Anatomy and Comparative Transcriptome Reveal the Mechanism of Male Sterility in Salvia miltiorrhiza
Abstract
1. Introduction
2. Results
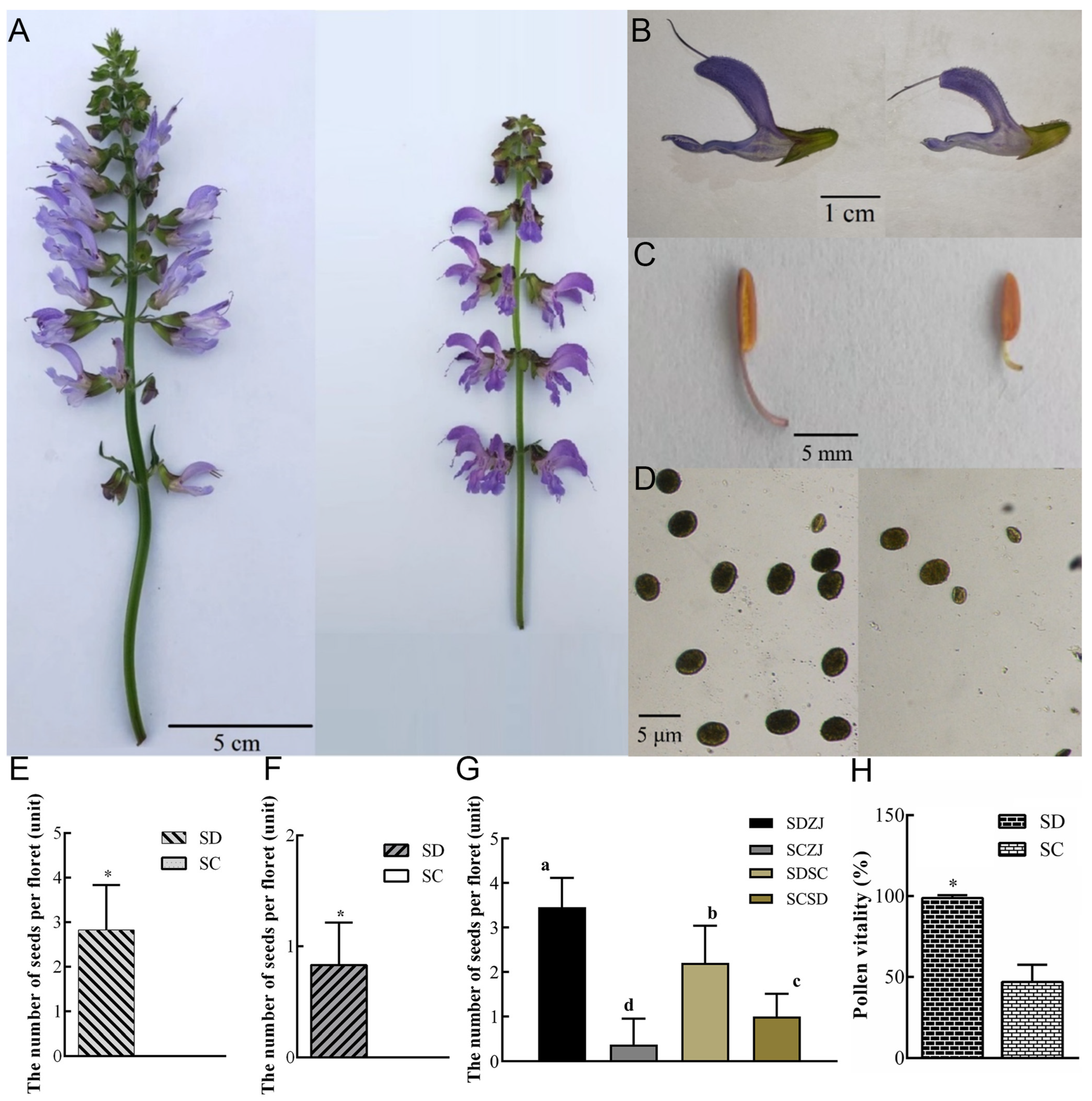
2.1. Defective Pistil and Pollen Grains Jointly Cause Sterility in SC
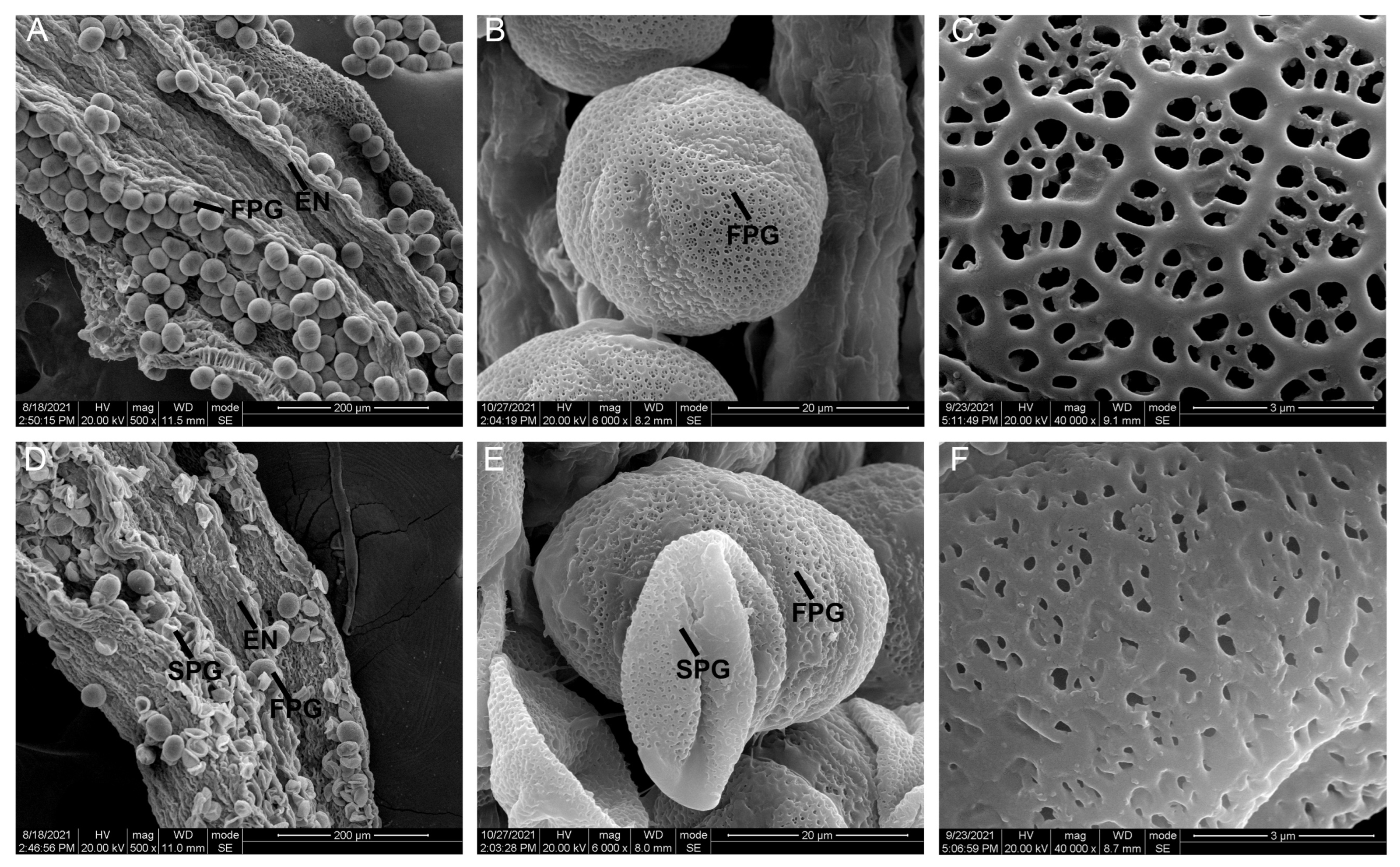
2.2. Sunken Pollen Grains and Impaired Pollen Walls Lead to Partial Abortion of SC Pollen Grains
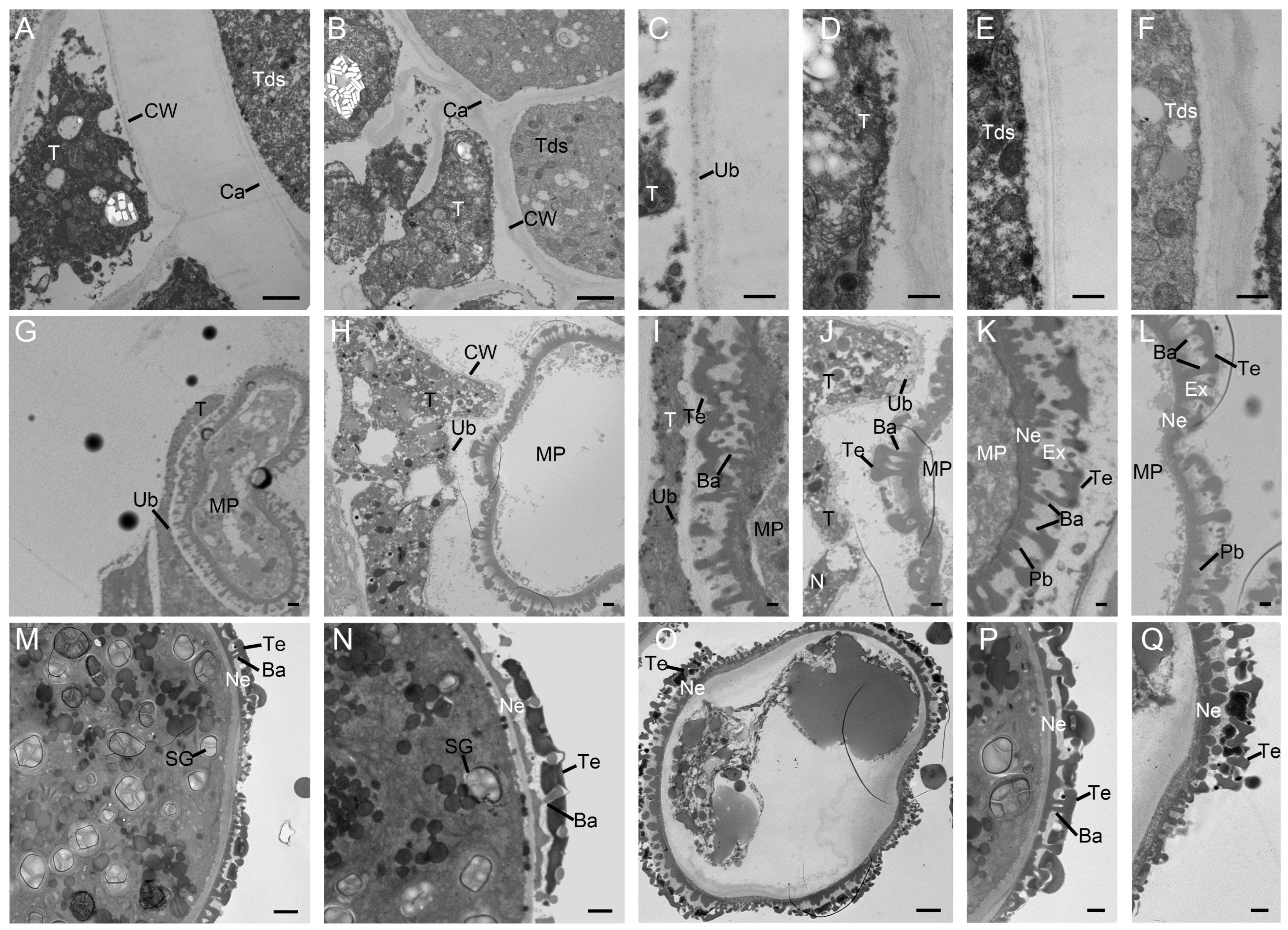
2.3. Defective Pollen Cell Walls in SC Caused by Delayed Degradation of Tapetum
2.4. Defects of the Microsporangium Wall and the Lack of Starch Lead to the Pollen Sterility
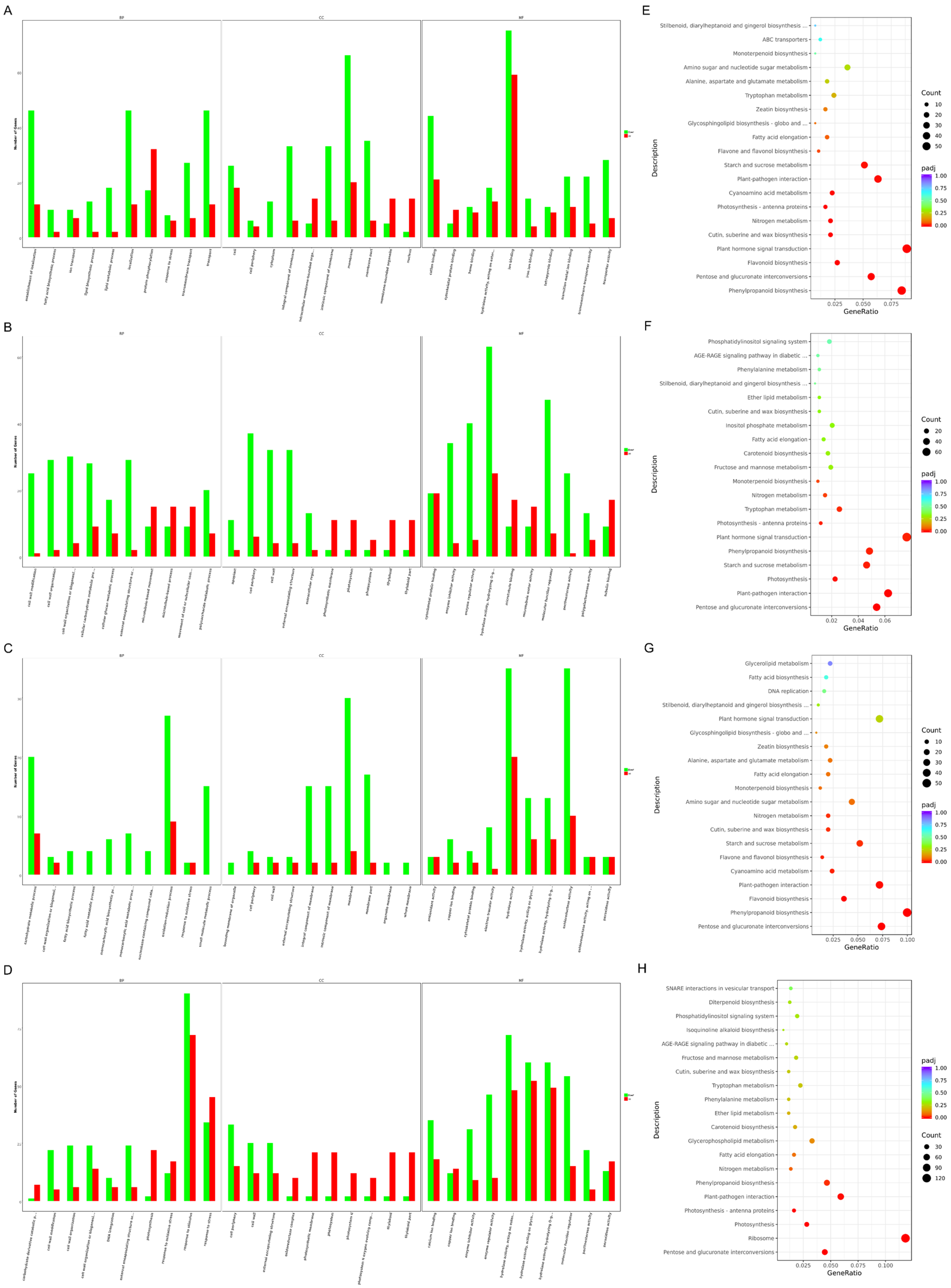
2.5. Identification of Differentially Expressed Genes (DEGs) between SD and SC at the Different Stages
2.6. Transcriptome Analysis Revealed Potential Pathways Related to Male Sterility
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Artificial Pollination
4.3. Phenotypic Analysis and Pollen Viability Analysis
4.4. SEM Analyses
4.5. Paraffin Section Observation
4.6. TEM Analyses
4.7. Transcriptome Sequencing Analysis
4.8. Q-PCR Assay/Quantitative Realtime-PCR (qRT-PCR) Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abarikwu, S.; Onuah, C.; Singh, S. Plants in the Management of Male Infertility. Andrologia 2020, 52, e13509. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Duan, W.; Song, X.; Shi, G.; Zhang, J.; Deng, X.; Zhang, S.; Hou, X. Instability in Mitochondrial Membranes in Polima Cytoplasmic Male Sterility of Brassica rapa ssp. chinensis. Funct. Integr. Genom. 2014, 14, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, W.; Chen, S.; Zhou, Y.; Ji, P.; Gong, Y.; Yang, Z.; Mao, Z.; Zhang, C.; Mu, F. TMT-Based Quantitative Proteomics Analyses of Sterile/Fertile Anthers from a Genic Male-Sterile Line and its Maintainer in Cotton (Gossypium hirsutum L.). J. Proteom. 2021, 232, 104026. [Google Scholar] [CrossRef]
