Antiangiogenic Effect of Dopamine and Dopaminergic Agonists as an Adjuvant Therapeutic Option in the Treatment of Cancer, Endometriosis, and Osteoarthritis
Abstract
1. Introduction
2. Methodology
3. Dopamine (DA) and Its Receptors
4. Dopamine (DA) and Dopamine Agonists (DA-Ag)
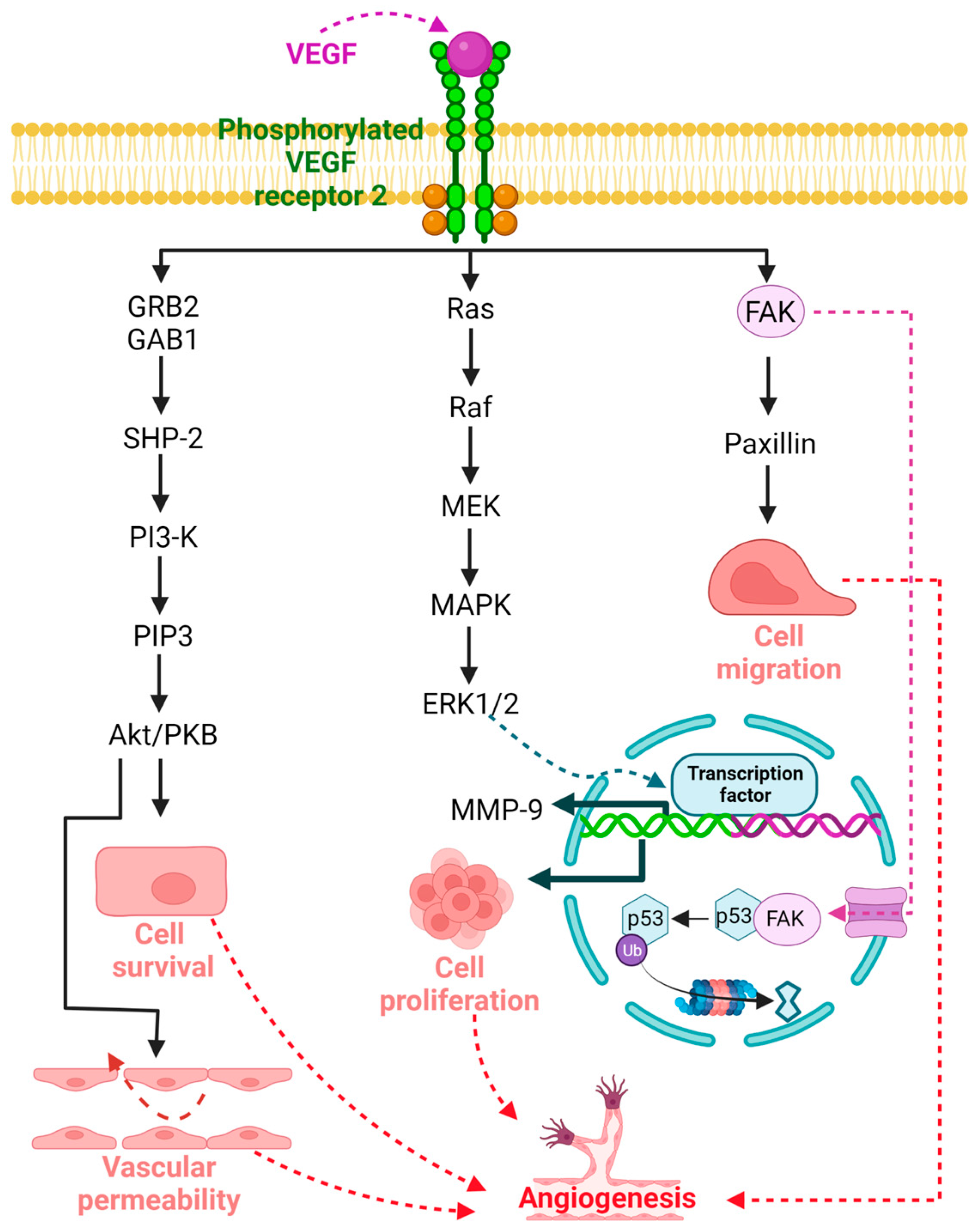
5. Antiangiogenic Capacity of Dopamine (DA) and Dopamine Agonists (DA-Ag) and the Mechanisms of Action
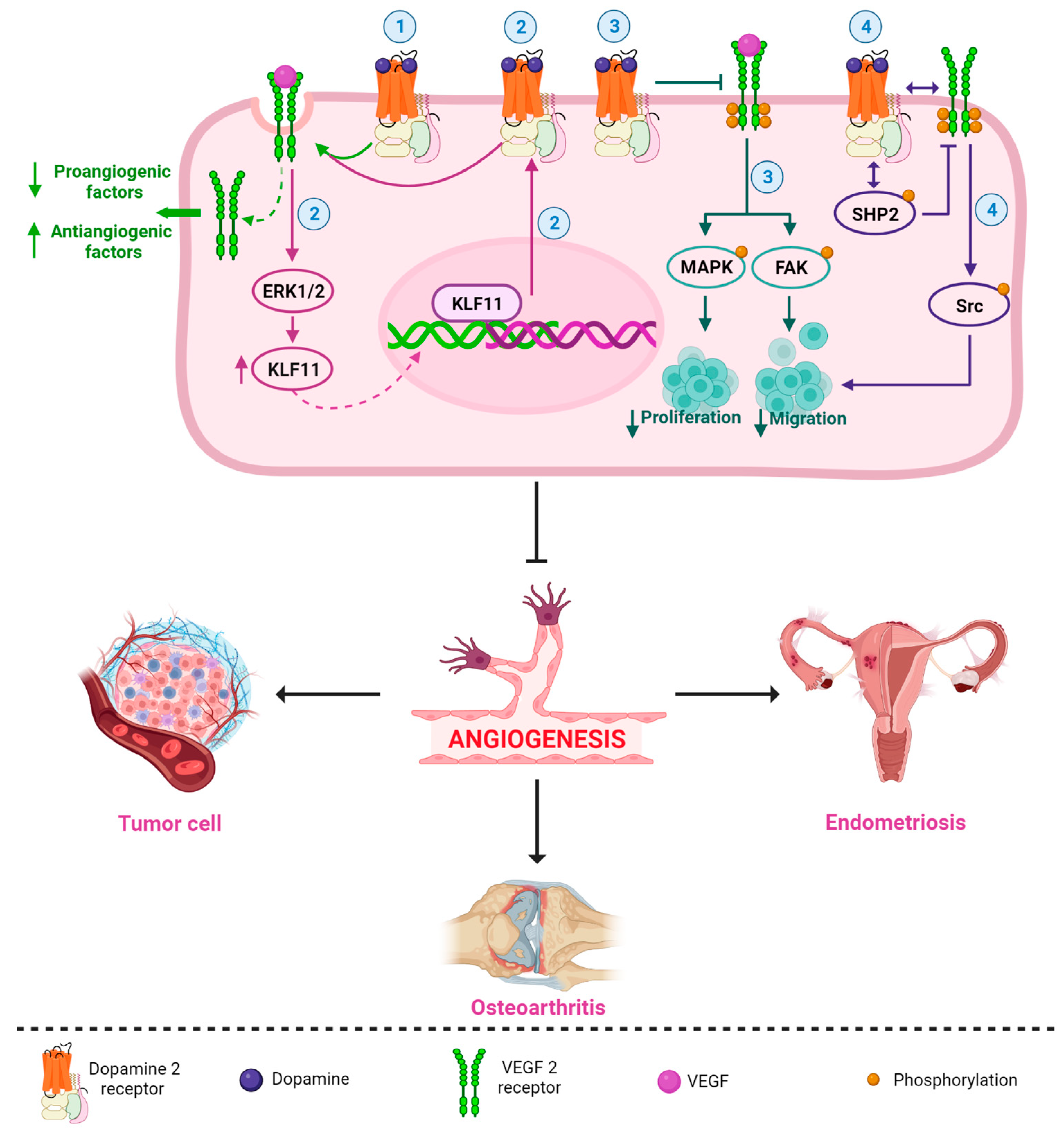
6. Therapeutic Potential of Dopamine (DA) and Dopamine Agonists (DA-Ag) as Antiangiogenic Agents
6.1. Therapeutic Potential of Dopamine and Dopamine Agonists in Endometriosis
6.2. Therapeutic Potential of Dopamine and Dopamine Agonists in Cancer
6.3. Therapeutic Potential of Dopamine and Dopamine Agonists in Osteoarthritis
7. Advantages and Disadvantages of Dopamine (DA) and Dopamine Agonists (DA-Ag) Compared to Monoclonal Antibody Inhibitors of the VEGF/VEGFR 2 Pathway
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOPAL | 3,4-dihydroxyphenylacetaldehyde |
| DOPET | 3,4-dihydroxyphenylethanol |
| HMPAL | 3-methoxy-4-hydroxyphenylacetaldehyde |
| 3-MT | 3-methoxytyramine |
| AC | Adenylyl cyclase |
| ADH | Alcohol dehydrogenase |
| AKT | Protein kinase B |
| ALDH | Aldehyde dehydrogenase |
| DOPAC | Carboxylic acid 3,4-dihydroxyphenylacetic acid |
| COMT | Catechol O-methyl-transferase |
| CNS | Central nervous system |
| CF | Cobalt ferrite |
| CF-DA-PEG | Cobalt ferrite-dopamine-polyethylene glycol |
| cAMP | Cyclic AMP |
| COX-2 | Cyclooxygenase-2 |
| DA | Dopamine |
| DA-Ag | Dopamine agonists |
| D2R | Dopamine receptor D2 |
| ER-β | Estrogen receptor beta |
| ERK | Extracellular-signal-regulated kinase |
| FAK | Focal adhesion kinase |
| GSK-3β | Glycogen synthase kinase-3 beta |
| HVA | Homovanillic acid |
| HIF | Hypoxia-inducible factor |
| iNOS | Inducible nitric oxide synthase |
| IL | Interleukin |
| JAK | Janus kinase |
| KLF11 | Krüppel-like factor 11 |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| L-amino acid decarboxylase | DOPA decarboxylase |
| MMPs | Matrix metalloproteases |
| MAPK | Mitogen-activated protein kinase |
| MAO | Monoamine oxidase |
| NGF | Nerve growth factor |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| OA | Osteoarthritis |
| PLR | Prolactin |
| SOX-9 | SRY-box transcription factor 9 |
| STAT | Signal transducer and activator of transcription |
| Shh | Sonic hedgehog |
| SHP-2 | Phosphatase-2 containing Src homology region 2 domain |
| TNFα | Tumor necrosis factor alpha |
| TH | Tyrosine hydroxylase enzyme |
| TKIs | Tyrosine kinase inhibitor |
| VEGF | Vascular endothelial growth factor |
| VEGFR 2 | Vascular endothelial growth factor receptor 2 |
References
- Elena, P.; Livia, B.; Annalisa, N.; Paraskevi, K.; Marcello, D. The role of dopamine in NLRP3 inflammasome inhibition: Implications for neurodegenerative diseases. Ageing Res. Rev. 2023, 87, 101907. [Google Scholar]
- Weissenrieder, J.S.; Neighbors, J.D.; Mailman, R.B.; Hohl, R.J. Cancer and the Dopamine D2 Receptor: A Pharmacological Perspective. J. Pharm. Exp. Ther. 2019, 370, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.Á.; Santos-Llamas, A.I.; Fernández-Ramírez, M.J.; Tarín, J.J.; Cano, A.; Gómez, R.A. Reassessment of the Therapeutic Potential of a Dopamine Receptor 2 Agonist (D2-AG) in Endometriosis by Comparison against a Standardized Antiangiogenic Treatment. Biomedicines 2021, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Wan, H.; Kaper, H.J.; Sharma, P.K. Dopamine-conjugated hyaluronic acid delivered via intra-articular injection provides articular cartilage lubrication and protection. J. Colloid Interface Sci. 2022, 619, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Zhao, H.C.; Hou, S.J.; Zhang, H.J.; Cheng, L.; Yuan, S.; Zhang, L.R.; Song, J.; Zhang, S.Y.; Chen, S.W. Recent development of multi-target VEGFR-2 inhibitors for the cancer therapy. Bioorg. Chem. 2023, 133, 106425. [Google Scholar] [CrossRef]
- Chung, M.S.; Han, S.J. Endometriosis-Associated Angiogenesis and Anti-angiogenic Therapy for Endometriosis. Front. Glob. Womens Health 2022, 3, 856316. [Google Scholar] [CrossRef]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Sato, E.; Furue, A.; Nawaz, A.; Hatta, H.; Fukushi, Y.; Wada, S.; Tobe, K.; et al. CD206+ macrophage is an accelerator of endometriotic-like lesion via promoting angiogenesis in the endometriosis mouse model. Sci. Rep. 2021, 11, 853. [Google Scholar] [CrossRef]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Takano, S.; Uchida, K.; Inoue, G.; Matsumoto, T.; Aikawa, J.; Iwase, D.; Mukai, M.; Miyagi, M.; Takaso, M. Vascular endothelial growth factor expression and their action in the synovial membranes of patients with painful knee osteoarthritis. BMC Musculoskelet Disord. 2018, 19, 204. [Google Scholar] [CrossRef]
- Jia, L.S.; Yi, T.W.; Jia, Y.O.; De, L.L.; Deng, H.X.; Hai, Y.Z.; Hang, F.; Dao, Z.C. The role of vascular endothelial growth factor in osteoarthritis: A review. Int. J. Clin. Exp. Med. 2019, 12, 194–200. [Google Scholar]
- Pei, Y.A.; Chen, S.; Pei, M. The essential anti-angiogenic strategies in cartilage engineering and osteoarthritic cartilage repair. Cell Mol. Life Sci. 2022, 79, 71. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Nagy, J.A.; Pal, S.; Vasile, E.; Eckelhoefer, I.A.; Bliss, V.S.; Manseau, E.J.; Dasgupta, P.S.; Dvorak, H.F.; Mukhopadhyay, D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 2001, 7, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Vohra, P.K.; Bhattacharya, R.; Dutta, S.; Sinha, S.; Mukhopadhyay, D. Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2. J. Cell Sci. 2009, 122, 3385–3392. [Google Scholar] [CrossRef] [PubMed]
- Kasica, N.; Święch, A.; Saładziak, K.; Mackiewicz, J.; Osęka, M. The Inhibitory Effect of Selected D2 Dopaminergic Receptor Agonists on VEGF-Dependent Neovascularization in Zebrafish Larvae: Potential New Therapy in Ophthalmic Diseases. Cells 2022, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Finley, B.; Liu, K.; Powell, D.; Kellner, J.; Kinder, D.H. Potential Use of Dopamine and Dopamine Agonists as Angiogenesis Inhibitors in the Treatment of Cancer. Pharm. Wellness Rev. 2015, 6, 12–16. [Google Scholar]
