Effect of Purslane (Portulaca oleracea L.) on Intestinal Morphology, Digestion Activity and Microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection
Abstract
1. Introduction
2. Results
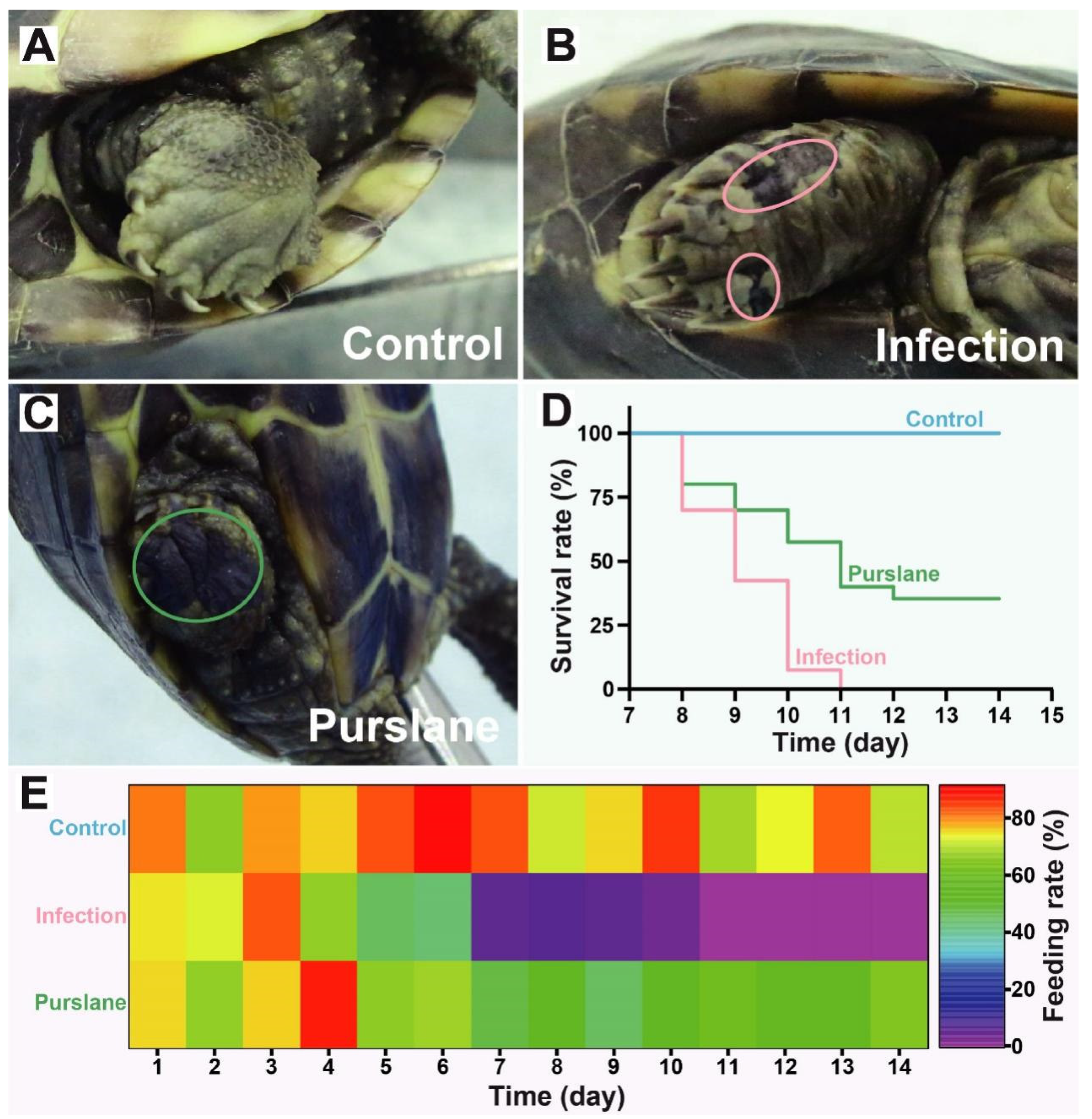
2.1. Purslane Mitigates the Symptoms and Increases Survival and Feeding Rates of Infected Turtles
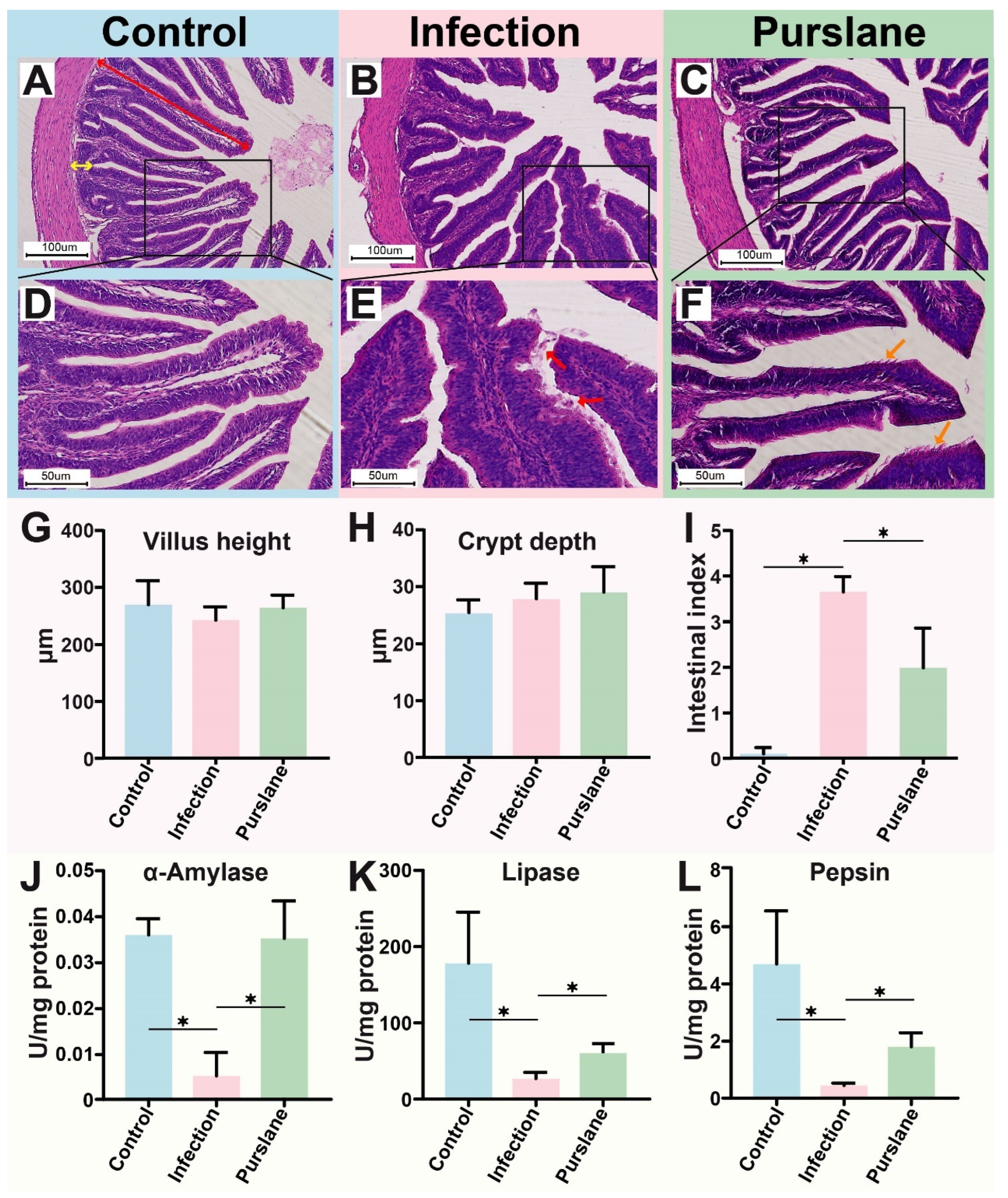
2.2. Purslane Improves the Intestinal Morphology and Digestive Enzyme Activities of Infected Turtles
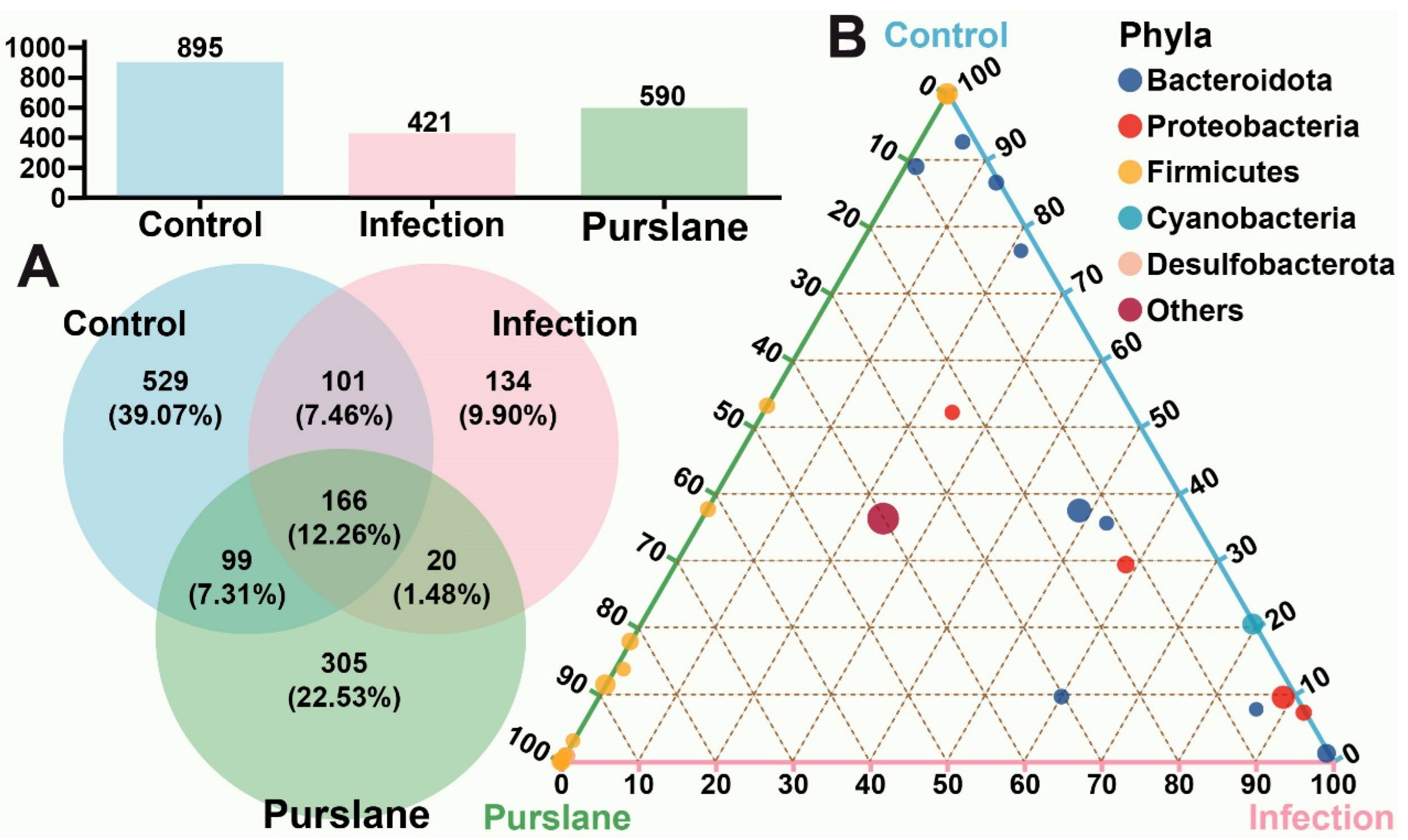
2.3. Global Analysis of OTUs
2.4. Purslane Alters the Diversity and Richness of the Intestinal Community of Infected Turtles
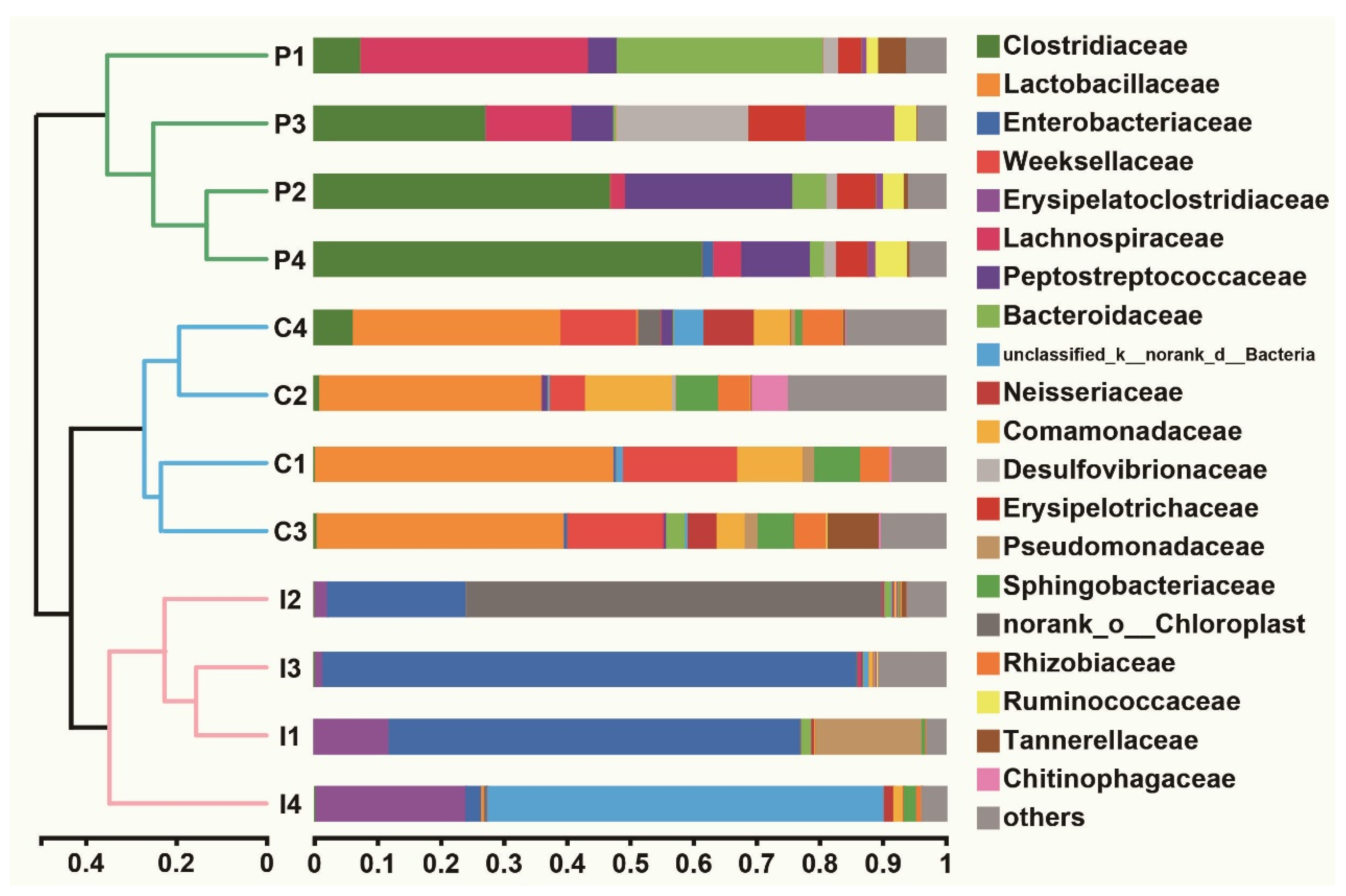
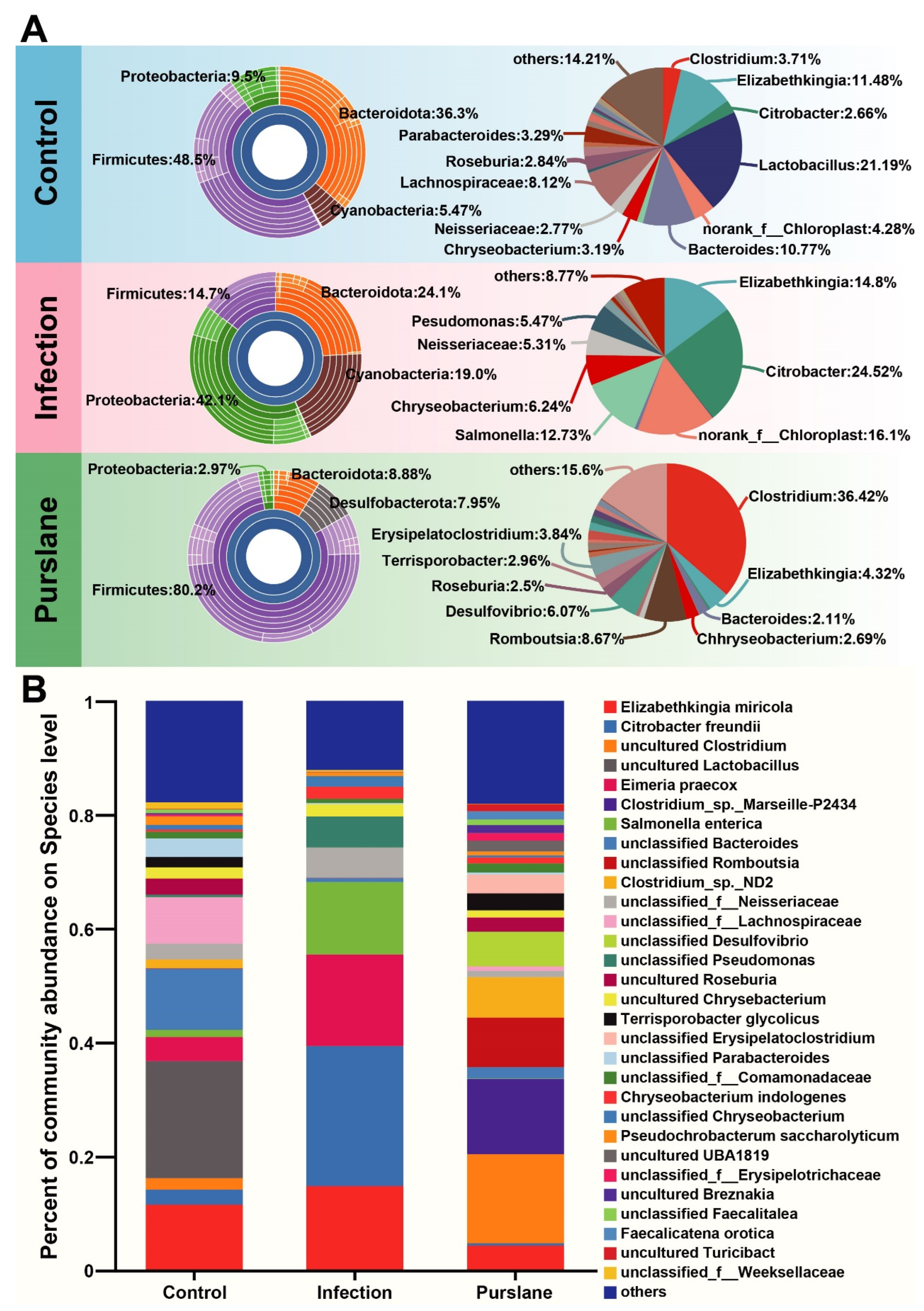
2.5. Effect of Purslane on the Abundance of Intestinal Microbiota of Infected Turtles
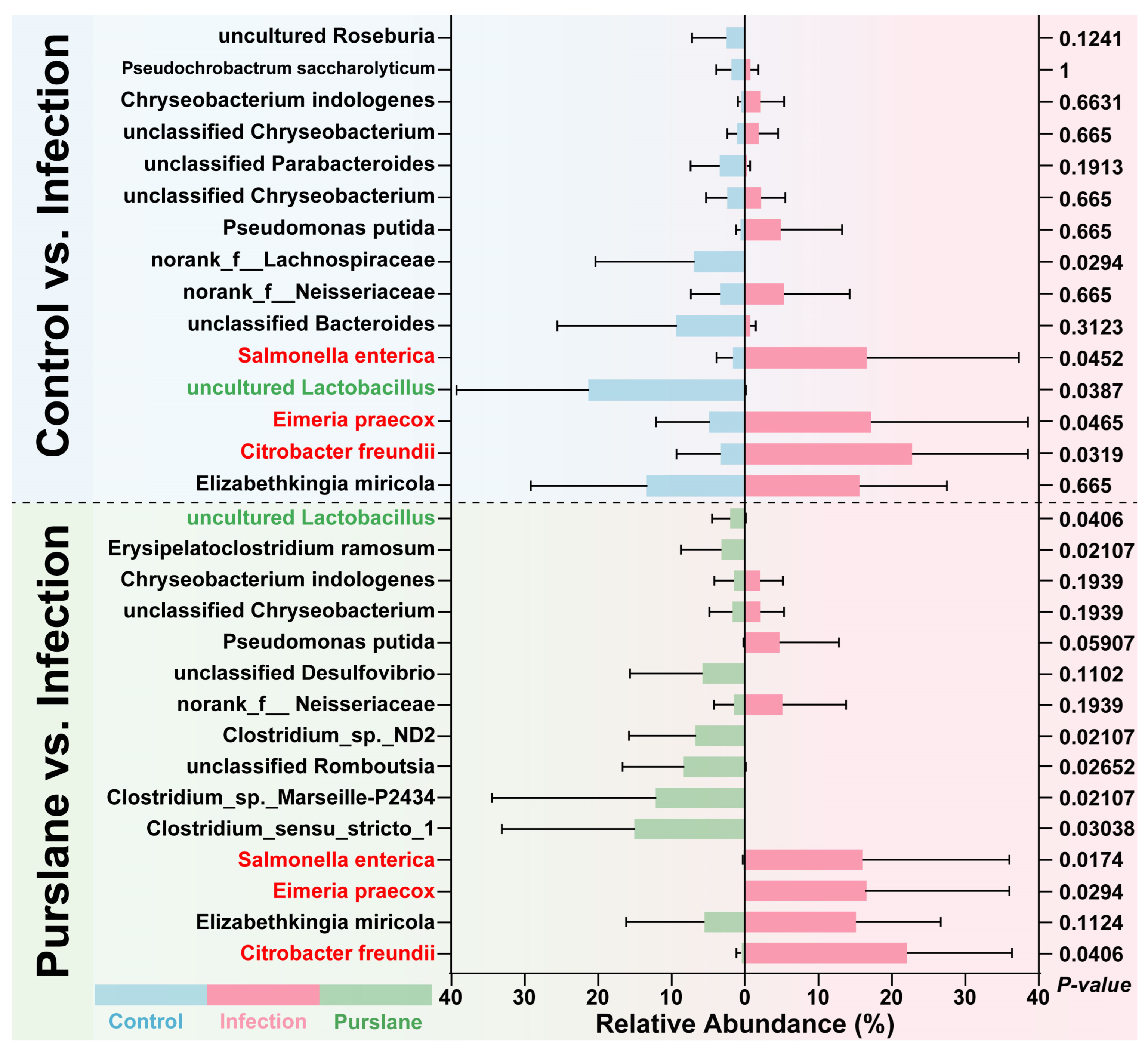
2.6. Purslane Reduces the Proportion of Potential Pathogens and Raises the Abundance of Probiotics of Infected Turtles
3. Discussion
4. Materials and Methods
4.1. Experimental Turtle and Design
4.2. Sample Collection
4.3. Histological Observation
4.4. Digestive Enzyme Activity Assays of Intestine
4.5. DNA Isolation, Library Construction, and Sequencing
4.6. Taxonomy Classification and Differential Abundance Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoshi, H.; Nakao, A. Molecular cloning of full-length Dmrt1 cDNA of Reeves turtle (Chinemys reevesii). J. Vet. Med. Sci. 2008, 70, 687–692. [Google Scholar] [CrossRef]
- Xu, C.; Xu, W.; Lu, H. Compensatory growth responses to food restriction in the Chinese three-keeled pond turtle, Chinemys reevesii. Springerplus 2014, 3, 687. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, Y.; Tan, J.; Wang, X.; Liu, X.; Zeng, C. Comparative Transcriptome Analysis Reveals the Molecular Immunopathogenesis of Chinese Soft-Shelled Turtle (Trionyx sinensis) Infected with A. hydrophila. Biology 2021, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Tekedar, H.C.; Waldbieser, G.C.; Karsi, A.; Liles, M.R.; Griffin, M.J.; Vamenta, S.; Sonstegard, T.; Hossain, M.; Schroeder, S.G.; Khoo, L.; et al. Complete Genome Sequence of a Channel Catfish Epidemic Isolate, A. hydrophila Strain ML09-119. Genome Announc. 2013, 1, e00755-13. [Google Scholar] [CrossRef] [PubMed]
