Abstract
Pulmonary hypertension (PH) is a disease which affects the cardiopulmonary system; it is defined as a mean pulmonary artery pressure (mPAP) > 20 mmHg as measured by right heart catheterization at rest, and is caused by complex and diverse mechanisms. In response to stimuli such as hypoxia and ischemia, the expression and synthesis of endothelin (ET) increase, leading to the activation of various signaling pathways downstream of it and producing effects such as the induction of abnormal vascular proliferation during the development of the disease. This paper reviews the regulation of endothelin receptors and their pathways in normal physiological processes and disease processes, and describes the mechanistic roles of ET receptor antagonists that are currently approved and used in clinical studies. Current clinical researches on ET are focused on the development of multi-target combinations and novel delivery methods to improve efficacy and patient compliance while reducing side effects. In this review, future research directions and trends of ET targets are described, including monotherapy and precision medicine.
1. Introduction
Pulmonary hypertension (PH) is a pathophysiological disorder in which the pressure in the pulmonary arteries rises due to increased pulmonary vascular resistance, most recently defined as a mean pulmonary artery pressure (mPAP) > 20 mmHg measured by right heart catheterization at rest at sea level (different altitudes can be converted to sea level). In order to distinguish PH with regard to different factors such as pulmonary vascular disease and left heart disease, the two indicators of pulmonary vascular resistance (PVR) and pulmonary arterial wedge pressure (PAWP) are usually introduced for definition. According to the 2022 ESC/ERS Guidelines, pre-capillary PH is hemodynamically defined as mPAP > 20 mmHg, PAWP ≤ 15 mmHg, and PVR > 2 Wood units (WU), while the post-capillary differences are PAWP > 15 mmHg and PVR ≤ 2 WU [1,2].
PH can lead to progressive dyspnea, fatigue, chest pain, syncope, oedema, palpitations, and eventually right heart failure [3]. This disease has complex mechanisms and diverse etiologies, and is currently classified into five main groups based on different pathological features: Group I—pulmonary arterial hypertension; Group II—PH due to heart disease; Group III—PH due to hypoxia and lung disease; Group IV—chronic thromboembolic PH; and Group V—PH due to other causes [4]. Based on PVR and PAWP levels, another classification method is to define patients with PH as pre-capillary PH, isolated post-capillary PH (IpcPH), or combined pre/post-capillary PH (CpcPH) [5]. Among these, PH associated with left heart disease is mainly linked with the two categories of IpcPH and CpcPH, while the rest of the categories belong to pre-capillary PH [1,5].
The etiology and pathogenesis of PH are diverse and complex, and are associated with many individual and environmental factors, usually leading to pulmonary vasoconstriction, the abnormal proliferation of pulmonary vascular smooth muscle, and pulmonary vascular remodeling [3]. Endothelin receptor antagonists (ERAs) are a powerful class of vasodilators and antiproliferative agents, and are currently commonly targeted for the treatment of PH. From the perspective of pathogenesis, in addition to targeting endothelin (ET) receptors to cause vasodilation, there are research advances being made in the fields of cell proliferation and anti-apoptosis, gene mutation, epigenetic dysregulation, metabolism, and immunity [6].
In addition to environmental factors (e.g., hypoxia, air pollution) and endothelial dysfunction, mutations in bone morphogenetic protein receptor 2 (BMPR2), activator receptor-like kinase 1 (ALK1), endoglin, and other genetic predispositions [7] are multiple factors contributing to PH, as are somatic circulatory factors such as pro-coagulation and inflammation and the expressions of many ion channels and receptors [8].
However, targeting endothelial function remains an important avenue for treatment, and this is currently the first choice for PH treatment. Combinations targeting the ET receptor pathway, the development of new formulations, and the expansion of indications remain advanced and are promising areas of research. Therefore, in this review we focus on the pathological changes in ET and its signaling pathway in the progression of PH as well as the drugs and latest clinical studies employed in achieving this target.
2. Background and Mechanism of PH
2.1. Pathological Process of PH
PH is a disease of the cardiopulmonary unit that affects pulmonary circulation and the right ventricle (RV). Although PH is divided into five major categories based on different and somewhat heterogeneous etiologies, in general their pathogeneses have many similarities. They are all characterized by the excessive proliferation of vascular cells, the increased deposition of the extracellular matrix, and the accumulation of inflammatory cells within the pulmonary vascular wall, which together lead to increased PVR [9]. In addition, other underlying causes such as congenital heart disease, systemic connective tissue disease, chronic lung disease, and left heart disease are potential factors that promote pulmonary vascular disease [10].
The pathological features of PH include endothelial cell (EC) dysfunction, abnormal signaling, and the abnormal proliferation of multiple cells and blood vessels, leading to the progressive evolution of arteriolar intimal proliferation with centripetal or eccentric laminar sclerosis, medial hypertrophy, and adventitial proliferation. It is also accompanied by variable inflammatory response and occasional fibrin-like necrosis [11], as shown in Figure 1. Currently, in addition to the widely recognized abnormalities in cell proliferation and function, the bioenergetics aspects of cell-related metabolic pathways as well as mitochondrial abnormalities are becoming increasingly important in PH [12]. Hypoxemia results in the need for higher cardiac output to maintain adequate tissue oxygenation, which is more difficult to achieve with a higher afterload [13]. Under normal conditions, the RV wall is thin compared to the left ventricle (LV), and low loads are sufficient for pulmonary oxygen circulation. Under normal physiological conditions, an appropriate increase in pulmonary artery pressure (PAP) facilitates systemic oxygen circulation [14]. When pressure increases slowly, RV first maintains output by compensating for the increase in wall thickness. However, this ventricular remodeling is not infinite and eventually leads to right heart failure [15]. The increase in resistance due to pulmonary vascular remodeling, which may be fourfold or higher, is the most important indicator of hemodynamic changes in PH [16].
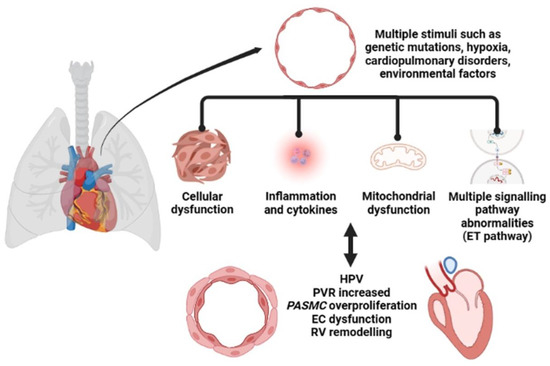
Figure 1.
The pathological process of pulmonary hypertension (PH).
Among the many stimuli, hypoxemia plays an important role in the production of PH. Hypoxic pulmonary vasoconstriction (HPV) due to acute hypoxia can lead to acute PH, and consequently to high altitude pulmonary edema. In contrast, chronic hypoxic exposure induces pulmonary vascular remodeling and the development of sustained PH, which increases the RV pressure load and eventually leads to right heart failure or even death [17]. HPV is a homeostatic mechanism that optimizes oxygen uptake by matching lung perfusion to ventilation. Local HPV is not accompanied by an increase in PAP; however, in the presence of total alveolar hypoxia, HPV involves the entire pulmonary circulation, resulting in increased PVR and elevated PAP [18]. Mitochondrial sensors present within the pulmonary artery smooth muscle cell (PASMC) respond to changes in oxygen tension by altering the production of reactive oxygen species (ROS), which are then rapidly and locally converted to H2O2 through mitochondrial superoxide dismutase 2 (SOD2) and regulate the activity of ion channels such as potassium (K+) and calcium (Ca2+). The simultaneous increase in combined nitrogen species inactivates various enzymes and vasodilatory factors in the nitric oxide (NO) pathway and promotes pulmonary vasoconstriction [19].
When pulmonary vasoconstriction persists over a long period of time, it causes vascular occlusion and the sustained elevation of PVP. This pathological status leads to the recruitment of perivascular inflammatory cells and factors with chronic inflammation and autoimmune features. In turn, certain inflammatory factors exacerbate pulmonary vasoconstriction and are closely associated with proliferation and remodeling of pulmonary vascular, ultimately leading to the development and progression of PH.
2.2. Signaling Pathways in PH
In the process of pulmonary endothelial dysfunction, endothelium-dependent vasodilatation becomes impaired. Many signaling pathways are involved in this process, with metabolic changes, ROS production, and disordered processes of different chemokines, cytokines, and growth factors. This eventually leads to impaired angiogenesis and repair mechanisms, which plays a major role in pulmonary vascular remodeling [20]. Many of these signaling pathways are associated with ET.
The small guanosine triphosphate binding protein ras homolog gene family member A (Rho A) and its major effector molecules, Rho-related kinases (ROCKs), play important roles in the cardiovascular system and have been found to regulate a wide range of essential cellular functions such as contraction, motility, proliferation, and apoptosis [21]. The Rho/ROCKs signaling pathway is a common signal transduction pathway in body tissues. Rho/ROCK can be activated by a variety of upstream stimulus signals, including ET-1, NO, and angiotensin II (Ang II), as well as by oxidative stress [22]. One study demonstrated the increased expression of Rho A, ROCK1, and ROCK2 in PH lung tissue and RV tissue, suggesting the activation of the Rho A/ROCK pathway and confirming that this pathway is associated with vascular remodeling in PH [23].
Additionally, factors such as hypoxia-inducible factor-1 (HIF-1α), which has an important role in PH, can regulate ET production and EC migration [24]. After a period of hypoxemia, a number of hypoxia-sensitive inflammatory responses and proliferative pathways are activated, leading to increased vascular resistance and even pulmonary vascular remodeling [25,26]. The exposure of pulmonary artery ECs to hypoxia in humans can cause an increase in HIF-1 expression, and the heterozygous expression of HIF-1 and HIF-2 in mice can have a protective effect against PH [27,28]. The increased expression of HIF-1α during hypoxia leads to the increased expression of the closely related transforming growth factor-beta 1 (TGF-β1), fibroblast growth factors (FGFs), and vascular endothelial growth factor A (VEGFA) along with its receptor vascular endothelial growth factor receptor 2 (VEGFR2), among others.
