Integrated Transcriptome and Metabolome Analysis Revealed the Causal Agent of Primary Bud Necrosis in ‘Summer Black’ Grape
Abstract
1. Introduction
2. Results
2.1. Primary Bud Necrosis Progression in ‘Summer Black’
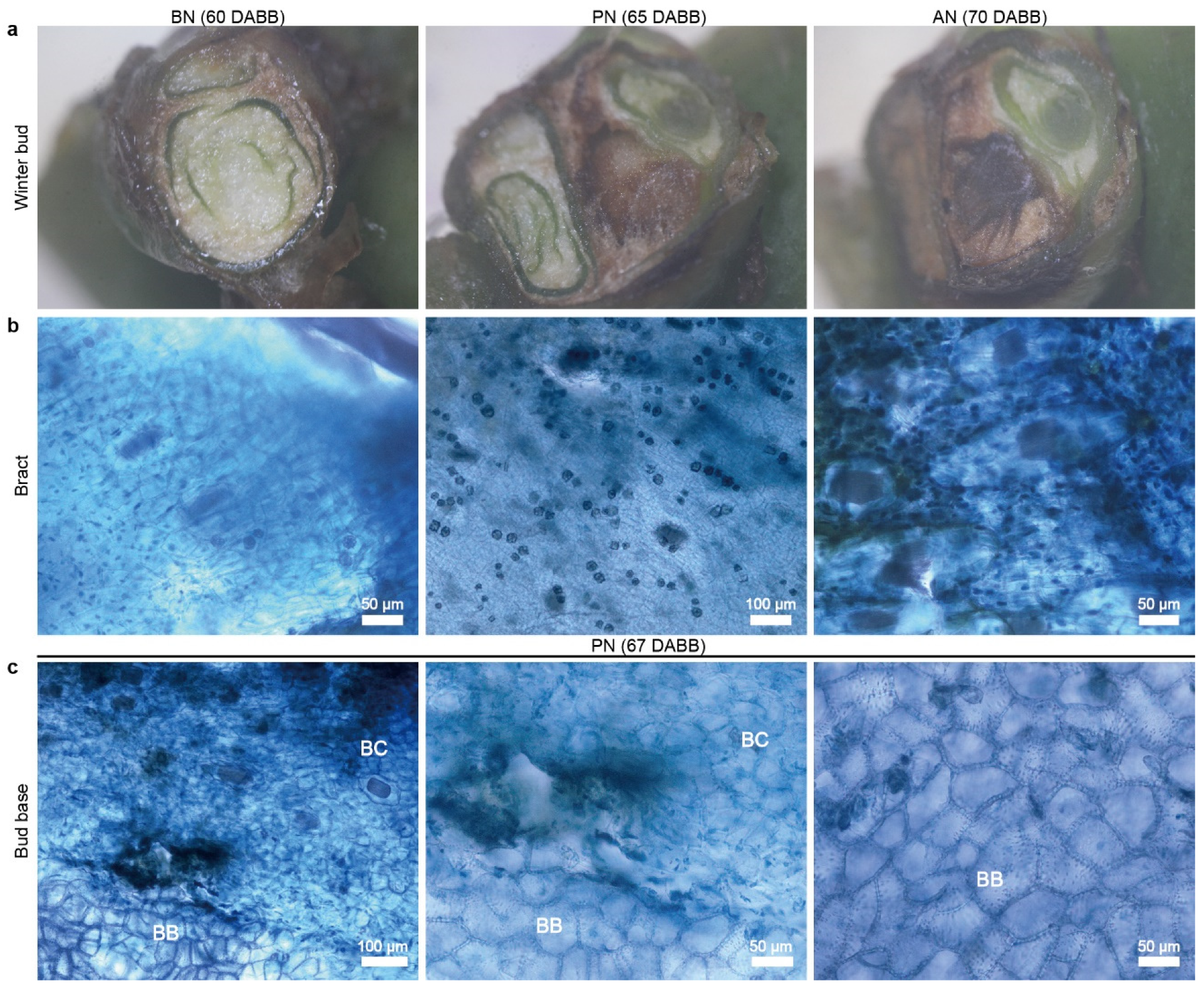
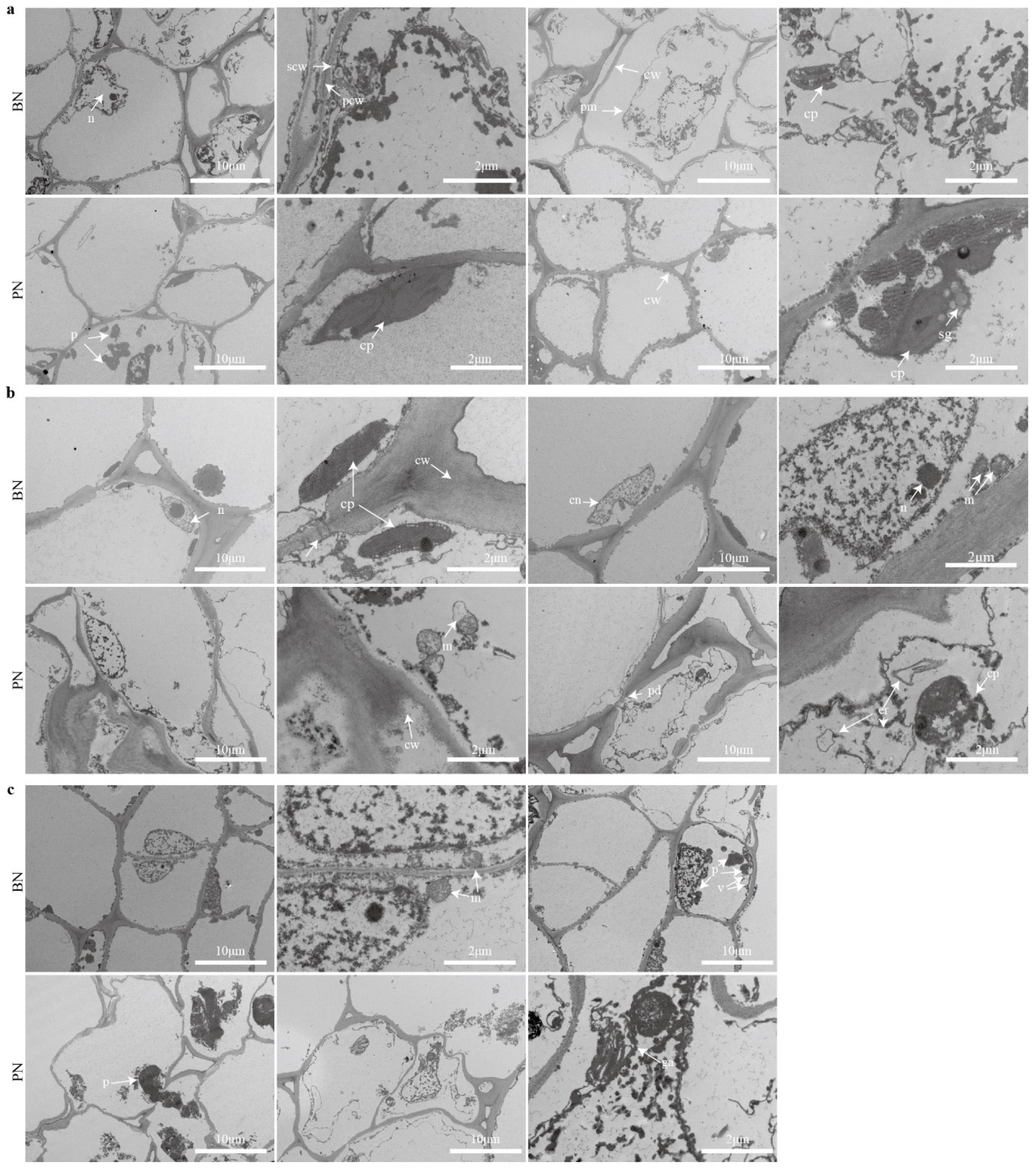
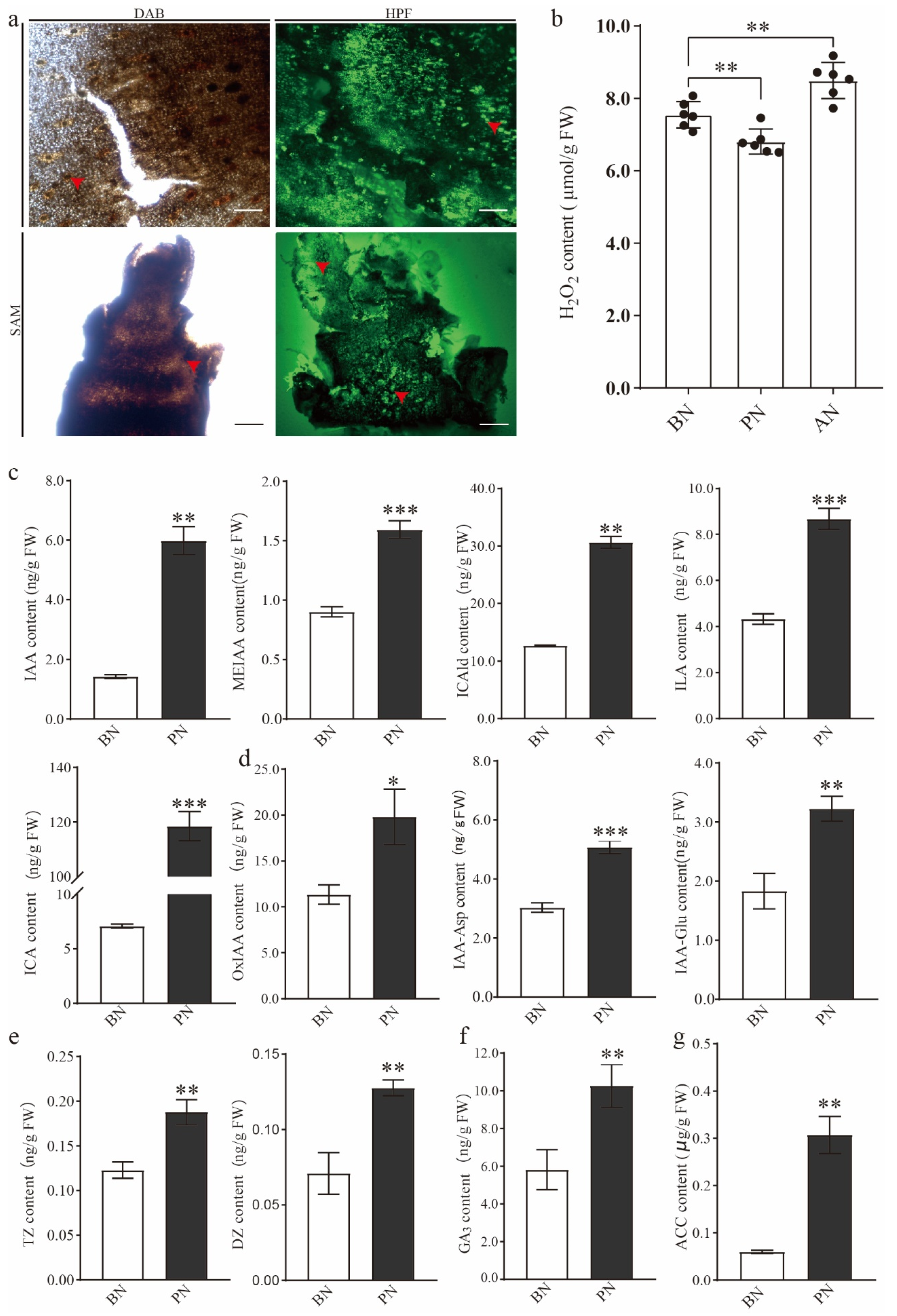
2.2. The Changes in Hormone and H2O2 Levels during PBN Progression in ‘Summer Black’
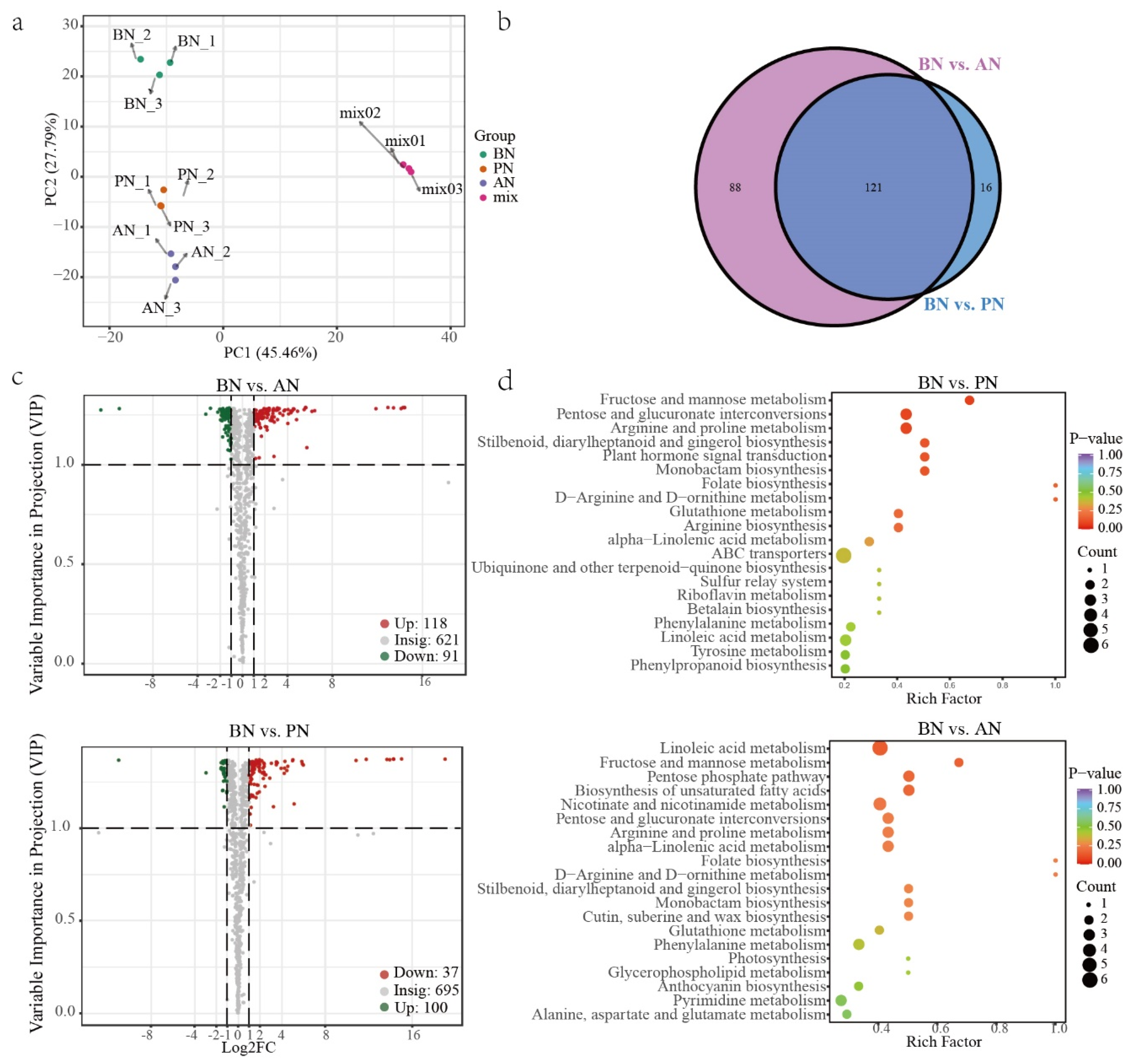
2.3. Differentially Expressed Genes during PBN Progression in ‘Summer Black’
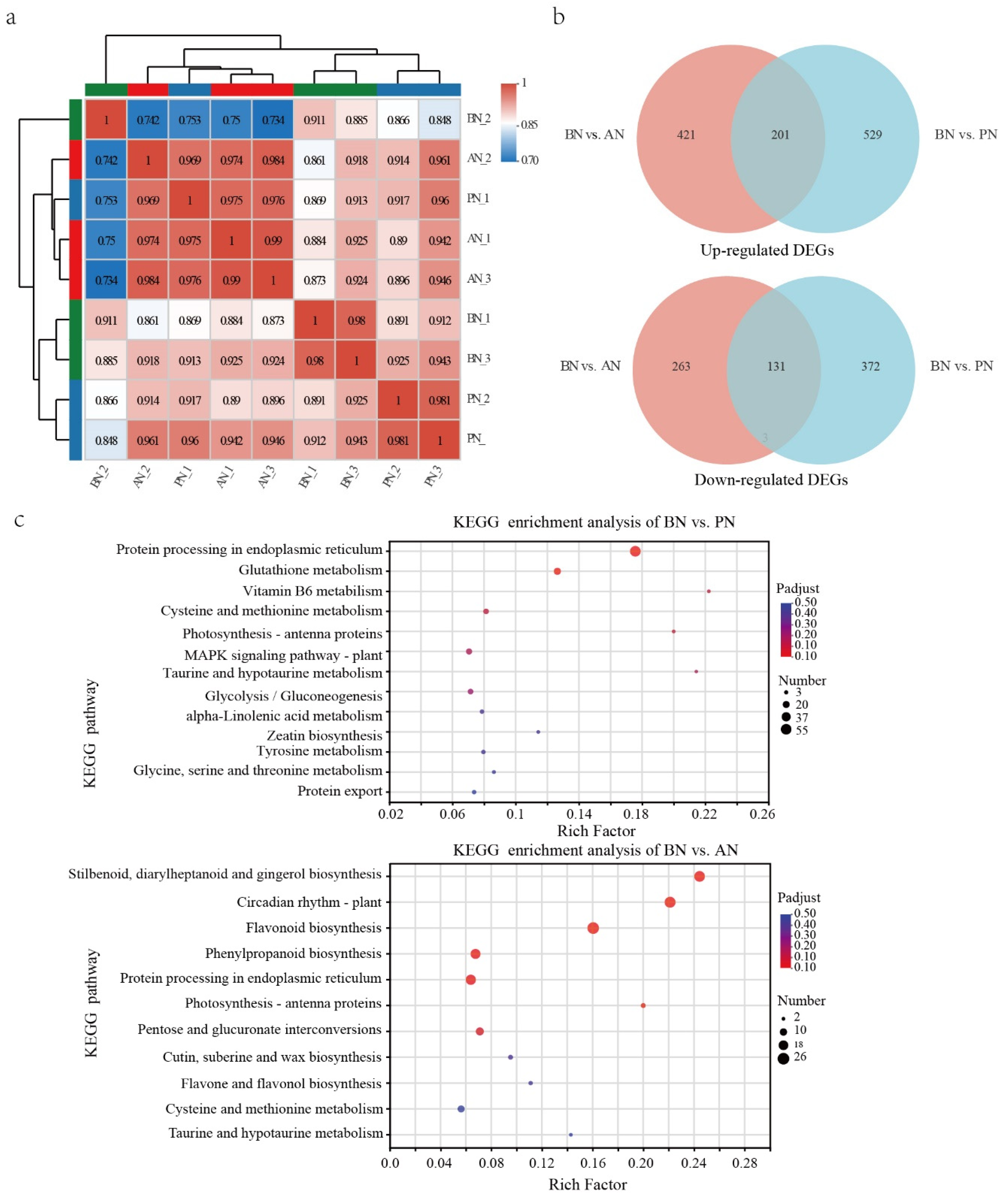
2.4. Differentially Accumulated Metabolites during PBN Progression in ‘Summer Black’
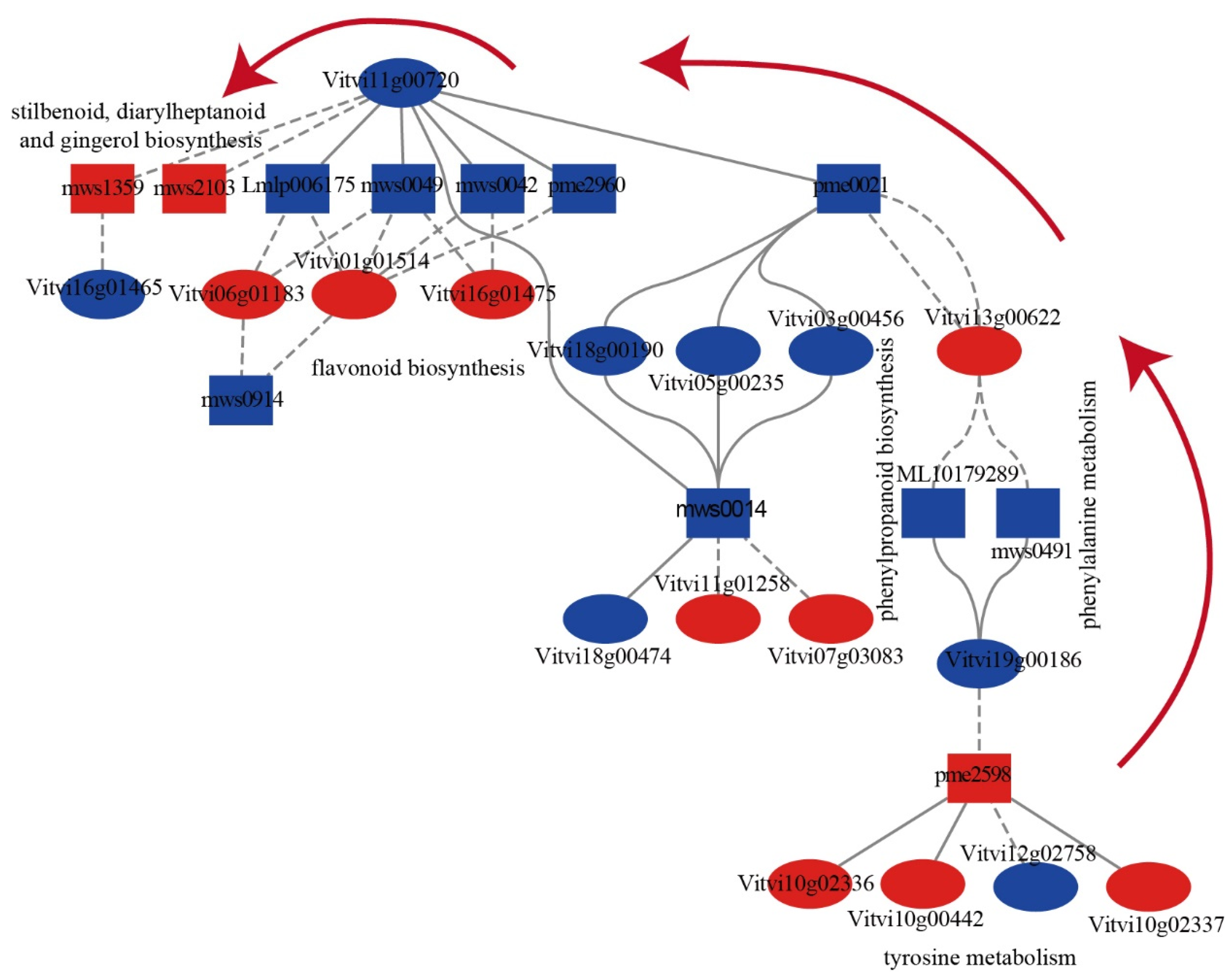
2.5. The Correlation between DAMs and DEGs during PBN Progression in ‘Summer Black’
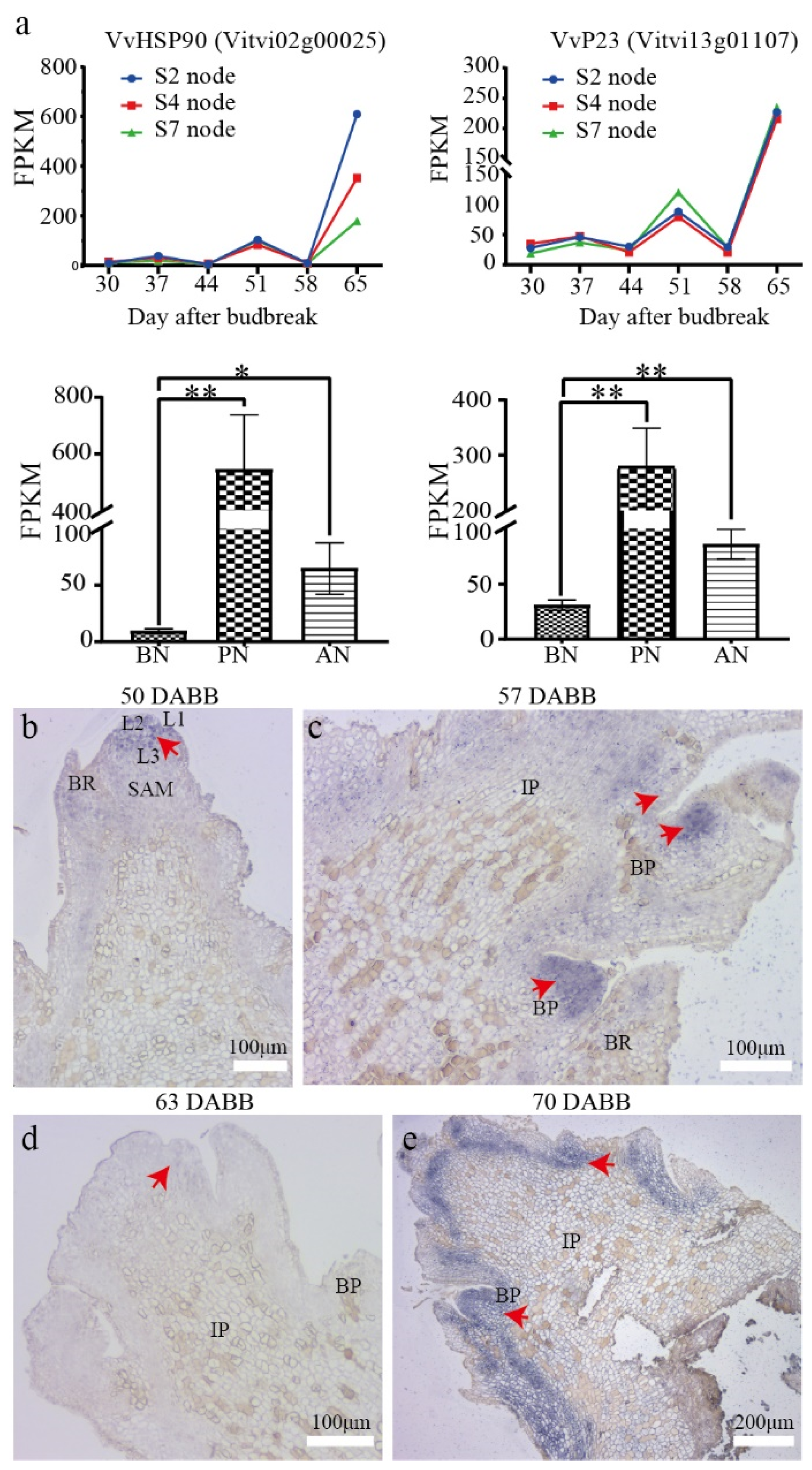
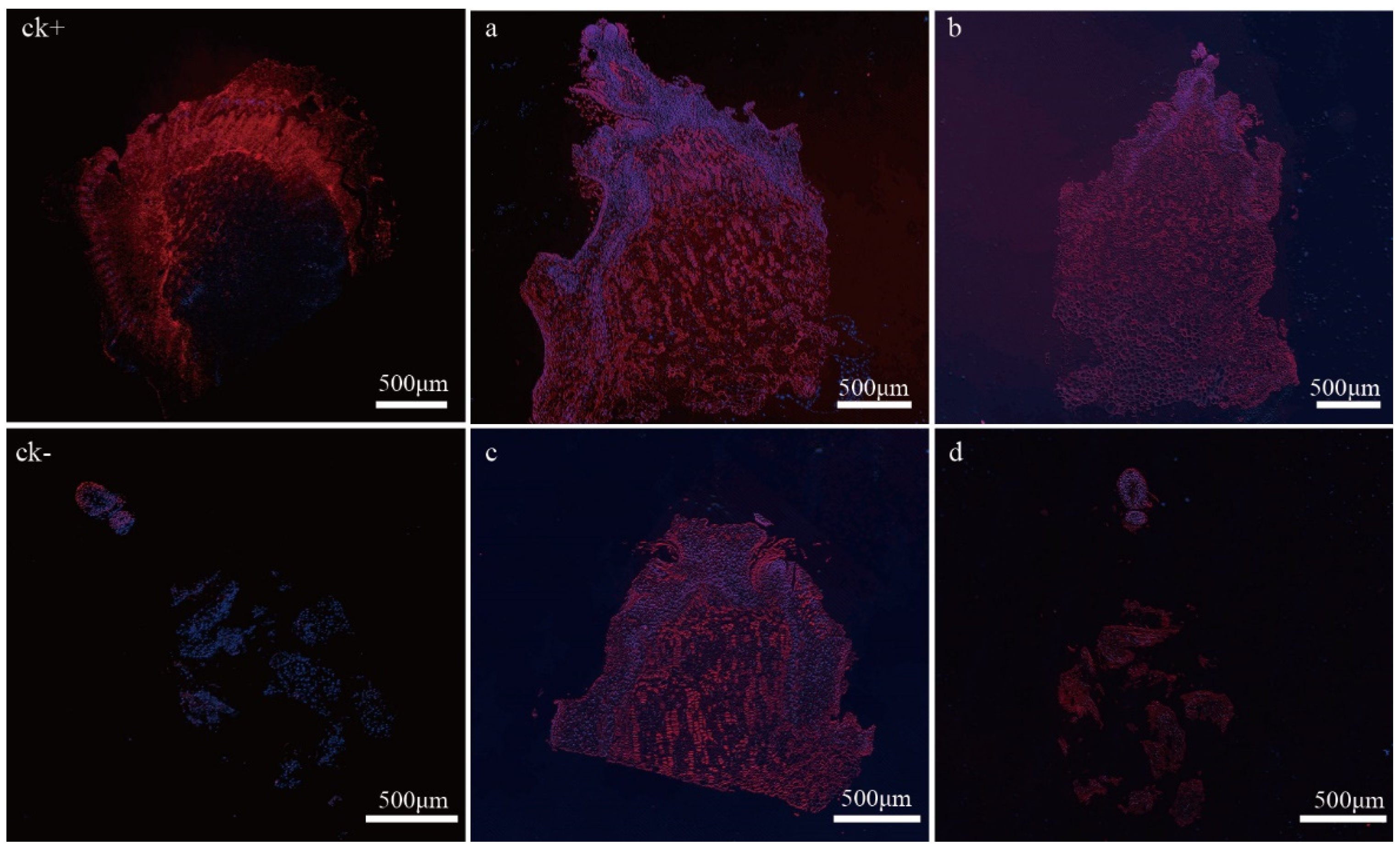
2.6. In Situ Expression of VvP23 during PBN Progression in ‘Summer Black’
3. Discussion
3.1. The Development Process and Irreversibility of Primary Bud Necrosis
3.2. The Crucial Role of ROS in Primary Bud Necrosis
3.3. Protein Misfolding and Aggregation Are Closely Related to Primary Bud Necrosis
3.4. Tissue Browning Is a Significant Characteristic of the Primary Bud Necrosis
3.5. The Role of Endogenous Hormones in Primary Bud Necrosis
4. Materials and Methods
4.1. Plant Material
4.2. Determining Bud Necrosis in the Field
4.3. Trypan Blue Staining
4.4. ROS Staining
4.5. TUNEL Assay
4.6. TEM Analysis
4.7. Detection of Phytohormones
4.8. Determination of H2O2
4.9. RNA Isolation and Transcriptome Analysis
4.10. Widely Targeted Metabolomics Assay
4.11. Integrative Analysis of Transcriptome and Metabolome
4.12. In Situ Hybridization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratt, C. Vegetative anatomy of cultivated grapes-a review. Am. J. Enol. Vitic. 1974, 25, 131. [Google Scholar] [CrossRef]
- Torregrosa, L.; Carbonneau, A.; Kelner, J.-J. The shoot system architecture of Vitis vinifera ssp. sativa. Sci. Hortic. 2021, 288, 110404. [Google Scholar] [CrossRef]
