Drought Eliminates the Difference in Root Trait Plasticity and Mycorrhizal Responsiveness of Two Semiarid Grassland Species with Contrasting Root System
Abstract
1. Introduction
2. Results
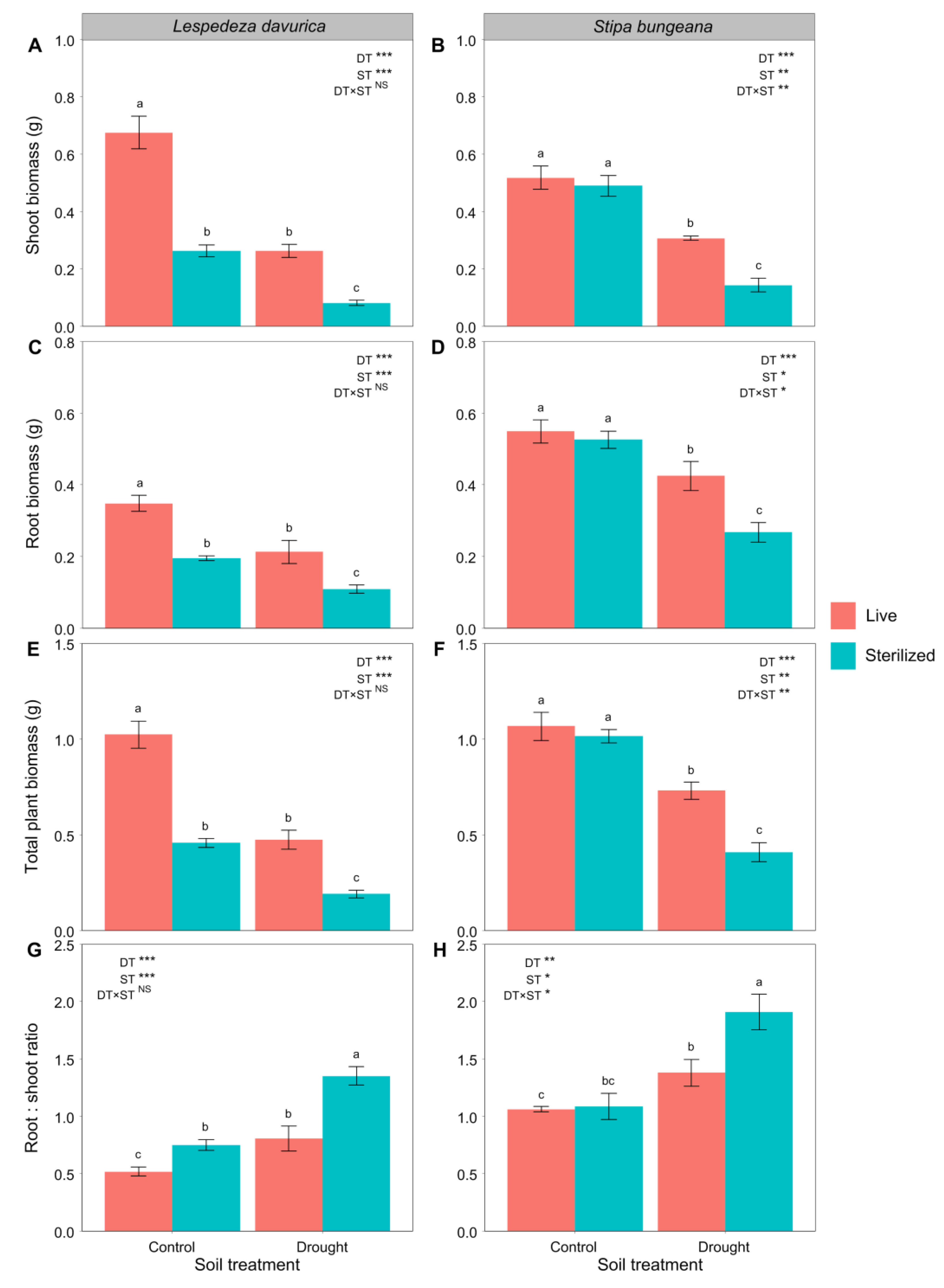
2.1. Plant Biomass and Biomass Allocation
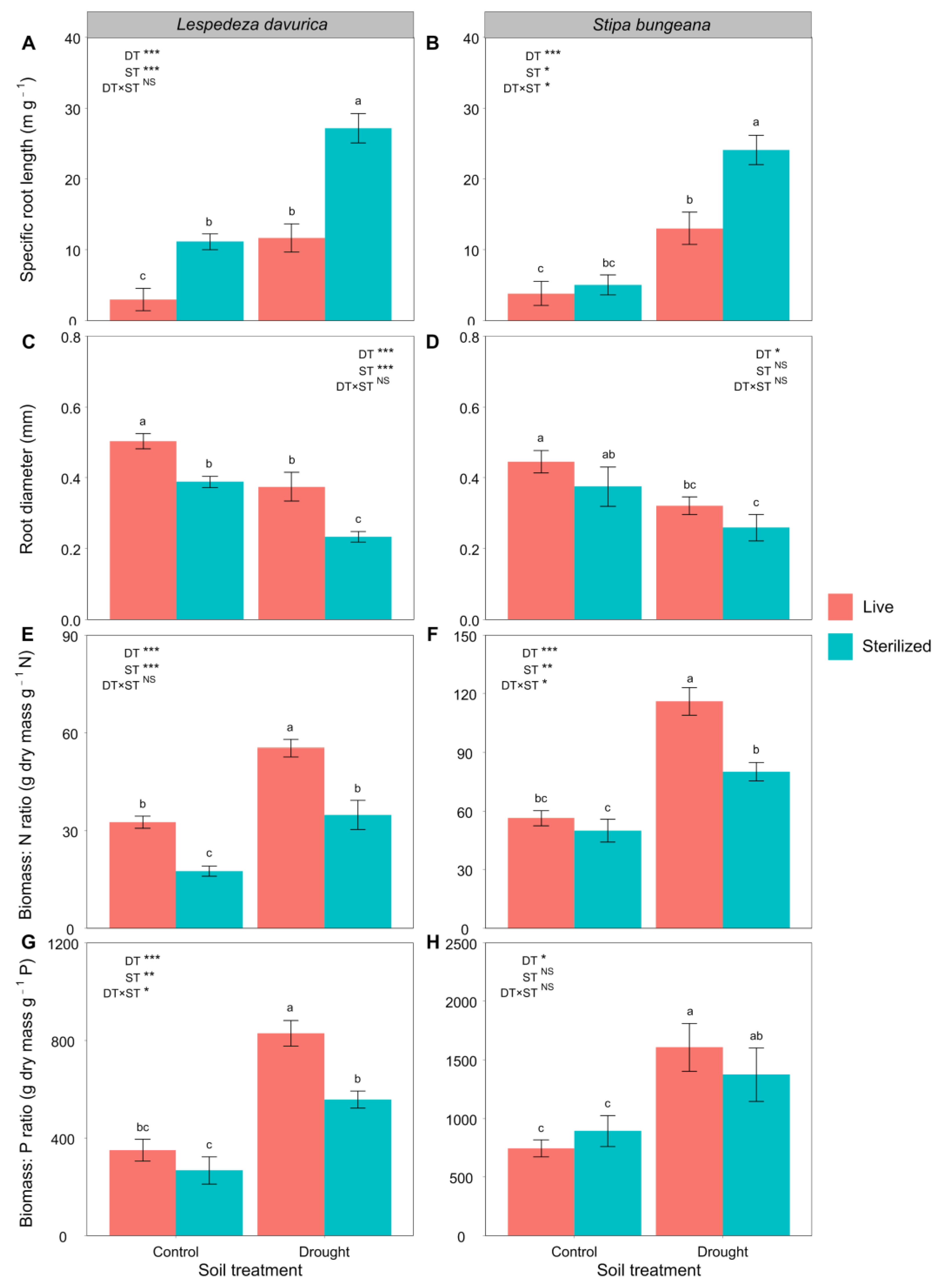
2.2. Root Traits
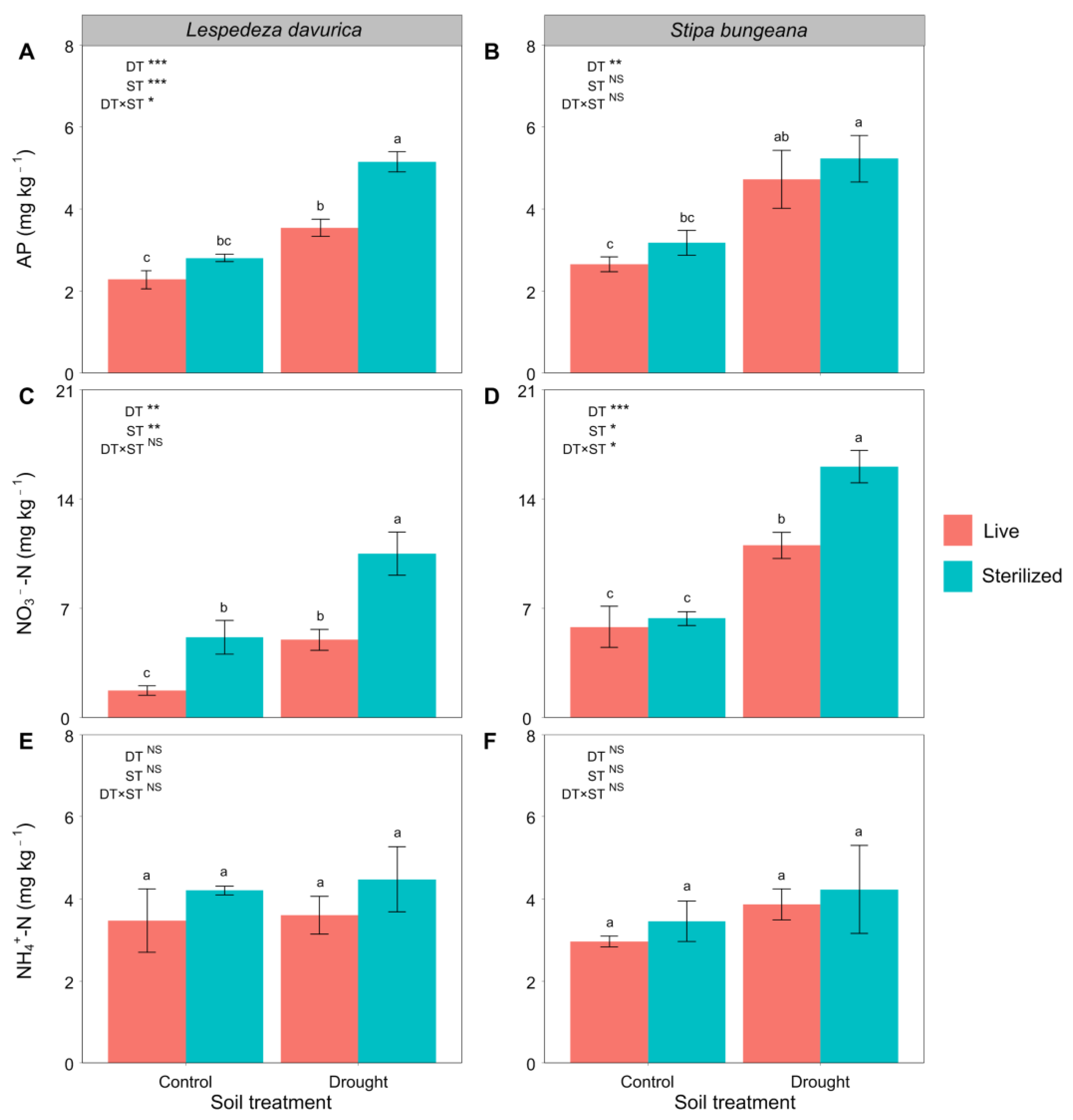
2.3. Soil Nutrients
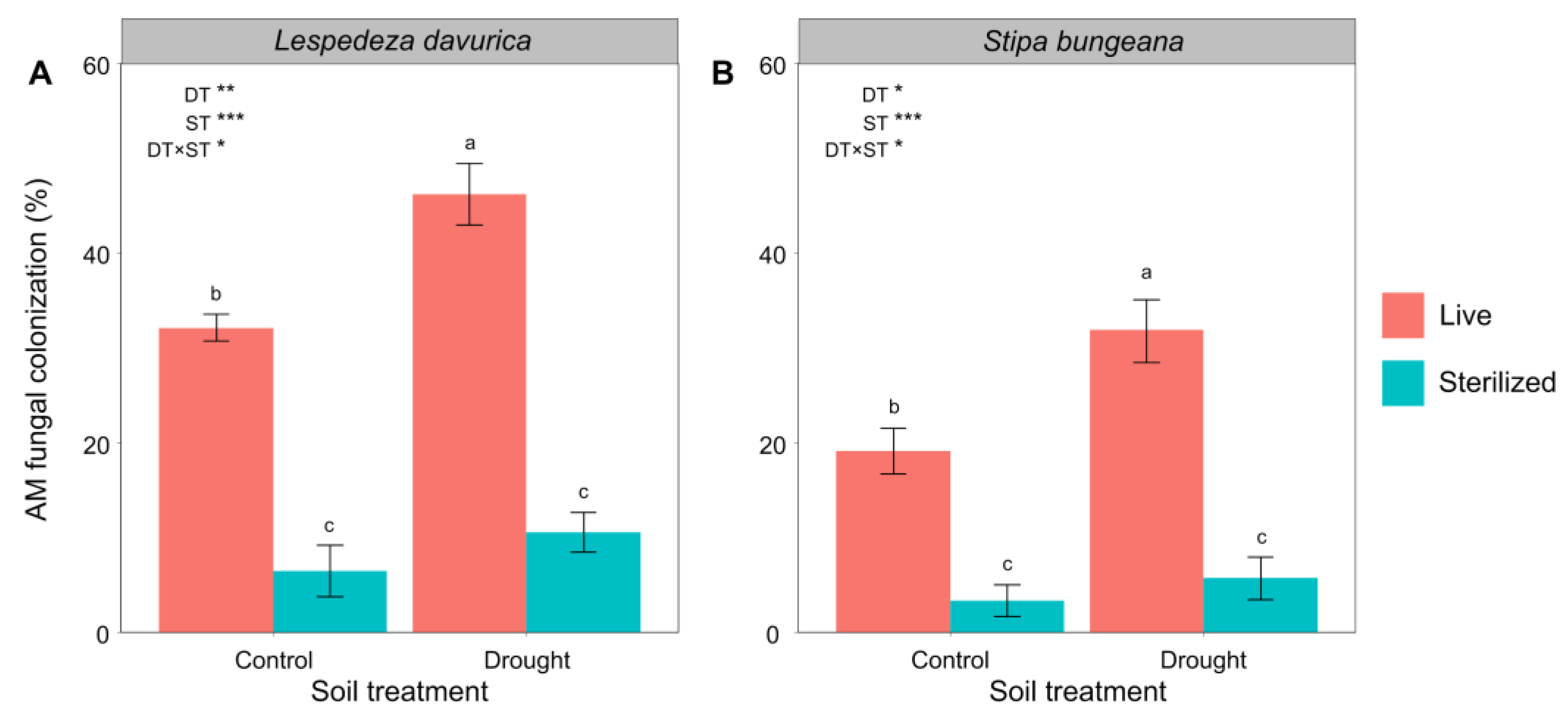
2.4. AM Fungal Colonization
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Study Plants and Seed Collection
4.3. Soil Sampling and Processing
4.4. Glasshouse Experiment
4.5. Plant Measurements
4.6. Soil Measurements
4.7. Arbuscular Mycorrhizal Fungi in Roots
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, W.M.; Wan, S.Q.; Niu, S.L.; Liu, W.X.; Chen, Q.S.; Wang, Q.B.; Zhang, W.H.; Han, X.G.; Li, L.H. Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: Implications for ecosystem C cycling. Glob. Chang. Biol. 2010, 16, 1306–1316. [Google Scholar] [CrossRef]
- Batbaatar, A.; Carlyle, C.N.; Bork, E.W.; Chang, S.X.; Cahill, J.F. Multi-year drought alters plant species composition more than productivity across northern temperate grasslands. J. Ecol. 2022, 110, 197–209. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogee, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Dudenhoffer, J.H.; Luecke, N.C.; Crawford, K.M. Changes in precipitation patterns can destabilize plant species coexistence via changes in plant-soil feedback. Nat. Ecol. Evol. 2022, 6, 546–554. [Google Scholar] [CrossRef]
- Yang, H.J.; Li, Y.; Wu, M.Y.; Zhang, Z.; Li, L.H.; Wan, S.Q. Plant community responses to nitrogen addition and increased precipitation: The importance of water availability and species traits. Glob. Chang. Biol. 2011, 17, 2936–2944. [Google Scholar] [CrossRef]
- Christensen, L.; Coughenour, M.B.; Ellis, J.E.; Chen, Z.Z. Vulnerability of the Asian typical steppe to grazing and climate change. Clim. Chang. 2004, 63, 351–368. [Google Scholar] [CrossRef]
- Sasaki, T.; Okayasu, T.; Jamsran, U.; Takeuchi, K. Threshold changes in vegetation along a grazing gradient in Mongolian rangelands. J. Ecol. 2008, 96, 145–154. [Google Scholar] [CrossRef]