On the other hand, the enhancement of TGF-β1 stimulates an increase in platelet-derived growth factor beta (PDGFβ), leading to over-proliferation of ECs by stimulating VEGFA expression, all of which has been demonstrated in the pulmonary artery vessels of patients [29]. Additionally, this can activate the Rho A/ROCK signaling pathway, thereby promoting the occurrence and development of PH [30]. This further includes the release of different chemokines, cytokines, and growth factors from the endothelium and the increased expression of adhesion molecules such as E-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecules [20].
In addition to the pathways highlighted above, the AMP-activated protein kinases (AMPK), Notch, extracellular-signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), and P38 mitogen-activated protein kinase (MAPK) signaling pathways, which are associated with cellular energy state and vascular morphogenesis, play important roles in vascular development and differentiation [31,32,33]. Similarly, upregulated TRB3 [34] and abnormal resistin-like molecule β gene expression [35] both have regulatory effects on the MAPK signaling pathway. The more studied pathways include the NO pathway, where established drugs including phosphodiesterase (PDE5) inhibitors block the breakdown of cGMP and soluble guanylate cyclase activators (sGCS) act synergistically with endogenous NO to directly stimulate sGC and raise cGMP, and the prostacyclin pathway, which increases cAMP, promotes vasodilation, and inhibits platelet aggregation and smooth muscle cell proliferation [36]. Representative drugs include prostacyclin analogues and prostacyclin receptor agonists. Additionally, there are multiple pathways related to hypoxia, stress, and energy metabolism.
Many signaling pathways play a role in determining disease progression during the development of PH, and a large number of them function as upstream or downstream molecules of the ET signaling pathway. This indicates the importance of ET and its pathways in PH; the potential regulatory role of modulating ET receptors for the disease is very broad, and a very worthy target for research. Therefore, we next elaborate on ET receptors and their pathways.
3. Endothelin Receptors and Their Pathway Mechanisms
3.1. Endothelin Subtypes and Distribution
ET was originally identified as a potent vasoconstrictor peptide from porcine aortic ECs, which consist of twenty-one amino acid residues with a hydrophobic C terminus linked by two sets of intra-chain disulfide bonds and having a vasopressor effect. Similar to many peptide hormones and neuropeptides, ET is produced in ECs from a pre-peptide of approximately two hundred amino acids [37]. Based on the deduced amino acid sequences, Akihiro Inoue et al. cloned three different human ET-related genes by screening a genomic DNA library with synthetic oligonucleotide probes encoding partial sequences of ET at low hybridization intensity: synthesized endothelin-1 (ET-1), endothelin-2 (ET-2), and endothelin-3 (ET-3). Their biological activity was tested by contraction assays in isolated porcine coronary artery strips and intravenous injection assays in anesthetized rats. The results showed that three isomers of human ET exist, differing in structure and activity, but all being antihypertensive in vivo and potent arterial smooth muscle constrictors in vitro. The vasoconstrictor activity in terms of the maximum tension induced was ET-2 > ET-1 > ET-3, an order that is correlated with the hydrophobicity of the peptide [38,39].
The ET family is widely distributed in a variety of organs and systems in the human body, although different isomers are mainly distributed in slightly different organs. Of the ET family, the mature ET-1 peptide is synthesized in almost all kinds of cells and is highly expressed in ECs [40]. In response to stimuli such as hypoxia, ischemia, and shear stress, the messenger RNA (mRNA) for ET-1 undergoes transcription and rapidly synthesizes ET-1 [41]. In contrast to ET-1, ET-2 is mainly distributed in the kidney and small intestine. It is involved in a variety of biological activities in different systems, such as alveolarization, intestinal contraction, thermoregulation, and ovulation [42,43]. ET-3 is found mainly in the brain, and may be involved in the functional regulation of many neurons and glial cells. In addition, it is distributed in the lungs, kidney, and gastrointestinal tract [44].
3.2. Classification of Endothelin Receptors
As ET is hydrophilic and cannot cross the plasma membrane, it must bind to a specific cell surface receptor to function. There are two main subtypes of ET receptors, namely, ETA and ETB; they belong to the G protein-coupled receptor superfamily, which consists of approximately four hundred amino acids, including seven transmembrane structural domains containing 22–26 hydrophobic amino acids [45]. The ETA receptor is ET isoform selective and binds ET-1 and ET-2 with higher affinity than ET-3, whereas ETB has the same affinity for all three isoforms and is not isoform selective [46]. There are two different basic functions of ETB receptors; one is located on ECs that activate NO release, the other on vascular smooth muscle cells that mediate vasoconstriction [47,48,49].
In the human body, both receptors are abundantly distributed in the kidney, while ETA receptors are found mainly in smooth muscle cells, cardiac muscle, hepatic stellate cells and hepatocytes, brain neurons, osteoblasts, etc. ETB receptors are found mainly in ECs, osteoblasts, neurons of the central and peripheral nervous system, and various cells of the reproductive tract [50]. In the pulmonary arterial system, ETA receptors mediate the proliferation of vascular smooth muscle cells and vasoconstriction, whereas ETB receptors are located primarily on ECs, where they mediate vasodilation through the release of NO and prostaglandin-I-2 [51,52]. Figure 2 shows the relationship between the different isoforms of ET and the receptor subtypes, including their differences.
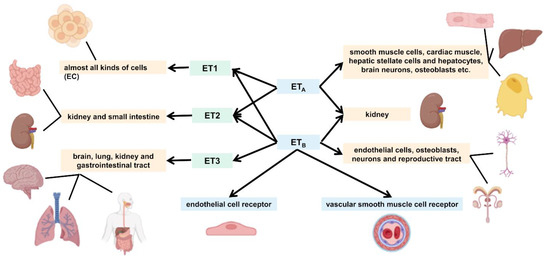
Figure 2.
Endothelin (ET) isoforms and ET receptor subtypes.
In addition, the ETB receptor can initiate a positive autocrine loop that allows ET-1 to regulate the expression of its own genes and act as a “scavenger receptor” to remove circulating ET-1 via the lysosomal pathway [53,54]. The activated receptor couples to the effector system to produce second messengers such as inositol phosphate, diglycerides and calcium, ultimately producing the corresponding biological effects.
3.3. Endothelin Synthesis
ET is able to produce a variety of effects in lung tissue, including the proliferation of smooth muscle cells, pulmonary vascular contraction, secretion of mucus, stimulation of DNA synthesis, and changes in vascular permeability [55]. As shown in Figure 3, after being transcribed into mRNA by PPET gene regulation, ET is first translated into pre-proendothelin (PPET), which is regulated by c-fos and c-jun, nuclear factor-1, AP-1, and GATA-2. PPET is post-translationally cleaved at dibasic sites by furin-like endopeptidase to form proendothelin, then the biologically inactive big endothelin (big ETs), which in turn is cleaved at the Trp-Val for ET-1 (amino-terminal fragment) and ET-2 or at the Trp-Ile for ET-3 and a detectable carboxy-terminal fragment [56]. The formation of proendothelin activation receives regulation by the following pathways: the human PPET-1 gene is directly regulated by intracellular signaling mediated by protein kinase C via the trans-acting transcription factors FOS and JUN, and NF-1 binding elements mediate the induction of PPET-1 mRNA by TGF-β [57].
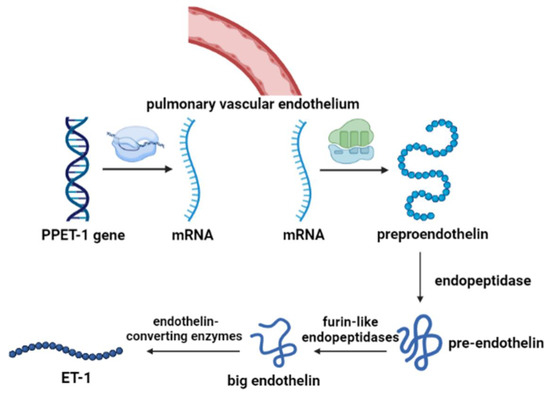
Figure 3.
The synthesis process of ET-1.
After synthesis, ET is secreted in two main ways. The first is through Weibel–Palade vesicles that act as storage sites for ET, fusing with the plasma membrane upon stimulation and releasing it via exocytosis. The other more dominant mechanism is the constitutive secretory pathway, which occurs in most endothelin-producing cell types. ET synthesized by vascular endothelial cells is released towards the basolateral side of the cell, and usually acts on smooth muscle cells. Therefore, tissue levels of ET are likely to be higher than plasma levels. ET is widely considered to be a paracrine/autocrine peptide rather than an “endocrine” peptide [58]. ET-1 can activate the PI3K/Akt pathway to stimulate endothelial NO synthase (eNOS) phosphorylation, leading to NO production, while NO can antagonize ET-1 by inhibiting preproET-1 transcription [59].
Because many ET-producing cells do not have storage vesicles or pathways to regulate secretion, the regulation of ET occurs primarily at the transcriptional stage [46,60]. At the molecular level, mRNA at the transcriptional level can be regulated to influence the synthesis of ET from ECs after intracellular processing. The synthesis of mRNA occurred in close proximity to the binding sites of ET in various tissues, and the binding sites of ET are evenly distributed in the lung, being distributed in decreasing order of frequency in the cardiovascular system, cardiac nerves, atria, ventricles, and coronary arteries. Of these, ET-1 and ET-3 are produced by the ECs of the large and small pulmonary vessels and the respiratory epithelium of the bronchiole, while ET-2 is mainly expressed by intestinal epithelial cells [60].