- Hu, C.; Sheng, O.; Dong, T.; Yang, Q.; Dou, T.; Li, C.; He, W.; Gao, H.; Yi, G.; Deng, G.; et al. Overexpression of MaTPD1A Impairs Fruit and Pollen Development by Modulating Some Regulators in Musa itinerans. BMC Plant. Biol. 2020, 20, 402. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, H.; Kang, S.; Silva, J.; Kim, E.; Park, S.; Jung, K.; Lee, C. Physiological Importance of Pectin Modifying Genes During Rice Pollen Development. Int. J. Mol. Sci. 2020, 21, 4840. [Google Scholar] [CrossRef]
- Verma, N. Transcriptional Regulation of Anther Development in Arabidopsis. Gene 2019, 20, 202–209. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; Mclntire, K.N.; Hsu, Y.C.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther Developmental Defects in Arabidopsis thaliana Male-Sterile Mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Ks, R.; Ra, L.; Rd, W.; Ha, O.; Merced, A. Callose in sporogenesis: Novel Composition of the Inner Spore Wall in Hornworts. Plant Syst. Evol. 2020, 306, 16. [Google Scholar]
- Varnier, A.; Mazeyrat-Gourbeyre, F.; Sangwan, R.; Clément, C. Programmed Cell Death Progressively Models the Development of Anther Sporophytic Tissues from the Tapetum and Is Triggered in Pollen Grains during Maturation. J. Struct. Biol. 2005, 152, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, H.; Bai, J.; Ren, F. The Regulatory Framework of Developmentally Programmed Cell Death in Floral Organs: A Review. Plant Physiol. Biochem. 2021, 158, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, Y.; Zhang, G. Formation Pattern and Regulatory Mechanisms of Pollen Wall in Arabidopsis. J. Plant Physiol. 2021, 260, 153388. [Google Scholar] [CrossRef]
