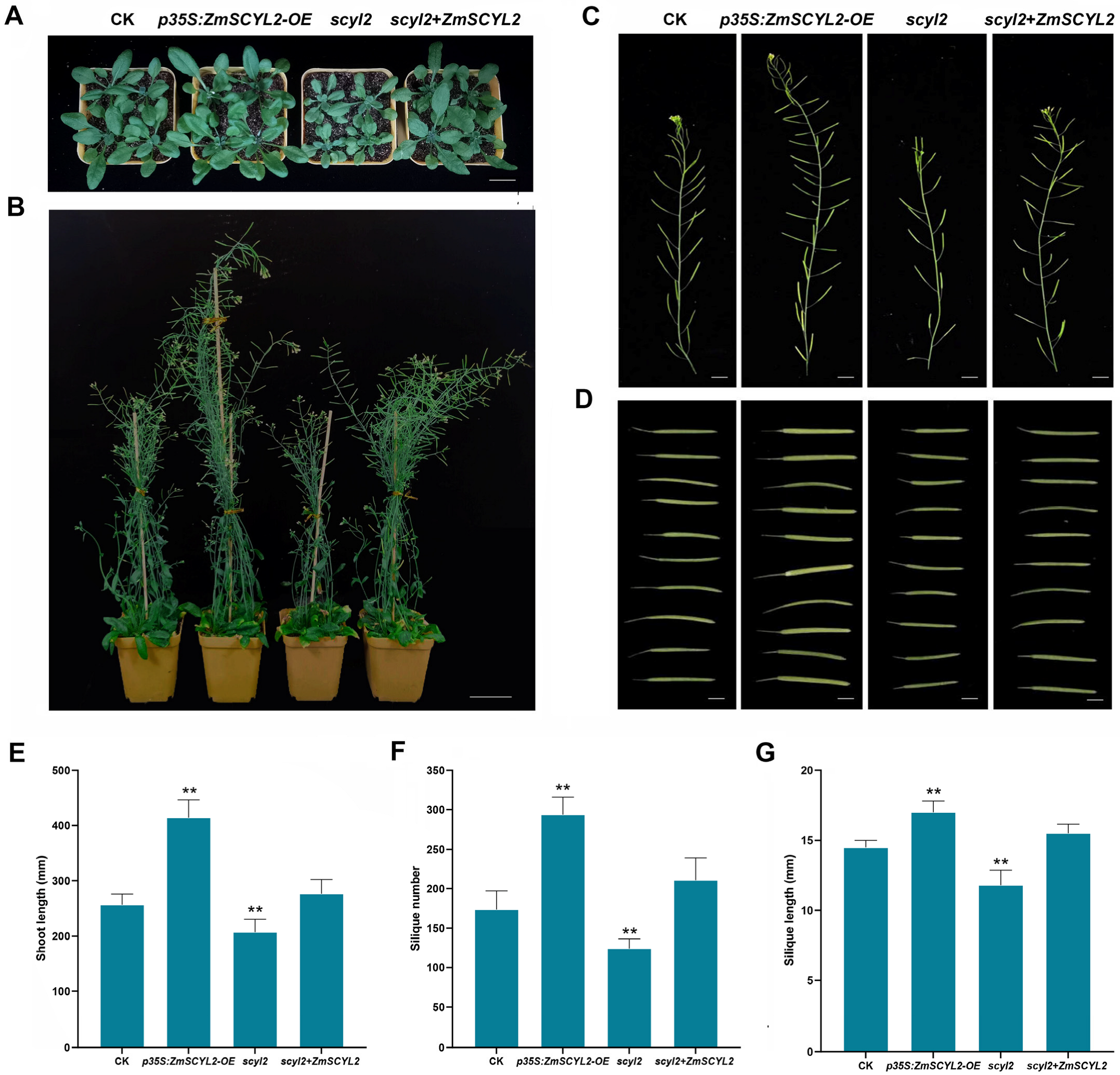
2.4. Expression of ZmSCYL2 Affects Phytosterol Content in Arabidopsis
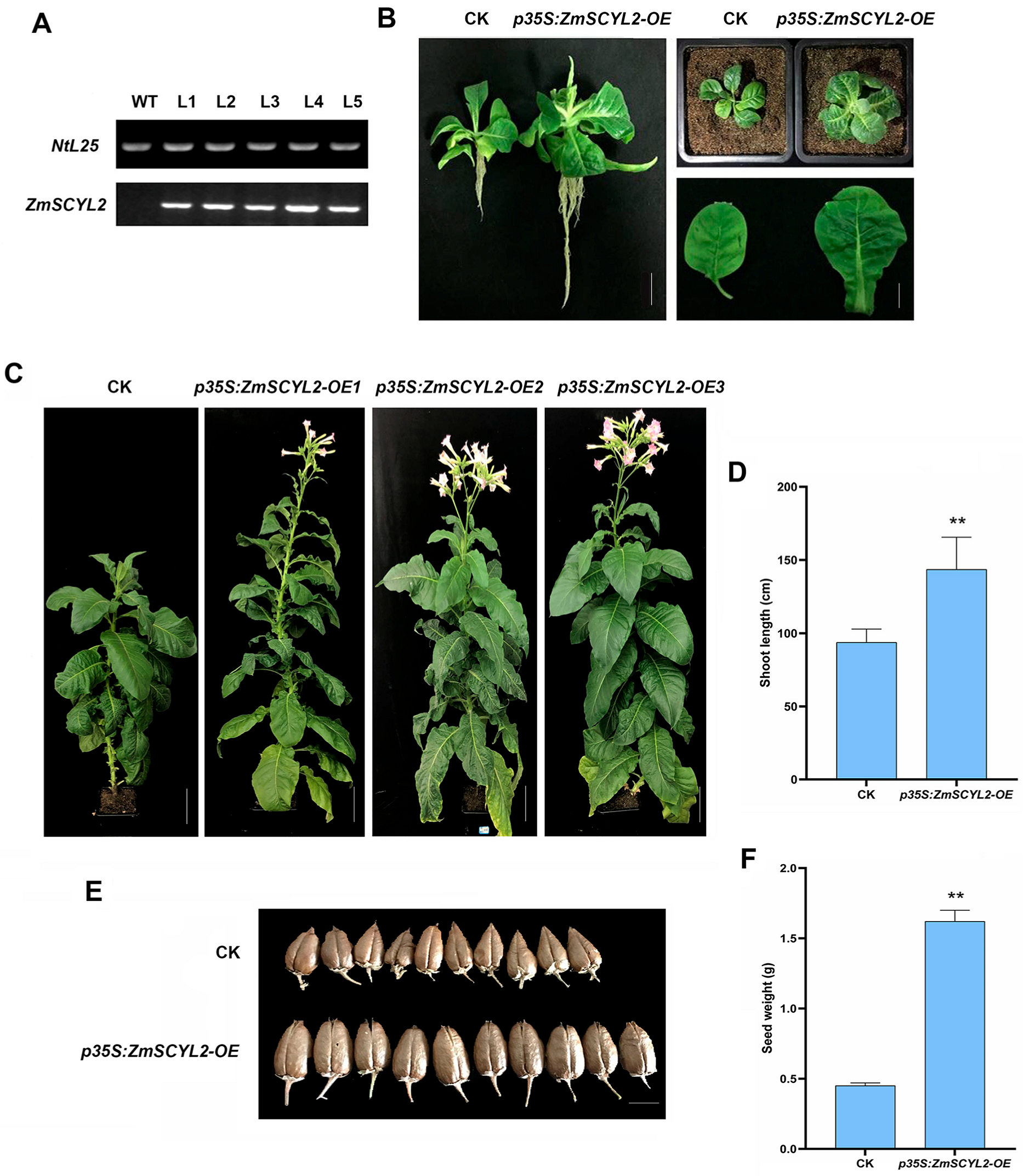
2.5. Effects of ZmSCYL2 on Growth and Phytosterol Content in Tobacco
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Extraction and Quantification of Phytosterols
4.3. GWAS Analysis
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Cloning of ZmSCYL2 and Bioinformatics Analysis
4.6. Subcellular Localization of ZmSCYL2-GFP Fusion Protein
4.7. Generation of Transgenic Plants for Arabidopsis and Tobacco
4.8. Identification and Complementation of Arabidopsis Mutants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miras-Moreno, B.; Sabater-Jara, A.B.; Pedreño, M.A.; Almagro, L. Bioactivity of phytosterols and their production in plant in vitro cultures. J. Agric. Food Chem. 2016, 64, 7049–7058. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Belwal, T.; Li, L.; Limwachiranon, J.; Liu, X.; Luo, Z. Affiliation phytosterols and their derivatives: Potential health-promoting uses against lipid metabolism and associated diseases, mechanism, and safety issues. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1243–1267. [Google Scholar] [CrossRef] [PubMed]
- Rahier, A.; Taton, M.; Bouvier-Navé, P.; Schmitt, P.; Benveniste, P.; Schuber, F.; Narula, A.S.; Cattel, L.; Anding, C.; Place, P. Affiliation design of high energy intermediate analogues to study sterol biosynthesis in higher plants. Lipids 1985, 21, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Bot, A. Phytosterols. In Encyclopedia of Food Chemistry, 1st ed.; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 225–228. [Google Scholar]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant sterols: Diversity, biosynthesis, and physiological functions. Biochem. Mosc. 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, S.R.; Dwivedi, V.; Rai, A.; Pal, S.; Shasany, A.K.; Nagegowda, D.A. A WRKY transcription factor from Withania somnifera regulates triterpenoid withanolide accumulation and biotic stress tolerance through modulation of phytosterol and defense pathways. New Phytol. 2017, 215, 1115–1131. [Google Scholar] [CrossRef]