3.4. Regulation of the Endothelin Pathway
ET can interact with a variety of substances to initiate the corresponding signaling pathways and produce multiple biological effects. Several of these pathways are shown in Figure 4. When ET binds to its specific receptor, it activates phospholipase C and produces inositol triphosphate (IP3) and diglycerides [61]. It has been demonstrated in isolated rabbit arteries that IP3 subsequently diffuses to specific receptors on the endoplasmic reticulum, which in turn promote the release of Ca2+ from intracellular calcium stores and the inward flow of calcium through voltage-dependent calcium channels [62] and can induce mitogenesis. At the same time, the accumulation of diglycerides in turn activates Rho-Kinase and PKC, leading to the inhibition of myosin light chain (MLC) phosphatase [63]. Subsequently, the sensitivity of myofilaments to Ca2+ increases and the phosphorylation of MLC initiates the contraction of smooth muscle cells [62,64]. ET causes cytoplasmic alkalinization through the activation of Na+/H− exchange, resulting in increased transcription of c-fos proto-oncogene and biochemical signals associated with proliferation [64]. In addition, ET-1 stimulates arachidonic acid production and prostaglandin release through the activation of phospholipase A2 and increased intracellular Ca2+, leading to the activation of the cAMP pathway. It can play an important role in lung diseases by mutually regulating protein expression through MAPK and AMPK signaling pathways, leading to the transcription of downstream genes and ultimately activating cell growth and migration [65,66].
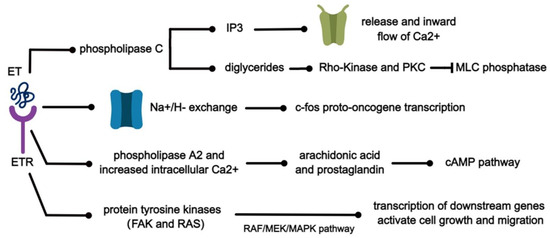
Figure 4.
Signal pathways related to the ET pathway.
ET can be affected by a wide range of substances in the body. Ang II can stimulate the release of immunoreactive ET from rat vascular smooth muscle cells via the AT1 receptor. Moreover, it shares a common receptor–effector transduction pathway with ET, and the two may promote vascular smooth muscle cell growth through a common intracellular signaling mechanism [67,68]. Its activity is potentiated by growth factors such as PDGF, epidermal growth factor (EGF), basic FGF, TGF-α, TGF-β, and insulin-like growth factor to synergistically stimulate DNA synthesis in vascular smooth muscle cells, fibroblasts, mesangial cells, melanocytes, osteoblasts, and ECs [60,69,70]. It has been shown that the full expression of ET-1 in vascular smooth muscle cells is potentiated by PDGF and EGF for pro-growth activity [71].
In ECs, TGF-β activates two different type I receptors, ALK5 and ALK1, through their respective ALK5/Smad2/3 and ALK1/Smad1/5 signaling pathways, with the former inhibiting and the latter stimulating EC proliferation and migration. TGF-β plays a key role in regulating the balance of ALK1 and ALK5 signaling in EC proliferation [72,73]. In studies targeting the TGF-b/ALK1/Endoglin signaling pathway, increased expression of ALK1 and ENG was found in the lung tissue and pulmonary ECs of patients with PH compared to the normal group. Increased TGF-β in the plasma and lungs led to the phosphorylation of Smad1/5/8, and induced pulmonary ECs to express ET-1, PDGFβ and FGF2. At the same time, despite reduced pulmonary vascular density, ENG-deficient mice were partially protected against chronic hypoxia-induced PH compared to wild-type mice [74].
4. Mechanism of the Endothelin Pathway and Its Antagonists in PH
4.1. The Role of Endothelin in the Disease Process
The importance of the ET pathway and its vital role in disease has been on the radar of researchers for three decades. In 1988, Masashi Yanagisawa and his team showed by in vitro and in vivo experiments with synthetic rat ET that it caused slow and sustained contraction of rat aortic strips and showed strong coronary constricting activity in isolated perfused rat hearts [75]. In humans, when high concentrations of ET-1 or ET-3 are provided via the forearm circulation, transient vasodilation occurs first, followed by sustained vasoconstriction, consistent with the effects on ETB receptors. The systemic circulation shows a dose-dependent significant sustained increase in blood pressure that is largely dependent on renal, mesenteric and muscle vasoconstriction, whereas the pulmonary circulation is far less sensitive. ET is not only a vasoconstrictor; it appears to be associated with vascular and cardiac hypertrophy as well as with cardiovascular diseases such as arterial hypertension, PH, atherosclerosis, coronary artery disease, and cardiac and renal failure [76]. Overexpression of the ET-1 gene in blood vessels exacerbates vascular hypertrophy [77], though not remodeling.
In addition, ET induces cell differentiation by activating PKC to inhibit EGF-stimulated DNA synthesis, stimulates protein and RNA synthesis in smooth and cardiac muscle, and may play an important role in the development of cardiac diseases such as vascular hypertrophy and cardiac hypertrophy. For example, it acts as a mitogen and dedifferentiation factor in smooth muscle cells at the onset of atherosclerosis. Plasma ET-1 concentrations are higher in atherosclerotic patients than in normal subjects, which may be related to oxidative LDL stimulating ET-1 production in ECs [78].
Patients with PH have elevated levels of ET-1 in plasma and elevated ET-1 expression in pulmonary vascular ECs. Additionally, hypoxic or monocrotaline-induced rat models exhibited a lung-specific increase in ET-1 peptide, ET-1, and ETA mRNA along with elevated PAP and RV hypertrophy. ETA/ETB receptor antagonists have been shown to prevent and delay plasma ET-1 levels in the 2-week hypoxic prophylactic model only, though they have exhibited results of effectively improved RV hypertrophy and PAP [79,80]. In the systemic circulation, the ETA-selective antagonist BQ123 abolished the pressor effect of ET-1 in rats, while the ETA/ETB antagonist PD145065 suppressed constriction of systemic vessels in response to ET. The impact of transcription factor signaling pathways on the disease process has been studied as well. In a Kruppel-like factor 4 knockout model (KLF4, a transcription factor expressed in the vascular endothelium) as well as at the protein level, it has been shown to have anti-inflammatory and antithrombotic effects [81,82] and to regulate the transcription of genes involved in three key pathways in the pathogenesis of PH, namely, the NO pathway, ET pathway and prostacyclin pathway [83].
4.2. ETA Receptor Antagonists
ERAs have a long history of research and their mechanisms of action have been well studied. In studies of ERAs, the first to be tested in humans was a natural byproduct of Streptomyces mistakii fermentation, but was less potent in terms of binding and functional analysis. Selective ETA receptor blockers reduced acute hypoxic PH in two models of hypoxia and group B streptococcal-induced acute PH in piglets, whereas they were not only ineffective but even exacerbated group B streptococcal-induced PH [84]. In acute hypoxia experiments with mature conscious horses, the ETA receptor antagonist TBC11251 was found to not significantly affect the hemodynamic or ventilatory response to acute (10 min) hypoxia. In isolated equine pulmonary arteries, ETA receptors mediated the contraction in response to ET-1, and TBC11251 effectively blocked ET-1-induced pulmonary vasoconstriction in horses, again demonstrating the role of ET in HPH at the animal level [85].
Similar results have been demonstrated in a variety of hypoxic and monocrotaline rat models, significantly reducing PH, RV hypertrophy, and RV remodeling [80], and in a post-reoxygenation neonatal piglet model showed decreased leukocyte-mediated injury and improved pulmonary function [86]. Additionally, in many studies decades ago, many antagonists showed similar protective effects on PH, such as the highly effective oral selective ETA receptor antagonist TA-0201 [87]. Research continues on its broad effects and potential side effects to expand the indications and improve the efficacy of this target.
4.3. ETB Receptor Antagonists
Similar results have been obtained when targeting ETB receptors. It has been found that ETB receptor deletion enhances the appearance of cellular and molecular markers associated with the pathobiology of PH and accelerates the disease progression, suggesting the regulation of the resting pulmonary vascular tone and an anti-proliferative protective role for ETB receptors in pulmonary vascular homeostasis [88]. There have been many studies on many different targeting strategies over the years. As an antagonist targeting the effects of ETB receptors on PH, Daniel S. Green’s team targeted receptor fragments via cell permeable peptides, which blocked ETB receptor-mediated vasoconstriction on smooth muscle cells while maintaining the integrity of other functions. The peptide was found to impede the ET-1-promoted phosphorylation of ERK and Akt in pulmonary artery smooth muscle, significantly reducing RV systolic pressure and RV hypertrophy as well as significantly reducing fully muscularized vessels and alleviating the symptoms of PH [89].
However, studies in EC-specific ETB knockout (KO) mice have shown that ETB-mediated NO/PGI2 release is reduced during hypoxia and that the concomitant absence of the NO/PGI2-mediated vasodilatory pathway may lead to disease progression, demonstrating the protective role of ETB [90]. Moreover, the absence of ETB signaling may further contribute to an increase in ET-1 [91].
5. Therapeutic Approaches
A variety of therapeutic modalities, such as exercise rehabilitation, oxygen therapy, anticoagulation, calcium channel blockers, diuretics, and anti-arrhythmic drugs, are currently used in clinical practice [92]. An imbalance between pulmonary vasodilator factors, including NO and PGI2, and vasoconstrictor factors such as ET-1, is considered to be an important factor in PH. Therefore, current therapeutic agents target the endothelial factors of vasodilation or constriction and proliferation.
The three main endothelial factor pathways currently used to target vasoconstriction and proliferation are the NO-cGMP signaling pathway (PDE5 inhibitors and soluble GC stimulators), ERAs, and the prostacyclin signaling pathway (prostacyclin analogs and IP receptor agonists) [13]. In addition to ERAs, drugs targeting other pathways can have a modulatory effect on ET. For example, both sildenafil and inhaled NO treatment can reduce HIF-1α and ET-1 levels in patients, demonstrating the broad role of ET targets [93]. The use of a single ERA or PDE5 inhibitor remains the initial treatment option for patients with PH [94].