- Hirsche, J.; García Fernández, J.M.; Stabentheiner, E.; Großkinsky, D.K.; Roitsch, T. Differential Effects of Carbohydrates on Arabidopsis Pollen Germination. Plant Cell Physiol. 2017, 58, 691–701. [Google Scholar] [CrossRef]
- Wang, J.; Kambhampati, S.; Allen, D.K.; Chen, L. Comparative Metabolic Analysis Reveals a Metabolic Switch in Mature, Hydrated, and Germinated Pollen in Arabidopsis thaliana. Front Plant Sci. 2022, 13, 836665. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Cao, J. Pollen Wall Development: The Associated Enzymes and Metabolic Pathways. Plant Biol. 2013, 15, 249–263. [Google Scholar] [CrossRef]
- Grienenberger, E.; Quilichini, T.D. The Toughest Material in the Plant Kingdom: An Update on Sporopollenin. Front Plant Sci. 2021, 12, 703864. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xiong, S.; Yin, W.; Teng, X.; Lou, Y.; Zhu, J.; Zhang, C.; Gu, J.; Wilson, Z.; Yang, Z. MS1, a Direct Target of MS188, Regulates the Expression of Key Sporophytic Pollen Coat Protein Genes in Arabidopsis. J. Exp. Bot. 2020, 71, 4877–4889. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.C.; Pearce, S.; Band, L.R.; Yang, C.; Ferjentsikova, I.; King, J.; Yuan, Z.; Zhang, D.; Wilson, Z.A. Biphasic Regulation of the Transcription Factor ABORTED MICROSPORES (AMS) Is Essential for Tapetum and Pollen Development in Arabidopsis. New Phytol. 2017, 213, 778–790. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, J.; Yu, Y.; Teng, X.; Lou, Y.; Xu, X.; Liu, J.; Yang, Z. DYT1 Directly Regulates the Expression of TDF1 for Tapetum Development and Pollen Wall Formation in Arabidopsis. Plant J. 2014, 80, 1005–1013. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Li, H.; Gao, J.F.; Jiang, H.; Wang, C.; Guan, Y.F.; Yang, Z.N. Defective in Tapetal Development and Function 1 is Essential for Anther Development and Tapetal Function for Microspore Maturation in Arabidopsis. Plant J. 2008, 55, 266–277. [Google Scholar] [CrossRef]