- Citterio, B.; Francesca, B.A. Aeromonas hydrophila virulence. Virulence 2015, 6, 417–418. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, Z.; Yao, J.; Jin, Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J. Adv. Res. 2022, 37, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y.; Yoon, S.S. Disruption of the Gut Ecosystem by Antibiotics. Yonsei Med. J. 2018, 59, 4–12. [Google Scholar] [CrossRef]
- Leser, T.D.; Mølbak, L. Better living through microbial action: The benefits of the mammalian gastrointestinal microbiota on the host. Environ. Microbiol. 2009, 11, 2194–2206. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Rolfe, R.D. Interactions among microorganisms of the indigenous intestinal flora and their influence on the host. Rev. Infect. Dis. 1984, 6 Suppl. 1, S73–S79. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, Y.; Cao, K.; Li, J.; Wang, S.; Zhu, J.; Zeng, X.; Zhang, H.; Qin, Z. Transcriptome, intestinal microbiome and histomorphology profiling of differences in the response of Chinese sea bass (Lateolabrax maculatus) to A. hydrophila infection. Front. Microbiol. 2023, 14, 1103412. [Google Scholar] [CrossRef]
- Liu, L.; Gong, Y.X.; Zhu, B.; Liu, G.L.; Wang, G.X.; Ling, F. Effect of a new recombinant A. hydrophila vaccine on the grass carp intestinal microbiota and correlations with immunological responses. Fish Shellfish Immunol. 2015, 45, 175–183. [Google Scholar] [CrossRef]