- Wolf, T.K.; Warren, M.K. Shoot growth rate and density affect bud necrosis of `Riesling’ grapevines. J. Am. Soc. Hort. Sci. 1995, 120, 989–996. [Google Scholar] [CrossRef]
- Morrison, J.C.; Iodi, M. The development of primary bud necrosis in Thompson Seedless and Flame Seedless grapevines. Vitis 1990, 29, 133–144. [Google Scholar]
- Collins, C.; Coles, R.; Conran, J.G.; Rawnsley, B. The progression of primary bud necrosis in the grapevine cv. Shiraz (Vitis vinifera L.): A histological analysis. Vitis 2006, 45, 57–62. [Google Scholar]
- Collins, C.; Rawnsley, B. Factors influencing primary bud necrosis (PBN) in australian vineyards. Acta Hortic. 2005, 689, 81–86. [Google Scholar] [CrossRef]
- Cheng, G.; Zhou, S.; Zhang, J.; Huang, X.; Bai, X.; Xie, T.; Guo, R.; Liu, J.; Yu, H.; Xie, L. Comparison of transcriptional expression patterns of phenols and carotenoids in ‘Kyoho’ grapes under a two-crop-a-year cultivation system. PLoS ONE 2019, 14, e0210322. [Google Scholar] [CrossRef]
- Vasudevan, L.; Wolf, T.K.; Welbaum, G.G.; Wisniewski, M.E. Anatomical developments and effects of artificial shade on bud necrosis of Riesling grapevines. Am. J. Enol. Vitic. 1998, 49, 429–439. [Google Scholar] [CrossRef]
- Naito, R.; Yamamura, H.; Yoshino, K. Effects of shoot vigor and foliar application of GA and SADH on the occurrence of bud necrosis in ‘Kyoho’ grape. J. Jpn. Soc. Hort. Sci. 1986, 55, 130–137. [Google Scholar] [CrossRef]
- Lavee, S.; Melamud, H.; Ziv, M.; Bernstein, Z. Necrosis in grapevine buds (Vitis vinifera cv. Queen of Vineyard) I. Relation to vegetative vigor1. Vitis 1981, 20, 8–14. [Google Scholar]
- Dry, P.R.; Coombe, B.G. Primary bud-axis necrosis of grapevines. I. Natural incidence and correlation with vigour. Vitis 1994, 33, 225–230. [Google Scholar]
- Choi, I.M.; Lee, C.H.; Hong, Y.P.; Park, H.S. Relation between shoot vigour and bud necrosis in ‘Campbell Early’ grapevines. Korean J. Hort. Sci. Technol. 2007, 25, 375–381. [Google Scholar]
- Avoosi, B.; Eshghi, S.; Tafazoli, E.; Rahemi, M.; Emam, Y. Primary bud necrosis (PBN) as affected by cane diameter, node position and sampling date in grapevine (Vitis vinifera L. ‘Askari’). Acta Hortic. 2012, 931, 453–460. [Google Scholar]