- Brunner, I.; Godbold, D.L. Tree roots in a changing world. J. For. Res. 2007, 12, 78–82. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.X.; Yang, L.D.; Guo, J.Y. Effects of drought and warming on biomass, nutrient allocation, and oxidative stress in Abies fabri in eastern Tibetan Plateau. J. Plant Growth Regul. 2013, 32, 298–306. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Flaig, I.C.; Rillig, M.C. Root trait responses to drought are more heterogeneous than leaf trait responses. Funct. Ecol. 2020, 34, 2224–2235. [Google Scholar] [CrossRef]
- Reich, P.B. Do plants increase resource acquisition potential in the face of resource shortfalls, and if so, how? New Phytol. 2018, 219, 1142–1144. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, J.; Bai, W.M.; Zhang, Y.S.; Zhang, W.H. The response of root traits to precipitation change of herbaceous species in temperate steppes. Funct. Ecol. 2019, 33, 2030–2041. [Google Scholar] [CrossRef]
- Larson, J.E.; Funk, J.L. Seedling root responses to soil moisture and the identification of a belowground trait spectrum across three growth forms. New Phytol. 2016, 210, 827–838. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Differences in seedling growth behaviour among species: Trait correlations across species, and trait shifts along nutrient compared to rainfall gradients. J. Ecol. 1999, 87, 85–97. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Debinski, D.M.; Wickham, H.; Kindscher, K.; Caruthers, J.C.; Germino, M. Montane meadow change during drought varies with background hydrologic regime and plant functional group. Ecology 2010, 91, 1672–1681. [Google Scholar] [CrossRef]
- Jiang, L.B.; Liu, H.Y.; Peng, Z.Y.; Dai, J.Y.; Zhao, F.J.; Chen, Z.T. Root system plays an important role in responses of plant to drought in the steppe of China. Land Degrad. Dev. 2021, 32, 3498–3506. [Google Scholar] [CrossRef]
- Torrey, J.G.; Clarkson, D.T. The Development and Function of Roots; Academic Press, Inc.: New York, NY, USA, 1975. [Google Scholar]
- Ye, C. Botany; Higher Education Press: Beijing, China, 2007; p. 89. [Google Scholar]
- Breitschwerdt, E.; Jandt, U.; Bruelheide, H. Trait-performance relationships of grassland plant species differ between common garden and field conditions. Ecol. Evol. 2019, 9, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Jhc, C.; Jalili, A. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fry, E.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil 2018, 433, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, D.; Kitt, D.G.; Wilson, G.T. Mycorrhizal dependence and growth habit of warm-season and cool-season tallgrass prairie plants. Can. J. Bot. 1988, 66, 1376–1380. [Google Scholar] [CrossRef]