- Geng, R.; Liu, H.; Tan, K.; Wang, Z.; Wang, W. RNase1 can modulate gut microbiota and metabolome after A. hydrophila infection in blunt snout bream. Environ. Microbiol. 2021, 23, 5258–5272. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Kayani, M.U.R.; Yu, K.; Qiu, Y.; Shen, Y.; Gao, C.; Feng, R.; Zeng, X.; Wang, W.; Chen, L.; Su, H.L. Environmental concentrations of antibiotics alter the zebrafish gut microbiome structure and potential functions. Environ. Pollut. 2021, 278, 116760. [Google Scholar] [CrossRef]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [PubMed]
- Karimi, G.; Aghasizadeh, M.; Razavi, M.; Taghiabadi, E. Protective effects of aqueous and ethanolic extracts of Nigella sativa L. and Portulaca oleracea L. on free radical induced hemolysis of RBCs. Daru 2011, 19, 295–300. [Google Scholar] [PubMed]
- Kim, J.Y.; Oh, H.M.; Kwak, S.C.; Cheon, Y.H.; Lee, M.S.; Rho, M.C.; Oh, J. Purslane suppresses osteoclast differentiation and bone resorbing activity via inhibition of Akt/GSK3β-c-Fos-NFATc1 signaling in vitro and prevents lipopolysaccharide-induced bone loss in vivo. Biol. Pharm. Bull. 2015, 38, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.B.; Ajam, F.; Rakhshandeh, H.; Askari, V.R. A Pharmacological Review on Portulaca oleracea L.: Focusing on Anti-Inflammatory, Anti-Oxidant, Immuno-Modulatory and Antitumor Activities. J. Pharmacopunct. 2019, 22, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhou, S.; Yu, M.; Lu, Y.; He, G.; Huang, X.; Huang, Y. Portulaca oleracea Polysaccharides Modulate Intestinal Microflora in Aged Rats in vitro. Front. Microbiol. 2022, 13, 841397. [Google Scholar] [CrossRef]
- Zhao, X.H.; He, X.; Yang, X.F.; Zhong, X.H. Effect of Portulaca oleracea extracts on growth performance and microbial populations in ceca of broilers. Poult. Sci. 2013, 92, 1343–1347. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, D.; Zhang, Y.; Chen, Q.; Chen, Y.; Tang, Y.; Que, R.; Chen, Y.; Zheng, L.; Dai, Y.; et al. Portulaca oleracea L. Extract Ameliorates Intestinal Inflammation by Regulating Endoplasmic Reticulum Stress and Autophagy. Mol. Nutr. Food Res. 2022, 66, e2100791. [Google Scholar] [CrossRef]