5.1. Treatments Targeting Endothelin Receptors
Selective and non-selective ERAs are classical drug candidates currently being developed for potential clinical use in various diseases, including GMA-306 in PAH and BQ-123 in congestive heart failure, PD-161721 for atherosclerosis, enrasentan in myocardial infarction, sovateltide in cerebrovascular and coronary artery spasm, atrasentan in renal diseases, and more [45]. Researchers conducting a systematic review of randomized and semi-randomized trials that included patients with PH found that the ERA group increased exercise capacity, improved subjects’ WHO functional class, and produced favorable changes in cardiopulmonary hemodynamic variables. However, they were less effective in reducing dyspnoea and mortality. The combined use of ERAs and phosphodiesterase inhibitors may provide more benefit in PH [95].
Table 1 summarizes drugs in development that target ET receptors, covering those which are marketed, in clinical studies, in preclinical studies, and have been withdrawn, including their targets, doses, development institutions, and other basic information. The main drugs currently approved to target the ET pathway are bosentan, ambrisentan, and macitentan [96]. The role of oral ERAs in this disease was first demonstrated back in 1995, when the oral non-selective antagonist bosentan was found to have a preventive and reversal effect on pulmonary vasoconstriction and pulmonary vascular remodeling caused by acute and chronic hypoxia-induced PH [97]. Similar results were obtained in experiments with the orally administered selective ETA receptor antagonist ZD1611 [98]. Most of the subsequent drug development was carried out following the ideas of bosentan-based structural modification and potency enhancement. Improvement and good tolerability of several secondary endpoints were evaluated in the Randomized Double-Blind Placebo-Controlled Multicenter Efficacy Study 1 and 2 (ARIES-1 and ARIES-2) randomized and controlled trials for the selective ETA receptor antagonist ambrisentan [99,100], which has a lower risk of liver injury and less potential for drug interactions compared with bosentan [101]. Macitentan, also called Actelion-1 or ACT-064992, is a dual ET-1 receptor antagonist obtained by modifying the structure of bosentan [102,103]. The efficacy of macitentan in PH was investigated in the phase III SERAPHIN trial (NCT00660179), which showed a significant reduction in morbidity and mortality and an improvement in 6 min walk distance (6MWD) in patients with PAH. Its therapeutic effect was comparable to that of bosentan and aniracetam, and patients exhibited fewer adverse effects [104]. It had higher lipophilicity, increased receptor affinity, prolonged receptor binding, enhanced tissue penetration, and sustained antagonism of the ET-1 receptor [105]. In addition, it had activity on both ETA and ETB receptors, though it was much more selective for the ETA receptor than the ETB receptor in vitro.
However, there have been failures along the road of ERAs development as well. The selective ETA receptor antagonist sitaxentan was withdrawn from the market in 2010 due to acute hepatotoxicity, and possible increased liver toxicity has been mentioned in systematic reviews [95,106]. In the Tsang JY trial, the non-selective ET receptor blocker tezosentan had minimal effect on post-acute pulmonary thromboembolism gas exchange, although it reduced the symptoms of PH in acute pulmonary thromboembolism-induced hypoxia PH [107]. Following the withdrawal of sitaxsentan, many ERA drug development projects have been aborted. PH and heart failure were the main indications in several clinical studies with tezosentan, and all were stopped by early 2010 for reasons including slow recruitment (NCT01094067, NCT01077297).
Currently, ambrisentan, bosentan, and macitentan have clinical applications and all improve symptoms and exercise capacity. Neither ambrisentan nor macitentan causes abnormal liver function such as the elevation of serum aminotransferase concentrations, though the former may cause peripheral edema and the latter may cause a decrease in hemoglobin in patients. Patients treated with bosentan should have monthly liver function tests, and it can produce many drug interactions [99,108,109].
5.2. Drugs under Clinical Investigation
In recent years, the combination of ERAs and PDE5 antagonists has become a hot topic in research and development to improve efficacy by targeting different pathways involved in disease pathogenesis. Studies of fixed-dose combinations (NCT03904693), combination formulations (CTR20211325), and tadalafil alone are all in clinical phase III. Two UK companies are conducting Phase 1 clinical trials of fixed-dose combinations of aniracetam and tadalafil (NCT02688387, JPRN-UMIN000005464).
Progress has been made in the study of a novel targeted drug, with preclinical studies by Zhang Cheng’s team on the ETA receptor antagonist monoclonal antibody (MABs) getagozumab demonstrating its long-lasting and high effective action and good safety profile. It has been granted orphan drug designation by the FDA, and is expected to become a new therapeutic option [110].
Compared to small molecules, MABs are highly selective for target proteins, have fewer off-target, longer plasma half-lives, and improve patient compliance. They offer a wider range of therapeutic strategies, though they may cause immunogenic reactions in patients, potentially reducing their therapeutic efficacy. Between 2018 and 2020, Gmax Biopharm LLC in China conducted four clinical trials to validate the safety, tolerability, and pharmacokinetic profile of this monoclonal antibody (NCT04503733, CTR20200854, NCT04505137, ACTRN12618000121268), all of which are currently in the Phase I clinical stage. Another small molecule drug, SC-0062, is undergoing phase I trials in oral capsules (CTR20201868) conducted by the same Chinese company, and has been granted patents relating to pyrimidine sulfonamide derivatives and their preparation and medical applications. In addition to PH, the indications under investigation include immunological, endocrine, and metabolic diseases such as diabetic nephropathy, immunoglobulin A nephropathy, and high-altitude disease.

Table 1.
Summary of clinical studies of endothelin receptor antagonists (ERA) drugs.
Table 1.
Summary of clinical studies of endothelin receptor antagonists (ERA) drugs.
| Drug | Dosage | Target | R&D Status | R&D Institutions | Country | Reference/Clinical Trial Identifier: |
|---|---|---|---|---|---|---|
| Macitentan | 10 mg qd p.o. | Non-selective | Approved | Actelion Pharmaceuticals Ltd. | Switzerland | [111] |
| Ambrisentan | 5 mg qd p.o. 10 mg qd p.o. if tolerated | ETA | Approved | Abbott Laboratories | United States | [112] |
| Bosentan | 62.5 mg bid p.o. (4w) 125 mg bid p.o. | Non-selective | Approved | F. Hoffmann-La Roche Ltd. | Switzerland | [113] |
| Macitentan/Tadalafil | Macitentan 10 mg Tadalafil 20 mg | Non-selective PDE5A | Clinical Phase III | Actelion Pharmaceuticals Ltd. | Switzerland | NCT05236231 |
| Ambrisentan/Tadalafil | Ambrisentan 10 mg Tadalafil 40 mg | ETA | Clinical Phase I | GSK Plc | United Kingdom | NCT02688387 |
| Getagozumab | 300–1800 mg i.v. | ETA | Clinical Phase I | Gmax Biopharm LLC | China | NCT04503733 |
| Recombinant anti-human ETA humanized monoclonal antibody | 1500–2000 mg i.v. | ETA | Clinical Phase I | Gmax Biopharm LLC | China | NCT04505137 |
| SC-0062 | 50 mg qd p.o. | ETA | Clinical Phase I | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd. | China | CTR20201868 |
| Sitaxentan Sodium | 100 mg qd p.o. | ETA | Pre-clinical | Pfizer Inc. | United States | NCT01210443 |
| Enrasentan | 60–90 mg qd p.o. | Non-selective | Terminated | GSK Plc | United Kingdom | [114] |
| PD-156707 | 40 mg/kg qd p.o. for rats | ETA | Terminated | Pfizer Inc. | United States | [115] |
| Tezosentan disodium | 5 mg/h i.v. | Non-selective | Terminated | F. Hoffmann-La Roche Ltd. | Switzerland | NCT01094067 |
| ZD-1611 | 1–3 mg/kg qid p.o. | ETA | Terminated | AstraZeneca Pharmaceutical Co., Ltd. | China | [98] |
Moreover, in Jin Cai’s work, a series of phenoxybutyric acid derivatives were designed and synthesized based on the characteristics of most non-peptide ERAs and it was demonstrated that one of these selective ETA antagonists was effective in alleviating hypoxia PH and RV hypertrophy index, which may have potential for further development [116].
5.3. Problems and Prospects in Research and Development
Although current treatments targeting the ET receptor pathway have antiproliferative effects on PASMCs and delay the disease process, they do not reverse the progression of PH and have not been successful in improving survival. The current mortality rate of PH patients remains high, the prognosis remains unsatisfactory, and the situation may have become worse under the impact of the COVID-19 pandemic in the last three years. In recent years, research into targeting ET receptors using chemical entities other than small molecules, such as monoclonal antibody antagonists and selective peptide agonists and antagonists, has expanded rapidly, diversifying the targets in the ET signaling pathway. Monoclonal antibodies have low oral bioavailability due to their large molecular weight and poor membrane permeability; thus, oral bioavailability is sacrificed to extend therapy to other pathophysiological conditions. The emerging strategy is to target the dual vasoconstrictor targets angiotensin AT1 receptor and ETA receptor and use the combination of two drugs with different targets (the ETA antagonist aniracetam and the PDE5 inhibitor tadalafil) to improve the treatment of PH [117]. In addition, the induction of mild acidosis reverses PASMC phenotype conversion improves pulmonary hemodynamics and vascular function and attenuates pulmonary artery remodeling, which may be a complementary method for enhancing the effectiveness of vasodilators for PH [63].
More popular PH target research areas include hypoxia signaling pathways, cell metabolism, inflammation, cell proliferation, and personalized therapy. Beginning in 2010, the NHLBI has proposed the application of systems biology and histological thinking to develop models that better reflect disease states as a means of improving molecular-clinical phenotypic coupling of disease. Throughout the decade, significant progress has been made in these areas [118]. PH shares similar characteristics with cancer in terms of aberrant proliferation; therefore, a number of the approaches that have been applied to cancer treatment could be potential research directions for the treatment of PH, such as targeting transcription factors [119]. Altered epigenetic histone acetylation modifications are a widespread feature in PH, and by defining interaction networks and hierarchies, bioinformatics approaches can help to identify potentially successful transcription factor targets [120]. The application of histological ideas to the study of the PH lung transcriptome can provide a method for the identification of many pathways and the regulators within them [121]. Investigators have suggested that future therapeutic targets for PH could focus more on clinical endpoints of symptoms, quality of life and exercise capacity, surrogate endpoints, the understanding of treatment heterogeneity, and a review of responder analysis [122]. In addition, as PH is caused by abnormalities in multiple pathways in patients and our existing animal models are relatively homogeneous, it is difficult to simulate the clinical situations; this variability between animals and humans is one of the reasons why many drugs currently fail when they enter clinical studies.
