Imbalance of Lymphocyte Subsets and CD45RA-Expressing Cells in Intrathoracic Lymph Nodes, Alveolar Compartment and Bloodstream of Pulmonary Sarcoidosis Patients
Abstract
1. Introduction
2. Results
2.1. Study Population
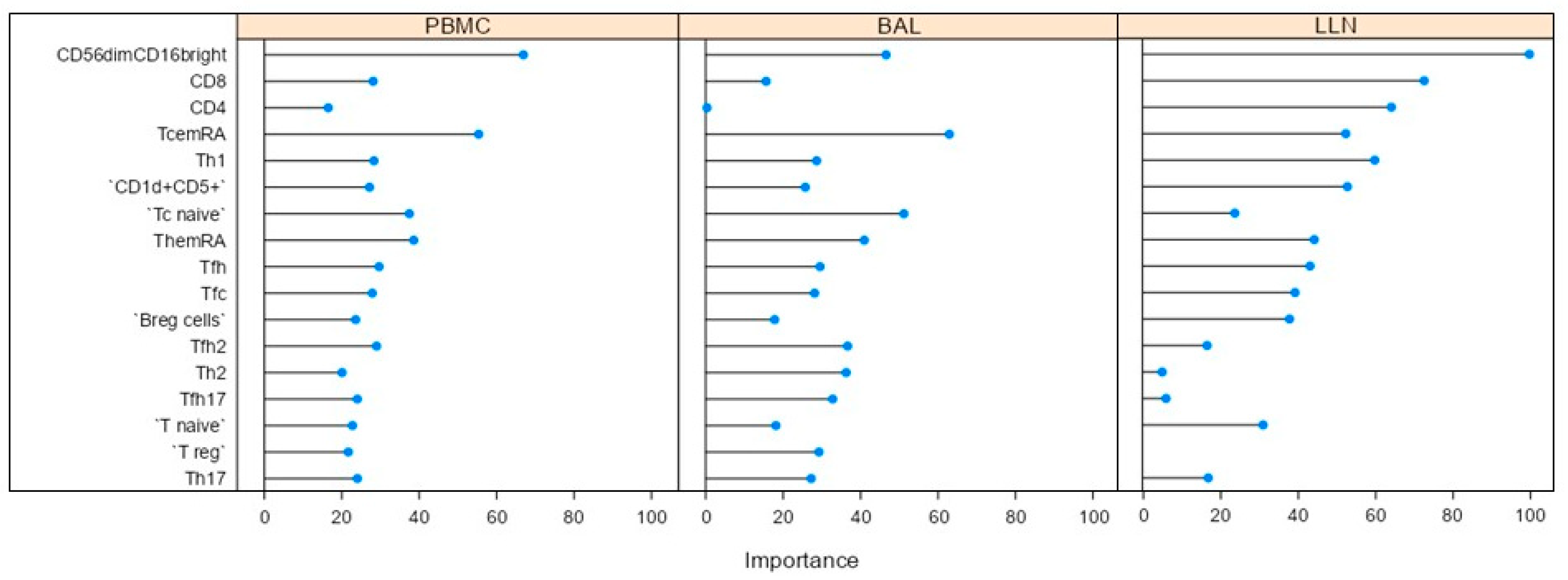
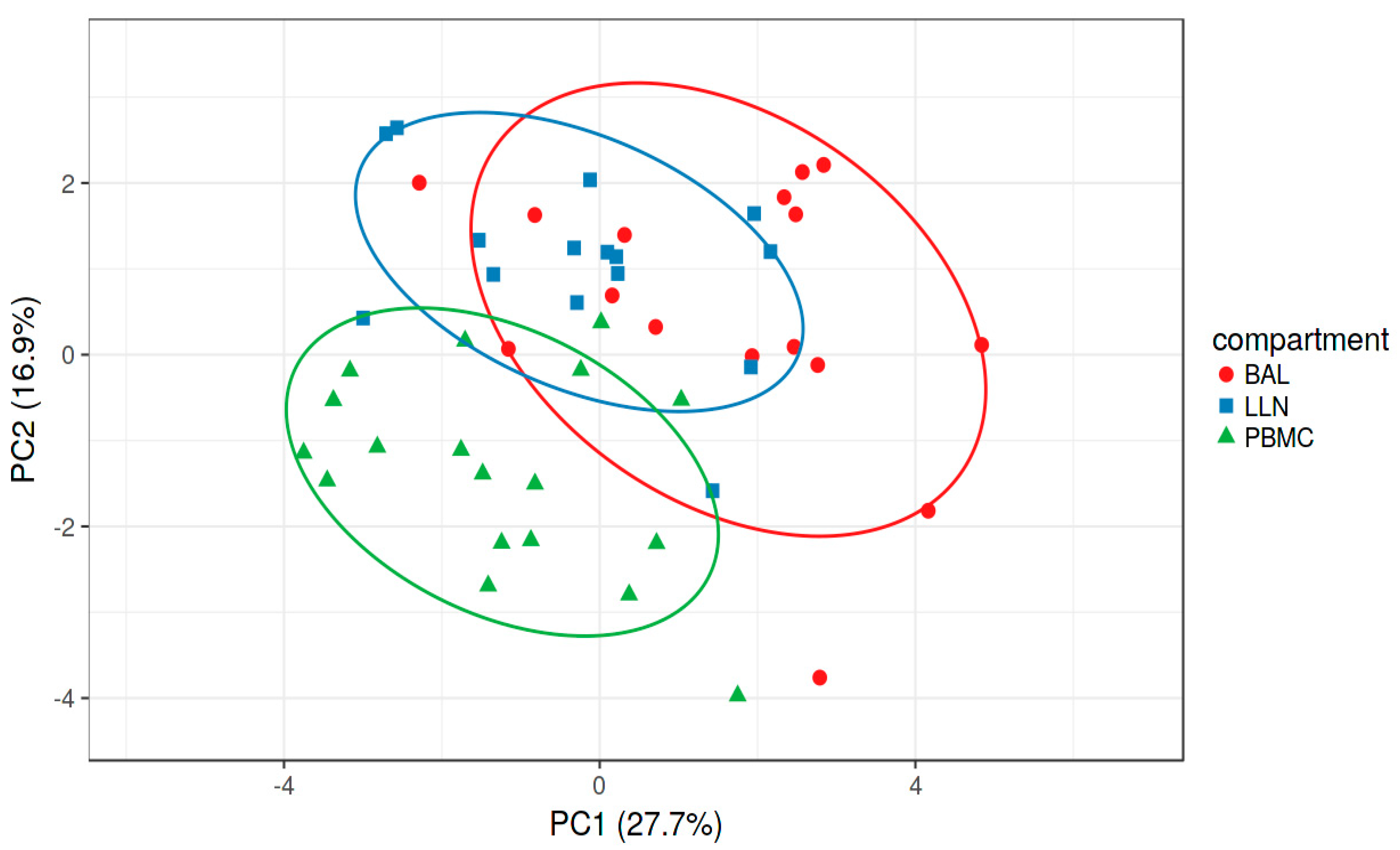
2.2. Multivariate Analysis
2.3. Comparative Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Single Cell Preparations from Blood, Bronchoalveolar Lavage Fluid and Lymph Nodes
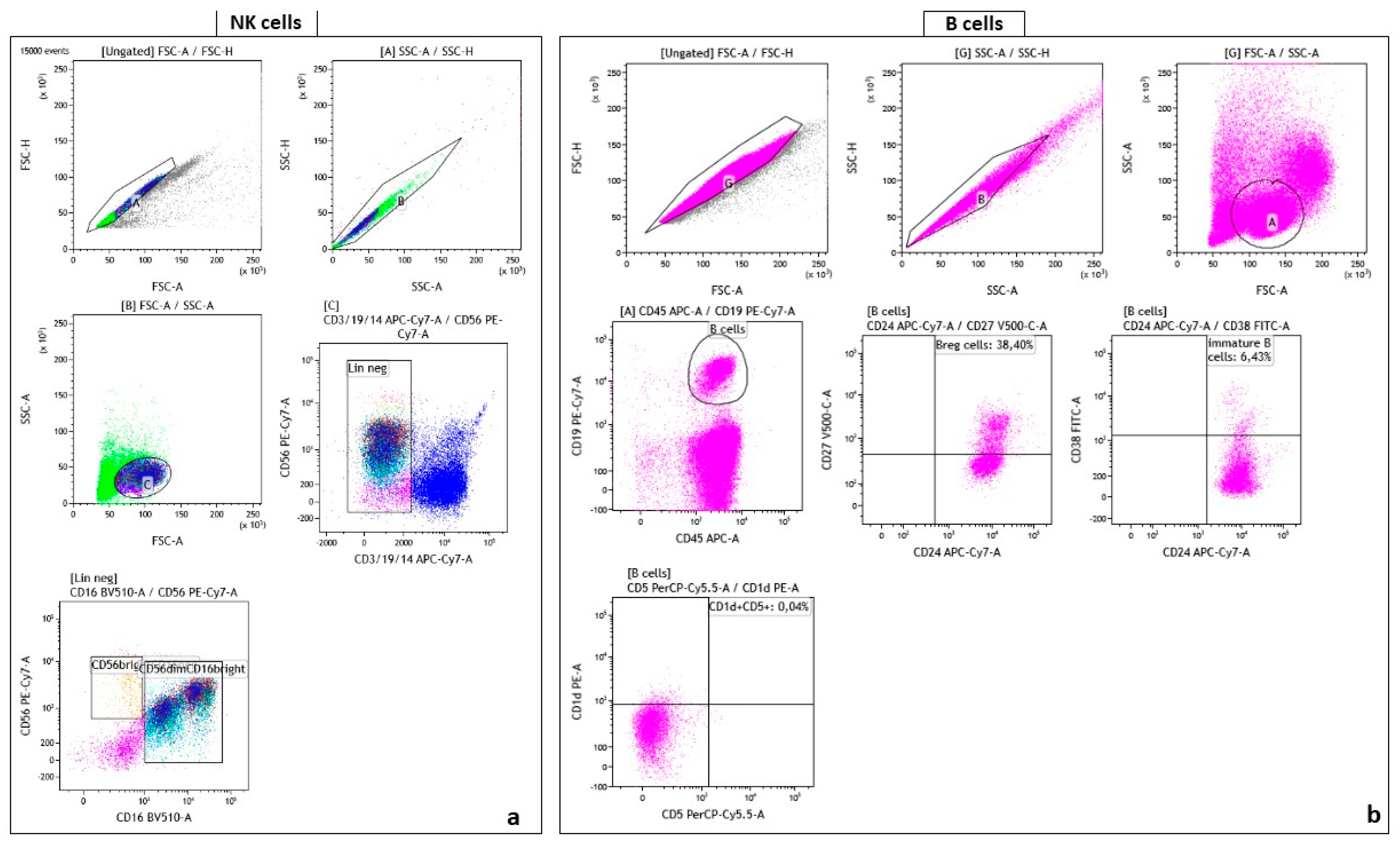
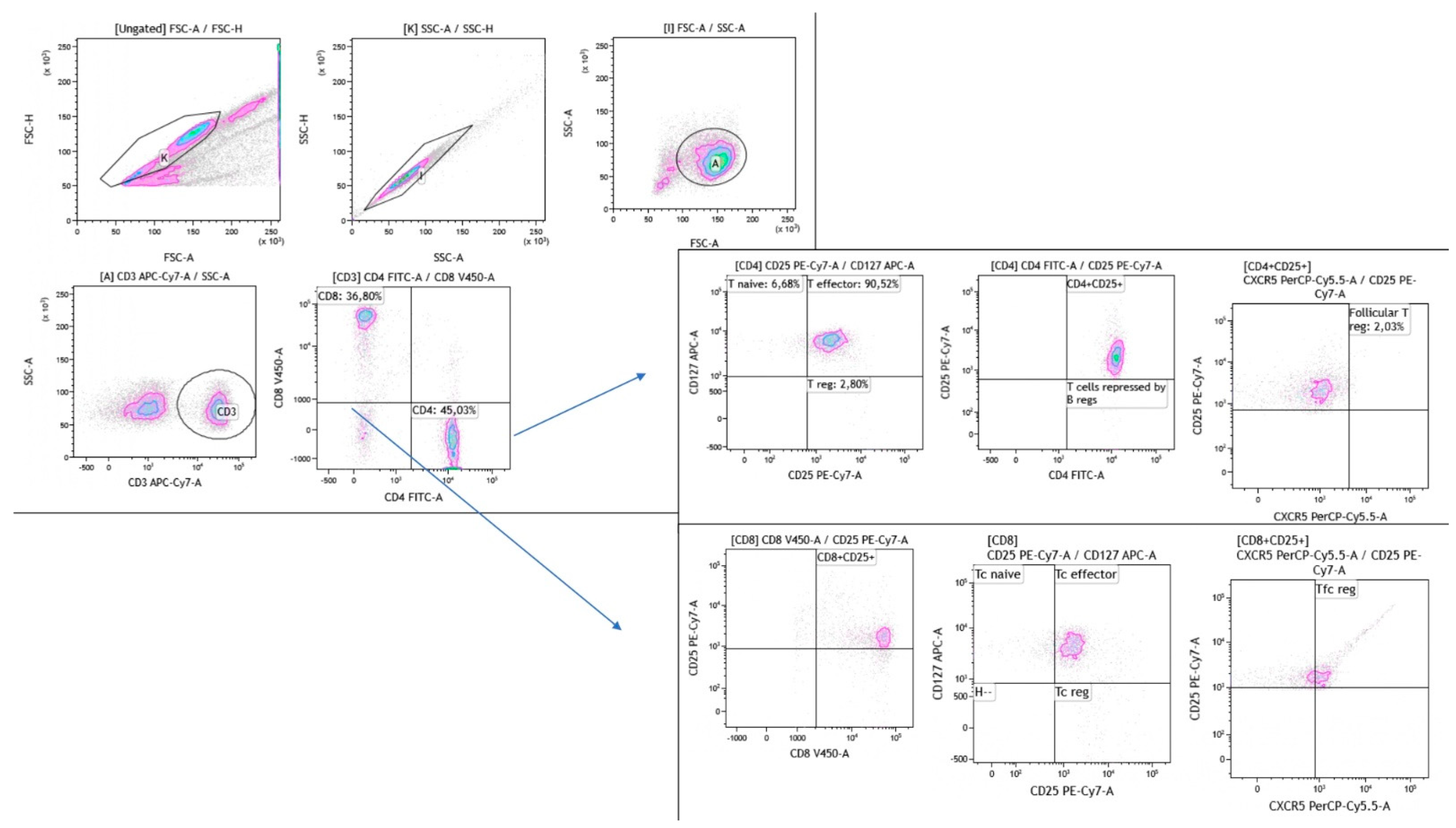
4.3. Flow Cytometry Gating Strategy
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Arkema, E.V.; Cozier, Y.C. Sarcoidosis Epidemiology: Recent Estimates of Incidence, Prevalence and Risk Factors. Curr. Opin. Pulm. Med. 2020, 26, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Sarcoidosis. Nat. Rev. Dis. Primers 2019, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Scadding, J.G. Prognosis of Intrathoracic Sarcoidosis in England. A Review of 136 Cases after Five Years’ Observation. Br. Med. J. 1961, 2, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, M.; Carleo, A.; Cameli, P.; Bergantini, L.; Perrone, A.; Vietri, L.; Lanzarone, N.; Vagaggini, C.; Sestini, P.; Bargagli, E. BAL Biomarkers’ Panel for Differential Diagnosis of Interstitial Lung Diseases. Clin. Exp. Med. 2020, 20, 207–216. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Bergantini, L.; Mezzasalma, F.; Cavallaro, D.; Gangi, S.; Baglioni, S.; Armati, M.; Abbritti, M.; Cattelan, S.; Cameli, P.; et al. Immune-Checkpoint Expression on CD4, CD8 and NK Cells in Blood, Bronchoalveolar Lavage and Lymph Nodes of Sarcoidosis. Mol. Diagn. Ther. 2022, 26, 437–449. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Gangi, S.; Cavallaro, D.; Bergantini, L.; Mezzasalma, F.; Cattelan, S.; Baglioni, S.; Abbritti, M.; Cameli, P.; Bargagli, E. CD103 Expression on Regulatory and Follicular T Cells in Lymph Nodes, Bronchoalveolar Lavage Fluid and Peripheral Blood of Sarcoidosis Patients. Life 2022, 12, 762. [Google Scholar] [CrossRef]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef]
- Asai, Y.; Chiba, H.; Nishikiori, H.; Kamekura, R.; Yabe, H.; Kondo, S.; Miyajima, S.; Shigehara, K.; Ichimiya, S.; Takahashi, H. Aberrant Populations of Circulating T Follicular Helper Cells and Regulatory B Cells Underlying Idiopathic Pulmonary Fibrosis. Respir. Res. 2019, 20, 244. [Google Scholar] [CrossRef]
- Achour, A.; Simon, Q.; Mohr, A.; Séité, J.-F.; Youinou, P.; Bendaoud, B.; Ghedira, I.; Pers, J.-O.; Jamin, C. Human Regulatory B Cells Control the TFH Cell Response. J. Allergy Clin. Immunol. 2017, 140, 215–222. [Google Scholar] [CrossRef]
- Broos, C.E.; Hendriks, R.W.; Kool, M. T-Cell Immunology in Sarcoidosis: Disruption of a Delicate Balance between Helper and Regulatory T-Cells. Curr. Opin. Pulm. Med. 2016, 22, 476–483. [Google Scholar] [CrossRef]
- Kudryavtsev, I.; Zinchenko, Y.; Starshinova, A.; Serebriakova, M.; Malkova, A.; Akisheva, T.; Kudlay, D.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Circulating Regulatory T Cell Subsets in Patients with Sarcoidosis. Diagnostics 2023, 13, 1378. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Bergantini, L.; Gangi, S.; Conticini, E.; Cavallaro, D.; Cameli, P.; Mezzasalma, F.; Cantarini, L.; Frediani, B.; Bargagli, E. Immunological Pathways in Sarcoidosis and Autoimmune Rheumatic Disorders—Similarities and Differences in an Italian Prospective Real-Life Preliminary Study. Biomedicines 2023, 11, 1532. [Google Scholar] [CrossRef]