- Zhao, H.; Han, B.; Li, X.; Sun, C.; Zhai, Y.; Li, M.; Jiang, M.; Zhang, W.; Liang, Y.; Kai, G. Salvia miltiorrhiza in Breast Cancer Treatment: A Review of Its Phytochemistry, Derivatives, Nanoparticles, and Potential Mechanisms. Front. Pharmacol. 2022, 13, 872085. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases. Drug Des. Devel. Ther. 2020, 14, 4735–4748. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Liu, P. Salvia miltiorrhiza Bunge (Danshen): A Golden Herbal Medicine in Cardiovascular Therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef]
- Yao, S.; Jiang, Y.; Ni, S.; Wang, L.; Feng, J.; Yang, R.W.; Yang, L.; Len, Q.; Zhang, L. Development of a Highly Efficient Virus-Free Regeneration System of Salvia miltiorrhiza from Sichuan Using Apical Meristem as Explants. Plant Methods 2022, 18, 50. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, H.; Zhou, Z.; Guo, H.; Dong, J. Physiological and Transcriptome Analysis Reveal Molecular Mechanism in Salvia miltiorrhiza Leaves of Near-isogenic Male Fertile Lines and Male Sterile Lines. BMC Genom. 2019, 20, 780. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, Y.; Wang, L.; Wu, Y.; Liao, J.; Zhong, M.; Yang, R.; Chen, X.; Li, Q.; Zhang, L. Comparative Transcriptome Analysis Reveals Key Insights into Male Sterility in Salvia miltiorrhiza Bunge. Peer J. 2021, 9, e11326. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, A.; Zhang, B. Salviae miltiorrhiza: A Model Organism for Chinese Traditional Medicine Genomic Studies. Acta Chin. Med. Pharmacol. 2009, 37, 1–3+101. [Google Scholar]