- Kari, Z.A.; Wee, W.; Sukri, S.A.M.; Harun, H.C.; Reduan, M.F.H.; Khoo, M.I.; Van Doan, H.; Goh, K.W.; Wei, L.S. Role of phytobiotics in relieving the impacts of A. hydrophila infection on aquatic animals: A mini-review. Front. Vet. Sci. 2022, 9, 1023784. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, N.; Awad, S.M.; Abdel-Tawwab, M. Effect of dietary purslane (Portulaca oleracea L.) leaves powder on growth, immunostimulation, and protection of Nile tilapia, Oreochromis niloticus against A. hydrophila infection. Fish Physiol. Biochem. 2019, 45, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Rashed, A.N.; Afifi, F.U.; Disi, A.M. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J. Ethnopharmacol. 2003, 88, 131–136. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, G.; Zhou, Z.; Dai, Z.; Sun, Y.; Ji, Y.; Li, W.; Wang, W.; Liu, C.; Han, F.; et al. Glutamine and intestinal barrier function. Amino Acids 2015, 47, 2143–2154. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef]
- Geibel, J.P. Secretion and absorption by colonic crypts. Annu. Rev. Physiol. 2005, 67, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lyu, W.; Liu, L.; Lv, K.; Zheng, F.; Wang, Y.; Chen, J.; Dai, B.; Yang, H.; Xiao, Y. Comparison of Digestive Enzyme Activities and Expression of Small Intestinal Transporter Genes in Jinhua and Landrace Pigs. Front. Physiol. 2021, 12, 669238. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alabdali, A.Y.M.; Aldhalmi, A.K.; Reda, F.M.; Bassiony, S.S.; Selim, S.; El-Saadony, M.T.; Alagawany, M. Impacts of Purslane (Portulaca oleracea) extract supplementation on growing Japanese quails’ growth, carcass traits, blood indices, nutrients digestibility and gut microbiota. Poult. Sci. 2022, 101, 102166. [Google Scholar] [CrossRef]
- Okuda, S.; Wajima, T.; Yamada, T.; Nakaminami, H.; Ikoshi, H.; Noguchi, N. In vitro growth-inhibitory effects of Portulaca oleracea L. formulation on intestinal pathogens. Access Microbiol. 2021, 3, 000208. [Google Scholar] [CrossRef]
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef]