- Huang, H.; Lu, Z.; Jiang, C.; Liu, J.; Wang, Y.; Xu, Z. Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis. Int. J. Mol. Sci. 2013, 14, 21463–21473. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, M. Editorial: Sarcoidosis and Autoimmunity: From Bench to Bedside. Front. Med. 2023, 10, 1147529. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.M.; Riddell, N.E.; Akbar, A.N. Properties of End-Stage Human T Cells Defined by CD45RA Re-Expression. Curr. Opin. Immunol. 2012, 24, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh, S.; Esmaeil, N.; Shaygannejad, V.; Mirmosayyeb, O. Comparison of Follicular T Helper Cells, Monocytes, and T Cells Priming between Newly Diagnosed and Rituximab-Treated MS Patients and Healthy Controls. Res. Pharm. Sci. 2022, 17, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; Cameli, P.; d’Alessandro, M.; Vagaggini, C.; Refini, R.M.; Landi, C.; Pieroni, M.G.; Spalletti, M.; Sestini, P.; Bargagli, E. NK and NKT-like Cells in Granulomatous and Fibrotic Lung Diseases. Clin. Exp. Med. 2019, 19, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, I.; Serebriakova, M.; Starshinova, A.; Zinchenko, Y.; Basantsova, N.; Malkova, A.; Soprun, L.; Churilov, L.P.; Toubi, E.; Yablonskiy, P.; et al. Imbalance in B Cell and T Follicular Helper Cell Subsets in Pulmonary Sarcoidosis. Sci. Rep. 2020, 10, 1059. [Google Scholar] [CrossRef]
- Bergantini, L.; d’Alessandro, M.; Del Zotto, G.; Marcenaro, E.; Bargagli, E. Characterization of Natural Killer and T Cells in Bronchoalveolar Lavage and Peripheral Blood of Sarcoidosis Patients. Front. Immunol. 2022, 13, 1080556. [Google Scholar] [CrossRef]