- Huang, Y.; Feng, C.; Ye, Q.; Wu, W.; Chen, Y. Arabidopsis WRKY6 Transcription Factor Acts as a Positive Regulator of Abscisic Acid Signaling during Seed Germination and Early Seedling Development. PLoS Genet. 2016, 12, e1005833. [Google Scholar] [CrossRef]
- Song, G.; Li, X.; Munir, R.; Khan, A.; Azhar, W.; Yasin, M.; Jiang, Q.; Bancroft, I.; Gan, Y. The WRKY6 Transcription Factor Affects Seed Oil Accumulation and Alters Fatty Acid Compositions in Arabidopsis thaliana. Physiol. Plant. 2020, 169, 612–624. [Google Scholar] [CrossRef]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.; Zhang, D. Genetic and Biochemical Mechanisms of Pollen Wall Development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Ariizumi, T.; Toriyama, K. Genetic Regulation of Sporopollenin Synthesis and Pollen Exine Development. Annu. Rev. Plant Biol. 2011, 62, 437–460. [Google Scholar] [CrossRef]
- Yang, H.; Liu, F.; Wang, W.; Rui, Q.; Li, G.; Tan, X.; Ye, J.; Shen, H.; Liu, Y.; Liu, W.; et al. Sporopollenin Biosynthesis in an OsTKPR2-Containg Metabolon Is Required for Rice Pollen Exine Formation. J. Exp. Bot. 2023, 74, 1911–1925. [Google Scholar] [CrossRef]
- Wan, X.; Wu, S.; Li, Z.; An, X.; Tian, Y. Lipid Metabolism: Critical Roles in Male Fertility and Other Aspects of Reproductive Development in Plants. Mol Plant. 2020, 13, 955–983. [Google Scholar] [CrossRef]
- Gabarayeva, N.; Polevova, S.; Grigorjeva, V.; Severova, E.; Volkova, O.; Blackmore, S. Suggested Mechanisms Underlying Pollen Wall Development in Ambrosia trifida (Asteraceae: Heliantheae). Protoplasma 2019, 256, 555–574. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J.; Ma, L.; Wang, H.; Zhou, H.; Ni, E.; Jiang, D.; Liu, Z.; Zhuang, C. OsAGO2 Controls ROS Production and the Initiation of Tapetal PCD by Epigenetically Regulating OsHXK1 Expression in Rice Anthers. Proc. Natl. Acad. Sci. USA 2019, 116, 7549–7558. [Google Scholar] [CrossRef]