- Perez, J.; Kliewer, W.M. Effect of shading on bud necrosis and bud fruitfulness of Thompson Seedless grapevines. Am. J. Enol. Vitic. 1990, 41, 168–175. [Google Scholar] [CrossRef]
- Vasudevan, L.; Wolf, T.K.; Welbaum, G.G.; Wisniewski, M. Reductions in bud carbohydrates are associated with grapevine bud necrosis. Vitis 1998, 37, 189–190. [Google Scholar]
- Ziv, M.; Melamud, H.; Bernstein, Z.; Lavee, S. Necrosis in grapevine buds (Vitis vinifera cv. Queen of Vineyard) II. Effect of gibberellic acid (GA3) application. Vitis 1981, 20, 105–114. [Google Scholar]
- Collins, C.; Rawnsley, B. Effect of gibberellic acid and paclobutrazol on the incidence of primary bud necrosis in cv. Shiraz. Am. J. Enol. Vitic. 2008, 59, 83–87. [Google Scholar] [CrossRef]
- De Almeida Junior, O.; de Souza, C.R.; Dias, F.A.N.; de Paula Fernandes, F.; Torregrosa, L.; Fernandes-Brum, C.N. Effect of pruning strategy on ‘Syrah’ bud necrosis and fruitfulness in Brazilian subtropical Southeast. Vitis 2019, 58, 87–94. [Google Scholar]
- Gonçalves, M.J.; Bogo, A.; Casa, R.T.; Pelizza, T.R.; Miquelluti, D.J.; Penter, F.; da Cunha, I.C. Behavior of European pear cultivars to floral bud necrosis in southern Brazil. Crop. Prot. 2014, 65, 95–99. [Google Scholar] [CrossRef]
- Seo, H.J.; Jin, Y.O.; Lee, C.L.; Roan, S.F.; Chen, I.Z. Effect of altitude on flower bud differentiation and necrosis in ‘Shinko’ pears in subtropical climates. Korean J. Hortic. Sci. 2015, 33, 18–23. [Google Scholar] [CrossRef]
- Leoni, C.; Duarte, F.; Speroni, G.; Silvera, M.; Iriarte, W.; Bonnecarrere, V. Etiology of pear flower bud necrosis in Uruguay. Acta Hortic. 2021, 1303, 359–366. [Google Scholar] [CrossRef]
- Kester, D.E.; Shackel, K.A.; Micke, W.C.; Viveros, M.; Gradziel, T.M. Noninfectious bud failure in ‘Carmel’ almond: I. Pattern of development in vegetative progeny trees. J. Am. Soc. Hort. Sci. 2004, 129, 244–249. [Google Scholar] [CrossRef]