- Solati, Z.; Moghadasian, M.H. Use of animal models in plant sterol and stanol research. J. AOAC Int. 2015, 98, 691–696. [Google Scholar] [CrossRef]
- Aldini, R.; Micucci, M.; Cevenini, M.; Fato, R.; Bergamini, C.; Nanni, C.; Cont, M.; Camborata, C.; Spinozzi, S.; Montagnani, M.; et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: Prevention, induction, remission study. PLoS ONE 2014, 9, 108–112. [Google Scholar] [CrossRef]
- Jones, P.J.H.; Shamloo, M.; MacKay, D.S.; Rideout, T.C.; Myrie, S.B.; Plat, J.; Roullet, J.B.; Baer, D.J.; Calkins, K.L.; Davis, H.R.; et al. Progress and perspectives in plant sterol and plant stanol research. Nutr. Rev. 2018, 76, 725–746. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E.; Ścibisz, I.; Rudzińska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef]
- Shahzad, N.; Khan, W.; Md, S.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F.; et al. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef]
- Ryan, E.; McCarthy, F.; Maguire, A.; O’Brien, N. Phytosterol oxidation products: Their formation, occurrence, and biological effects. Food Rev. Int. 2009, 25, 157–174. [Google Scholar] [CrossRef]
- Vriese, K.D.; Pollier, J.; Goossens, A.; Beeckman, T.; Vanneste, S. Dissecting cholesterol and phytosterol biosynthesis via mutants and inhibitors. J. Exp. Bot. 2021, 72, 241–253. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Lung, S.C.; Liao, P.; Yeung, E.C.; Hsiao, A.S.; Xue, Y.; Chye, M.L. Acyl-CoA-binding protein ACBP1 modulates sterol synthesis during embryogenesis. Plant Physiol. 2017, 174, 1420–1435. [Google Scholar] [CrossRef]
- Kumar, K.; Gibbs, H.C.; Yeh, A.T.; Griffing, L.R. The sterol trafficking pathway in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 616631. [Google Scholar] [CrossRef]
- Plummer, A.M.; Culbertson, A.T.; Liao, M. The ABCs of sterol transport. Annu. Rev. Physiol. 2020, 83, 153–181. [Google Scholar] [CrossRef]
- Edqvist, J.; Rönnberg, E.; Rosenquist, S.; Blomqvist, K.; Viitanen, L.; Salminen, T.A.; Nylund, M.; Tuuf, J.; Mattjus, P. Plants express a lipid transfer protein with high similarity to mammalian sterol carrier protein2. J. Biol. Chem. 2004, 279, 53544–53553. [Google Scholar] [CrossRef]