- Ansari, M.J.; Bokov, D.; Markov, A.; Jalil, A.T.; Shalaby, M.N.; Suksatan, W.; Chupradit, S.; Al-Ghamdi, H.S.; Shomali, N.; Zamani, A.; et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun. Signal 2022, 20, 49. [Google Scholar] [CrossRef]
- Zirlik, K.; Duyster, J. Anti-Angiogenics: Current Situation and Future Perspectives. Oncol. Res. Treat. 2018, 41, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1548S. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Aslanoglou, D.; Bertera, S.; Sánchez-Soto, M.; Free, B.R.; Lee, J.; Zong, W.; Xue, X.; Shrestha, S.; Brissova, M.; Logan, R.W.; et al. Dopamine regulates pancreatic glucagon and insulin secretion via adrenergic and dopaminergic receptors. Transl. Psychiatr. 2021, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Bunzow, J.R.; Van Tol, H.H.; Grandy, D.K.; Albert, P.; Salon, J.; Christie, M.; Machida, C.A.; Neve, K.A.; Civelli, O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature 1988, 336, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Kebabian, J.W.; Petzold, G.L.; Greengard, P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the “dopamine receptor”. Proc. Natl. Acad. Sci. USA 1972, 69, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, A.C.; Malenka, R.C. Striatal plasticity and basal ganglia circuit function. Neuron 2008, 60, 543–554. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef]
- Wei, X.; Ma, T.; Cheng, Y.; Huang, C.C.Y.; Wang, X.; Lu, J.; Wang, J. Dopamine D1 or D2 receptor-expressing neurons in the central nervous system. Addict. Biol. 2018, 23, 569–584. [Google Scholar] [CrossRef]
- Baldwin, J.M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993, 12, 1693–1703. [Google Scholar] [CrossRef]
- Bone, N.B.; Liu, Z.; Pittet, J.F.; Zmijewski, J.W. Frontline Science: D1 dopaminergic receptor signaling activates the AMPK-bioenergetic pathway in macrophages and alveolar epithelial cells and reduces endotoxin-induced ALI. J. Leukoc. Biol. 2017, 101, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef] [PubMed]
- Sadana, R.; Dessauer, C.W. Physiological roles for G protein-regulated adenylyl cyclase isoforms: Insights from knockout and overexpression studies. Neurosignals 2009, 17, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Bandala, C.; Cárdenas-Rodríguez, N.; Mendoza-Torreblanca, J.G.; Contreras-García, I.J.; Martínez-López, V.; Cruz-Hernández, T.R.; Carro-Rodríguez, J.; Vargas-Hernández, M.A.; Ignacio-Mejía, I.; Alfaro-Rodriguez, A.; et al. Therapeutic Potential of Dopamine and Related Drugs as Anti-Inflammatories and Antioxidants in Neuronal and Non-Neuronal Pathologies. Pharmaceutics 2023, 15, 693. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J.L.; Free, R.B.; Sibley, D.R. Identification of G protein-biased agonists that fail to recruit β-arrestin or promote internalization of the D1 dopamine receptor. ACS Chem. Neurosci. 2015, 6, 681–692. [Google Scholar] [CrossRef]
- Kostrzewa, R.M.; Wydra, K.; Filip, M.; Crawford, C.A.; McDougall, S.A.; Brown, R.W.; Borroto-Escuela, D.O.; Fuxe, K.; Gainetdinov, R.R. Dopamine D2 Receptor Supersensitivity as a Spectrum of Neurotoxicity and Status in Psychiatric Disorders. J. Pharmacol. Exp. Ther. 2018, 366, 519–526. [Google Scholar] [CrossRef]
- Vijayraghavan, S.; Wang, M.; Birnbaum, S.G.; Williams, G.V.; Arnsten, A.F. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007, 10, 376–384. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Mawlawi, O.; Lombardo, I.; Gil, R.; Martinez, D.; Huang, Y.; Hwang, D.R.; Keilp, J.; Kochan, L.; Van Heertum, R.; et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 2002, 22, 3708–3719. [Google Scholar] [CrossRef]
- Lemon, N.; Manahan-Vaughan, D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J. Neurosci. 2006, 26, 7723–7729. [Google Scholar] [CrossRef]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Hall, W.C.; Lamantia, A.S.; McNamara, J.O.; Williams, S.M. Neurotransmitters and Their Receptors. In Neuroscience, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2004. [Google Scholar]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal 2013, 11, 34. [Google Scholar] [CrossRef]
- Kamal, S.; Lappin, S.L. Biochemistry, Catecholamine Degradation; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Hasbi, A.; O’Dowd, B.F.; George, S.R. Dopamine D1-D2 receptor heteromer signaling pathway in the brain: Emerging physiological relevance. Mol. Brain 2011, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Ledonne, A.; Mercuri, N.B. Current Concepts on the Physiopathological Relevance of Dopaminergic Receptors. Front. Cell Neurosci. 2017, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Boyd, K.N.; Mailman, R.B. Dopamine receptor signaling and current and future antipsychotic drugs. Handb. Exp. Pharmacol. 2012, 212, 53–86. [Google Scholar]
- Gandhi, K.R.; Saadabadi, A. Levodopa (L-Dopa); StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Alabsi, A.; Khoudary, A.C.; Abdelwahed, W. The Antidepressant Effect of L-Tyrosine-Loaded Nanoparticles: Behavioral Aspects. Ann. Neurosci. 2016, 23, 89–99. [Google Scholar] [CrossRef]
- Carbone, F.; Djamshidian, A.; Seppi, K.; Poewe, W. Apomorphine for Parkinson’s Disease: Efficacy and Safety of Current and New Formulations. CNS Drugs 2019, 33, 905–918. [Google Scholar] [CrossRef]
- Colao, A.; Lombardi, G.; Annunziato, L. Cabergoline. Expert Opin. Pharmacother. 2000, 1, 555–574. [Google Scholar] [CrossRef]
- Curran, M.P.; Perry, C.M. Cabergoline: A review of its use in the treatment of Parkinson’s disease. Drugs 2004, 64, 2125–2141. [Google Scholar] [CrossRef]
- DrugBank Online. 2022. Available online: https://go.drugbank.com (accessed on 30 April 2023).
- Peihua, L.; Jianqin, W. Clinical Effects of Piribedil in Adjuvant Treatment of Parkinson’s Disease: A Meta-Analysis. Open Med. 2018, 13, 270–277. [Google Scholar] [CrossRef]