- D’Amico-Willman, K.M.; Sideli, G.M.; Allen, B.J.; Anderson, E.S.; Gradziel, T.M.; Fresnedo-Ramírez, J. Identification of putative markers of Non-infectious bud failure in almond [Prunus dulcis (Mill.) D.A. Webb] through genome wide DNA methylation profiling and gene expression analysis in an almond x peach hybrid population. Front. Plant Sci. 2022, 13, 804145. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.H.C.; Fernandes-Brum, C.N.; de Souza, C.R.; Dias, F.A.N.; de Almeida-Junior, O.; Regina, M.D.A.; de Oliveira, K.K.P.; dos Reis, G.L.; Oliveira, L.M.; Fernandes, F.D.P.; et al. Transcriptome analyses suggest that changes in fungal endophyte lifestyle could be involved in grapevine bud necrosis. Sci. Rep. 2020, 10, 9514. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, H.; Zhou, J.-M.; Smith, S.M.; Li, J. Malate Circulation: Linking chloroplast metabolism to mitochondrial ROS. Trends Plant Sci. 2020, 25, 446–454. [Google Scholar] [CrossRef]
- Golstein, P.; Kroemer, G. Cell death by necrosis: Towards a molecular definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef]
- Biebl, M.M.; Lopez, A.; Rehn, A.; Freiburger, L.; Lawatscheck, J.; Blank, B.; Sattler, M.; Buchner, J. Structural elements in the flexible tail of the co-chaperone p23 coordinate client binding and progression of the Hsp90 chaperone cycle. Nat. Commun. 2021, 12, 828. [Google Scholar] [CrossRef]
- D’alessandro, S.; Golin, S.; Hardtke, C.S.; Lo Schiavo, F.; Zottini, M. The co-chaperone p23 controls root development through the modulation of auxin distribution in the Arabidopsis root meristem. J. Exp. Bot. 2015, 66, 5113–5122. [Google Scholar] [CrossRef]
- Samakovli, D.; Roka, L.; Dimopoulou, A.; Plitsi, P.K.; Zŭkauskaitė, A.; Georgopoulou, P.; Novák, O.; Milioni, D.; Hatzopoulos, P. HSP90 affects root growth in Arabidopsis by regulating the polar distribution of PIN1. New Phytol. 2021, 231, 1814–1831. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Berghe, T.V.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Waszczak, C.; Hunter, K.; Wrzaczek, M. Bound by fate: The role of reactive oxygen species in receptor-like kinase signaling. Plant Cell 2017, 29, 638–654. [Google Scholar] [CrossRef]