- Zissel, G. Cellular Activation in the Immune Response of Sarcoidosis. Semin. Respir. Crit. Care Med. 2014, 35, 307–315. [Google Scholar] [CrossRef]
- Santosa, A.; Wong, C.F.; Koh, L.W. Multisystemic Sarcoidosis—Important Lessons Learnt from One of the Great Imitators. BMJ Case Reports CP 2019, 12, e227929. [Google Scholar] [CrossRef] [PubMed]
- Georas, S.N.; Chapman, T.J.; Crouser, E.D. Sarcoidosis and T-Helper Cells. Th1, Th17, or Th17.1? Am. J. Respir. Crit. Care Med. 2016, 193, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Facco, M.; Cabrelle, A.; Teramo, A.; Olivieri, V.; Gnoato, M.; Teolato, S.; Ave, E.; Gattazzo, C.; Fadini, G.P.; Calabrese, F.; et al. Sarcoidosis Is a Th1/Th17 Multisystem Disorder. Thorax 2011, 66, 144–150. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 Expression Inversely Correlates with FoxP3 and Suppressive Function of Human CD4+ T Reg Cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Wang, Y.-F. Phenotypic Identification of CD19+CD5+CD1d+ Regulatory B Cells That Produce Interleukin 10 and Transforming Growth Factor Β1 in Human Peripheral Blood. Arch. Med. Sci. 2019, 15, 1176–1183. [Google Scholar] [CrossRef]
- Dauphinée, M.; Tovar, Z.; Talal, N. B Cells Expressing CD5 Are Increased in Sjögren’s Syndrome. Arthritis Rheum. 1988, 31, 642–647. [Google Scholar] [CrossRef]
- Bauer, L.; Müller, L.J.; Volkers, S.M.; Heinrich, F.; Mashreghi, M.-F.; Ruppert, C.; Sander, L.E.; Hutloff, A. Follicular Helper–like T Cells in the Lung Highlight a Novel Role of B Cells in Sarcoidosis. Am. J. Respir. Crit. Care Med. 204 2021, 204, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Doan Ngoc, T.