- Lai, W.L.; Wang, S.Q.; Peng, C.L.; Chen, Z.H. Root features related to plant growth and nutrient removal of 35 wetland plants. Water Res. 2011, 45, 3941–3950. [Google Scholar] [PubMed]
- McCormack, M.L.; Kaproth, M.A.; Cavender-Bares, J.; Carlson, E.; Hipp, A.L.; Han, Y.; Kennedy, P.G. Climate and phylogenetic history structure morphological and architectural trait variation among fine-root orders. New Phytol. 2020, 228, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Zadworny, M.; Eissenstat, D.M. Contrasting the morphology, anatomy and fungal colonization of new pioneer and fibrous roots. New Phytol. 2011, 190, 213–221. [Google Scholar] [CrossRef]
- Hetrick, B. Mycorrhizas and root architecture. Cell. Mol. Life Sci. 1991, 47, 355–362. [Google Scholar] [CrossRef]
- Newsham, K.K.; Fitter, A.H.; Watkinson, A.R. Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol. Evol. 1995, 10, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Gianinazzi-Pearson, V. Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 221–244. [Google Scholar] [CrossRef]
- Baylis, G. The magnolioid mycorrhiza and mycotrophy in root systems derived from it. In Endomycorrhizas; Sanders, F.E., Mosse, B., Tinker, P.B., Eds.; Academic Press: New York, NY, USA, 1975; pp. 373–389. [Google Scholar]
- Smith SE, R.D. Mycorrhizal Symbiosis; Academic Press Inc.: San Diego, CA, USA, 2008. [Google Scholar]
- Mariotte, P.; Canarini, A.; Dijkstra, F.A. Stoichiometric N:P flexibility and mycorrhizal symbiosis favour plant resistance against drought. J. Ecol. 2017, 105, 958–967. [Google Scholar] [CrossRef]
- Remke, M.J.; Johnson, N.C.; Wright, J.; Williamson, M.; Bowker, M.A. Sympatric pairings of dryland grass populations, mycorrhizal fungi and associated soil biota enhance mutualism and ameliorate drought stress. J. Ecol. 2021, 109, 1210–1223. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Mathimaran, N.; Boller, T.; Kahmen, A. Bioirrigation: A common mycorrhizal network facilitates the water transfer from deep-rooted pigeon pea to shallow-rooted finger millet under drought. Plant Soil 2019, 440, 277–292. [Google Scholar] [CrossRef]