- Burbridge, E.; Diamond, M.; Dix, P.J.; Mccabe, P.F. Use of cell morphology to evaluate the effect of a peroxidase gene on cell death induction thresholds in tobacco. Plant Sci. 2006, 171, 139–146. [Google Scholar] [CrossRef]
- Mccabe, P.F.; Levine, A.; Meijer, P.-J.; Tapon, N.A.; Pennell, R.I. A programmed cell death pathway activated in carrot cells cultured at low cell density. Plant J. 1997, 12, 267–280. [Google Scholar] [CrossRef]
- Tiwari, B.S.; Belenghi, B.; Levine, A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002, 128, 1271–1281. [Google Scholar] [CrossRef]
- Lennon, S.V.; Martin, S.J.; Cotter, T.G. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991, 24, 203–214. [Google Scholar] [CrossRef]
- Kale, R.S.; Seep, J.L.; Sallans, L.; Frankel, L.K.; Bricker, T.M. Oxidative modification of LHC II associated with photosystem II and PS I-LHC I-LHC II membranes. Photosynth. Res. 2022, 152, 261–274. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; Kapralov, A.A.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef] [PubMed]
- Balch, W.E.; Morimoto, R.I.; Dillin, A.; Kelly, J.W. Adapting proteostasis for disease intervention. Science 2008, 319, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, F.; Xie, Q. Insights into endoplasmic reticulum-associated degradation in plants. New Phytol. 2020, 226, 345–350. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Li, Y.; Xue, Y.; Xu, X.; Wang, G.; Liu, Y.; Wu, H.A.; Li, W.; Wang, W.; Chen, Z.; Zhang, W.; et al. mitochondrial FUNDC1/HSC70 interaction organizes the proteostatic stress response at the risk of cell morbidity. EMBO J. 2019, 38, e98786. [Google Scholar] [CrossRef]
- Ruan, L.; Zhou, C.; Jin, E.; Kucharavy, A.; Zhang, Y.; Wen, Z.; Florens, L.; Li, R. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 2017, 543, 443–446. [Google Scholar] [CrossRef]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In vivo aspects of protein folding and quality control. Science 2016, 353, aac4354. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xu, M.; Zhou, Z.; Zhu, X.; Ma, X.; Hou, J.; Tan, L.; Zhu, Z.; Cai, H.; et al. A common wild rice-derived BOC1 allele reduces callus browning in indica rice transformation. Nat. Commun. 2020, 11, 443. [Google Scholar] [CrossRef]
