Standardized Extract from Wastes of Edible Flowers and Snail Mucus Ameliorate Ultraviolet B-Induced Damage in Keratinocytes
Abstract
1. Introduction
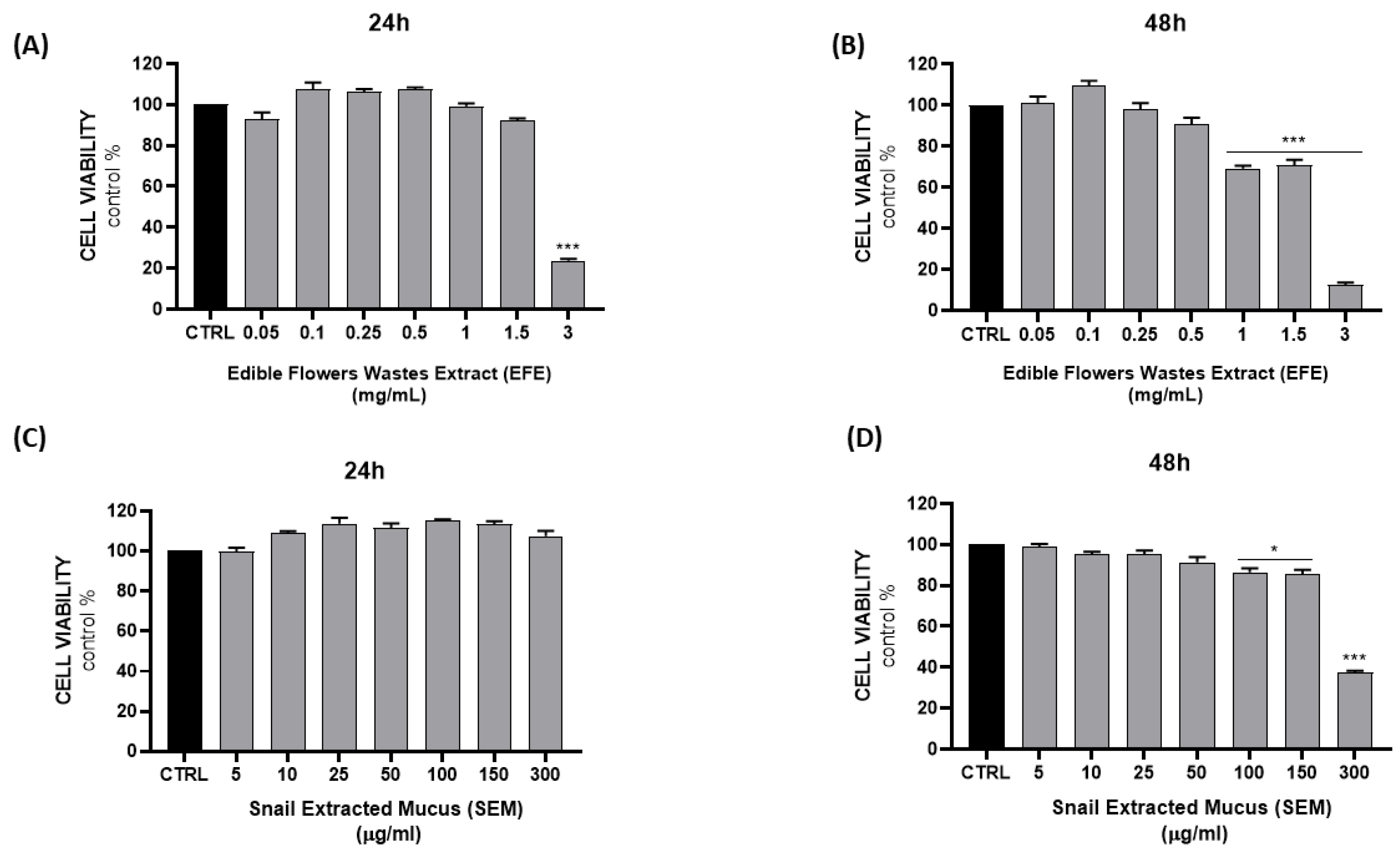
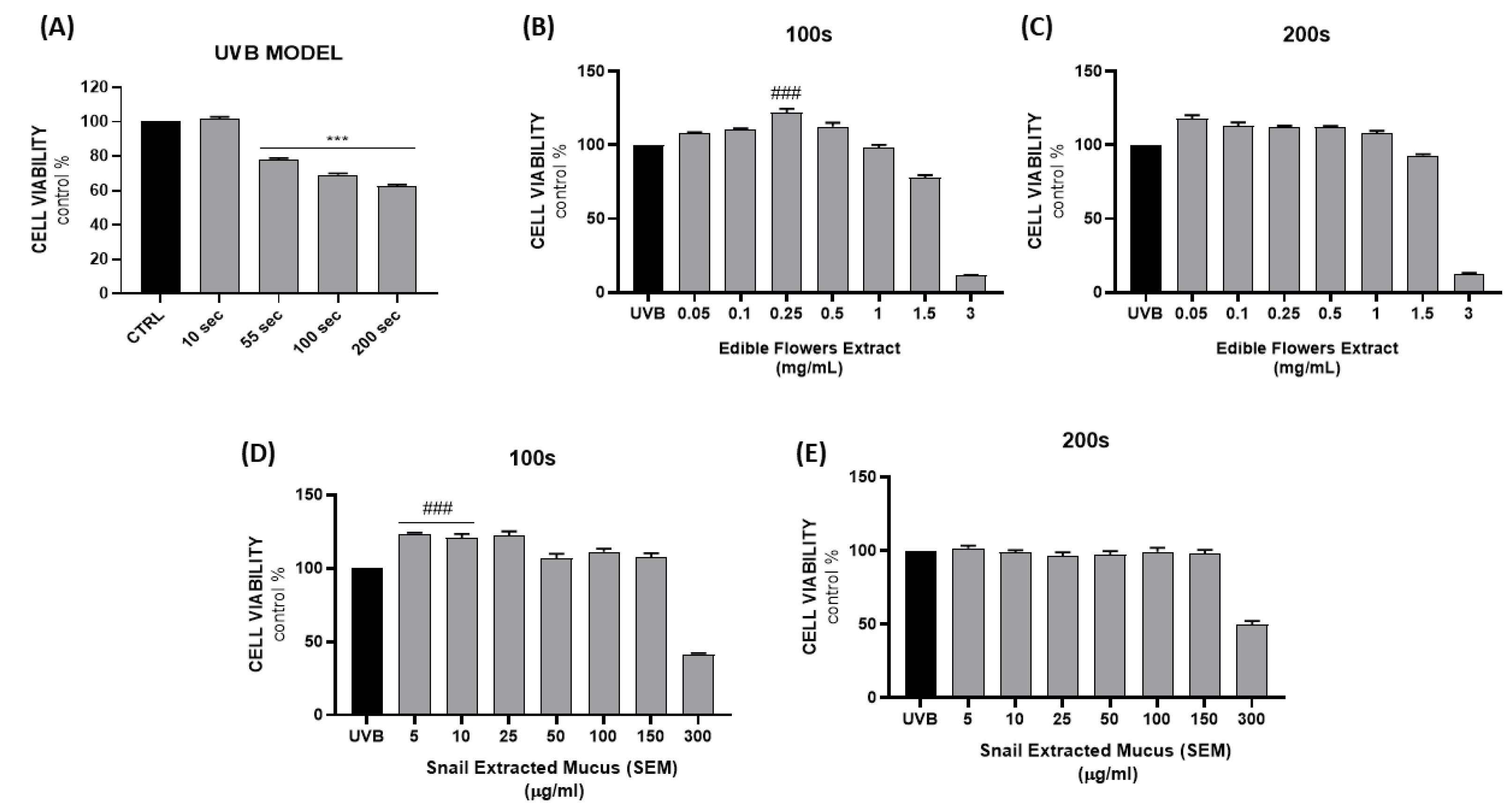
2. Results and Discussion
3. Materials and Methods
3.1. EFE Composition
3.2. Determination of Total Polyphenol Content of EFE
3.3. Determination of Total Carotenoid Content of EFE
3.4. Helix Mucus Collection and Composition Evaluation
3.5. DPPH Assay
3.6. SOD-Like Activity
3.7. UV Spectra
3.8. Cell Culture and Viability Assay
3.9. Cell Treatments and UV Model Establishment
3.10. Measurement of ROS Levels
3.11. Thiol (RSH) Group Determination
3.12. Measurement of Lipid Peroxidation
3.13. RNA Extraction and Quantitative Real-Time PCR Analysis
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, H.R.; Mistry, P.; Herbert, K.E.; Lunec, J. Molecular and cellular effects of ultraviolet light-induced genotoxicity. Crit. Rev. Clin. Lab. Sci. 1998, 35, 189–237. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; Acquaviva, R.; La Mantia, A.; Di Giacomo, C. Phytocomplex of a Standardized Extract from Red Orange. Cells 2022, 11, 1447. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Jia, J.; Zheng, Y. UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer. Adv. Exp. Med. Biol. 2017, 996, 27–40. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free. Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Newar, J.; Ghatak, A. Studies on the Adhesive Property of Snail Adhesive Mucus. Langmuir 2015, 31, 12155–12160. [Google Scholar] [CrossRef]
- Gentili, V.; Bortolotti, D.; Benedusi, M.; Alogna, A.; Fantinati, A.; Guiotto, A.; Turrin, G.; Cervellati, C.; Trapella, C.; Rizzo, R.; et al. HelixComplex snail mucus as a potential technology against O3 induced skin damage. PLoS ONE 2020, 15, e0229613. [Google Scholar] [CrossRef]