-M.; Tilly, G.; Danger, R.; Bonizec, O.; Masset, C.; Guérif, P.; Bruneau, S.; Glemain, A.; Harb, J.; Cadoux, M.; et al. Effector Memory-Expressing CD45RA (TEMRA) CD8+ T Cells from Kidney Transplant Recipients Exhibit Enhanced Purinergic P2X4 Receptor-Dependent Proinflammatory and Migratory Responses. J. Am. Soc. Nephrol. 2022, 33, 2211–2231. [Google Scholar] [CrossRef]
- Perl, A.; Morel, L. Expanding Scope of TEMRA in Autoimmunity. eBioMedicine 2023, 90, 104520. [Google Scholar] [CrossRef]
- Ding, T.; Su, R.; Wu, R.; Xue, H.; Wang, Y.; Su, R.; Gao, C.; Li, X.; Wang, C. Frontiers of Autoantibodies in Autoimmune Disorders: Crosstalk Between Tfh/Tfr and Regulatory B Cells. Front. Immunol. 2021, 12, 641013. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Costabel, U.; Ando, M.; Baughman, R.; Cordier, J.F.; du Bois, R.; Eklund, A.; Kitaichi, M.; Lynch, J.; Rizzato, G.; et al. ATS/ERS/WASOG Statement on Sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and Other Granulomatous Disorders. Sarcoidosis Vasc. Diffuse Lung Dis. 1999, 16, 149–173. [Google Scholar] [PubMed]
- Costabel, U.; Hunninghake, G.W. ATS/ERS/WASOG Statement on Sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur. Respir. J. 1999, 14, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Riedhammer, C.; Halbritter, D.; Weissert, R. Peripheral Blood Mononuclear Cells: Isolation, Freezing, Thawing, and Culture. Methods Mol. Biol. 2016, 1304, 53–61. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Sarcoidosis Patients n = 32 |
|---|---|
| Gender (F/M) | 19/13 |
| Median age (IQR), years | 57 (52–58) |
| Smoke (never/smoker) | 21/11 |
| Radiological Scadding stage II | 32/32 |
| BAL cellular pattern: | |
| Macrophages | 72 ± 12 |