- Paravisini, L.; Peterson, D.G. Mechanisms non-enzymatic browning in orange juice during storage. Food Chem. 2019, 289, 320–327. [Google Scholar] [CrossRef]
- Zuo, W.; Lu, L.; Su, M.; Zhang, J.; Li, Y.; Huang, S.; Li, C.; Wang, N.; Zhang, Z.; Chen, X. Analysis of differentially expressed genes and differentially abundant metabolites associated with the browning of Meihong red-fleshed apple fruit. Postharvest Biol. Technol. 2021, 174, 111437. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, J.H.; Wen, X.; Ni, Y.Y. Purification and structural analysis of membrane-bound polyphenol oxidase from Fuji apple. Food Chem. 2015, 183, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Iqbal, A.; Li, J.; Liu, C.; Murtaza, A.; Xu, X.; Pan, S.; Hu, W. Changes in browning degree and reducibility of polyphenols during autoxidation and enzymatic oxidation. Antioxidants 2021, 10, 1089. [Google Scholar] [CrossRef]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, B.; Zhang, Y.; Zhang, X.; Xu, Y.; Li, C. Evolutionary dynamic analyses on monocot flavonoid 3′-hydroxylase gene family reveal evidence of plant-environment interaction. BMC Plant Biol. 2019, 19, 347. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Leon-Reyes, A.; Van Der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Christofferson, D.E.; Yuan, J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 2010, 22, 263–268. [Google Scholar] [CrossRef]
- Wang, C.; Dai, S.; Zhang, Z.; Lao, W.; Wang, R.; Meng, X.; Zhou, X. Ethylene and salicylic acid synergistically accelerate leaf senescence in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 828–833. [Google Scholar] [CrossRef]
- Huysmans, M.; Lema, A.S.; Coll, N.S.; Nowack, M.K. Dying two deaths—Programmed cell death regulation in development and disease. Curr. Opin. Plant Biol. 2017, 35, 37–44. [Google Scholar] [CrossRef]