- Sikes, B.A.; Cottenie, K.; Klironomos, J.N. Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J. Ecol. 2009, 97, 1274–1280. [Google Scholar] [CrossRef]
- Wilson, G.; Hartnett, D.C. Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am. J. Bot. 1998, 85, 1732–1738. [Google Scholar] [CrossRef]
- Yang, H.S.; Zhang, Q.; Dai, Y.J.; Liu, Q.; Tang, J.J.; Bian, X.M.; Chen, X. Effects of arbuscular mycorrhizal fungi on plant growth depend on root system: A meta-analysis. Plant Soil 2015, 389, 361–374. [Google Scholar] [CrossRef]
- Chen, T.; Christensen, M.; Nan, Z.B.; Hou, F.J. The effects of different intensities of long-term grazing on the direction and strength of plant-soil feedback in a semiarid grassland of Northwest China. Plant Soil 2017, 413, 303–317. [Google Scholar] [CrossRef]
- Cheng, J.; Cheng, J.; Hu, T. Distribution responses of Lespedeza davurica community on Loess Plateau to climate change. Chin. J. Appl. Ecol. 2011, 22, 35–40. [Google Scholar]
- Zhang, J. A study on relations of vegetation, climate and soils in Shanxi province, China. Plant Ecol. 2002, 162, 23–31. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, Z.B.; Dong, K.H.; Yang, W.D.; Zhu, H.S.; Liang, P.F. Effects of drought stress and rewatering on enzymatic defensive system in Lespedeza davurica (Laxm.) Schindl. Acta Agrestia Sin. 2010, 18, 199–204,211. [Google Scholar]
- Bell, D.L.; Sultan, S.E. Dynamic phenotypic plasticity for root growth in Polygonum: A comparative study. Am. J. Bot. 1999, 86, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.M.; Miranda, J.D.; Jorquera, M.J.; Pugnaire, F.I. Variability in amount and frequency of water supply affects roots but not growth of arid shrubs. Plant Ecol. 2009, 204, 261–270. [Google Scholar] [CrossRef]
- Williams, D.G.; Black, R.A. Drought response of a native and introduced Hawaiian grass. Oecologia 1994, 97, 512–519. [Google Scholar] [CrossRef]
- Chapin, I. The Mineral Nutrition of Wild Plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.B.; Tian, D.; Han, W.X.; Tang, Z.Y.; Fang, J.Y. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Packer, A.; Clay, K. Soil pathogens and Prunus serotina seedling and sapling growth near conspecific trees. Ecology 2003, 84, 108–119. [Google Scholar] [CrossRef]