- Chwen, L.T.; Foo, H.L.; Thanh, N.T.; Choe, D.W. Growth performance, plasma Fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol. Asian Australas. J. Anim. Sci. 2013, 26, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef]
- Zheng, J.; Gänzle, M.G.; Lin, X.B.; Ruan, L.; Sun, M. Diversity and dynamics of bacteriocins from human microbiome. Environ. Microbiol. 2015, 17, 2133–2143. [Google Scholar] [CrossRef]
- Eichmiller, J.J.; Hamilton, M.J.; Staley, C.; Sadowsky, M.J.; Sorensen, P.W. Environment shapes the fecal microbiome of invasive carp species. Microbiome 2016, 4, 44. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Sylvain, F.; Cheaib, B.; Llewellyn, M.; Gabriel Correia, T.; Barros Fagundes, D.; Luis Val, A.; Derome, N. pH drop impacts differentially skin and gut microbiota of the Amazonian fish tambaqui (Colossoma macropomum). Sci. Rep. 2016, 6, 32032. [Google Scholar] [CrossRef] [PubMed]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’Connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Sourani, Z.; Shirian, S.; Shafiei, S.; Mosayebi, N.; Nematollahi, A. Modulation of Immune-Related Gene Expressions in Zebrafish (Danio rerio) by Dietary Purslane (Portulaca oleracea) Extract. Mar. Biotechnol. 2023, 25, 214–221. [Google Scholar] [CrossRef]
- Tschape, H.; Prager, R.; Streckel, W.; Fruth, A.; Tietze, E.; Böhme, G. Verotoxinogenic Citrobacter freundii associated with severe gastroenteritis and cases of haemolytic uraemic syndrome in a nursery school: Green butter as the infection source. Epidemiol. Infect. 1995, 114, 441–450. [Google Scholar] [CrossRef]
- Parida, S.N.; Verma, I.C.; Deb, M.; Bhujwala, R.A. An outbreak of diarrhea due to citrobacter freundii in a neonatal special care nursery. Indian J. Pediatr. 1980, 47, 81–84. [Google Scholar] [CrossRef]