- Casanova-Saez, R.; Voss, U. Auxin metabolism controls developmental decisions in land plants. Trends Plant Sci. 2019, 24, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E. The tryptophan conjugates of jasmonic and indole-3-acetic acids are endogenous auxin inhibitors. Plant Physiol. 2009, 150, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Porco, S.; Pěnčík, A.; Rashed, A.; Voß, U.; Casanova-Sáez, R.; Bishopp, A.; Golebiowska, A.; Bhosale, R.; Swarup, R.; Swarup, K.; et al. Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 11016–11021. [Google Scholar] [CrossRef]
- Noyce, P.W.; Harper, J.D.I.; Steel, C.C.; Wood, R.M. A practical method for staging grapevine inflorescence primordia in season 1, with improved description of stages. Am. J. Enol. Vitic. 2015, 66, 492–501. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, P.; Claus, L.A.; Savatin, D.V.; Xu, G.; Wu, S.; Meng, X.; Russinova, E.; He, P.; Shan, L.; et al. Proteolytic processing of SERK3/BAK1 regulates plant immunity, development, and cell death. Plant Physiol. 2019, 180, 543–558. [Google Scholar] [CrossRef]
- Gupta, A.; Dixit, S.K.; Senthil-Kumar, M. Drought stress predominantly endures Arabidopsis thaliana to Pseudomonas syringae infection. Front. Plant Sci. 2016, 7, 808. [Google Scholar] [CrossRef] [PubMed]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012, 24, 275. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Tran, D.; Zhao, T.; Arbelet-Bonnin, D.; Kadono, T.; Meimoun, P.; Cangémi, S.; Kawano, K.; Errakhi, R.; Bouteau, F. Early cellular responses induced by sedimentary calcite-processed particles in bright yellow 2 tobacco cultured cells. Int. J. Mol. Sci. 2020, 21, 4279. [Google Scholar] [CrossRef]
- Dunand, C.; Crevecoeur, M.; Penel, C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 2007, 174, 332–341. [Google Scholar] [CrossRef]