| Lymphocytes | 19 ± 4 |
| Neutrophils | 4 ± 1 |
| Eosinophils | 1.5 ± 1.3 |
| CD4/CD8 ratio | 4.21 ± 2 |
| CD | Alternative Name | Clone | Isotype | Fluorochrome | Company |
|---|---|---|---|---|---|
| CD3 | T3, CD3ε | UCHT1 | IgG1 | APC-Cy7 | Biolegend (San Diego, CA, USA) |
| CD14 | Leu-M3, LPS-R | HCD14 | IgG1 | APC-Cy7 | Biolegend |
| CD16 | FCRγIII | 3G8 | IgG1 | BV510 | Biolegend |
| CD19 | B4 | HIB19 | IgG1 | APC-Cy7 | Biolegend |
| 7-AAD | PerCPCy5 | Miltenyi (Bergisch Gladbach, Germay) | |||
| CD56 | NCAM | HCD56 | IgG1 | PE-Cy7 | Biolegend |
| CXCR3 | CXCR3, CD183, CKR-L2, CMKAR3, GPR9, IP10-R, Mig-R, MigR | REA 232 | IgG1 | FITC | Miltenyi |
| CCR4 | CKR4, K5-5, CMKBR4, ChemR13, CC-CKR-4, MGC88293, HGCN:14099 | L291H4 | Mouse IgG1, κ | PE | BD (Franklin Lakes, NJ, USA) |
| CD45RA | PECy7 | ||||
| CCR6 | CCR6, BN-1, CKRL3, CMKBR6, DCR2, DRY6, GPR29, GPRCY4, STRL22 | REA 190 | IgG1 | APC | Miltenyi |
| CD4 | T4, Leu-3, CD4mut | REA 623 | IgG1 | APC-Cy7 | Miltenyi |
| CD8 | Leu-2, T8 | REA734 | IgG1 | VioBlue | Miltenyi |
| CXCR5 | J252D4 | PerCPCy5 | Biolegend | ||
| Treg-cocktail (CD4/25/127) | FITC/PE/PECy7 | BD | |||
| CD38 | T10; ADP-ribosyl cyclase 1; Cyclic ADP-ribose hydrolase 1; OKT10 | HB-7 | Mouse IgG1, κ | FITC | BD |
| CD1d | R3G1 | SK9 | Mouse IgG2b, κ | PE | BD |
| CD19 | B4, Leu-12 | REA 675 | IgG1 | PECy7 | Miltenyi |
| CD45 | Ptprc, GP180, L-CA, LY5, T200 | REA 747 | IgG1 | APC | Miltenyi |
| CD24 | Heat Stable Antigen Homologue (HSA); Ba-1; CD24A | ML5 | Mouse IgG2a, κ | APC-Cy7 | BD |
| CD27 | S152, T14, TNFRSF7 | M-T271 | Mouse IgG1, κ | BV510 | Biolegend |