- Ryalls, J.M.W.; Moore, B.D.; Johnson, S.N.; Connor, M.; Hiltpold, I. Root responses to domestication, precipitation and silicification: Weeping meadow grass simplifies and alters toughness. Plant Soil 2018, 427, 291–304. [Google Scholar] [CrossRef]
- Holdaway, R.J.; Richardson, S.J.; Dickie, I.A.; Peltzer, D.A.; Coomes, D.A. Species- and community-level patterns in fine root traits along a 120000-year soil chronosequence in temperate rain forest. J. Ecol. 2011, 99, 954–963. [Google Scholar] [CrossRef]
- Prieto, I.; Roumet, C.; Cardinael, R.; Dupraz, C.; Jourdan, C.; Kim, J.H.; Maeght, J.L.; Mao, Z.; Pierret, A.; Portillo, N.; et al. Root functional parameters along a land-use gradient: Evidence of a community-level economics spectrum. J. Ecol. 2015, 103, 361–373. [Google Scholar] [CrossRef]
- Weemstra, M.; Sterck, F.J.; Visser, E.J.W.; Kuyper, T.W.; Goudzwaard, L.; Mommer, L. Fine-root trait plasticity of beech (Fagus sylvatica) and spruce (Picea abies) forests on two contrasting soils. Plant Soil 2017, 415, 175–188. [Google Scholar] [CrossRef]
- Florian, F.; Claire, J.; Pablo, C. Root and leaf functional trait relations in Poaceae species: Implications of differing resource-acquisition strategies. J. Plant Ecol. 2013, 6, 211–219. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Craine, J.M.; Wedin, D.; Reich, P.B.; Tilman, D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol. 2005, 167, 493–508. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Flaig, I.C.; Rillig, M.C. Root trait responses to drought depend on plant functional group. bioRxiv 2019. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.I.; Vilagrosa, A.; Pausas, J.G.; Bellot, J. Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecol. 2010, 207, 233–244. [Google Scholar] [CrossRef]
- Read, D.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems–a journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Cheng, L.; Booker, F.L.; Tu, C.; Burkey, K.O.; Zhou, L.S.; Shew, H.D.; Rufty, T.W.; Hu, S.J. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 2012, 337, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Koide, R.T.; Fernandez, C.; Malcolm, G. Determining place and process: Functional traits of ectomycorrhizal fungi that affect both community structure and ecosystem function. New Phytol. 2014, 201, 433–439. [Google Scholar] [CrossRef]