- Xie, D.; Zheng, X.; Zhou, C.; Kanwar, M.; Zhou, J. Functions of Redox Signaling in Pollen Development and Stress Response. Antioxidants 2022, 11, 287. [Google Scholar] [CrossRef]
- Bai, Z.; Ding, X.; Zhang, R.; Yang, Y.; Wei, B.; Yang, S.; Gai, J. Transcriptome Analysis Reveals the Genes Related to Pollen Abortion in a Cytoplasmic Male-Sterile Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2022, 23, 12227. [Google Scholar] [CrossRef]
- Paul, W.; Hodge, R.; Smartt, S.; Draper, J.; Scott, R. The Isolation and Characterisation of the Tapetum-Specific Arabidopsis thaliana A9 Gene. Plant Mol. Biol. 1992, 19, 611–622. [Google Scholar] [CrossRef]
- Verma, N.; Burma, P.K. Regulation of Tapetum-Specific A9 Promoter by Transcription Factors AtMYB80, AtMYB1 and AtMYB4 in Arabidopsis thaliana and Nicotiana tabacum. Plant J. 2017, 92, 481–494. [Google Scholar] [CrossRef]
- Matsushima, R.; Hisano, H. Imaging Amyloplasts in the Developing Endosperm of Barley and Rice. Sci Rep. 2019, 9, 3745. [Google Scholar] [CrossRef]
- Yue, J.; Ren, Y.; Wu, S.; Zhang, X.; Wang, H.; Tang, C. Differential Proteomic Studies of the Genic Male-Sterile Line and Fertile Line Anthers of Upland Cotton (Gossypium hirsutum L.). Genes Genom. 2014, 36, 415–426. [Google Scholar] [CrossRef]
- Sun, W.; Cheng, S.; Chao, Y.; Lin, S.; Yang, T.; Ho, Y.; Shih, M.; Ko, S. Sugars and Sucrose Transporters in Pollinia of Phalaenopsis aphrodite (Orchidaceae). J. Exp. Bot. 2023, 74, 2556–2571. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Cho, J.; Nguyen, C.; Moo, S.; Park, J.; Park, H.; Huh, J.; Jung, K.; Guiderdoni, E.; et al. Deficiency of Rice Hexokinase HXK5 Impairs Synthesis and Utilization of Starch in Pollen Grains and Causes Male Sterility. J. Exp. Bot. 2020, 71, 116–125. [Google Scholar] [CrossRef]