- Cilia, G.; Fratini, F. Antimicrobial properties of terrestrial snail and slug mucus. J. Complement. Integr. Med. 2018, 15, 20170168. [Google Scholar] [CrossRef]
- Pitt, S.J.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181. [Google Scholar] [CrossRef]
- Trapella, C.; Rizzo, R.; Gallo, S.; Alogna, A.; Bortolotti, D.; Casciano, F.; Zauli, G.; Secchiero, P.; Voltan, R. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci. Rep. 2018, 8, 17665. [Google Scholar] [CrossRef]
- López Angulo, D.E.; do Amaral Sobral, P.J. Characterization of gelatin/chitosan scaffold blended with aloe vera and snail mucus for biomedical purpose. Int. J. Biol. Macromol. 2016, 92, 645–653. [Google Scholar] [CrossRef]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional composition and antioxidant capacity in edible flowers: Characterisation of phenolic compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2014, 16, 805–822. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S. Nutraceutical potential of dietary phytochemicals in edible flowers-A review. J. Food Biochem. 2021, 45, e13642. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products-A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
- Wu, L.C.; Fan, N.C.; Lin, M.H.; Chu, I.R.; Huang, S.J.; Hu, C.Y.; Han, S.Y. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J. Agric. Food Chem. 2008, 56, 2341–2349. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, S.Y.; Valan Arasu, M.; Al-Dhabi, N.A.; Ahn, H.G.; Kim, J.K.; Park, S.U. Accumulation of Carotenoids and Metabolic Profiling in Different Cultivars of Tagetes Flowers. Molecules 2017, 22, 313. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Escher, G.B.; Santos, J.S.; Rosso, N.D.; Marques, M.B.; Azevedo, L.; do Carmo, M.A.V.; Daguer, H.; Molognoni, L.; Prado-Silva, L.D.; Sant’Ana, A.S.; et al. Chemical study, antioxidant, anti-hypertensive, and cytotoxic/cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food Chem. Toxicol. 