- Li, T.; Zou, S.; Zhang, Z.; Liu, M.; Liang, Z. Efficacy of pramipexole on quality of life in patients with Parkinson’s disease: A systematic review and meta-analysis. BMC Neurol. 2022, 22, 320. [Google Scholar] [CrossRef]
- Shill, H.A.; Stacy, M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2009, 5, 33–36. [Google Scholar] [PubMed]
- Rewane, A.; Nagalli, S. Ropinirole; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Singh, R.; Parmar, M. Pramipexole; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Szymanski, M.W.; Richards, J.R. Fenoldopam; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Salmi, P.; Isacson, R.; Kull, B. Dihydrexidine--the first full dopamine D1 receptor agonist. CNS Drug Rev. 2004, 10, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Mailman, R.B.; Yang, Y.; Huang, X. D1, not D2, dopamine receptor activation dramatically improves MPTP-induced parkinsonism unresponsive to levodopa. Eur. J. Pharmacol. 2021, 892, 173760. [Google Scholar] [CrossRef] [PubMed]
- Dajas-Bailador, F.A.; Asencio, M.; Bonilla, C.; Scorza, M.C.; Echeverry, C.; Reyes-Parada, M.; Silveira, R.; Protais, P.; Russell, G.; Cassels, B.K.; et al. Dopaminergic pharmacology and antioxidant properties of pukateine, a natural product lead for the design of agents increasing dopamine neurotransmission. Gen. Pharmacol. 1999, 32, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Dalefield, R. Veterinary Toxicology for Australia and New Zealand, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Boulougouris, V.; Castañé, A.; Robbins, T.W. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: Investigation of D3 receptor involvement in persistent behavior. Psychopharmacology 2009, 202, 611–620. [Google Scholar] [CrossRef] [PubMed]
- van Reij, R.R.I.; Salmans, M.M.A.; Eijkenboom, I.; van den Hoogen, N.J.; Joosten, E.A.J.; Vanoevelen, J.M. Dopamine-neurotransmission and nociception in zebrafish: An anti-nociceptive role of dopamine receptor drd2a. Eur. J. Pharmacol. 2021, 912, 174517. [Google Scholar] [CrossRef]
- Tobaldini, G.; Reis, R.A.; Sardi, N.F.; Lazzarim, M.K.; Tomim, D.H.; Lima, M.M.S.; Fischer, L. Dopaminergic mechanisms in periaqueductal gray-mediated antinociception. Behav. Pharmacol. 2018, 29, 225–233. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Olofsson, B.; Pajusola, K.; Kaipainen, A.; von Euler, G.; Joukov, V.; Saksela, O.; Orpana, A.; Pettersson, R.F.; Alitalo, K.; Eriksson, U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2576–2581. [Google Scholar] [CrossRef]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996, 15, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Mäkinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Maglione, D.; Guerriero, V.; Viglietto, G.; Delli-Bovi, P.; Persico, M.G. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl. Acad. Sci. USA 1991, 88, 9267–9271. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Bio. 2006, 39, 469–478. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Cerezo, A.B.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C. Anti-VEGF Effect of Bioactive Indolic Compounds and Hydroxytyrosol Metabolites. Foods 2022, 11, 526. [Google Scholar] [CrossRef]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signaling by VEGF. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2000–2013. [Google Scholar]
- Torres, J.S.S.; Cerón, L.F.Z.; Bernal, S.I.F.; Ordoñez, G.W.M.; Salguero, C. El rol de VEGF en la angiogénesis fisiológica y tumoral. Medicina 2017, 39, 190–209. [Google Scholar]
- Sarkar, C.; Chakroborty, D.; Mitra, R.B.; Banerjee, S.; Dasgupta, P.S.; Basu, S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1554–H1560. [Google Scholar] [CrossRef]
- Sarkar, C.; Chakroborty, D.; Goswami, S.; Fan, H.; Mo, X.; Basu, S. VEGF-A controls the expression of its regulator of angiogenic functions, dopamine D2 receptor, on endothelial cells. J. Cell Sci. 2022, 135, jcs259617. [Google Scholar] [CrossRef]
- Pellicer, N.; Galliano, D.; Herraiz, S.; Bagger, Y.Z.; Arce, J.C.; Pellicer, A. Use of dopamine agonists to target angiogenesis in women with endometriosis. Hum. Reprod. 2021, 36, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Novella-Maestre, E.; Carda, C.; Noguera, I.; Ruiz-Sauri, A.; Garcia-Velasco, J.A.; Simon, C.; Pellicer, A. Dopamine agonist administration causes a reduction in endometrial implants through modulation of angiogenesis in experimentally induced endometriosis. Hum. Reprod. 2009, 24, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Novella-Maestre, E.; Carda, C.; Ruiz-Sauri, A.; Garcia-Velasco, J.A.; Simon, C.; Pellicer, A. Identification and Quantification of Dopamine Receptor 2 in Human Eutopic and Ectopic Endometrium: A Novel Molecular Target for Endometriosis Therapy. Biol. Reprod. 2010, 83, 866–873. [Google Scholar] [CrossRef]
- Sarkar, C.; Chakroborty, D.; Dasgupta, P.S.; Basu, S. Dopamine is a safe antiangiogenic drug which can also prevent 5-fluorouracil induced neutropenia. Int. J. Cancer 2015, 137, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, D.; Sarkar, C.; Mitra, R.B.; Banerjee, S.; Dasgupta, P.S.; Basu, S. Depleted dopamine in gastric cancer tissues: Dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin. Cancer Res. 2004, 10, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Chakroborty, D.; Chowdhury, U.R.; Dasgupta, P.S.; Basu, S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin. Cancer Res. 2008, 14, 2502–2510. [Google Scholar] [CrossRef]
- Hoeppner, L.H.; Wang, Y.; Sharma, A.; Javeed, N.; Van Keulen, V.P.; Wang, E.; Yang, P.; Roden, A.C.; Peikert, T.; Molina, J.R.; et al. Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells. Mol. Oncol. 2014, 9, 270–281. [Google Scholar] [CrossRef]
- Lan, Y.L.; Wang, X.; Xing, J.S.; Lou, J.C.; Ma, X.C.; Zhang, B. The potential roles of dopamine in malignant glioma. Acta Neurol. Belg. 2016, 117, 613–621. [Google Scholar] [CrossRef]
