Cyanidin-3-O-glucoside as a Nutrigenomic Factor in Type 2 Diabetes and Its Prominent Impact on Health
Abstract
1. Introduction
2. Civilization Diseases
3. Diabetes
3.1. Lifestyle Factors and T2D
3.2. Genetic and Epigenetic Factors and T2D
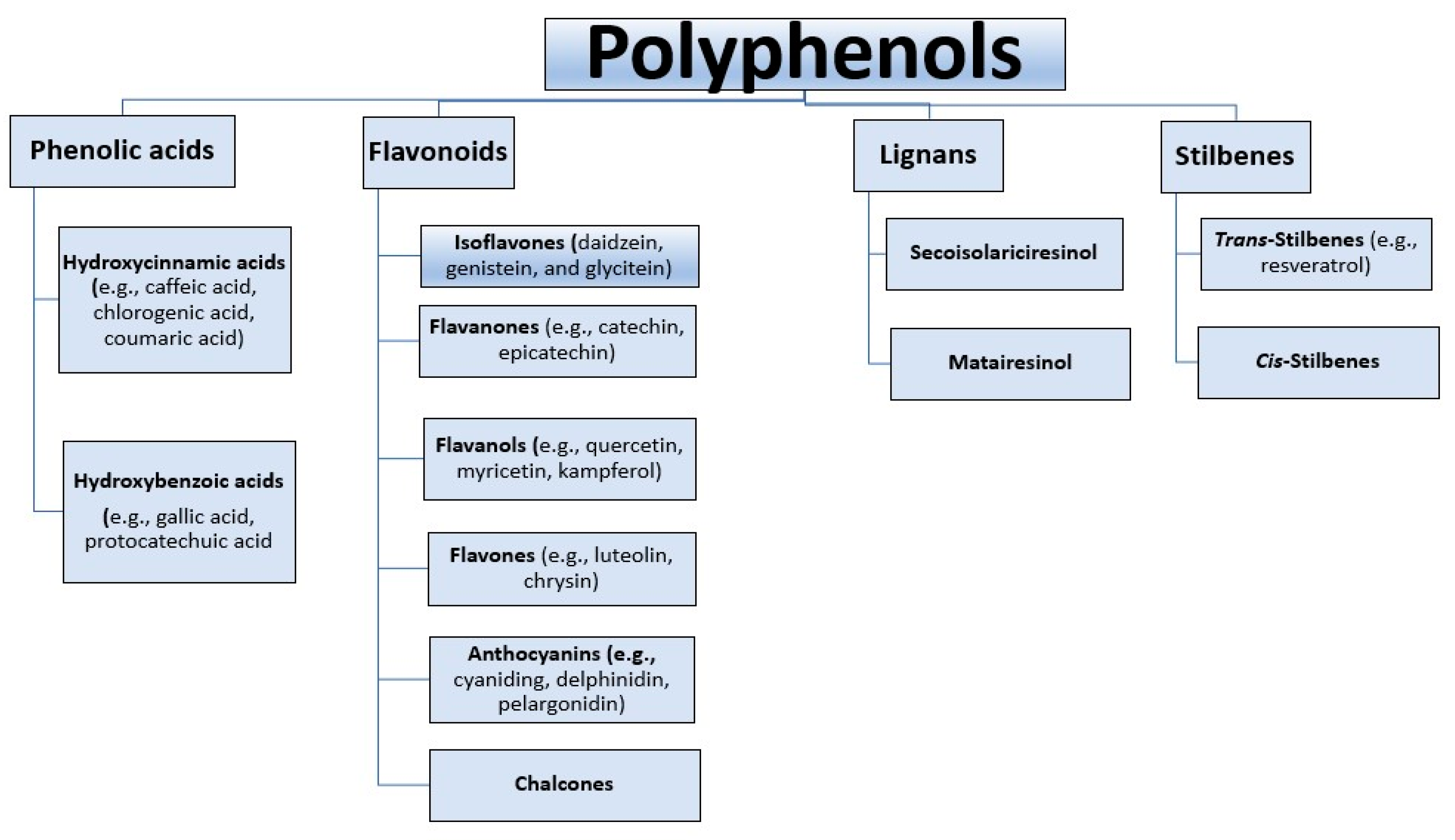
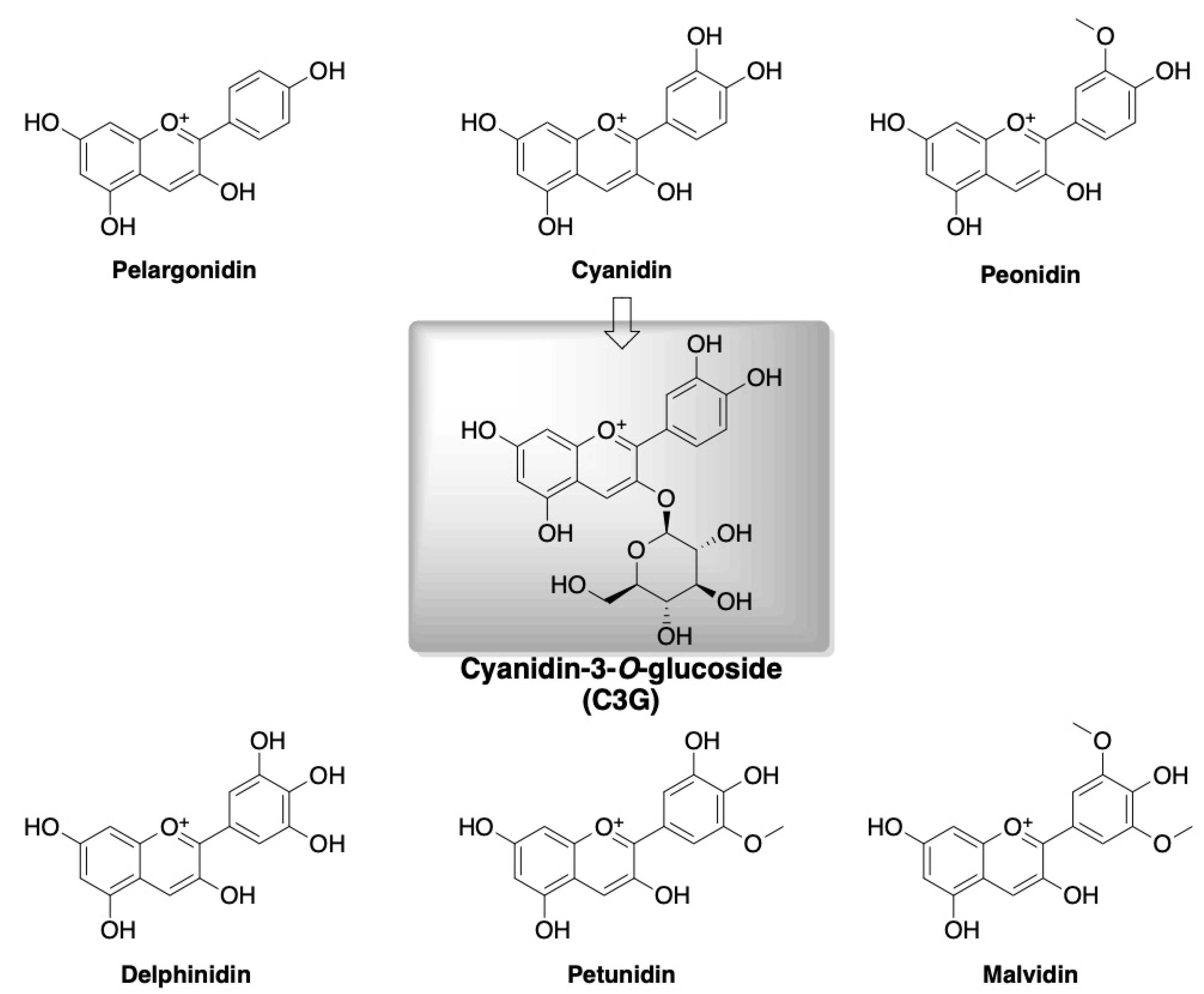
4. Polyphenols and Cyanidin-3-O-glucoside
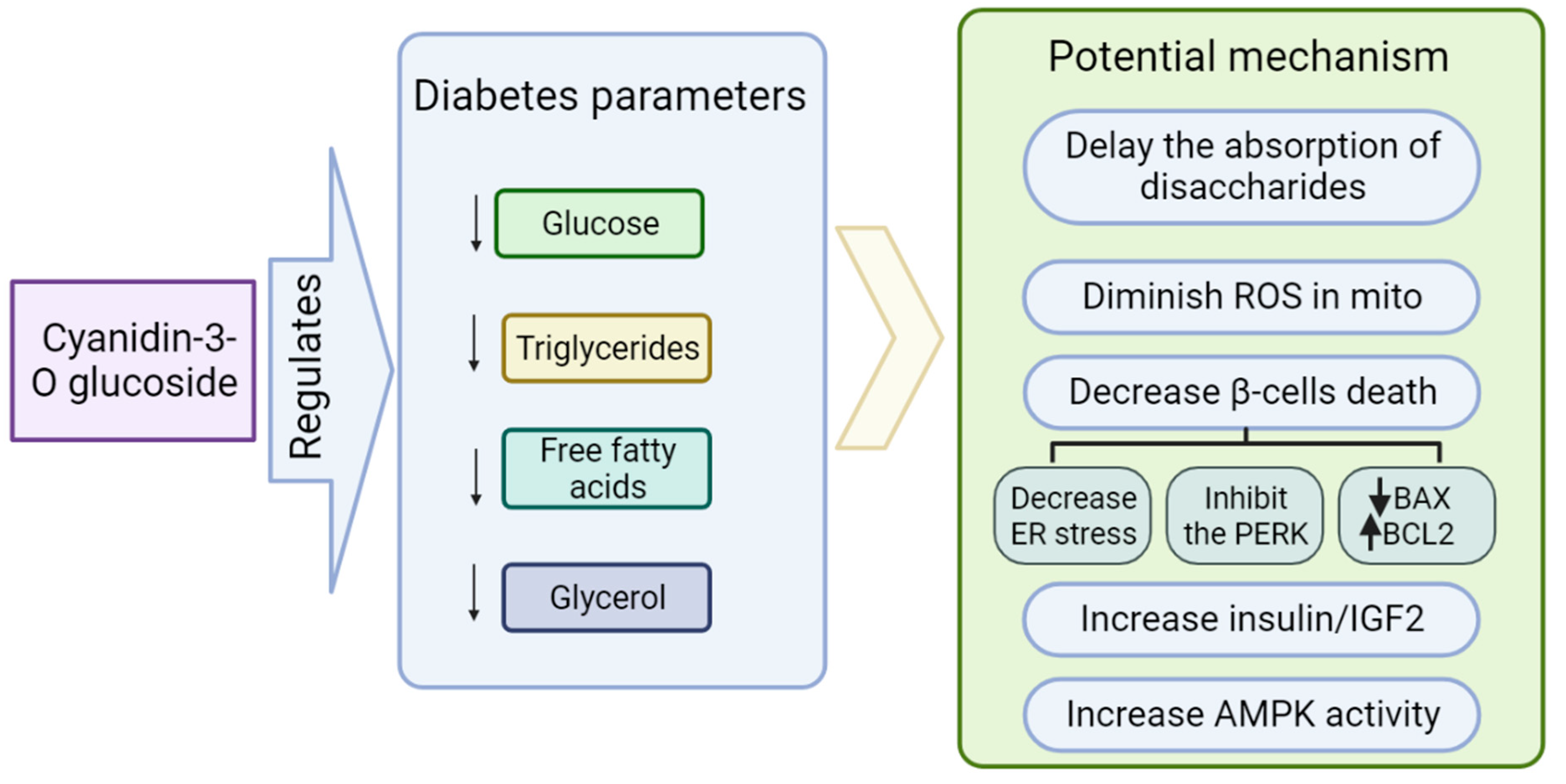
Role of Cyanidin-3-O-glucoside (C3G) in Diabetes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tucker, K.L.; Smith, C.E.; Lai, C.Q.; Ordovas, J.M. Quantifying diet for nutrigenomic studies. Ann. Rev. Nutr. 2013, 33, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Bastos, P.; Fontes-Villalba, M.; HO’Keefe, J.; Lindeberg, S.; Cordain, L. The western diet and lifestyle and diseases of civilization. Clin. Res. Cardio. 2011, 2, 15–35. [Google Scholar] [CrossRef]
- Temelkova-Kurktschiev, T.; Stefanov, T. Lifestyle and genetics in obesity and type 2 diabetes. Exp. Clin. Endocrinol. Diabetes. 2012, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ali, O. Genetics of type 2 diabetes. World J. Diabetes 2013, 4, 114. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Hou, D.-X.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Rupasinghe, H.P. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef] [PubMed]
- Dryden, G.W.; Song, M.; McClain, C. Polyphenols and gastrointestinal diseases. Curr. Opin. Gastroenterol. 2006, 22, 165–170. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Tseng, P.S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of side chain conformation on the activity of glycosidase inhibitors. Angew. Chem. Int. 2023, 135, e202217809. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5, 6 & 6, 6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 2022, 5–23. [Google Scholar] [CrossRef]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of glycals into vicinal-1,2-diazides and 1, 2-(or 2,1)-azidoacetates using hypervalent iodine reagents and Me 3 SiN 3. Application in the synthesis of N-glycopeptides, pseudo-trisaccharides and an iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 27 April 2023).