- Stefan, C.J.; Trimble, W.S.; Grinstein, S.; Drin, G.; Reinisch, K.; Camilli, P.D.; Cohen, S.; Valm, A.M.; Schwartz, J.L.; Levine, T.P.; et al. Membrane dynamics and organelle biogenesis-lipid pipelines and vesicular carriers. BMC Biol. 2017, 15, 102. [Google Scholar] [CrossRef]
- Singh, M.K.; Jürgens, G. Specificity of plant membrane trafficking—ARFs, regulators and coat proteins. Semin. Cell Dev. Biol. 2018, 80, 85–93. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Law, K.C.; Chung, K.K.; Zhuang, X.H. An update on coat protein complexes for vesicle formation in plant post-Golgi trafficking. Front. Plant Sci. 2022, 13, 826007. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Li, S.; Zhao, X.Y.; Zhou, L.Z.; Huang, G.Q.; Feng, C.; Zhang, Y. Hapless13, the Arabidopsis μ1 adaptin, is essential for protein sorting at the trans-Golgi network/early endosome. Plant Physiol. 2013, 162, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Béthune, J.; Wieland, F.T. Assembly of COPI and COPII vesicular coat proteins on membranes. Annu. Rev. Biophys. 2018, 21, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Gorelova, V. A roadmap of plant clathrin-mediated vesicle trafficking. Plant Cell 2022, 34, 2106–2107. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I. Revisiting the role of clathrin-mediated endoytosis in synaptic vesicle recycling. Front. Cell. Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef]
- Pelletier, S. SCYL pseudokinases in neuronal function and survival. Neural Regen. Res. 2016, 11, 42–44. [Google Scholar] [CrossRef]
- Duwel, M.; Ungewickell, E.J. Clathrin-dependent association of CVAK104 with endosomes and the trans-Golgi network. Mol. Biol. Cell 2006, 17, 4513–4525. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. CVAK104 is a novel poly-L-lysine-stimulated kinase that targets the beta2-subunit of AP2. J. Biol. Chem. 2005, 280, 21539–21544. [Google Scholar] [CrossRef]
- Borner, G.H.H.; Rana, A.A.; Forster, R.; Harbour, M.; Smith, J.C.; Robinson, M.S. CVAK104 is a novel regulator of clathrin-mediated SNARE sorting. Traffic 2007, 8, 893–903. [Google Scholar] [CrossRef]