- Faille, C.; Fontaine, F.; Lelièvre, C.; Bénézech, T. Adhesion of Escherichia coli, Citrobacter freundii and Klebsiella pneumoniae isolated from milk: Consequence on the efficiency of sanitation procedures. Water Sci. Technol. 2003, 47, 225–231. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Semreen, M.H.; El-Keblawy, A.A.; Abdullah, A.; Uppuluri, P.; Ibrahim, A.S. Assessment of herbal drugs for promising anti-Candida activity. BMC Complement. Altern. Med. 2017, 17, 257. [Google Scholar] [CrossRef]
- Hernandez, S.M.; Maurer, J.J.; Yabsley, M.J.; Peters, V.E.; Presotto, A.; Murray, M.H.; Curry, S.; Sanchez, S.; Gerner-Smidt, P.; Hise, K.; et al. Free-Living Aquatic Turtles as Sentinels of Salmonella spp. for Water Bodies. Front. Vet. Sci. 2021, 8, 674973. [Google Scholar] [CrossRef]
- Raidal, S.R.; Ohara, M.; Hobbs, R.P.; Prince, R.I. Gram-negative bacterial infections and cardiovascular parasitism in green sea turtles (Chelonia mydas). Aust. Vet. J. 1998, 76, 415–417. [Google Scholar] [CrossRef]
- Répérant, J.M.; Dardi, M.; Pagès, M.; Thomas-Hénaff, M. Pathogenicity of Eimeria praecox alone or associated with Eimeria acervulina in experimentally infected broiler chickens. Vet. Parasitol. 2012, 187, 333–336. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.; O’Toole, P.W. Lactobacillus: Host-microbe relationships. Curr. Top. Microbiol. Immunol. 2013, 358, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.N.; Zhang, J.L.; Guan, W.C.; Zhang, X.F.; Guan, S.H.; Zeng, Q.H.; Cheng, G.F.; Cui, W. Effects of Lactobacillus delbrueckii on immune response, disease resistance against A. hydrophila, antioxidant capability and growth performance of Cyprinus carpio Huanghe var. Fish Shellfish Immunol. 2017, 68, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tian, X.L.; Xu, X.B.; Li, H.; Tian, Y.; Ma, Y.H.; Li, X.F.; Li, N.; Zhang, T.T.; Sheng, Y.D.; et al. Dietary supplementation of probiotics fermented Chinese herbal medicine Sanguisorba officinalis cultures enhanced immune response and disease resistance of crucian carp (Carassius auratus) against A. hydrophila. Fish Shellfish Immunol. 2022, 131, 682–696. [Google Scholar] [CrossRef]