- Koide, R. Nutrient supply, nutrient demand and plant-response to mycorrhizal infection. New Phytol. 1991, 117, 365–386. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; He, M.Z.; Johansen, M.P.; Harrison, J.J.; Keitel, C. Plant and microbial uptake of nitrogen and phosphorus affected by drought using N-15 and P-32 tracers. Soil Biol. Biochem. 2015, 82, 135–142. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Koide, R.T. Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol. 2000, 147, 233–235. [Google Scholar] [CrossRef]
- Tedersoo, L.; Smith, M.E. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef]
- Puy, J.; Carmona, C.P.; Hiiesalu, I.; Opik, M.; de Bello, F.; Moora, M. Mycorrhizal symbiosis alleviates plant water deficit within and across generations via phenotypic plasticity. J. Ecol. 2022, 110, 262–276. [Google Scholar] [CrossRef]
- Auge, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource limitation in plants-an economic analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Martinez-Garcia, L.B.; Miranda, J.D.; Pugnaire, F.I. Impacts of changing rainfall patterns on mycorrhizal status of a shrub from arid environments. Eur. J. Soil Biol. 2012, 50, 64–67. [Google Scholar] [CrossRef]
- Pozo, M.J.; Lopez-Raez, J.A.; Azcon-Aguilar, C.; Garcia-Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Hardie, K. The effect of removal of extraradical hyphae on water uptake by vesicular-arbuscular mycorrhizal plants. New Phytol. 1985, 101, 677–684. [Google Scholar] [CrossRef]
- Allen, M.F.; Sexton, J.C.; Moore, T.S.J.; Christensen, M. Influence of phosphate source on vesicular-arbuscular mycorrhizae of Bouteloua gracilis. New Phytol. 1981, 87, 687–694. [Google Scholar] [CrossRef]
- Bowles, T.M.; Jackson, L.E.; Cavagnaro, T.R. Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Chang. Biol. 2018, 24, e171–e182. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreno, A.M.; Molina, S.; Andreo-Jimenez, B.; Porcel, R.; Garcia-Mina, J.M.; Ruyter-Spira, C.; Lopez-Raez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Knapp, D.G.; Kovacs, G.M.; Zajta, E.; Groenewald, J.Z.; Crous, P.W. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 2015, 35, 87–100. [Google Scholar] [CrossRef]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Bloemberg, G.V.; Lugtenberg, B.J. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 2001, 4, 343–350. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Park, J.M.; Lazarovits, G.; Huner, N.P.A. Involvement of plant stress hormones in Burkholderia phytofirmans-induced shoot and root growth promotion. Plant Growth Regul. 2015, 77, 179–187. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W.J. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current Perspectives on Plant Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Selvadurai, E.L.; Brown, A.E.; Hamilton, J. Production of indole-3-acetic acid analogues by strains of Bacillus cereus in relation to their influence on seedling development. Soil Biol. Biochem. 1991, 23, 401–403. [Google Scholar] [CrossRef]
- Lebuhn, M. Production of auxin and other indolic and phenolic compounds by Paenibacillus polymyxa strains isolated from different proximity to plant roots. FEMS Microbiol. Ecol. 1997, 22, 325–334. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Pare, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Ruan, J.J.; Zhou, Y.X.; Zhou, M.L.; Yan, J.; Khurshid, M.; Weng, W.F.; Cheng, J.P.; Zhang, K.X. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Chinese Soil Taxonomy Research Group. Chinese Soil Taxonomy (Revised Proposal); Chinese Agricultural Science and Technology Press: Beijing, China, 1995. [Google Scholar]
- Chen, T.; Nan, Z.B.; Kardol, P.; Duan, T.Y.; Song, H.; Wang, J.F.; Li, C.H.; Hou, F.J. Effects of interspecific competition on plant-soil feedbacks generated by long-term grazing. Soil Biol. Biochem. 2018, 126, 133–143. [Google Scholar] [CrossRef]
- Brinkman, E.P.; Van der Putten, W.H.; Bakker, E.J.; Verhoeven, K.J.F. Plant-soil feedback: Experimental approaches, statistical analyses and ecological interpretations. J. Ecol. 2010, 98, 1063–1073. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Zhang, K.R.; Cheng, X.L.; Dang, H.S.; Zhang, Q.F. Biomass:N:K:Ca:Mg:P ratios in forest stands world-wide: Biogeographical variations and environmental controls. Glob. Ecol. Biogeogr. 2020, 29, 2176–2189. [Google Scholar] [CrossRef]
- Nelson., D.; Sommers., L. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties 9; Wiley: Hoboken, NJ, USA, 1983; pp. 539–579. [Google Scholar]
- Olsen, S. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 94, 486–488. [Google Scholar] [CrossRef]
- Mcgonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.r-project.org/ (accessed on 9 October 2021).