- Gingras, S.; Earls, L.R.; Howell, S.; Smeyne, R.J.; Zakharenko, S.S.; Pelletier, S. SCYL2 protects CA3 pyramidal neurons from excitotoxicity during functional maturation of the mouse hippocampus. J. Neurosci. 2015, 35, 10510–10522. [Google Scholar] [CrossRef]
- Seidahmed, M.Z.; Al-Kindi, A.; Alsaif, H.; Miqdad, A.; Alabbad, N.; Alfifi, A.; Abdelbasit, O.; Alhussein, K.; Alsamadi, A.; Ibrahim, N.; et al. Recessive mutations in SCYL2 cause a novel syndromic form of arthrogryposis in humans. Hum. Genet. 2020, 139, 513–519. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, D.W.; Ryu, S.B.; Hwang, I.; Schachtman, D.P. SCYL2 genes are involved in clathrin-mediated vesicle trafficking and essential for plant growth. Plant Physiol. 2017, 175, 194–209. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, J.H.; Cheng, C.; Niu, F.; Zhang, A.P.; Sun, B.; Tu, R.J.; Wan, J.N.; Li, Y.; Huang, Y.W.; et al. A conserved clathrin-coated vesicle component, OsSCYL2, regulates plant innate immunity in rice. Plant Cell Environ. 2022, 45, 542–555. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.H.; Zhang, X.Q.; Li, Y.X.; Zhang, D.F.; Shi, Y.S.; Song, Y.C.; Li, Y.; Yang, D.J.; Wang, T.Y. Transcriptome and GWAS analyses reveal candidate gene for seminal root length of maize seedlings under drought stress. Plant Sci. 2020, 292, 110380. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.Y.; Xu, Q.Y.; Wang, D.D.; Di, H.; Huang, J.; Yang, X.W.; Wang, Z.F.; Zhang, L.; Dong, L.; et al. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seq approaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef]
- Zhang, X.; Julien-David, D.; Miesch, M.; Geoffroy, P.; Raul, F.; Roussi, S.; Aoudé-Werner, D. Identification and quantitative analysis of β-sitosterol oxides in vegetable oils by capillary gas chromatography-mass spectrometry. Steroids 2005, 6, 896–906. [Google Scholar] [CrossRef]
- Fu, J.; Cheng, Y.; Linghu, J.; Yang, X.; Kang, L.; Zhang, Z.; Zhang, J.; He, C.; Du, X.; Peng, Z.; et al. RNA sequencing reveals the complex regulatory network in the maize kernel. Nat. Commun. 2013, 4, 2832. [Google Scholar] [CrossRef]