- Cecchetti, V.; Altamura, M.; Falasca, G.; Costantino, P.; Cardarelli, M. Auxin Regulates Arabidopsis Anther Dehiscence, Pollen Maturation, and Filament Elongation. Plant Cell. 2008, 20, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Li, Y.; Hu, Q.; Zhu, L.; Gao, W.; Wu, Y.; Ding, Y.; Liu, S.; Yang, X.; Zhang, X. Sugar and Auxin Signaling Pathways Respond to High-Temperature Stress during Anther Development as Revealed by Transcript Profiling Analysis in Cotton. Plant Physiol. 2014, 164, 1293–1308. [Google Scholar] [CrossRef]
- Oliver, S.; Dennis, E.; Dolferus, R. ABA Regulates Apoplastic Sugar Transport and Is a Potential Signal for Cold-induced Pollen Sterility in Rice. Plant Cell Physiol. 2007, 48, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic acid Prevents Pollen Abortion under High-Temperature Stress by Mediating Sugar Metabolism in Rice Spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar] [CrossRef]
- Wang, M.; Hoekstra, S.; van Bergen, S.; Lamers, G.E.; Oppedijk, B.J.; van der Heijden, M.W.; de Priester, W.; Schilperoort, R.A. Apoptosis in Developing Anthers and the Role of ABA in this Process during Androgenesis in Hordeum vulgare L. Plant Mol. Biol. 1999, 39, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Zhang, J.; Shen, F.C.; Huang, Y.X.; Wu, Z.W. Characterization and Mapping of a New Male Sterility Mutant of Anther Advanced Dehiscence (t) in Rice. J. Genet. Genom. 2008, 35, 177–182. [Google Scholar] [CrossRef]