| Variables | Lespedeza davurica | Stipa bungeana | ||||
|---|---|---|---|---|---|---|
| Drought Treatment (DT) | Soil Sterilization (ST) | DT × ST | Drought Treatment (DT) | Soil Sterilization (ST) | DT × ST | |
| Shoot biomass † | 141.60 *** | 141.60 *** | 1.71 | 83.93 *** | 19.13 ** | 14.45 ** |
| Root biomass † | 26.06 *** | 32.04 *** | 0.11 | 34.21 *** | 9.75 * | 6.78 * |
| Total plant biomass † | 89.45 *** | 95.98 *** | 0.39 | 69.00 *** | 16.55 ** | 12.10 ** |
| Root:shoot ratio | 36.26 *** | 27.46 *** | 4.49 | 25.24 ** | 5.98 * | 5.95 * |
| Specific root length | 50.69 *** | 46.44 *** | 4.44 | 56.63 *** | 10.69 * | 6.88 * |
| Root diameter | 31.26 *** | 25.46 *** | 0.29 | 9.49 * | 2.89 | 0.01 |
| Biomass:N ratio | 47.91 *** | 37.93 *** | 0.77 | 64.61 *** | 15.20 ** | 6.96 * |
| Biomass:P ratio | 66.92 *** | 14.45 ** | 6.15 * | 5.48 * | 0.02 | 0.43 |
| Available P | 86.55 *** | 30.18 *** | 7.55 * | 19.23 ** | 1.22 | 0.00 |
| NO3−-N | 20.68 ** | 22.31 ** | 1.23 | 59.71 *** | 8.96 * | 5.37 * |
| NH4+-N | 0.11 | 1.78 | 0.01 | 1.80 | 0.46 | 0.01 |
| AM fungal colonization | 13.70 ** | 155.97 *** | 5.15 * | 10.09 * | 76.29 *** | 5.85 * |
| Plant Species | Family | Category | Life History | Cotyledon Type | Root System Type | Mycorrhizal Dependence |
|---|---|---|---|---|---|---|
| Lespedeza davurica | Leguminosae | Semi-shrub | Perennial | Bicotyledon | Tap-root | Yes |
| Stipa bungeana | Gramineae | Bunch grass | Perennial | Monocotyledon | Fibrous root | Yes |
| Soil Treatment | Soil Organic C (g/kg) | Total N (g/kg) | Total P (g/kg) | Available P (mg/kg) | NO3−-N (mg/kg) | NH4+-N (mg/kg) |
|---|---|---|---|---|---|---|
| Unsterilized | 4.96 (0.29) | 0.64 (0.04) | 0.53 (0.01) | 3.14 (0.17) | 2.38 (0.02) | 11.54 (0.07) |
| Sterilized | 5.36 (0.45) | 0.67 (0.05) | 0.50 (0.05) | 3.58 (0.09) | 2.56 (0.05) | 10.59 (0.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, D.; Feng, X.; Wu, N.; Tian, Z.; Dong, X.; Liu, H.; Nan, Z.; Chen, T. Drought Eliminates the Difference in Root Trait Plasticity and Mycorrhizal Responsiveness of Two Semiarid Grassland Species with Contrasting Root System. Int. J. Mol. Sci. 2023, 24, 10262. https://doi.org/10.3390/ijms241210262
Duan D, Feng X, Wu N, Tian Z, Dong X, Liu H, Nan Z, Chen T. Drought Eliminates the Difference in Root Trait Plasticity and Mycorrhizal Responsiveness of Two Semiarid Grassland Species with Contrasting Root System. International Journal of Molecular Sciences. 2023; 24(12):10262. https://doi.org/10.3390/ijms241210262
Chicago/Turabian StyleDuan, Dongdong, Xiaoxuan Feng, Nana Wu, Zhen Tian, Xin Dong, Huining Liu, Zhibiao Nan, and Tao Chen. 2023. "Drought Eliminates the Difference in Root Trait Plasticity and Mycorrhizal Responsiveness of Two Semiarid Grassland Species with Contrasting Root System" International Journal of Molecular Sciences 24, no. 12: 10262. https://doi.org/10.3390/ijms241210262
APA StyleDuan, D., Feng, X., Wu, N., Tian, Z., Dong, X., Liu, H., Nan, Z., & Chen, T. (2023). Drought Eliminates the Difference in Root Trait Plasticity and Mycorrhizal Responsiveness of Two Semiarid Grassland Species with Contrasting Root System. International Journal of Molecular Sciences, 24(12), 10262. https://doi.org/10.3390/ijms241210262