- Çetin, M.; Bakirtaş, I. Does Urbanization Induce the Health Expenditures? A Dynamic Macro-Panel Analysis for Developing Countries. Dumlupınar Üniversitesi Sos. Bilimler Derg. 2019, 61, 208–222. [Google Scholar]
- Goryakin, Y.; Rocco, L.; Suhrcke, M. The contribution of urbanization to non-communicable diseases: Evidence from 173 countries from 1980 to 2008. Econ. Hum. Biol. 2017, 26, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Zhang, S.; Zeng, H.; Zuo, T.; Xia, C.; Yang, Z.; He, J. Cancer incidence and mortality in China in 2013: An analysis based on urbanization level. Chin. J. Cancer Res. 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Voss, J.D.; Masuoka, P.; Webber, B.J.; Scher, A.I.; Atkinson, R.L. Association of elevation, urbanization and ambient temperature with obesity prevalence in the United States. Int. J. Obes. 2013, 37, 1407–1412. [Google Scholar] [CrossRef]
- Akpan, E.E.; Ekpenyong, C.E. Urbanization Drift and Obesity Epidemic in Sub-Saharan Africa: A Review of the Situation in Nigeria. Eur. J. Sustain. Dev. 2013, 2, 141. [Google Scholar] [CrossRef]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Aoki, Y.; Ogden, C.L. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013–2016. JAMA 2018, 319, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Fryar, C.D.; Hales, C.M.; Carroll, M.D.; Aoki, Y.; Freedman, D.S. Differences in obesity prevalence by demographics and urbanization in US children and adolescents, 2013–2016. JAMA 2018, 319, 2410–2418. [Google Scholar] [CrossRef]
- Hossain, M.; Khan, J.R.; Adhikary, A.C.; Anwar AH, M.; Raheem, E.; Siddiqee, M.H.; Hossain, M.S. Association between childhood overweight/obesity and urbanization in developing countries: Evidence from Bangladesh. J. Public Health 2022, 30, 2819–2828. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 5 January 2023).
- Internation Diabetes Federation. Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 7 January 2023).
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef] [PubMed]
- Bunney, P.E.; Zink, A.N.; Holm, A.A.; Billington, C.J.; Kotz, C.M. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.C.; Obimba, K.C.; Belonwu, C.D.; Unakalamba, C.B. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.E.; Moreira, N.C.; Sakamoto-Hojo, E.T. Mechanisms underlying the pathophysiology of type 2 diabetes: From risk factors to oxidative stress, metabolic dysfunction, and hyperglycemia. Mutat. Res. Toxicol. Environ. Mutagen. 2022, 874, 503437. [Google Scholar] [CrossRef]
- Ralph, A.D. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Devaraj, S.; Wang-Polagruto, J.; Polagruto, J.; Keen, C.L.; Jialal, I. High-fat, energy-dense, fast-food–style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism 2008, 57, 867–870. [Google Scholar] [CrossRef]
- Dali-Youcef, N.; Mecili, M.; Ricci, R.; Andres, E. Metabolic inflammation: Connecting obesity and insulin resistance. Ann. Med. 2013, 45, 242–253. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef]
- Szczepańska, E.; Białek-Dratwa, A.; Janota, B.; Kowalski, O. Dietary Therapy in Prevention of Cardiovascular Disease (CVD)—Tradition or Modernity? A Review of the Latest Approaches to Nutrition in CVD. Nutrients 2022, 14, 2649. [Google Scholar] [CrossRef]
- Rahati, S.; Shahraki, M.; Arjomand, G.; Shahraki, T. Food pattern, lifestyle and diabetes mellitus. Int. J. High Risk Behav. Addict. 2014, 10, e8725. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Schulze, M.B.; Hoffmann, K.; Manson, J.E.; Willett, W.C.; Meigs, J.B.; Weikert, C.; Heidemann, C.; Colditz, G.A.; Hu, F.B. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am. J. Clin. 2005, 82, 675–684. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.-Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O.; et al. Natural products in diabetes research: Quantitative literature analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress− activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scivittaro, V.; Ganz, M.B.; Weiss, M.F. AGEs induce oxidative stress and activate protein kinase C-βII in neonatal mesangial cells. Am. J. Physiol. 2000, 278, F676–F683. [Google Scholar] [CrossRef]
