Galectin-3, Inflammation, and the Risk of Atrial High-Rate Episodes in Patients with Dual Chamber Pacemakers
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
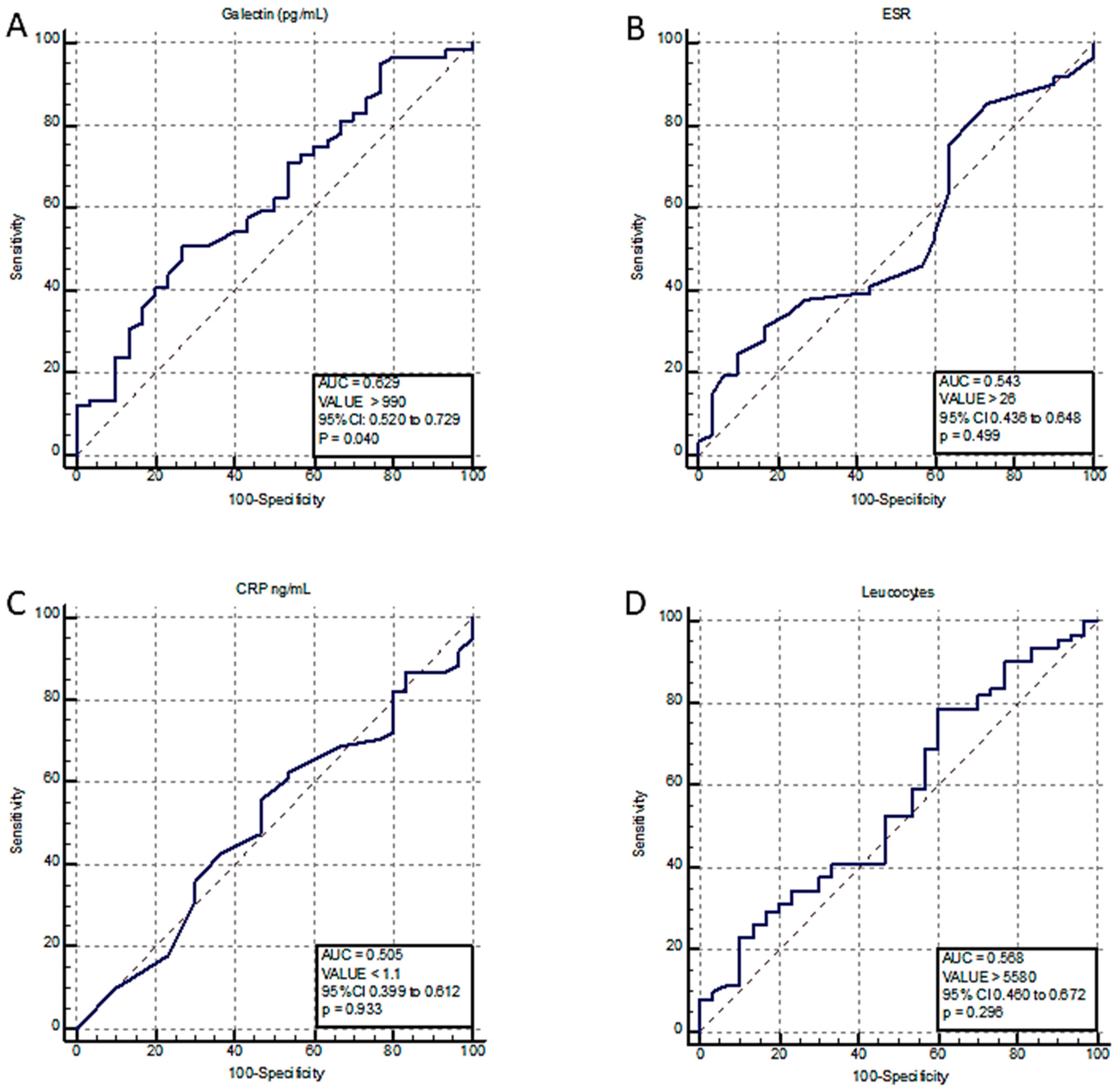
2.2. AHRE Incidence and Predictors
3. Discussion
Limitations
4. Materials and Methods
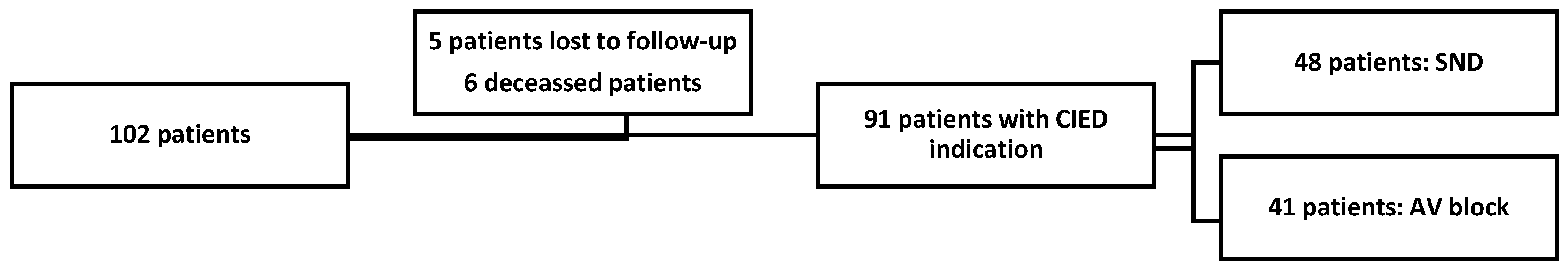
4.1. Study Population
4.2. Implant Procedure
4.3. Follow-Up
4.4. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Glotzer, T.V.; Daoud, E.G.; Wyse, D.G.; Singer, D.E.; Ezekowitz, M.D.; Hilker, C.; Miller, C.; Qi, D.; Ziegler, P.D. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: The TRENDS study. Circ. Arrhyth. Electrophysiol. 2009, 2, 474–480. [Google Scholar] [CrossRef]
- Vanassche, T.; Lauw, M.N.; Eikelboom, J.W.; Healey, J.S.; Hart, R.G.; Alings, M.; Avezum, A.; Díaz, R.; Hohnloser, S.H.; Lewis, B.S.; et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: Analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur. Heart J. 2015, 36, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.A.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; et al. Temporal Relationship Between Subclinical Atrial Fibrillation and Embolic Events. Circulation 2014, 129, 2094–2099. [Google Scholar] [CrossRef]
- Daoud, E.G.; Glotzer, T.V.; Wyse, D.G.; Ezekowitz, M.D.; Hilker, C.; Koehler, J.; Ziegler, P.D.; Investigators, T. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: A subgroup analysis of TRENDS. Heart Rhythm 2011, 8, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.; Boriani, G.; Glotzer, T.V.; Healey, J.S.; Kirchhof, P.; Potpara, T.S. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat. Rev. Cardiol. 2017, 14, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Chen, T.-W.; Lu, W.-D. HATCH Score and Left Atrial Size Predict Atrial High-Rate Episodes in Patients With Cardiac Implantable Electronic Devices. Front. Cardiovasc. Med. 2021, 8, 746225. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; González, A.; Pizard, A.; Papageorgiou, A.P.; López-Andrés, N.; Jaisser, F.; Thum, T.; Zannad, F.; Díez, J. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur. J. Heart Fail. 2015, 17, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering with Myocardial Fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.U.; Gurses, K.M.; Kocyigit, D.; Canpinar, H.; Canpolat, U.; Evranos, B.; Yorgun, H.; Sahiner, M.L.; Kaya, E.B.; Hazirolan, T.; et al. The Association of Serum Galectin-3 Levels with Atrial Electrical and Structural Remodeling. J. Cardiovasc. Electrophysiol. 2015, 26, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, M.U.; Koçyiğit, D.; Canpınar, H.; Ateş, A.H.; Canpolat, U.; Yorgun, H.; Aytemir, K. Serum galectin-3 level predicts early recurrence following successful direct current cardioversion in persistent atrial fibrillation patients. Turk. Kardiyol. Dernegi Arsivi-Arch. Turk. Soc. Cardiol. 2019, 47, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Aksan, G.; Yanık, A.; Yontar, O.C.; Gedikli, Ö.; Arslan, U.; Soylu, K. Galectin-3 levels and the prediction of atrial high-rate episodes in patients with cardiac resynchronization therapy. J. Investig. Med. 2021, 69, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Miyazawa, K.; Li, Y.; Shahid, F.; Hado, H.; Lip, G.Y.H. Inflammation and the risk of atrial high-rate episodes (AHREs) in patients with cardiac implantable electronic devices. Clin. Res. Cardiol. 2018, 107, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, T.H.; Yu, H.T.; Choi, E.K.; Park, H.S.; Park, J.; Lee, Y.S.; Kang, K.-W.; Shim, J.; Sung, J.-H.; et al. Prevalence and predictors of clinically relevant atrial highrate episodes in patients with cardiac implantable electronic devices. Korean Circ. J. 2021, 51, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
| AHRE > 5 min No | AHRE > 5 min Yes | p-Value | |
|---|---|---|---|
| AF any type, n, (%) | 5 (16.7%) | 41 (67.3%) | <0.0001 |
| Age, mean (SD), years | 69 ± 8.2 | 74 ± 8.3 | 0.0084 |
| Left atrium diameter, mm | 39.03 | 44.95 | <0.0001 |
| BMI, mean (SD), kg/m2 | 29.36 ± 5.5 | 29.02 ± 5.0 | 0.7747 |