In the future, personalized precision medicine targeting ET aspects may become a hot topic of research. By linking variations in patient genetics, i.e., DNA sequence variations that occur when individual nucleotides in the genome differ between individuals (single nucleotide polymorphisms, SNPs), drug targets such as disease-associated transmitter systems and drug treatment regimens can be tailored to individual patients. A key advance in this field is the experimental validation of genome-wide significant SNPs in five vascular diseases, with individuals carrying different levels of the minor allele having different levels of ET-1 and differing responsiveness to ETA antagonism [123]. This study provides a theoretical basis for stratifying patients with regard to ET compound therapy by testing for SNPs, exemplifying the potential for the use of precision medicine in ET.
6. Conclusions
Due to multiple factors, PH is a complex disease of the cardiopulmonary vascular system that can lead to right heart failure and death. ET is a very important vasoconstrictor peptide that is widely distributed throughout the body and plays multiple roles in human physiological activities. An elevated expression of ET and elevated plasma levels acts through multiple signaling pathways to ultimately lead to elevated pulmonary vascular pressure, remodeling, and subsequent disease progression. In this review, we have discussed the pathological process of PH, focusing on the signaling pathways and mechanisms related to ET in disease development, and reviewed the synthesis, distribution, and effects of ET. In the clinical treatment of PH, ERAs are among the most common first-line drugs, and are pharmacologically effective in relieving symptoms. As a multi-causal and highly lethal disease, most current treatment options target symptoms and other related cardiopulmonary diseases while failing to reverse disease processes such as vascular remodeling.
ERAs have a relatively long history of development and use, and are a relatively mature treatment option in clinical practice. Clinical studies on ERAs are numerous, focusing on structural modifications of existing drugs, development of new targeted drugs, dosage forms, and multi-target combination studies with the aim of improving patient compliance and reducing side effects. With the rapid development of targeted immunotherapy in recent years, humanized monoclonal antibodies for ET receptors have made relatively good progress, resulting in a new turnaround of ET in the field of targeted therapy. As the development of genomics, transcriptomics, and other multidimensional genomics has progressed, researchers have discovered that suboptimal clinical efficacy may be related to individual differences, which in turn has led to a surge in research on personalized treatment for individual patients. In addition, with the development of bioinformatics such as system biology and network pharmacology, it is possible to obtain multi-target information on the disease and the target network relationship with ET. This is a very important non-experimental approach for target screening, multi-target combinations, and expansion of ET indications. For example, research on inflammatory pathways, metabolic disorders, and immune abnormalities has progressed in recent years, providing new potential targets for the development of new drugs for PH.
Overall, ET receptors play an important role in PH research as a classical target. Considering both efficacy and side effects, future studies on ERAs should always consider factors such as the patient’s age, gender, body mass index, ethnicity, and genetic susceptibility, as well as any comorbidities and their severity. Many new techniques and theoretical approaches that have been applied to other diseases can be considered as a reference for future research in this field. Moreover, the development of more clinically appropriate animal models is a key issue in improving the quality of drug development, and it is particularly important to review past studies in this regard and learn from them.
Author Contributions
R.L.: Writing—original draft preparation, writing—review and editing. T.Y.: Preparation, writing—review and editing. R.W.: Preparation of the tables and figures. D.G.: Preparation of the tables and figures. S.W.: Guidance in the cardiovascular direction G.D.: Conceptualization, supervision, writing—review and editing, guiding the writing of the manuscript. L.F.: Conceptualization, supervision, writing—review and editing, guiding the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82073853, 82141204) and the CAMS Innovation Fund for Medical Sciences (2022-I2M-JB-010, 2021-I2M-1-005).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef]
- Galiè, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An Overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef] [PubMed]
- Poch, D.; Mandel, J. Pulmonary Hypertension. Ann. Intern. Med. 2021, 174, ITC49–ITC64. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic Definitions and Updated Clinical Classification of Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Maron, B.A.; Kovacs, G.; Vaidya, A.; Bhatt, D.L.; Nishimura, R.A.; Mak, S.; Guazzi, M.; Tedford, R.J. Cardiopulmonary Hemodynamics in Pulmonary Hypertension and Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary Arterial Hypertension: Pathogenesis and Clinical Management. BMJ 2018, j5492. [Google Scholar] [CrossRef]
- Machado, R.D.; Eickelberg, O.; Elliott, C.G.; Geraci, M.W.; Hanaoka, M.; Loyd, J.E.; Newman, J.H.; Phillips, J.A.; Soubrier, F.; Trembath, R.C.; et al. Genetics and Genomics of Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2009, 54, S32–S42. [Google Scholar] [CrossRef]
- Sommer, N.; Ghofrani, H.A.; Pak, O.; Bonnet, S.; Provencher, S.; Sitbon, O.; Rosenkranz, S.; Hoeper, M.M.; Kiely, D.G. Current and Future Treatments of Pulmonary Arterial Hypertension. Br. J. Pharm. 2021, 178, 6–30. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Savai, R.; Seeger, W.; Goncharova, E.A. From Cancer Biology to New Pulmonary Arterial Hypertension Therapeutics. Targeting Cell Growth and Proliferation Signaling Hubs. Am. J. Respir. Crit. Care Med. 2017, 195, 425–437. [Google Scholar] [CrossRef]
- Sydykov, A.; Mamazhakypov, A.; Maripov, A.; Kosanovic, D.; Weissmann, N.; Ghofrani, H.A.; Sarybaev, A.S.; Schermuly, R.T. Pulmonary Hypertension in Acute and Chronic High Altitude Maladaptation Disorders. Int. J. Envrion. Res. Public Health 2021, 18, 1692. [Google Scholar] [CrossRef]
- Naeije, R.; Richter, M.J.; Rubin, L.J. The Physiologic Basis of Pulmonary Arterial Hypertension. Eur. Respir. J. 2022, 59, 2102334. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Janocha, A.J.; Erzurum, S.C. Metabolism in Pulmonary Hypertension. Annu. Rev. Physiol. 2021, 83, 551–576. [Google Scholar] [CrossRef]
- Mandras, S.A.; Mehta, H.S.; Vaidya, A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin. Proc. 2020, 95, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Cassady, S.J.; Ramani, G.V. Right Heart Failure in Pulmonary Hypertension. Cardiol. Clin. 2020, 38, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, B.E.; Saouti, N.; van der Laarse, W.J.; Westerhof, N.; Vonk Noordegraaf, A. Treatment Strategies for the Right Heart in Pulmonary Hypertension. Cardiovasc. Res. 2017, 113, 1465–1473. [Google Scholar] [CrossRef]
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and Its Load in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef]
- Luks, A.M.; Swenson, E.R.; Bärtsch, P. Acute High-Altitude Sickness. Eur. Respir. Rev. 2017, 26, 160096. [Google Scholar] [CrossRef]
- Wu, D.; Dasgupta, A.; Read, A.D.; Bentley, R.E.T.; Motamed, M.; Chen, K.-H.; Al-Qazazi, R.; Mewburn, J.D.; Dunham-Snary, K.J.; Alizadeh, E.; et al. Oxygen Sensing, Mitochondrial Biology and Experimental Therapeutics for Pulmonary Hypertension and Cancer. Free Radic. Biol. Med. 2021, 170, 150–178. [Google Scholar] [CrossRef]
- Rawat, M.; Lakshminrusimha, S.; Vento, M. Pulmonary Hypertension and Oxidative Stress: Where Is the Link? Semin. Fetal Neonatal Med. 2022, 27, 101347. [Google Scholar] [CrossRef]
- Huertas, A.; Guignabert, C.; Barberà, J.A.; Bärtsch, P.; Bhattacharya, J.; Bhattacharya, S.; Bonsignore, M.R.; Dewachter, L.; Dinh-Xuan, A.T.; Dorfmüller, P.; et al. Pulmonary Vascular Endothelium: The Orchestra Conductor in Respiratory Diseases: Highlights from Basic Research to Therapy. Eur. Respir. J. 2018, 51, 1700745. [Google Scholar] [CrossRef]