- Chakroborty, D.; Sarkar, C.; Basu, B.; Dasgupta, P.S.; Basu, S. Catecholamines Regulate Tumor Angiogenesis. Cancer Res. 2009, 69, 3727–3730. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 250. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the Nervous System in Tumor Angiogenesis. Cancer Microenviron. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Roy, S.; Lu, K.; Nayak, M.K.; Bhuniya, A.; Ghosh, T.; Kundu, S.; Ghosh, S.; Baral, R.; Dasgupta, P.S.; Basu, S. Activation of D2Dopamine Receptors in CD133+ve Cancer Stem Cells in Non-small Cell Lung Carcinoma Inhibits Proliferation, Clonogenic Ability, and Invasiveness of These Cells. J. Biol. Chem. 2016, 292, 435–445. [Google Scholar] [CrossRef]
- Sakurai, Y.; Ohgimoto, K.; Kataoka, Y.; Yoshida, N.; Shibuya, M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sanyal, S.; Mukhopadhyay, D. Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J. Biol. Chem. 2001, 276, 32714–32719. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Smith, M.; Lu, C.; Shahzad, M.M.; Pena, G.N.; Allen, J.K.; Stone, R.L.; Mangala, L.S.; Han, H.D.; Kim, H.S.; Farley, D.; et al. Dopamine blocks stress-mediated ovarian carcinoma growth. Clin. Cancer Res. 2011, 17, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rosas, F.; Gómez, R.; Ferrero, H.; Gaytan, F.; Garcia-Velasco, J.; Simón, C.; Pellicer, A. The effects of ergot and non-ergot-derived dopamine agonists in an experimental mouse model of endometriosis. Reproduction 2011, 142, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum. Reprod. Update 2012, 18, 682–702. [Google Scholar] [CrossRef]
- Motyl, K.J.; Beauchemin, M.; Barlow, D.; Le, P.T.; Nagano, K.; Treyball, A.; Contractor, A.; Baron, R.; Rosen, C.J.; Houseknecht, K.L. A novel role for dopamine signaling in the pathogenesis of bone loss from the atypical antipsychotic drug risperidone in female mice. Bone 2017, 103, 168–176. [Google Scholar] [CrossRef]
- Soares, S.R.; Martínez-Varea, A.; Hidalgo-Mora, J.J.; Pellicer, A. Pharmacologic therapies in endometriosis: A systematic review. Fertil. Steril. 2012, 98, 529–555. [Google Scholar] [CrossRef]
- Grothey, A.; Ellis, L.M. Targeting angiogenesis driven by vascular endothelial growth factors using antibody-based therapies. Cancer J. (Sudbury Mass) 2008, 14, 170–177. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Wakelee, H.A. Monoclonal antibodies targeting vascular endothelial growth factor: Current status and future challenges in cancer therapy. BioDrugs 2009, 23, 289–304. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum. Reprod. Update 2018, 24, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Akyol, A.; Kavak, E.; Akyol, H.; Pala, Ş.; Gürsu, F. The Non-Ergot Derived Dopamine Agonist Quinagolide as an Anti-Endometriotic Agent. Gynecol. Obstet. Investig. 2017, 82, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Thanatsis, N.; Filindris, T.; Siampalis, A.; Papageorgiou, E.; Panagodimou, E.; Adonakis, G.; Kaponis, A. The Effect of Novel Medical Nonhormonal Treatments on the Angiogenesis of Endometriotic Lesions. Obstetr. Gynecol. Surv. 2021, 76, 281–291. [Google Scholar] [CrossRef]
- Andersson, J.K.; Khan, Z.; Weaver, A.L.; Vaughan, L.E.; Gemzell-Danielsson, K.; Stewart, E.A. Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: A pilot study. Acta Obstet. Gynecol. Scand. 2019, 98, 1341–1350. [Google Scholar] [CrossRef]
- Iampietro, C.; Brossa, A.; Canosa, S.; Tritta, S.; Croston, G.E.; Reinheimer, T.M.; Bonelli, F.; Carosso, A.R.; Gennarelli, G.; Cosma, S.; et al. Quinagolide Treatment Reduces Invasive and Angiogenic Properties of Endometrial Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2022, 23, 1775. [Google Scholar] [CrossRef] [PubMed]
- Karslıoglu, T.; Karasu, A.; Yildiz, P. The Effects of Micronized Progesterone and Cabergoline On a Rat Autotransplantation Endometriosis Model: A Placebo Controlled Randomized Trial. J. Investig. Surg. 2021, 34, 897–901. [Google Scholar] [CrossRef]
- Ercan, C.M.; Kayaalp, O.; Cengiz, M.; Keskin, U.; Yumusak, N.; Aydogan, U.; Ide, T.; Ergun, A. Comparison of efficacy of bromocriptine and cabergoline to GnRH agonist in a rat endometriosis model. Arch. Gynecol. Obstet. 2015, 291, 1103–1111. [Google Scholar] [CrossRef]
- Jouhari, S.; Mohammadzadeh, A.; Soltanghoraee, H.; Mohammadi, Z.; Khazali, S.; Mirzadegan, E.; Lakpour, N.; Fatemi, F.; Zafardoust, S.; Mohazzab, A.; et al. Effects of silymarin, cabergoline and letrozole on rat model of endometriosis, Taiwan. J. Obstet. Gynecol. 2018, 57, 830–835. [Google Scholar]
- Barbe, A.; Berbets, A.; Davydenko, I.; Yuzko, V.; Yuzko, O. The effects of certain angioneogenesis inhibitors in experimental endometriosis in rats. CAL 2019, 49, 50. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.J.; Kim, M.J.; Lee, S.J.; Kwon, H.; Lee, J.H. Novel Medicine for Endometriosis and Its Therapeutic Effect in a Mouse Model. Biomedicines 2020, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.M.; Madkour, W.A.; Moawad, A.; Elzaher, M.A.; Roberts, M.P. Does cabergoline help in decreasing endometrioma size compared to LHRH agonist? A prospective randomized study. Arch. Obstet. Gynaecol. 2014, 290, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinskaya, M.; Suslova, E.; Tkachenko, N.; Molotkov, A.; Kogan, I. Dopamine agonists as genital endometriosis target therapy. J. Gynaecol. Endocrinol. 2020, 36, 7–11. [Google Scholar] [CrossRef]
- Gómez, R.; Abad, A.; Delgado, F.; Tamarit, S.; Simón, C.; Pellicer, A. Effects of hyperprolactinemia treatment with the dopamine agonist quinagolide on endometriotic lesions in patients with endometriosis-associated hyperprolactinemia. Fertil. Steril. 2011, 95, 882–888.e1. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). (September 2015–February 2020) Dopamine Receptor Agonist Therapy for Pain Relief in Women Suffering from Endometriosis: A Pilot Study. Identifier: NCT02542410. Available online: https://clinicaltrials.gov/ct2/show/NCT02542410 (accessed on 5 April 2023).
- National Library of Medicine (U.S.). (November 2018–September 2022) Quinagolide Vaginal Ring on Lesion Reduction Assessed by MRI in Women with Endometriosis/Adenomyosis (QLARITY). Identifier: NCT03749109. Available online: https://clinicaltrials.gov/ct2/show/NCT03749109 (accessed on 5 April 2023).