| CRP, mean (SD), mg/mL | 2.2 ± 3.3 | 2.8 ± 4.4 | 0.5822 |
| Galectin-3, mean (SD), pg/mL | 790 ± 411.7 | 1007.5 ± 447.3 | 0.0286 |
| Creatinine, mg/dl | 0.95 [42.8] | 1.09 [47.6] | 0.3983 |
| EF, mean (SD), (%) | 57.7 ± 8.4 | 53.7 ± 11.5 | 0.0964 |
| LAVi, mean (SD), mL/m2 | 31.7 ± 8.4 | 47.9 | <0.0001 |
| ESR, mean (SD), mm/hr | 15.2 ± 12.4 | 19.6 ± 18.9 | 0.2477 |
| Amiodarone (%) | 2.5 | 45 | 0.0003 |
| 1C antiarrhythmic (%) | 5.12 | 15 | 0.3579 |
| Anticoagulant therapy (%) | 2.5 | 77.3 | <0.0001 |
| Betablocker (%) | 38.4 | 96.2 | 0.0008 |
| Coronary artery disease (%) | 20.5 | 18.8 | 0.2501 |
| Dilated cardiomyopathy (%) | 2.5 | 13.2 | 0.1997 |
| COPD (%) | 10.2 | 13.2 | 0.7994 |
| Diuretic (%) | 10.2 | 38.9 | 0.0173 |
| Male (%) | 35.8 | 54.7 | 0.9377 |
| Arterial hypertension (%) | 64.1 | 98.1 | 0.8131 |
| HFrEF (%) | 12.82 | 49 | 0.0.146 |
| ACEi (%) | 35.8 | 62.2 | 0.5072 |
| Valvular heart disease (%) | 61 | 60.3 | 0.1378 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Galectin-3 | 1.0012 | 1.0001 to 1.0023 | 0.0328 |
| CRP | 1.0326 | 0.9220 to 1.1565 | 0.5789 |
| ESR | 1.0176 | 0.9878 to 1.0483 | 0.2508 |
| Leucocytes | 1.0001 | 0.9999 to 1.0004 | 0.2085 |
| Age | 1.0755 | 1.0150 to 1.1397 | 0.0137 |
| LAVi | 1.1002 | 1.0484 to 1.1545 | 0.0001 |
| Gender = “M” | 1.4097 | 0.3340 to 5.9493 | 0.6402 |
| Arterial hypertension | 0.7416 | 0.1087 to 5.0583 | 0.7603 |
| BMI | 0.9793 | 0.8640 to 1.1100 | 0.7437 |
| CHADSVASC score | 1.2327 | 0.8028 to 1.8930 | 0.3390 |
| Ischemic heart disease | 0.9665 | 0.2258 to 4.1381 | 0.9634 |
| Amiodarone | 2.9726 | 0.2609 to 33.8725 | 0.3802 |
| Class 1C antiarrhythmics | 1.4505 | 0.1439 to 14.6192 | 0.7524 |
| ACEi | 0.5604 | 0.1652 to 1.9004 | 0.3526 |
| ARB | 0.6636 | 0.2268 to 1.9418 | 0.4541 |
| Betablocker | 0.1593 | 0.0535 to 0.4743 | 0.0010 |
| Creatinine | 0.9985 | 0.9882 to 1.0089 | 0.7761 |
| Dyslipidemia | 1.4866 | 0.5379 to 4.1084 | 0.4446 |
| E/e′ | 0.9866 | 0.8407 to 1.1578 | 0.8690 |
| Ejection fraction | 0.9864 | 0.9290 to 1.0473 | 0.6535 |