- Li, H.; Peng, Z.; Yang, X.; Wang, W.; Fu, J.; Wang, J.; Han, Y.; Chai, Y.; Guo, T.; Yang, N.; et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 2013, 45, 43–50. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real–time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, S.Z.; Song, Z.Z.; Liu, X.Q.; Zhou, X.J.; Yang, W.Z.; Chen, J.T.; Chen, R.M. Mediation of zinc and iron accumulation in maize by ZmIRT2, a novel iron-regulated transporter. Plant Cell Physiol. 2022, 63, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.R.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 97, 641–646. [Google Scholar]
- Xie, C.; Kúc, J. Induction of resistance to Peronospora tabacinain tobacco leaf disks by leaf disks with induced resistance. Physiol. Mol. Plant Pathol. 1997, 51, 279–286. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Ma, W.; Xu, M.; Li, T.; Han, G.; Gu, L.; Chen, M.; Zhang, M.; Cheng, B.; Zhang, X. Identification and Functional Characterization of ZmSCYL2 Involved in Phytosterol Accumulation in Plants. Int. J. Mol. Sci. 2023, 24, 10411. https://doi.org/10.3390/ijms241210411
Zhang C, Ma W, Xu M, Li T, Han G, Gu L, Chen M, Zhang M, Cheng B, Zhang X. Identification and Functional Characterization of ZmSCYL2 Involved in Phytosterol Accumulation in Plants. International Journal of Molecular Sciences. 2023; 24(12):10411. https://doi.org/10.3390/ijms241210411
Chicago/Turabian StyleZhang, Chenchen, Wanlu Ma, Minyan Xu, Tao Li, Guomin Han, Longjiang Gu, Meng Chen, Mengting Zhang, Beijiu Cheng, and Xin Zhang. 2023. "Identification and Functional Characterization of ZmSCYL2 Involved in Phytosterol Accumulation in Plants" International Journal of Molecular Sciences 24, no. 12: 10411. https://doi.org/10.3390/ijms241210411
APA StyleZhang, C., Ma, W., Xu, M., Li, T., Han, G., Gu, L., Chen, M., Zhang, M., Cheng, B., & Zhang, X. (2023). Identification and Functional Characterization of ZmSCYL2 Involved in Phytosterol Accumulation in Plants. International Journal of Molecular Sciences, 24(12), 10411. https://doi.org/10.3390/ijms241210411