- Butprom, S.; Phumkhachorn, P.; Rattanachaikunsopon, P. Effect of Lactobacillus plantarum C014 on innate immune response and disease resistance against A. hydrophila in hybrid catfish. Sci. World J. 2013, 2013, 392523. [Google Scholar] [CrossRef]
- Ngamkala, S.; Satchasataporn, K.; Setthawongsin, C.; Raksajit, W. Histopathological study and intestinal mucous cell responses against A. hydrophila in Nile tilapia administered with Lactobacillus rhamnosus GG. Vet. World 2020, 13, 967–974. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Ye, F.; Tang, H.; Xiong, Y.; Wu, Y.; Wang, L.; Feng, X.; Zhang, S.; Wan, Y.; et al. Dietary purslane (Portulaca oleracea L.) promotes the growth performance of broilers by modulation of gut microbiota. AMB Express 2021, 11, 31. [Google Scholar] [CrossRef]
- Muñoz, F.A.; Estrada-Parra, S.; Romero-Rojas, A.; Gonzalez-Ballesteros, E.; Work, T.M.; Villaseñor-Gaona, H.; Estrada-Garcia, I. Immunological evaluation of captive green sea turtle (Chelonia mydas) with ulcerative dermatitis. J. Zoo Wildl. Med. 2013, 44, 837–844. [Google Scholar] [CrossRef]
- Baums, C.G.; Hermeyer, K.; Leimbach, S.; Adamek, M.; Czerny, C.P.; Hörstgen-Schwark, G.; Valentin-Weigand, P.; Baumgärtner, W.; Steinhagen, D. Establishment of a model of Streptococcus iniae meningoencephalitis in Nile tilapia (Oreochromis niloticus). J. Comp. Pathol. 2013, 149, 94–102. [Google Scholar] [CrossRef]
- Dai, T.; Wen, D.; Bates, C.T.; Wu, L.; Guo, X.; Liu, S.; Su, Y.; Lei, J.; Zhou, J.; Yang, Y. Nutrient supply controls the linkage between species abundance and ecological interactions in marine bacterial communities. Nat. Commun. 2022, 13, 175. [Google Scholar] [CrossRef] [PubMed]

| Sample Category | Replicate | Seq_Num | Base_Num | Mean_Length | Min_Length | Max_Length |
|---|---|---|---|---|---|---|
| Control | C1 | 42,201 | 17,744,244 | 420.46 | 208 | 528 |
| C2 | 46,657 | 19,623,401 | 420.58 | 254 | 486 | |
| C3 | 44,444 | 18,657,768 | 419.80 | 205 | 486 | |
| C4 | 48,767 | 20,723,904 | 424.95 | 208 | 458 | |