- Canaguier, A.; Grimplet, J.; Di Gaspero, G.; Scalabrin, S.; Duchêne, E.; Choisne, N.; Mohellibi, N.; Guichard, C.; Rombauts, S.; Le Clainche, I.; et al. A new version of the grapevine reference genome assembly (12X.v2) and of its annotation (VCost.v3). Genom. Data 2017, 14, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A Comparative metabolomics study of flavonoids in radish with different skin and flesh colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ruan, Z.; Fei, Z.; Yan, J.; Tang, G. Integrated transcriptome and metabolome analysis revealed that flavonoid biosynthesis may dominate the resistance of Zanthoxylum bungeanum against Stem Canker. J. Agric. Food Chem. 2021, 69, 6360–6378. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, Q.; Zheng, J.; Zhang, A.; Wang, H. Transcriptomic and metabolomic analyses reveal that melatonin promotes melon root development under copper stress by inhibiting jasmonic acid biosynthesis. Hortic. Res. 2020, 7, 79. [Google Scholar] [CrossRef]
- Yang, C.; Shen, S.; Zhou, S.; Li, Y.; Mao, Y.; Zhou, J.; Shi, Y.; An, L.; Zhou, Q.; Peng, W.; et al. Rice metabolic regulatory network spanning the entire life cycle. Mol. Plant 2022, 15, 258–275. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Xu, Y.; Bai, M.; Luo, F.; Yu, J.; Yang, G. Integrated Transcriptome and Metabolome Analysis Revealed the Causal Agent of Primary Bud Necrosis in ‘Summer Black’ Grape. Int. J. Mol. Sci. 2023, 24, 10410. https://doi.org/10.3390/ijms241210410
Fan S, Xu Y, Bai M, Luo F, Yu J, Yang G. Integrated Transcriptome and Metabolome Analysis Revealed the Causal Agent of Primary Bud Necrosis in ‘Summer Black’ Grape. International Journal of Molecular Sciences. 2023; 24(12):10410. https://doi.org/10.3390/ijms241210410
Chicago/Turabian StyleFan, Shaogang, Yanshuai Xu, Miao Bai, Feixiong Luo, Jun Yu, and Guoshun Yang. 2023. "Integrated Transcriptome and Metabolome Analysis Revealed the Causal Agent of Primary Bud Necrosis in ‘Summer Black’ Grape" International Journal of Molecular Sciences 24, no. 12: 10410. https://doi.org/10.3390/ijms241210410
APA StyleFan, S., Xu, Y., Bai, M., Luo, F., Yu, J., & Yang, G. (2023). Integrated Transcriptome and Metabolome Analysis Revealed the Causal Agent of Primary Bud Necrosis in ‘Summer Black’ Grape. International Journal of Molecular Sciences, 24(12), 10410. https://doi.org/10.3390/ijms241210410