- Cai, A.; Li, L.; Zhou, Y. Pathophysiological Effects of RhoA and Rho-Associated Kinase on Cardiovascular System. J. Hypertens. 2016, 34, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Z.; Li, S.-Y.; Tian, X.-Y.; Hong, Z.; Li, J.-X. Effect of Rho Kinase Inhibitor Fasudil on the Expression ET-1 and NO in Rats with Hypoxic Pulmonary Hypertension. Clin. Hemorheol. Microcirc. 2019, 71, 3–8. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, P.; Wang, J.; Xu, Q.; Fan, J.; Yan, L.; Ma, P.; Zhou, R. Betaine Alleviates Right Ventricular Failure via Regulation of Rho A/ROCK Signaling Pathway in Rats with Pulmonary Arterial Hypertension. Eur. J. Pharmacol. 2021, 910, 174311. [Google Scholar] [CrossRef] [PubMed]
- Morand, J.; Briançon-Marjollet, A.; Lemarie, E.; Gonthier, B.; Arnaud, J.; Korichneva, I.; Godin-Ribuot, D. Zinc Deficiency Promotes Endothelin Secretion and Endothelial Cell Migration through Nuclear Hypoxia-Inducible Factor-1 Translocation. Am. J. Physiol.-Cell Physiol. 2019, 317, C270–C276. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiao, Y.; Guo, L.; Ma, Y.; Zhao, R.; Li, X.; Shen, L.; Zhou, Z.; Kim, S.; Liu, J. Astragaloside IV Blocks Monocrotaline-induced Pulmonary Arterial Hypertension by Improving Inflammation and Pulmonary Artery Remodeling. Int. J. Mol. Med. 2020, 47, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ji, Z.; Fu, J.; Wang, X.-F.; Zhang, L.-S. Endosulfan Induces Endothelial Inflammation and Dysfunction via IRE1α/NF-ΚB Signaling Pathway. Environ. Sci. Pollut. Res. 2020, 27, 26163–26171. [Google Scholar] [CrossRef]
- Barnes, E.A.; Chen, C.; Sedan, O.; Cornfield, D.N. Loss of Smooth Muscle Cell Hypoxia Inducible Factor-1α Underlies Increased Vascular Contractility in Pulmonary Hypertension. FASEB J. 2017, 31, 650–662. [Google Scholar] [CrossRef]
- Hu, C.-J.; Poth, J.M.; Zhang, H.; Flockton, A.; Laux, A.; Kumar, S.; McKeon, B.; Frid, M.G.; Mouradian, G.; Li, M.; et al. Suppression of HIF2 Signalling Attenuates the Initiation of Hypoxia-Induced Pulmonary Hypertension. Eur. Respir. J. 2019, 54, 1900378. [Google Scholar] [CrossRef]
- Singh, N.; Dorfmüller, P.; Shlobin, O.A.; Ventetuolo, C.E. Group 3 Pulmonary Hypertension: From Bench to Bedside. Circ. Res. 2022, 130, 1404–1422. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, R.X.; Bao, D. TGF-Β1 Promotes Pulmonary Arterial Hypertension in Rats via Activating RhoA/ROCK Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4988–4996. [Google Scholar]
- Guo, M.; Zhang, M.; Cao, X.; Fang, X.; Li, K.; Qin, L.; He, Y.; Zhao, J.; Xu, Y.; Liu, X.; et al. Notch4 Mediates Vascular Remodeling via ERK/JNK/P38 MAPK Signaling Pathways in Hypoxic Pulmonary Hypertension. Respir. Res. 2022, 23, 6. [Google Scholar] [CrossRef]
- Morris, H.E.; Neves, K.B.; Montezano, A.C.; MacLean, M.R.; Touyz, R.M. Notch3 Signalling and Vascular Remodelling in Pulmonary Arterial Hypertension. Clin. Sci. 2019, 133, 2481–2498. [Google Scholar] [CrossRef] [PubMed]
- Carling, D. AMPK Signalling in Health and Disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Fang, X.; Guo, M.; Li, X.; He, Y.; Xie, M.; Xu, Y.; Liu, X. TRB3 Mediates Vascular Remodeling by Activating the MAPK Signaling Pathway in Hypoxic Pulmonary Hypertension. Respir. Res. 2021, 22, 312. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, L.; Wu, Y.; Wang, R.; Jiang, Y.; Hu, R.; Zhu, L.; Li, L.; Fang, Y.; Yang, C.; et al. Resistin-like Molecule β Acts as a Mitogenic Factor in Hypoxic Pulmonary Hypertension via the Ca2+-Dependent PI3K/Akt/MTOR and PKC/MAPK Signaling Pathways. Respir. Res. 2021, 22, 8. [Google Scholar] [CrossRef]
- Coons, J.C.; Pogue, K.; Kolodziej, A.R.; Hirsch, G.A.; George, M.P. Pulmonary Arterial Hypertension: A Pharmacotherapeutic Update. Curr. Cardiol. Rep. 2019, 21, 141. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A Novel Potent Vasoconstrictor Peptide Produced by Vascular Endothelial Cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Inoue, A.; Yanagisawa, M.; Kimura, S.; Kasuya, Y.; Miyauchi, T.; Goto, K.; Masaki, T. The Human Endothelin Family: Three Structurally and Pharmacologically Distinct Isopeptides Predicted by Three Separate Genes. Proc. Natl. Acad. Sci. USA 1989, 86, 2863–2867. [Google Scholar] [CrossRef]
- Sakurai, T.; Yanagisawa, M.; Takuwat, Y.; Miyazakit, H.; Kimura, S.; Goto, K.; Masaki, T. Cloning of a CDNA Encoding a Non-Isopeptide-Selective Subtype of the Endothelin Receptor. Nature 1990, 348, 732–735. [Google Scholar] [CrossRef]
- Mouchtouri, E.-T.; Konstantinou, T.; Lekkas, P.; Kolettis, T.M. Endothelin System and Ischemia-Induced Ventricular Tachyarrhythmias. Life 2022, 12, 1627. [Google Scholar] [CrossRef]
- Raevens, S.; Boret, M.; Fallon, M.B. Hepatopulmonary Syndrome. JHEP Rep. 2022, 4, 100527. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.J.; Cho, Y.M.; Ham, E.; Cacioppo, J.A.; Park, C.J. Endothelin 2: A Key Player in Ovulation and Fertility. Reproduction 2022, 163, R71–R80. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Bramall, A.N.; Baynash, A.G.; Rattner, A.; Rakheja, D.; Post, M.; Joza, S.; McKerlie, C.; Stewart, D.J.; McInnes, R.R.; et al. Endothelin-2 Deficiency Causes Growth Retardation, Hypothermia, and Emphysema in Mice. J. Clin. Investig. 2013, 123, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Siao, A.-C.; Shih, L.-J.; Lin, Y.-Y.; Tsuei, Y.-W.; Kuo, Y.-C.; Ku, H.-C.; Chuu, C.-P.; Hsiao, P.-J.; Kao, Y.-H. Investigation of the Molecular Mechanisms by Which Endothelin-3 Stimulates Preadipocyte Growth. Front. Endocrinol. 2021, 12, 661828. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Sakai, S. Endothelin and the Heart in Health and Diseases. Peptides 2019, 111, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Maguire, J.J.; Davenport, A.P. Endothelin-2, the Forgotten Isoform: Emerging Role in the Cardiovascular System, Ovarian Development, Immunology and Cancer: Emerging Role of ET-2. Br. J. Pharm. 2013, 168, 283–295. [Google Scholar] [CrossRef]
- Czopek, A.; Moorhouse, R.; Webb, D.J.; Dhaun, N. Therapeutic Potential of Endothelin Receptor Antagonism in Kidney Disease. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R388–R397. [Google Scholar] [CrossRef]
- Lüscher, T.F.; Barton, M. Endothelins and Endothelin Receptor Antagonists: Therapeutic Considerations for a Novel Class of Cardiovascular Drugs. Circulation 2000, 102, 2434–2440. [Google Scholar] [CrossRef]
- Maguire, J.J.; Davenport, A.P. Endothelin@25—New Agonists, Antagonists, Inhibitors and Emerging Research Frontiers: IUPHAR Review 12. Br. J. Pharm. 2014, 171, 5555–5572. [Google Scholar] [CrossRef]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharm. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.-B. The Roles of Endothelin and Its Receptors in Cigarette Smoke-Associated Pulmonary Hypertension with Chronic Lung Disease. Pathol.-Res. Pract. 2020, 216, 153083. [Google Scholar] [CrossRef]
- Ziegler, J.W.; Ivy, D.D.; Kinsella, J.P.; Abman, S.H. The Role of Nitric Oxide, Endothelin, and Prostaglandins in the Transition of the Pulmonary Circulation. Clin. Perinatol. 1995, 22, 387–403. [Google Scholar] [CrossRef]
- Bremnes, T.; Paasche, J.D.; Mehlum, A.; Sandberg, C.; Bremnes, B.; Attramadal, H. Regulation and Intracellular Trafficking Pathways of the Endothelin Receptors. J. Biol. Chem. 2000, 275, 17596–17604. [Google Scholar] [CrossRef]
- Shihoya, W.; Nishizawa, T.; Okuta, A.; Tani, K.; Dohmae, N.; Fujiyoshi, Y.; Nureki, O.; Doi, T. Activation Mechanism of Endothelin ETB Receptor by Endothelin-1. Nature 2016, 537, 363–368. [Google Scholar] [CrossRef]
- Barton, M.; Yanagisawa, M. Endothelin: 30 Years from Discovery to Therapy. Hypertension 2019, 74, 1232–1265. [Google Scholar] [CrossRef]
- Lerman, A.; Hildebrand, F.L.; Margulies, K.B.; O’Murchu, B.; Perrella, M.A.; Heublein, D.M.; Schwab, T.R.; Burnett, J.C. Endothelin: A New Cardiovascular Regulatory Peptide. Mayo Clin. Proc. 1990, 65, 1441–1455. [Google Scholar] [CrossRef]
- Inoue, A.; Yanagisawa, M.; Takuwa, Y.; Mitsui, Y.; Kobayashi, M.; Masaki, T. The Human Preproendothelin-1 Gene. J. Biol. Chem. 1989, 264, 14954–14959. [Google Scholar] [CrossRef]
- Khimji, A.; Rockey, D.C. Endothelin—Biology and Disease. Cell. Signal. 2010, 22, 1615–1625. [Google Scholar] [CrossRef]