- National Library of Medicine (U.S.). (April 2019–September 2022) Cabergoline for the Treatment of Chronic Pain Due to Endometriosis. Identifier: NCT03928288. Available online: https://clinicaltrials.gov/ct2/show/NCT03928288 (accessed on 5 April 2023).
- Beaulieu, J.M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef]
- Gatto, F.; Hofland, L.J. The role of somatostatin and dopamine D2 receptors in endocrine tumors. Endocr. Relat. Cancer 2011, 18, R233–R251. [Google Scholar] [CrossRef]
- Chi, J.; Li, A.; Zou, M.; Wang, S.; Liu, C.; Hu, R.; Jiang, Z.; Liu, W.; Sun, R.; Han, B. Novel dopamine-modified oxidized sodium alginate hydrogels promote angiogenesis and accelerate healing of chronic diabetic wounds. Int. J. Biol. Macromol. 2022, 203, 492–504. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Jia, Y.; Qu, C.; Li, J. Covalently Assembled Dopamine Nanoparticle as an Intrinsic Photosensitizer and pH-Responsive Nanocarrier for Potential Application of Anticancer Therapy. Chem. Commun. 2019, 55, 15057–15060. [Google Scholar] [CrossRef]
- Tang, H.; Mourad, S.; Zhai, S.-D.; Hart, R.J. Dopamine agonists for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst. Rev. 2016, 11, CD008605. [Google Scholar] [CrossRef]
- Sarkar, C.; Chakroborty, D.; Basu, S. Neurotransmitters as Regulators of Tumor Angiogenesis and Immunity: The Role of Catecholamines. J. Neuroimmune Pharmacol. 2012, 8, 7–14. [Google Scholar] [CrossRef]
- Chakroborty, D.; Chowdhury, U.R.; Sarkar, C.; Baral, R.; Dasgupta, P.S.; Basu, S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J. Clin. Investig. 2008, 118, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, R.S.; Pirchio, R.; De Alcubierre, D.; Pivonello, R.; Colao, A. Dopamine Agonists: From the 1970s to Today. Neuroendocrinology 2019, 109, 34–41. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Upadhyay, P.; Das, A.; Ghosh, A.; Adhikary, A.; Goswami, M.M. Studies on cancer cell death through delivery of dopamine as anti-cancer drug by a newly functionalized cobalt ferrite nano-carrier. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127202. [Google Scholar] [CrossRef]
- Taleb, M.; Ding, Y.; Wang, B.; Yang, N.; Han, X.; Du, C.; Qi, Y.; Zhang, Y.; Sabet, Z.F.; Alanagh, H.R.; et al. Dopamine Delivery via pH-Sensitive Nanoparticles for Tumor Blood Vessel Normalization and an Improved Effect of Cancer Chemotherapeutic Drugs. Adv. Healthc. Mater. 2019, 8, 1900283. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, R.; Zhang, X.; Liu, J.; Wu, H.; Li, Y.; Cui, M.; Li, T.; Song, H.; Gao, J.; et al. Dopamine improves chemotherapeutic efficacy for pancreatic cancer by regulating macrophage-derived inflammations. Cancer Immunol. Immunother. 2021, 70, 2165–2177. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Cabergoline in the Management of Residual Nonfunctioning Pituitary Adenoma: NCT03271918. Available online: https://clinicaltrials.gov/ct2/show/NCT03271918?cond=NCT03271918 (accessed on 31 March 2023).
- Costa, R.; Santa-Maria, C.A.; Scholtens, D.M.; Jain, S.; Flaum, L.; Gradishar, W.J.; Clevenger, C.V.; Kaklamani, V.G. A pilot study of cabergoline for the treatment of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 165, 585–592. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). (September 2014–September 2014) The Effect of Norepinephrine and Dopamine on Radial Forearm Free Flap Tissue Oxygen Pressure and Microdialysate Metabolite Measurements. Identifier: NCT02241083. Available online: https://clinicaltrials.gov/ct2/show/NCT02241083 (accessed on 31 March 2023).
- National Library of Medicine (U.S.). (September 2019–July 2020) PRolaCT—Three Prolactinoma RCTs. Identifier: NCT04107480. Available online: https://clinicaltrials.gov/ct2/show/NCT04107480 (accessed on 31 March 2023).
- National Library of Medicine (U.S.). (January 2017–July 2021) Treatment of Hyperprolactinemia with the Nonergoline Dopamine Agonist Ropinirole. Identifier: NCT03038308. Available online: https://clinicaltrials.gov/ct2/show/NCT03038308 (accessed on 31 March 2023).
- National Library of Medicine (U.S.). (November 2012–September 2019) Cabergoline in Metastatic Breast Cancer. Identifier: NCT01730729. Available online: https://clinicaltrials.gov/ct2/show/NCT01730729 (accessed on 31 March 2023).
- Flugsrud, G.B.; Nordsletten, L.; Reinholt, F.P.; Risberg, M.A.; Rydevik, K.; Uhlig, T. Artrose [Osteoarthritis]. Tidsskr. Nor. Laegeforen. 2010, 130, 2136–2140. [Google Scholar] [CrossRef]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef]
- Decker, R.S. Articular cartilage and joint development from embryogenesis to adulthood. Semin. Cell Dev. Biol. 2017, 62, 50–56. [Google Scholar] [CrossRef]
- Pitsillides, A.A.; Beier, F. Cartilage biology in osteoarthritis-lessons from developmental biology. Nat. Rev. Rheumatol. 2011, 7, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ding, Z.; Liu, F.; Shan, W.; Cheng, C.; Xu, J.; He, W.; Huang, W.; Ma, J.; Yin, Z. Dopamine delays articular cartilage degradation in osteoarthritis by negative regulation of the NF-κB and JAK2/STAT3 signaling pathways. Biomed Pharmacother. 2019, 119, 109419. [Google Scholar] [CrossRef] [PubMed]
- Schwendich, E.; Salinas Tejedor, L.; Schmitz, G.; Rickert, M.; Steinmeyer, J.; Rehart, S.; Tsiami, S.; Braun, J.; Baraliakos, X.; Reinders, J.; et al. Modulation of Dopamine Receptors on Osteoblasts as a Possible Therapeutic Strategy for Inducing Bone Formation in Arthritis. Cells 2022, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Bagdadi, K.; Zaucke, F.; Meurer, A.; Straub, R.H.; Jenei-Lanzl, Z. Norepinephrine Inhibits Synovial Adipose Stem Cell Chondrogenesis via α2a-Adrenoceptor-Mediated ERK1/2 Activation. Int. J. Mol. Sci. 2019, 20, 3127. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Schäfer, N.; Bauer, R.; Jenei-Lanzl, Z.; Springorum, R.H.; Grässel, S. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthr. Cartil. 2016, 24, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Muschter, D. Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology. Int. J. Mol. Sci. 2017, 18, 931. [Google Scholar] [CrossRef]
- Speichert, S.; Molotkov, N.; El Bagdadi, K.; Meurer, A.; Zaucke, F.; Jenei-Lanzl, Z. Role of Norepinephrine in IL-1β-Induced Chondrocyte Dedifferentiation under Physioxia. Int. J. Mol. Sci. 2019, 20, 1212. [Google Scholar] [CrossRef]
- Kuang, Z.; Chen, Z.; Tu, S.; Mai, Z.; Chen, L.; Kang, X.; Chen, X.; Wei, J.; Wang, Y.; Peng, Y.; et al. Dopamine Suppresses Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells via AKT/GSK-3β/β-Catenin Signaling Pathway. Stem Cells Int. 2022, 2022, 4154440. [Google Scholar] [CrossRef]
- Lee, D.J.; Tseng, H.C.; Wong, S.W.; Wang, Z.; Deng, M.; Ko, C.C. Dopaminergic effects on in vitro osteogenesis. Bone Res. 2015, 3, 15020. [Google Scholar] [CrossRef]
- Daghestani, H.N.; Kraus, V.B. Inflammatory biomarkers in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1890–1896. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Walsh, D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology 2005, 44, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.B.; Luo, C.; Mao, X.Y.; Li, X.; Yin, J.Y.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. The Prospective Value of Dopamine Receptors on Bio-Behavior of Tumor. J. Cancer 2019, 10, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Walsh, D.A. Angiogenesis in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Haywood, L.; McWilliams, D.F.; Pearson, C.I.; Gill, S.E.; Ganesan, A.; Wilson, D.; Walsh, D.A. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003, 48, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Lange-Asschenfeldt, B.; Velasco, P.; Hirakawa, S.; Kunstfeld, R.; Brown, L.F.; Bohlen, P.; Senger, D.R.; Detmar, M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the α1β1 and α2β1 integrins. FASEB J. 2004, 18, 1111–1113. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Zhang, X.; Wang, C.; Huang, Y.; Dai, K.; Zhang, X. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 2017, 8, e2715. [Google Scholar] [CrossRef]