2018, 118, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Fanelli, F.; Sibillano, T.; Corriero, N.; Cosma, P. Chitosan/snail slime films as multifunctional platforms for potential biomedical and cosmetic applications: Physical and chemical characterization. J. Mater. Chem. B 2023, 11, 2638–2649. [Google Scholar] [CrossRef]
- Xian, D.; Xiong, X.; Xu, J.; Xian, L.; Lei, Q.; Song, J.; Zhong, J. Nrf2 Overexpression for the Protective Effect of Skin-Derived Precursors against UV-Induced Damage: Evidence from a Three-Dimensional Skin Model. Oxid. Med. Cell. Longev. 2019, 2019, 7021428. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Yu, S.M.; Kang, C.H.; Kim, G.Y. Protective Effect of Anthocyanin-Enriched Polyphenols from. Antioxidants 2021, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liu, J.; Zhang, X.; Zhou, J.; Liu, H.; Liang, J.; Jiang, L.; Hu, J.; Zhang, Y.; Ma, L.; et al. Protective effect of γ-glutamylcysteine against UVB radiation in NIH-3T3 cells. Photodermatol. Photoimmunol. Photomed. 2022, 38, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventόs, R.M. 14 Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Consoli, V.; Sorrenti, V.; Burò, I.; Modica, M.N.; Vanella, L. Antiproliferative Effect of Plant-Derived Bioactive Compounds Endowed with Antioxidant Activity on Breast Cancer Cells. Nutraceuticals 2022, 2, 246–252. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Salerno, L.; Vanella, L.; Sorrenti, V.; Consoli, V.; Ciaffaglione, V.; Fallica, A.N.; Canale, V.; Zajdel, P.; Pignatello, R.; Intagliata, S. Novel mutual prodrug of 5-fluorouracil and heme oxygenase-1 inhibitor (5-FU/HO-1 hybrid): Design and preliminary. J. Enzyme Inhib. Med. Chem. 2021, 36, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; D’Amico, A.G.; Barbagallo, I.; Consoli, V.; Grosso, S.; Vanella, L. Tin Mesoporphyrin Selectively Reduces Non-Small-Cell Lung Cancer Cell Line A549 Proliferation by Interfering with Heme Oxygenase and Glutathione Systems. Biomolecules 2021, 11, 917. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Pittalà, V.; Greish, K.; D’Amico, A.G.; Romeo, G.; Intagliata, S.; Salerno, L.; Vanella, L. Heme Oxygenase Modulation Drives Ferroptosis in TNBC Cells. Int. J. Mol. Sci. 2022, 23, 5709. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanella, L.; Consoli, V.; Burò, I.; Gulisano, M.; Giglio, M.S.; Maugeri, L.; Petralia, S.; Castellano, A.; Sorrenti, V. Standardized Extract from Wastes of Edible Flowers and Snail Mucus Ameliorate Ultraviolet B-Induced Damage in Keratinocytes. Int. J. Mol. Sci. 2023, 24, 10185. https://doi.org/10.3390/ijms241210185
Vanella L, Consoli V, Burò I, Gulisano M, Giglio MS, Maugeri L, Petralia S, Castellano A, Sorrenti V. Standardized Extract from Wastes of Edible Flowers and Snail Mucus Ameliorate Ultraviolet B-Induced Damage in Keratinocytes. International Journal of Molecular Sciences. 2023; 24(12):10185. https://doi.org/10.3390/ijms241210185
Chicago/Turabian StyleVanella, Luca, Valeria Consoli, Ilaria Burò, Maria Gulisano, Manuela Stefania Giglio, Ludovica Maugeri, Salvatore Petralia, Angela Castellano, and Valeria Sorrenti. 2023. "Standardized Extract from Wastes of Edible Flowers and Snail Mucus Ameliorate Ultraviolet B-Induced Damage in Keratinocytes" International Journal of Molecular Sciences 24, no. 12: 10185. https://doi.org/10.3390/ijms241210185
APA StyleVanella, L., Consoli, V., Burò, I., Gulisano, M., Giglio, M. S., Maugeri, L., Petralia, S., Castellano, A., & Sorrenti, V. (2023). Standardized Extract from Wastes of Edible Flowers and Snail Mucus Ameliorate Ultraviolet B-Induced Damage in Keratinocytes. International Journal of Molecular Sciences, 24(12), 10185. https://doi.org/10.3390/ijms241210185