- Feng, M.; Wang, D.; Wang, X.; Yang, Y.; Zhang, S. Bai-Hu-Tang Regulates Endothelin-1 and Its Signalling Pathway in Vascular Endothelial Cells. J. Ethnopharmacol. 2022, 284, 114812. [Google Scholar] [CrossRef]
- Kedzierski, R.M.; Yanagisawa, M. Endothelin System: The Double-Edged Sword in Health and Disease. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 851–876. [Google Scholar] [CrossRef]
- Almikhlafi, M.A.; Haghayeghi, K.; Gardner, A. Endothelin A (ETA) and Endothelin B (ETB) Receptor Subtypes Potentiate Epidermal Growth Factor (EGF)-Mediated Proliferation in Human Asthmatic Bronchial Airway Smooth Muscle. Cureus 2022. [Google Scholar] [CrossRef]
- Marsden, P.A.; Raju Danthuluri, N.; Brenner, B.M.; Ballermann, B.J.; Brock, T.A. Endothelin Action on Vascular Smooth Muscle Involves Inositol Trisphosphate and Calcium Mobilization. Biochem. Biophys. Res. Commun. 1989, 158, 86–93. [Google Scholar] [CrossRef]
- Christou, H.; Khalil, R.A. Mechanisms of Pulmonary Vascular Dysfunction in Pulmonary Hypertension and Implications for Novel Therapies. Am. J. Physiol.-Heart Circ. Physiol. 2022, 322, H702–H724. [Google Scholar] [CrossRef]
- Simonson, M.S.; Wann, S.; Mené, P.; Dubyak, G.R.; Kester, M.; Nakazato, Y.; Sedor, J.R.; Dunn, M.J. Endothelin Stimulates Phospholipase C, Na+/H+ Exchange, c-Fos Expression, and Mitogenesis in Rat Mesangial Cells. J. Clin. Investig. 1989, 83, 708–712. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Wang, T.; Yao, D.; Shi, Y.; Liu, J.; Wang, B.; Wei, H.; Liu, W.; Xu, C.; et al. DMSO-Soluble Smoking Particles up-Regulate the Vascular Endothelin Receptors through AMPK-SIRT1 and MAPK Pathways. Chem.-Biol. Interact. 2022, 368, 110203. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Fagan, K.A.; Frid, M.G. Hypoxia-Induced Pulmonary Vascular Remodeling: Cellular and Molecular Mechanisms. Circ. Res. 2006, 99, 675–691. [Google Scholar] [CrossRef]
- Arleth, J.; Storer, L.; Ohlstein, H. Angiotensin Type 1 Receptors Mediate Smooth Muscle Proliferation and Endothelin Biosynthesis in Rat Vascular Smooth Muscle. J. Pharmacol. Exp. Ther. 1994, 271, 9. [Google Scholar]
- Speck, D.; Kleinau, G.; Szczepek, M.; Kwiatkowski, D.; Catar, R.; Philippe, A.; Scheerer, P. Angiotensin and Endothelin Receptor Structures with Implications for Signaling Regulation and Pharmacological Targeting. Front. Endocrinol. 2022, 13, 880002. [Google Scholar] [CrossRef]
- Bellaye, P.-S.; Yanagihara, T.; Granton, E.; Sato, S.; Shimbori, C.; Upagupta, C.; Imani, J.; Hambly, N.; Ask, K.; Gauldie, J.; et al. Macitentan Reduces Progression of TGF-Β1-Induced Pulmonary Fibrosis and Pulmonary Hypertension. Eur. Respir. J. 2018, 52, 1701857. [Google Scholar] [CrossRef]
- Kemp, S.S.; Aguera, K.N.; Cha, B.; Davis, G.E. Defining Endothelial Cell-Derived Factors That Promote Pericyte Recruitment and Capillary Network Assembly. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2632–2648. [Google Scholar] [CrossRef]
- Masaki, T. Endothelin in Vascular Biology. Ann. N. Y. Acad. Sci. 1994, 714, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bofarid, S.; Hosman, A.E.; Mager, J.J.; Snijder, R.J.; Post, M.C. Pulmonary Vascular Complications in Hereditary Hemorrhagic Telangiectasia and the Underlying Pathophysiology. Int. J. Mol. Sci. 2021, 22, 3471. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Inamura, H.; Sugaya, T.; Matsuoka, M. Blockade of ALK4/5 Signaling Suppresses Cadmium- and Erastin-Induced Cell Death in Renal Proximal Tubular Epithelial Cells via Distinct Signaling Mechanisms. Cell Death Differ. 2019, 26, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Gore, B.; Izikki, M.; Mercier, O.; Dewachter, L.; Fadel, E.; Humbert, M.; Dartevelle, P.; Simonneau, G.; Naeije, R.; Lebrin, F.; et al. Key Role of the Endothelial TGF-β/ALK1/Endoglin Signaling Pathway in Humans and Rodents Pulmonary Hypertension. PLoS ONE 2014, 9, e100310. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Inoue, A.; Ishikawa, T.; Kasuya, Y.; Kimura, S.; Kumagaye, S.; Nakajima, K.; Watanabe, T.X.; Sakakibara, S.; Goto, K. Primary Structure, Synthesis, and Biological Activity of Rat Endothelin, an Endothelium-Derived Vasoconstrictor Peptide. Proc. Natl. Acad. Sci. USA 1988, 85, 6964–6967. [Google Scholar] [CrossRef]
- Dhaun, N.; Webb, D.J. Endothelins in Cardiovascular Biology and Therapeutics. Nat. Rev. Cardiol. 2019, 16, 491–502. [Google Scholar] [CrossRef]
- Brewster, L.M.; Garcia, V.P.; Levy, M.V.; Stockelman, K.A.; Goulding, A.; DeSouza, N.M.; Greiner, J.J.; Hijmans, J.G.; DeSouza, C.A. Endothelin-1-Induced Endothelial Microvesicles Impair Endothelial Cell Function. J. Appl. Physiol. 2020, 128, 1497–1505. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Asadikaram, G.; Mohammadi, A.; Jahani, Y.; Moridi, M.; Masoumi, M. The Association of Endothelin-1 Gene Polymorphism and Its Plasma Levels with Hypertension and Coronary Atherosclerosis. Arch. Med. Sci. 2021, 17, 613–620. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Bai, Y.; Li, X.; Gao, X.; Li, C.; Guo, G.; Chen, S.; Sun, M.; Liu, K.; et al. Pipersentan: A De Novo Synthetic Endothelin Receptor Antagonist That Inhibits Monocrotaline- and Hypoxia-Induced Pulmonary Hypertension. Front. Pharmacol. 2022, 13, 920222. [Google Scholar] [CrossRef]
- Tilton, R.G.; Munsch, C.L.; Sherwood, S.J.; Chen, S.-J.; Chen, Y.-F.; Wu, C.; Block, N.; Dixon, R.A.F.; Brock, T.A. Attenuation of Pulmonary Vascular Hypertension and Cardiac Hypertrophy with Sitaxsentan Sodium, an Orally Active ETAreceptor Antagonist. Pulm. Pharmacol. Ther. 2000, 13, 87–97. [Google Scholar] [CrossRef]
- Ban, Y.; Liu, Y.; Li, Y.; Zhang, Y.; Xiao, L.; Gu, Y.; Chen, S.; Zhao, B.; Chen, C.; Wang, N. S-Nitrosation Impairs KLF4 Activity and Instigates Endothelial Dysfunction in Pulmonary Arterial Hypertension. Redox. Biol. 2019, 21, 101099. [Google Scholar] [CrossRef] [PubMed]
- Shatat, M.A.; Tian, H.; Zhang, R.; Tandon, G.; Hale, A.; Fritz, J.S.; Zhou, G.; Martínez-González, J.; Rodríguez, C.; Champion, H.C.; et al. Endothelial Krüppel-Like Factor 4 Modulates Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2014, 50, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Nava, E.; Llorens, S. The Local Regulation of Vascular Function: From an Inside-Outside to an Outside-Inside Model. Front. Physiol. 2019, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Ambalavanan, N.; Philips, J.B.; Bulger, A.; Oparil, S.; Chen, Y.-F. Endothelin-A Receptor Blockade in Porcine Pulmonary Hypertension. Pediatr. Res. 2002, 52, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Benamou, A.E.; Marlin, D.J.; Lekeux, P. Endothelin in the Equine Hypoxic Pulmonary Vasoconstrictive Response to Acute Hypoxia. Equine Vet. J. 2010, 33, 345–353. [Google Scholar] [CrossRef]
- Pearl, J.M.; Wellmann, S.A.; McNamara, J.L.; Lombardi, J.P.; Wagner, C.J.; Raake, J.L.; Nelson, D.P. Bosentan Prevents Hypoxia-Reoxygenation–Induced Pulmonary Hypertension and Improves Pulmonary Function. Ann. Thorac. Surg. 1999, 68, 1714–1721. [Google Scholar] [CrossRef]
- Itoh, H.; Yokochi, A.; Yamauchi-Kohno, R.; Maruyama, K. Effects of the Endothelin ETA Receptor Antagonist, TA-0201, on Pulmonary Arteries Isolated from Hypoxic Rats. Eur. J. Pharmacol. 1999, 376, 233–238. [Google Scholar] [CrossRef]
- Tabeling, C.; González Calera, C.R.; Lienau, J.; Höppner, J.; Tschernig, T.; Kershaw, O.; Gutbier, B.; Naujoks, J.; Herbert, J.; Opitz, B.; et al. Endothelin B Receptor Immunodynamics in Pulmonary Arterial Hypertension. Front. Immunol. 2022, 13, 895501. [Google Scholar] [CrossRef]
- Green, D.S.; Rupasinghe, C.; Warburton, R.; Wilson, J.L.; Sallum, C.O.; Taylor, L.; Yatawara, A.; Mierke, D.; Polgar, P.; Hill, N. A Cell Permeable Peptide Targeting the Intracellular Loop 2 of Endothelin B Receptor Reduces Pulmonary Hypertension in a Hypoxic Rat Model. PLoS ONE 2013, 8, e81309. [Google Scholar] [CrossRef]
- Kelland, N.F.; Bagnall, A.J.; Morecroft, I.; Gulliver-Sloan, F.H.; Dempsie, Y.; Nilsen, M.; Yanagisawa, M.; MacLean, M.R.; Kotelevtsev, Y.V.; Webb, D.J. Endothelial ETB Limits Vascular Remodelling and Development of Pulmonary Hypertension during Hypoxia. J. Vasc. Res. 2010, 47, 16–22. [Google Scholar] [CrossRef]