- Westacott, C.I.; Barakat, A.F.; Wood, L.; Perry, M.J.; Neison, P.; Bisbinas, I.; Armstrong, L.; Millar, A.B.; Elson, C.J. Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 213–221. [Google Scholar] [CrossRef]
- Horner, A.; Bord, S.; Kelsall, A.W.; Coleman, N.; Compston, J.E. Tie2 ligands angiopoietin-1 and angiopoietin-2 are coexpressed with vascular endothelial cell growth factor in growing human bone. Bone 2001, 28, 65–71. [Google Scholar] [CrossRef]
- Davidson, E.N.; Remst, D.F.; Vitters, E.L.; van Beuningen, H.M.; Blom, A.B.; Goumans, M.J.; van den Berg, W.B.; van der Kraan, P.M. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009, 182, 7937–7945. [Google Scholar] [CrossRef]
- Engsig, M.T.; Chen, Q.J.; Vu, T.H.; Pedersen, A.C.; Therkidsen, B.; Lund, L.R.; Henriksen, K.; Lenhard, T.; Foged, N.T.; Werb, Z.; et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J. Cell Biol. 2000, 151, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Kevorkian, L.; Young, D.A.; Darrah, C.; Donell, S.T.; Shepstone, L.; Porter, S.; Brockbank, S.M.; Edwards, D.R.; Parker, A.E.; Clark, I.M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004, 50, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Barai, A.; Burkhardt, D.; Smith, S.M.; Fosang, A.J.; Werb, Z.; Shah, M.; Thompson, E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009, 60, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, F.M.; Last, K.; Golub, S.B.; Gauci, S.J.; Stanton, H.; Bell, K.M.; Fosang, A.J. ADAMTS-9 in Mouse Cartilage Has Aggrecanase Activity That Is Distinct from ADAMTS-4 and ADAMTS-5. Int. J. Mol. Sci. 2019, 20, 573. [Google Scholar] [CrossRef]
- Ismail, H.M.; Yamamoto, K.; Vincent, T.L.; Nagase, H.; Troeberg, L.; Saklatvala, J. Interleukin-1 Acts via the JNK-2 Signaling Pathway to Induce Aggrecan Degradation by Human Chondrocytes. Arthritis Rheumatol. 2015, 67, 1826–1836. [Google Scholar] [CrossRef]
- Shakibaei, M.; Schulze-Tanzil, G.; Mobasheri, A.; Beichler, T.; Dressler, J.; Schwab, W. Expression of the VEGF receptor-3 in osteoarthritic chondrocytes: Stimulation by interleukin-1 beta and association with beta 1-integrins. Histochem. Cell Biol. 2003, 120, 235–241. [Google Scholar] [CrossRef]
- Vinall, R.L.; Lo, S.H.; Reddi, A.H. Regulation of articular chondrocyte phenotype by bone morphogenetic protein 7, interleukin 1, and cellular context is dependent on the cytoskeleton. Exp. Cell Res. 2002, 272, 32–44. [Google Scholar] [CrossRef]
- Setoguchi, K.; Misaki, Y.; Kawahata, K.; Shimada, K.; Juji, T.; Tanaka, S.; Oda, H.; Shukunami, C.; Nishizaki, Y.; Hiraki, Y.; et al. Suppression of T-cell responses by chondromodulin I, a cartilage-derived angiogenesis inhibitory factor: Therapeutic potential in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 828–839. [Google Scholar] [CrossRef]
- Shukunami, C.; Hiraki, Y. Role of cartilage-derived anti-angiogenic factor, chondromodulin-I, during endochondral bone formation. Osteoarthr. Cartil. 2001, 9, S91–S101. [Google Scholar] [CrossRef]
- Poulet, B.; Liu, K.; Plumb, D.; Vo, P.; Shah, M.; Staines, K.; Sampson, A.; Nakamura, H.; Nagase, H.; Carriero, A.; et al. Overexpression of TIMP-3 in Chondrocytes Produces Transient Reduction in Growth Plate Length but Permanently Reduces Adult Bone Quality and Quantity. PLoS ONE 2016, 11, e0167971. [Google Scholar] [CrossRef]
- Johannessen, M.K.; Skretting, G.; Ytrehus, B.; Røed, K.H. Neonatal growth cartilage: Equine tissue specific gene expression. Biochem. Biophys. Res. Commun. 2007, 354, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Torres, J.; Martínez-Nava, G.A.; Gutiérrez-Ruíz, M.C.; Gómez-Quiroz, L.E.; Gutiérrez, M. Role of HIF-1α signaling pathway in osteoarthritis: A systematic review. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Pfander, D.; Swoboda, B.; Cramer, T. The role of HIF-1alpha in maintaining cartilage homeostasis and during the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2006, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Amarilio, R.; Viukov, S.V.; Sharir, A.; Eshkar-Oren, I.; Johnson, R.S.; Zelzer, E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 2007, 134, 3917–3928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zheng, Q.; Engin, F.; Munivez, E.; Chen, Y.; Sebald, E.; Krakow, D.; Lee, B. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19004–19009. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Liu, S.C.; Chung, W.H.; Wang, S.W.; Wu, M.H.; Tang, C.H. Visfatin Increases VEGF-dependent Angiogenesis of Endothelial Progenitor Cells during Osteoarthritis Progression. Cells 2020, 9, 1315. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Sato, M.; Kobayashi, M.; Yokoyama, M.; Tani, Y.; Mochida, J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res. Ther. 2014, 16, 427. [Google Scholar] [CrossRef]
- Vadalà, G.; Ambrosio, L.; Cattani, C.; Bernardini, R.; Giacalone, A.; Papalia, R.; Denaro, V. Bevacizumab Arrests Osteoarthritis Progression in a Rabbit Model: A Dose-Escalation Study. J. Clin. Med. 2021, 10, 2825. [Google Scholar] [CrossRef]
- Hajifathali, A.; Saba, F.; Atashi, A.; Soleimani, M.; Mortaz, E.; Rasekhi, M. The role of catecholamines in mesenchymal stem cell fate. Cell Tissue Res. 2014, 358, 651–665. [Google Scholar] [CrossRef]
- Hynes, M.; Porter, J.A.; Chiang, C.; Chang, D.; Tessier-Lavigne, M.; Beachy, P.A.; Rosenthal, A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron 1995, 15, 35–44. [Google Scholar] [CrossRef]
- Wang, M.Z.; Jin, P.; Bumcrot, D.A.; Marigo, V.; McMahon, A.P.; Wang, E.A.; Woolf, T.; Pang, K. Induction of dopaminergic neuron phenotype in the midbrain by Sonic hedgehog protein. Nat. Med. 1995, 1, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Gambassi, S.; Geminiani, M.; Thorpe, S.D.; Bernardini, G.; Millucci, L.; Braconi, D.; Orlandini, M.; Thompson, C.L.; Petricci, E.; Manetti, F.; et al. Smoothened-antagonists reverse homogentisic acid-induced alterations of Hedgehog signaling and primary cilium length in alkaptonuria. J. Cell Physiol. 2017, 232, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wei, X.; Chen, C.; Cao, K.; Li, Y.; Jiao, Q.; Ding, J.; Zhou, J.; Fleming, B.C.; Chen, Q.; et al. Indian hedgehog in synovial fluid is a novel marker for early cartilage lesions in human knee joint. Int. J. Mol. Sci. 2014, 15, 7250–7265. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, X.; Wei, L. Indian Hedgehog, a critical modulator in osteoarthritis, could be a potential therapeutic target for attenuating cartilage degeneration disease. Connect. Tissue Res. 2014, 55, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Buckland, J. Osteoarthritis: Blocking hedgehog signaling might have therapeutic potential in OA. Nat. Rev. Rheumatol. 2010, 6, 61. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.; Han, Y.; Wang, H.; Sun, Y.; Zhang, H. Dopamine/Phosphorylcholine Copolymer as an Efficient Joint Lubricant and ROS Scavenger for the Treatment of Osteoarthritis. ACS Appl. Mater. Interfaces 2019, 12, 51236–51248. [Google Scholar] [CrossRef]
- Zhong, G.; Yang, X.; Jiang, X.; Kumar, A.; Long, H.; Xie, J.; Zheng, L.; Zhao, J. Dopamine-melanin nanoparticles scavenge reactive oxygen and nitrogen species and activate autophagy for osteoarthritis therapy. Nanoscale 2019, 11, 11605–11616. [Google Scholar] [CrossRef]
- Zhang, F.X.; Liu, P.; Ding, W.; Meng, Q.B.; Su, D.H.; Zhang, Q.C.; Lian, R.X.; Yu, B.Q.; Zhao, M.D.; Dong, J.; et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