| CD5 | LEU1; Leu-1; Lymphocyte antigen T1; T1; LY1; Tp67 | L17F12 | Mouse BALB/c IgG2a, κ | PerCPCy5 | BD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Alessandro, M.; Bergantini, L.; Gangi, S.; Cameli, P.; Armati, M.; Fanetti, M.; Mezzasalma, F.; Baglioni, S.; SARC-SI Study Group; Bargagli, E. Imbalance of Lymphocyte Subsets and CD45RA-Expressing Cells in Intrathoracic Lymph Nodes, Alveolar Compartment and Bloodstream of Pulmonary Sarcoidosis Patients. Int. J. Mol. Sci. 2023, 24, 10344. https://doi.org/10.3390/ijms241210344
d’Alessandro M, Bergantini L, Gangi S, Cameli P, Armati M, Fanetti M, Mezzasalma F, Baglioni S, SARC-SI Study Group, Bargagli E. Imbalance of Lymphocyte Subsets and CD45RA-Expressing Cells in Intrathoracic Lymph Nodes, Alveolar Compartment and Bloodstream of Pulmonary Sarcoidosis Patients. International Journal of Molecular Sciences. 2023; 24(12):10344. https://doi.org/10.3390/ijms241210344
Chicago/Turabian Styled’Alessandro, Miriana, Laura Bergantini, Sara Gangi, Paolo Cameli, Martina Armati, Matteo Fanetti, Fabrizio Mezzasalma, Stefano Baglioni, SARC-SI Study Group, and Elena Bargagli. 2023. "Imbalance of Lymphocyte Subsets and CD45RA-Expressing Cells in Intrathoracic Lymph Nodes, Alveolar Compartment and Bloodstream of Pulmonary Sarcoidosis Patients" International Journal of Molecular Sciences 24, no. 12: 10344. https://doi.org/10.3390/ijms241210344
APA Styled’Alessandro, M., Bergantini, L., Gangi, S., Cameli, P., Armati, M., Fanetti, M., Mezzasalma, F., Baglioni, S., SARC-SI Study Group, & Bargagli, E. (2023). Imbalance of Lymphocyte Subsets and CD45RA-Expressing Cells in Intrathoracic Lymph Nodes, Alveolar Compartment and Bloodstream of Pulmonary Sarcoidosis Patients. International Journal of Molecular Sciences, 24(12), 10344. https://doi.org/10.3390/ijms241210344