- Naomi, S.; Iwaoka, T.; Disashi, T.; Inoue, J.; Kanesaka, Y.; Tokunaga, H.; Tomita, K. Endothelin-1 Inhibits Endothelin-Converting Enzyme-1 Expression in Cultured Rat Pulmonary Endothelial Cells. Circulation 1998, 97, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Zolty, R. Pulmonary Arterial Hypertension Specific Therapy: The Old and the New. Pharmacol. Ther. 2020, 214, 107576. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, H.-W.; Li, Z.-G. The Effect of Sildenafil Combined with Inhalational Nitric Oxide Therapy in Neonatal Pulmonary Hypertension. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4683–4690. [Google Scholar] [PubMed]
- Kuntz, M.; Leiva-Juarez, M.M.; Luthra, S. Systematic Review of Randomized Controlled Trials of Endothelin Receptor Antagonists for Pulmonary Arterial Hypertension. Lung 2016, 194, 723–732. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Gao, Y.; Deng, B.; Liu, K. Endothelin Receptor Antagonists for Pulmonary Arterial Hypertension. Cochrane Database Syst. Rev. 2021, 2021, CD004434. [Google Scholar] [CrossRef]
- Humbert, M.; Lau, E.M.T.; Montani, D.; Jaïs, X.; Sitbon, O.; Simonneau, G. Advances in Therapeutic Interventions for Patients with Pulmonary Arterial Hypertension. Circulation 2014, 130, 2189–2208. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, Y.F.; Meng, Q.C.; Durand, J.; Dicarlo, V.S.; Oparil, S. Endothelin-Receptor Antagonist Bosentan Prevents and Reverses Hypoxic Pulmonary Hypertension in Rats. J. Appl. Physiol. 1995, 79, 2122–2131. [Google Scholar] [CrossRef]
- Bialecki, R.A.; Fisher, C.S.; Abbott, B.M.; Barthlow, H.G.; Caccese, R.G.; Stow, R.B.; Rumsey, J.; Rumsey, W. ZD1611, an Orally Active Endothelin-A Receptor Antagonist, Prevents Chronic Hypoxia-Induced Pulmonary Hypertension in the Rat. Pulm. Pharmacol. Ther. 1999, 12, 303–312. [Google Scholar] [CrossRef]
- Galiè, N.; Olschewski, H.; Oudiz, R.J.; Torres, F.; Frost, A.; Ghofrani, H.A.; Badesch, D.B.; McGoon, M.D.; McLaughlin, V.V.; Roecker, E.B.; et al. Ambrisentan for the Treatment of Pulmonary Arterial Hypertension: Results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation 2008, 117, 3010–3019. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, N.; Chen, J.; Parks, D.; Tian, Z. Comparative Assessment of Efficacy and Safety of Ambrisentan and Bosentan in Patients with Pulmonary Arterial Hypertension: A Meta-analysis. Clin. Pharm. Ther. 2022, 47, 146–156. [Google Scholar] [CrossRef]
- Vizza, C.D.; Fedele, F.; Pezzuto, B.; Rubin, L.J. Safety and Efficacy Evaluation of Ambrisentan in Pulmonary Hypertension. Expert Opin. Drug Saf. 2012, 11, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Iglarz, M.; Binkert, C.; Morrison, K.; Fischli, W.; Gatfield, J.; Treiber, A.; Weller, T.; Bolli, M.H.; Boss, C.; Buchmann, S.; et al. Pharmacology of Macitentan, an Orally Active Tissue-Targeting Dual Endothelin Receptor Antagonist. J. Pharm. Exp. Ther. 2008, 327, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.; Bhardwaj, A.; Nair, A. Pharmacotherapy for Pulmonary Arterial Hypertension. J. Thorac. Dis. 2019, 11, S1767–S1781. [Google Scholar] [CrossRef] [PubMed]
- Bedan, M.; Grimm, D.; Wehland, M.; Simonsen, U.; Infanger, M.; Krüger, M. A Focus on Macitentan in the Treatment of Pulmonary Arterial Hypertension. Basic Clin. Pharm. Toxicol. 2018, 123, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kummer, O.; Haschke, M.; Hammann, F.; Bodmer, M.; Bruderer, S.; Regnault, Y.; Dingemanse, J.; Krähenbühl, S. Comparison of the Dissolution and Pharmacokinetic Profiles of Two Galenical Formulations of the Endothelin Receptor Antagonist Macitentan. Eur. J. Pharm. Sci. 2009, 38, 384–388. [Google Scholar] [CrossRef]
- Lavelle, A.; Sugrue, R.; Lawler, G.; Mulligan, N.; Kelleher, B.; Murphy, D.M.; Gaine, S.P. Sitaxentan-Induced Hepatic Failure in Two Patients with Pulmonary Arterial Hypertension. Eur. Respir. J. 2009, 34, 770–771. [Google Scholar] [CrossRef]
- Tsang, J.Y.C.; Lamm, W.J.E.; Neradilek, B.; Polissar, N.L.; Hlastala, M.P. Endothelin Receptor Blockade Does Not Improve Hypoxemia Following Acute Pulmonary Thromboembolism. J. Appl. Physiol. 2007, 102, 762–771. [Google Scholar] [CrossRef]
- Humbert, M.; Segal, E.S.; Kiely, D.G.; Carlsen, J.; Schwierin, B.; Hoeper, M.M. Results of European Post-Marketing Surveillance of Bosentan in Pulmonary Hypertension. Eur. Respir. J. 2007, 30, 338–344. [Google Scholar] [CrossRef]
- Pulido, T.; Adzerikho, I.; Channick, R.N.; Delcroix, M.; Galiè, N.; Ghofrani, H.-A.; Jansa, P.; Jing, Z.-C.; Le Brun, F.-O.; Mehta, S.; et al. Macitentan and Morbidity and Mortality in Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013, 369, 809–818. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Zhang, H.; Yao, C.; Pan, H.; Guo, Y.; Fan, K.; Jing, S. Therapeutic Monoclonal Antibody Antagonizing Endothelin Receptor A for Pulmonary Arterial Hypertension. J. Pharm. Exp. Ther. 2019, 370, 54–61. [Google Scholar] [CrossRef]
- Krause, A.; Zisowsky, J.; Dingemanse, J. Modeling of Pharmacokinetics, Efficacy, and Hemodynamic Effects of Macitentan in Patients with Pulmonary Arterial Hypertension. Pulm. Pharmacol. Ther. 2018, 49, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.; Kingman, M.; Duncan, M.; Berngard, S.C.; Fernandes, T. Titration of Pulmonary Arterial Hypertension Therapeutics: Experience-Based Recommendations. Respir. Med. 2018, 143, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Li, D.; Hui, Z.; McLachlan, C.S.; Zhang, Y. Chronic Dosing with Metformin plus Bosentan Decreases in Vitro Pulmonary Artery Contraction from Isolated Arteries in Adults with Pulmonary Hypertension. J. Cardiovasc. Thorac. Res. 2019, 11, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.K. Comparison of the Dual Receptor Endothelin Antagonist Enrasentan with Enalapril in Asymptomatic Left Ventricular Systolic Dysfunction: A Cardiovascular Magnetic Resonance Study. Heart 2005, 92, 798–803. [Google Scholar] [CrossRef]
- Haleen, S.; Schroeder, R.; Walker, D.; Quenby-Brown, E.; Welch, K.; Hallak, H.; Uprichard, A.; Keiser, J. Efficacy of CI-1020, an Endothelin-A Receptor Antagonist, in Hypoxic Pulmonary Hypertension. J. Cardiovasc. Pharmacol. 1998, 31, S331–S335. [Google Scholar] [CrossRef]
- Cai, J.; Liu, L.; Hong, K.H.; Wang, P.; Li, L.; Cao, M.; Sun, C.; Wu, X.; Zong, X.; Chen, J.; et al. Discovery of Phenoxybutanoic Acid Derivatives as Potent Endothelin Antagonists with Antihypertensive Activity. Bioorganic Med. Chem. 2015, 23, 657–667. [Google Scholar] [CrossRef]
- Davenport, A.P.; Kuc, R.E.; Southan, C.; Maguire, J.J. New Drugs and Emerging Therapeutic Targets in the Endothelin Signaling Pathway and Prospects for Personalized Precision Medicine. Physiol. Res. 2018, S37–S54. [Google Scholar] [CrossRef]
- Erzurum, S.; Rounds, S.I.; Stevens, T.; Aldred, M.; Aliotta, J.; Archer, S.L.; Asosingh, K.; Balaban, R.; Bauer, N.; Bhattacharya, J.; et al. Strategic Plan for Lung Vascular Research: An NHLBI-ORDR Workshop Report. Am. J. Respir. Crit. Care Med. 2010, 182, 1554–1562. [Google Scholar] [CrossRef]
- Van der Feen, D.E.; Kurakula, K.; Tremblay, E.; Boucherat, O.; Bossers, G.P.L.; Szulcek, R.; Bourgeois, A.; Lampron, M.-C.; Habbout, K.; Martineau, S.; et al. Multicenter Preclinical Validation of BET Inhibition for the Treatment of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2019, 200, 910–920. [Google Scholar] [CrossRef]
- Churko, J.M.; Garg, P.; Treutlein, B.; Venkatasubramanian, M.; Wu, H.; Lee, J.; Wessells, Q.N.; Chen, S.-Y.; Chen, W.-Y.; Chetal, K.; et al. Defining Human Cardiac Transcription Factor Hierarchies Using Integrated Single-Cell Heterogeneity Analysis. Nat. Commun. 2018, 9, 4906. [Google Scholar] [CrossRef]
- Sung, Y.K.; Yuan, K.; de Jesus Perez, V.A. Novel Approaches to Pulmonary Arterial Hypertension Drug Discovery. Expert Opin. Drug Discov. 2016, 11, 407–414. [Google Scholar] [CrossRef] [PubMed]
- George, M.P.; Gladwin, M.T.; Graham, B.B. Exploring New Therapeutic Pathways in Pulmonary Hypertension. Metabolism, Proliferation, and Personalized Medicine. Am. J. Respir. Cell Mol. Biol. 2020, 63, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.M.; Hadaya, J.; Trehan, A.; Zekavat, S.M.; Roselli, C.; Klarin, D.; Emdin, C.A.; Hilvering, C.R.E.; Bianchi, V.; Mueller, C.; et al. A Genetic Variant Associated with Five Vascular Diseases Is a Distal Regulator of Endothelin-1 Gene Expression. Cell 2017, 170, 522–533.e15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).