- Bano, A.; Rashid, S.; Ahmad, M.; Bhatti, G.R.; Yaseen, G.; Anjum, F.; Ahmed, S.N.; Zafar, M.; Asma, M.; Sultana, S.; et al. Comparative Pollen and Foliar Micromorphological Studies Using Light Microscopy and Scanning Electron Microscopy of Some Selected Species of Lamiaceae from Alpine Zone of Deosai Plateau, Western Himalayas. Microsc. Res. Tech. 2020, 83, 579–588. [Google Scholar] [CrossRef]
- Ellis, E.A. Staining Sectioned Biological Specimens for Transmission Electron Microscopy: Conventional and En bloc stains. Methods Mol. Biol. 2014, 1117, 57–72. [Google Scholar]
- Livak, K.; Schmittgen, T. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Zhang, Z.; Shang, Y.; Jiang, Y.; Su, Z.; Deng, X.; Pu, X.; Yang, R.; Zhang, L. Anatomy and Comparative Transcriptome Reveal the Mechanism of Male Sterility in Salvia miltiorrhiza. Int. J. Mol. Sci. 2023, 24, 10259. https://doi.org/10.3390/ijms241210259
Liao J, Zhang Z, Shang Y, Jiang Y, Su Z, Deng X, Pu X, Yang R, Zhang L. Anatomy and Comparative Transcriptome Reveal the Mechanism of Male Sterility in Salvia miltiorrhiza. International Journal of Molecular Sciences. 2023; 24(12):10259. https://doi.org/10.3390/ijms241210259
Chicago/Turabian StyleLiao, Jinqiu, Zhizhou Zhang, Yukun Shang, Yuanyuan Jiang, Zixuan Su, Xuexue Deng, Xiang Pu, Ruiwu Yang, and Li Zhang. 2023. "Anatomy and Comparative Transcriptome Reveal the Mechanism of Male Sterility in Salvia miltiorrhiza" International Journal of Molecular Sciences 24, no. 12: 10259. https://doi.org/10.3390/ijms241210259
APA StyleLiao, J., Zhang, Z., Shang, Y., Jiang, Y., Su, Z., Deng, X., Pu, X., Yang, R., & Zhang, L. (2023). Anatomy and Comparative Transcriptome Reveal the Mechanism of Male Sterility in Salvia miltiorrhiza. International Journal of Molecular Sciences, 24(12), 10259. https://doi.org/10.3390/ijms241210259