- van Nie, L.; Salinas-Tejedor, L.; Dychus, N.; Fasbender, F.; Hülser, M.L.; Cutolo, M.; Rehart, S.; Neumann, E.; Müller-Ladner, U.; Capellino, S. Dopamine induces in vitro migration of synovial fibroblast from patients with rheumatoid arthritis. Sci. Rep. 2020, 10, 11928. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Ruggieri, S.; Tamma, R.; Crivellato, E. Limitations of Anti-Angiogenic Treatment of Tumors. Transl. Oncol. 2019, 12, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.L.; Chintharlapalli, S.; Uhlik, M.T.; Pytowski, B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol. Ther. 2016, 164, 204–225. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, M.R.; Jang, J.H.; Na, H.J.; Lee, S. A Review of Anti-Angiogenic Targets for Monoclonal Antibody Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1786. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Leung, L.H.; Liu, L.; Yang, F.; Yao, X. Efficacy and safety of angiogenesis inhibitors in advanced gastric cancer: A systematic review and meta-analysis. J. Hematol. Oncol. 2016, 9, 111. [Google Scholar] [CrossRef]
- Sullivan, L.A.; Brekken, R.A. The VEGF family in cancer and antibody-based strategies for their inhibition. mAbs 2010, 2, 165–175. [Google Scholar] [CrossRef]
- McIntosh, E.; Kent, S.; Gray, A.; Clarke, C.E.; Williams, A.; Jenkinson, C.; Ives, N.; Patel, S.; Rick, C.; Wheatley, K.; et al. Cost-Effectiveness of Dopamine Agonists and Monoamine Oxidase B Inhibitors in Early Parkinson’s Disease. Mov. Disord. 2021, 36, 2136–2143. [Google Scholar] [CrossRef]
- Chauvet, N.; Romanò, N.; Lafont, C.; Guillou, A.; Galibert, E.; Bonnefont, X.; Le Tissier, P.; Fedele, M.; Fusco, A.; Mollard, P.; et al. Complementary actions of dopamine D2 receptor agonist and anti-vegf therapy on tumoral vessel normalization in a transgenic mouse model. Int. J. Cancer 2017, 140, 2150–2161. [Google Scholar] [CrossRef]
- Gomez, R.; Gonzalez-Izquierdo, M.; Zimmermann, R.C.; Novella-Maestre, E.; Alonso-Muriel, I.; Sanchez-Criado, J.; Remohi, J.; Simon, C.; Pellicer, A. Low-dose dopamine agonist administration blocks vascular endothelial growth factor (VEGF)-mediated vascular hyperpermeability without altering VEGF receptor 2-dependent luteal angiogenesis in a rat ovarian hyperstimulation model. Endocrinology 2006, 147, 5400–5411. [Google Scholar] [CrossRef]
- Liu, C.; Tyrrell, J.B. Successful treatment of a large macroprolactinoma with cabergoline during pregnancy. Pituitary 2001, 4, 179–185. [Google Scholar] [CrossRef]
- Robert, E.; Musatti, L.; Piscitelli, G.; Ferrari, C.I. Pregnancy outcome after treatment with the ergot derivative, cabergoline. Reprod. Toxicol. 1996, 10, 333–337. [Google Scholar] [CrossRef]
- Webster, J. A comparative review of the tolerability profiles of dopamine agonists in the treatment of hyperprolactinaemia and inhibition of lactation. Drug Saf. 1996, 14, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Barbieri, C.; Caldara, R.; Mucci, M.; Codecasa, F.; Paracchi, A.; Romano, C.; Boghen, M.; Dubini, A. Long-lasting prolactin-lowering effect of cabergoline, a new dopamine agonist, in hyperprolactinemic patients. J. Clin. Endocrinol. Metab. 1986, 63, 941–945. [Google Scholar] [CrossRef]
- Colao, A.; Di Sarno, A.; Sarnacchiaro, F.; Ferone, D.; Di Renzo, G.; Merola, B.; Annunziato, L.; Lombardi, G. Prolactinomas resistant to standard dopamine agonists respond to chronic cabergoline treatment. J. Clin. Endocrinol. Metab. 1997, 82, 876–883. [Google Scholar] [CrossRef]
- Duh, M.S.; Dial, E.; Choueiri, T.K.; Fournier, A.A.; Antras, L.; Rodermund, D.; Neary, M.P.; Oh, W.K. Cost implications of IV versus oral anti-angiogenesis therapies in patients with advanced renal cell carcinoma: Retrospective claims database analysis. Curr. Med. Res. Opin. 2009, 25, 2081–2090. [Google Scholar] [PubMed]
- Choueiri, T.K.; McDermott, D.; Sheng Duh, M.; Sarda, S.P.; Neary, M.P.; Oh, W.K. Costs associated with angiogenesis inhibitor therapies for metastatic renal cell carcinoma in clinical practice: Results from a medical chart review study. Urol. Oncol. 2012, 30, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Smala, A.M.; Spottke, E.A.; Machat, O.; Siebert, U.; Meyer, D.; Köhne-Volland, R.; Reuther, M.; DuChane, J.; Oertel, W.H.; Berger, K.B.; et al. Cabergoline versus levodopa monotherapy: A decision analysis. Mov. Disord. 2003, 18, 898–905. [Google Scholar] [CrossRef]
| Intervention | Model Analyzed | Observations |
|---|---|---|
| Cabergoline every three days at 50 µg/kg by oral gavage [3] | Heterologous mouse model | Cabergoline significantly decreased the lesion size, vascular density, and innervation in DA-Ag and anti-VEGF groups in comparison with control. |
| Cabergoline at 0.05 or 0.1 mg/kg/day orally for 14 days [81] | Implantation of human endometrium in female mice | The formation of new blood vessels was suppressed in endometriosis lesions, and a decrease in cellular proliferation was observed. VEGFR 2 phosphorylation was significantly lower in cabergoline-treated animals than controls. |
| Cabergoline at 0.05 (low dose) and 0.1 (high dose) mg/kg per day for 14 days [82] | Implantation of human endometrium (with mild or severe endometriosis) in female mice | D2R gene and protein expression was observed in human endometrial implants. Moreover, VEGF gene and VEGF and VEGFR 2 protein expressions were significantly lower in endometrial lesions treated with cabergoline than in controls. |
| Cabergoline 50 mg/kg per day orally or quinagolide 50 or 200 mg/kg per day for 14 days [95] | Nude mice with eutopic human endometrial fragments | Quinagolide and cabergoline both were effective at decreasing endometriotic lesion size and its cellular proliferation. Additionally, a reduction in VEGFR 2 and VEGF gene expression was observed. |
| Quinagolide at 200 μg/kg/day [102] | Wistar rats with Endometriosis was surgically induced by transplantation of autologous endometrial tissue | Quinagolide induced a significant regression in endometriotic implants and reduced the interleukin (IL)-6 and VEGF levels in peritoneal fluid. |
| Vaginal bromocriptine at a dose of 5 mg daily for 6 months of treatment [104] | Women with adenomyosis | Bromocriptine induced a significant improvement in menstrual bleeding and pain. |
| Cabergoline at 0.1 mg/kg/day by oral gavage for 4 weeks [106] | Autotransplantation of endometrial tissue on adult Sprague–Dawley rats | Cabergoline was not effective at endometriotic implant regression. |
| Cabergoline and Bromocriptine at 0.1 mg/kg/day orally for 30 days [107] | Induced endometriosis in Wistar rats | Bromocriptine and cabergoline significantly decreased the area (stromal and glandular tissue) of the endometriotic implants in comparison with controls. |
| Cabergoline at 0.5 mg/kg/day subcutaneously for 21 days [108] | Sprague–Dawley rats with endometriosis implantation | Cabergoline decreased the size and histopathological grade of the induced endometrial lesions. |
| Cabergoline at 0.075 mg/kg for 22 days [109] | Wistar rats with induction of endometriosis | Cabergoline produced a pronounced inhibitory effect on ectopic endometrioid formation. |
| Cabergoline at 0.05 mg/kg for 14 days [110] | C57BL/6 mice and ICR mice with induced endometriosis | Treatment with cabergoline diminished the inflammation in the uterus, peritoneum, and intestine in the recipient mice. Additionally, cabergoline decreased the expression pattern and localization of estrogen receptor beta (ER-β) and nerve growth factor (NGF). |
| Cabergoline 0.5 mg tablets, twice a week for 12 weeks [111] | Women with endometriosis | Cabergoline decreased the size of endometrioma. |
| Cabergoline 0.25 mg twice weekly for 6 months [112] | Women with endometriosis-associated pain syndrome | Cabergoline combined with hormone therapy standard schemes reduced the pain syndrome in patients with genital I–III endometriosis degree. |
| Quinagolide at 50 or 200 mg/kg per day for 14 days [113] | Women with endometriosis | Quinagolide induced a 69.5% reduction in the size of the lesions. |