| Pulmonary hypertension | 0.7187 | 0.1415 to 3.6506 | 0.6904 |
| HFrEF | 2.8392 | 0.7435 to 10.8429 | 0.1269 |
| Mitral regurcitation | 2.2642 | 0.8142 to 6.2962 | 0.1173 |
| Valvular heart disease | 1.9740 | 0.8032 to 4.8517 | 0.2151 |
| Variable | Coefficient | Std. Error | p-Value | OR | 95% CI |
|---|---|---|---|---|---|
| Galectin-3 | 0.0016302 | 0.00098842 | 0.0991 | 1.0016 | 0.9997 to 1.0036 |
| Age | −0.031035 | 0.058569 | 0.5962 | 0.9694 | 0.8643 to 1.0874 |
| LAVi | 0.12206 | 0.040942 | 0.0029 | 1.1298 | 1.0427 to 1.2242 |
| Betablocker | −0.88367 | 0.77187 | 0.2523 | 0.4133 | 0.0910 to 1.8761 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simu, G.R.; Tomoaia, R.; Rosu, R.O.; Gusetu, G.; Puiu, M.; Cismaru, G.; Caloian, B.; Terec, A.; Buliga, T.; Boer, A.; et al. Galectin-3, Inflammation, and the Risk of Atrial High-Rate Episodes in Patients with Dual Chamber Pacemakers. Int. J. Mol. Sci. 2023, 24, 7710. https://doi.org/10.3390/ijms24097710
Simu GR, Tomoaia R, Rosu RO, Gusetu G, Puiu M, Cismaru G, Caloian B, Terec A, Buliga T, Boer A, et al. Galectin-3, Inflammation, and the Risk of Atrial High-Rate Episodes in Patients with Dual Chamber Pacemakers. International Journal of Molecular Sciences. 2023; 24(9):7710. https://doi.org/10.3390/ijms24097710
Chicago/Turabian StyleSimu, Gelu Radu, Raluca Tomoaia, Radu Ovidiu Rosu, Gabriel Gusetu, Mihai Puiu, Gabriel Cismaru, Bogdan Caloian, Andreea Terec, Teodor Buliga, Armand Boer, and et al. 2023. "Galectin-3, Inflammation, and the Risk of Atrial High-Rate Episodes in Patients with Dual Chamber Pacemakers" International Journal of Molecular Sciences 24, no. 9: 7710. https://doi.org/10.3390/ijms24097710
APA StyleSimu, G. R., Tomoaia, R., Rosu, R. O., Gusetu, G., Puiu, M., Cismaru, G., Caloian, B., Terec, A., Buliga, T., Boer, A., Minciuna, I. A., Bodizs, G., Zdrenghea, D., & Pop, D. (2023). Galectin-3, Inflammation, and the Risk of Atrial High-Rate Episodes in Patients with Dual Chamber Pacemakers. International Journal of Molecular Sciences, 24(9), 7710. https://doi.org/10.3390/ijms24097710