| Infection | I1 | 46,145 | 18,921,528 | 410.04 | 247 | 490 |
| I2 | 51,282 | 21,729,312 | 423.72 | 208 | 499 | |
| I3 | 37,434 | 14,148,899 | 377.96 | 201 | 525 | |
| I4 | 58,329 | 24,967,742 | 428.05 | 221 | 486 | |
| Purslane | P1 | 65,251 | 26,607,757 | 407.77 | 240 | 486 |
| P2 | 73,938 | 30,679,329 | 414.93 | 227 | 433 | |
| P3 | 63,494 | 26,246,140 | 413.36 | 219 | 486 | |
| P4 | 73,640 | 30,037,311 | 407.89 | 214 | 433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Feng, L.; Zhang, J.; Yan, C.; Zhang, C.; Huang, Y.; Li, M.; Luo, W.; Huang, X.; Wu, J.; et al. Effect of Purslane (Portulaca oleracea L.) on Intestinal Morphology, Digestion Activity and Microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection. Int. J. Mol. Sci. 2023, 24, 10260. https://doi.org/10.3390/ijms241210260
Yang S, Feng L, Zhang J, Yan C, Zhang C, Huang Y, Li M, Luo W, Huang X, Wu J, et al. Effect of Purslane (Portulaca oleracea L.) on Intestinal Morphology, Digestion Activity and Microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection. International Journal of Molecular Sciences. 2023; 24(12):10260. https://doi.org/10.3390/ijms241210260
Chicago/Turabian StyleYang, Shiyong, Langkun Feng, Jiajin Zhang, Chaozhan Yan, Chaoyang Zhang, Yanbo Huang, Minghao Li, Wei Luo, Xiaoli Huang, Jiayun Wu, and et al. 2023. "Effect of Purslane (Portulaca oleracea L.) on Intestinal Morphology, Digestion Activity and Microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection" International Journal of Molecular Sciences 24, no. 12: 10260. https://doi.org/10.3390/ijms241210260
APA StyleYang, S., Feng, L., Zhang, J., Yan, C., Zhang, C., Huang, Y., Li, M., Luo, W., Huang, X., Wu, J., Du, X., & Li, Y. (2023). Effect of Purslane (Portulaca oleracea L.) on Intestinal Morphology, Digestion Activity and Microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection. International Journal of Molecular Sciences, 24(12), 10260. https://doi.org/10.3390/ijms241210260