| Cabergoline (0.5 mg twice weekly × 6 months) [114] Clinical trial NCT02542410. Status: completed | Women with endometriosis | In this pilot study, the change in the worst pain score (time frame: 6 months) after receiving cabergoline was measured. Moreover, changes in the sizes (mm) of endometrioma, deep infiltrating endometriosis, and adenomyosis lesions summed by type on magnetic resonance images at cycle 4 were also measured (time frame: at baseline and at menstrual cycle 4). Cabergoline decreased the pain score, and changes in the endometrial lesions inhibiting size and blood vessel growth was observed. |
| Quinagolide (1080 µg with daily target release rate of 13.5 µg) [115]. Clinical trial NCT03749109. Status: completed | Women with endometriosis | In this clinical trial, changes in the sizes (mm) of endometrioma, deep infiltrating endometriosis, and adenomyosis lesions (time frame: at baseline and at menstrual cycle 4) were measured via magnetic resonance at cycle 4. Quinagolide decreased the number and size of the endometrial and adenomyosis lesions. |
| Cabergoline (0.5 mg twice weekly for 6 months) [116]. Clinical trial NCT03928288. Status: recruiting | Women with endometriosis | In this clinical trial, the authors will measure changes in pain severity with different scales: the brief pain inventory interference scale (BPI), visual analog scale (VAS), and Biberoglu and Behrman patient ratings scale (B&B) over 6 months (time frame: every 6 weeks for 6 months). |
| Intervention | Model Analyzed | Observations |
|---|---|---|
| Cancer therapy using cobalt ferrite (CF) nanoparticles as a DA delivery agent by functionalizing CF-DA-polyethylene glycol (PEG) [125] | Human A549 cells lung cancer | CF-DA-PEG nanoparticles showed an anticancer effect by inducing apoptosis through activating the cytochrome-c and caspase-dependent apoptotic pathway and reactive oxygen species generation. |
| DA delivery via pH-sensitive nanoparticles [126] | Breast cancer mouse model | Nanoparticles induce tumor blood vessel normalization, improving the antitumor chemotherapeutic efficacy of doxorubicin. |
| DA 25 mg/kg twice a week [127] | Mouse model (C57BL/6) of pancreatic cancer | DA has synergistic roles with chemotherapy for pancreatic cancer by suppressing tumor-associated macrophages-derived inflammations. |
| Cabergoline (total week dose of 3.5 mg, starting 6 months after transsphenoidal surgery) [128]. Clinical trial NCT03271918. Status: completed | Subjects with pituitary adenoma | Tumor shrinkage, tumor rest stabilization, and cardiovascular safety (time frame: 24 months). |
| Cabergoline at a dose of 1 mg orally, twice a week for 4 weeks [129] | Women with breast cancer | Cabergoline was well tolerated, and although the overall response rate was low, a small subgroup of patients experienced prolonged disease control. |
| DA vasopressor dose individually titrated according to mean arterial pressure [130]. Clinical trial NCT02241083 Status: completed | Subjects with head and neck cancer | Evidence of clinically definite ischemia (time frame: 72 h). |
| Cabergoline, bromocriptine, or quinagolide (DA-Ag) [131]. Clinical trial NCT04107480. Status: recruiting | Subjects with prolactinoma | Health-related quality of life (time frame: 12 months) and long-term remission (time frame: 36 months). |
| Ropirinole (0.25 mg/day–6.0 mg/day oral) [132]. Clinical trial: NCT03038308. Status: Completed | Subjects with prolactinoma | Percentage of subjects that achieved stable PRL normalization (time frame: 6–12 months). A dose-dependent PRL nadir occurred 4.4 ± 1.2 h after drug intake, and PRL concentrations transiently normalized. |
| Cabergoline (twice weekly for weeks 1–4. Courses repeat every 4 weeks in the absence of disease progression or unacceptable toxicity) [133]. Clinical trial NCT01730729 Status: Completed | Women with breast cancer | Overall response rate at 2 months (time frame: after 8 weeks (2 cycles) of treatment). |
| Intervention | Model Analyzed | Observations |
|---|---|---|
| In vitro: DA 100 μM In vivo: DA was administered by intra-articular injection once a week for 12 weeks [139] | C28/I2 cells and primary cell culture of human chondrocytes Eight-week-old C57BL/6 male mice with surgically induced destabilization of the medial meniscus | In vitro, DA treatment inhibited the production of inducible nitric oxide synthase, COX-2, MMP-1, MMP-3, and MMP-13. DA reversed IL-1β-treated nuclear factor-kappa B activation and JAK2/STAT3 phosphorylation. In vivo, DA suppressed the degradation of cartilage matrix and reduced OA. |
| Copolymer P(DMA-co-MPC) with DA hydrochloride (5 g, 26.5 mmol) [183] | Mouse MC3T3-E1 osteoblastic cells | Improved lubrication and decreased reactive oxygen species in joint inflammation. |
| DA–melanin nanoparticles [184] | Primary chondrocytes were isolated from knee joint cartilage of 3-day-old Sprague–Dawley rats | DA–melanin nanoparticles have excellent anti-inflammatory and chondroprotective effects by inhibiting intracellular reactive oxygen species and reactive nitrogen species in vitro and in vivo. |
| Injectable hydrogel (alginate–DA, chondroitin sulfate, and regenerated silk fibroin) [185] | The adhesive strength of the material was measured by using a porcine skin interface and porcine cartilage ex vivo model | Hydrogel enhanced bone-marrow-derived mesenchymal stem cells recruitment, proliferation, and differentiation, as well as cartilage regeneration in a rat model. |
| D1R stimulation with fenoldopam D2R stimulation with ropinirole [186] | Cell culture and synovial fibroblasts from knee tissue from patients with rheumatoid arthritis and OA who underwent knee joint replacement surgery | Showed the involvement of the dopaminergic pathway in migration of synovial fibroblasts, supporting the therapeutic potential of the dopaminergic pathway in RA and in OA. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Torreblanca, J.G.; Cárdenas-Rodríguez, N.; Carro-Rodríguez, J.; Contreras-García, I.J.; Garciadiego-Cázares, D.; Ortega-Cuellar, D.; Martínez-López, V.; Alfaro-Rodríguez, A.; Evia-Ramírez, A.N.; Ignacio-Mejía, I.; et al. Antiangiogenic Effect of Dopamine and Dopaminergic Agonists as an Adjuvant Therapeutic Option in the Treatment of Cancer, Endometriosis, and Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 10199. https://doi.org/10.3390/ijms241210199
Mendoza-Torreblanca JG, Cárdenas-Rodríguez N, Carro-Rodríguez J, Contreras-García IJ, Garciadiego-Cázares D, Ortega-Cuellar D, Martínez-López V, Alfaro-Rodríguez A, Evia-Ramírez AN, Ignacio-Mejía I, et al. Antiangiogenic Effect of Dopamine and Dopaminergic Agonists as an Adjuvant Therapeutic Option in the Treatment of Cancer, Endometriosis, and Osteoarthritis. International Journal of Molecular Sciences. 2023; 24(12):10199. https://doi.org/10.3390/ijms241210199
Chicago/Turabian StyleMendoza-Torreblanca, Julieta Griselda, Noemi Cárdenas-Rodríguez, Jazmín Carro-Rodríguez, Itzel Jatziri Contreras-García, David Garciadiego-Cázares, Daniel Ortega-Cuellar, Valentín Martínez-López, Alfonso Alfaro-Rodríguez, Alberto Nayib Evia-Ramírez, Iván Ignacio-Mejía, and et al. 2023. "Antiangiogenic Effect of Dopamine and Dopaminergic Agonists as an Adjuvant Therapeutic Option in the Treatment of Cancer, Endometriosis, and Osteoarthritis" International Journal of Molecular Sciences 24, no. 12: 10199. https://doi.org/10.3390/ijms241210199
APA StyleMendoza-Torreblanca, J. G., Cárdenas-Rodríguez, N., Carro-Rodríguez, J., Contreras-García, I. J., Garciadiego-Cázares, D., Ortega-Cuellar, D., Martínez-López, V., Alfaro-Rodríguez, A., Evia-Ramírez, A. N., Ignacio-Mejía, I., Vargas-Hernández, M. A., & Bandala, C. (2023). Antiangiogenic Effect of Dopamine and Dopaminergic Agonists as an Adjuvant Therapeutic Option in the Treatment of Cancer, Endometriosis, and Osteoarthritis. International Journal of Molecular Sciences, 24(12), 10199. https://doi.org/10.3390/ijms241210199