- Association, A.D. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.H. Globalization of Diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef]
- Groop, L.; Forsblom, C.; Lehtovirta, M.; Tuomi, T.; Karanko, S.; Nissén, M.; Ehrnström, B.O.; Forsén, B.; Isomaa, B.; Snickars, B.; et al. Metabolic consequences of a family history of NIDDM (the Botnia study): Evidence for sex-specific parental effects. Diabetes 1996, 45, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.P.; Voight, B.F.; Teslovich, T.M.; Ferreira, T.; Segrè, A.V.; Steinthorsdottir, V.; Strawbridge, R.J.; Khan, H.; Grallert, H.; Mahajan, A.; et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 2012, 44, 981–990. [Google Scholar] [CrossRef]
- Lyssenko, V.; Nagorny, C.L.; Erdos, M.R.; Wierup, N.; Jonsson, A.; Spégel, P.; Bugliani, M.; Saxena, R.; Fex, M.; Pulizzi, N.; et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nature Genet. 2009, 41, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, H.; Loos, R.J.; Yu, Z.; Ye, X.; Chen, L.; Pan, A.; Hu, F.B.; Lin, X. Common Variants in CDKAL1, CDKN2A/B, IGF2BP2, SLC30A8, and HHEX/IDE Genes Are Associated With Type 2 Diabetes and Impaired Fasting Glucose in a Chinese Han Population. Diabetes 2008, 57, 2834–2842. [Google Scholar] [CrossRef]
- Ng, M.C.; Park, K.S.; Oh, B.; Tam, C.H.; Cho, Y.M.; Shin, H.D.; Lam, V.K.; Ma, R.C.; So, W.Y.; Cho, Y.S.; et al. Implication of Genetic Variants Near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in Type 2 Diabetes and Obesity in 6,719 Asians. Diabetes 2008, 57, 2226–2233. [Google Scholar] [CrossRef]
- Yasuda, K.; Miyake, K.; Horikawa, Y.; Hara, K.; Osawa, H.; Furuta, H.; Hirota, Y.; Mori, H.; Jonsson, A.; Sato, Y.; et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat. Genet. 2008, 40, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Liu, M.; Park, S. High genetic risk scores of SLIT3, PLEKHA5 and PPP2R2C variants increased insulin resistance and interacted with coffee and caffeine consumption in middle-aged adults. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 79–89. [Google Scholar] [CrossRef]
- Jin, T.; Youn, J.; Kim, A.N.; Kang, M.; Kim, K.; Sung, J.; Lee, J.E. Interactions of habitual coffee consumption by genetic polymorphisms with the risk of prediabetes and Type 2 diabetes combined. Nutrients 2020, 12, 2228. [Google Scholar] [CrossRef]
- Go, M.J.; Hwang, J.-Y.; Kim, Y.J.; Oh, J.H.; Kim, Y.-J.; Kwak, S.H.; Park, K.S.; Lee, J.; Kim, B.-J.; Han, B.-G.; et al. New susceptibility loci in MYL2, C12orf51 and OAS1 associated with 1-h plasma glucose as predisposing risk factors for type 2 diabetes in the Korean population. J. Hum. Genet. 2013, 58, 362–365. [Google Scholar] [CrossRef]
- Karachanak-Yankova, S.; Dimova, R.; Nikolova, D.; Nesheva, D.; Koprinarova, M.; Maslyankov, S.; Tafradjiska, R.; Gateva, P.; Velizarova, M.; Hammoudeh, Z.; et al. Epigenetic alterations in patients with type 2 diabetes mellitus. Balk. J. Med. Genet. 2015, 18, 15–24. [Google Scholar] [CrossRef]
- Ding, G.-L.; Wang, F.-F.; Shu, J.; Tian, S.; Jiang, Y.; Zhang, D.; Wang, N.; Luo, Q.; Zhang, Y.; Jin, F.; et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine Hyperglycemia. Diabetes 2012, 61, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.Z.; O’dea, K.; Gomez, M.; Girgis, S.; Colagiuri, R. Diet and exercise in the prevention of diabetes. J. Hum. Nutr. Diet. 2010, 23, 344–352. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Choudhary, N.; Tewari, D.; El-Demerdash, A.; Horbanczuk, O.K.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; et al. Quercetin: Total-scale literature landscape analysis of a valuable nutraceutical with numerous potential applications in the promotion of human and animal health a review. Anim. Sci. Pap. Rep. 2021, 39, 199–212. [Google Scholar]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. CMBL 2022, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Choudhary, N.; Tewari, D.; El-Demerdash, A.; Tomczyk, M.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; et al. Lycopene: Total-scale literature landscape analysis of a valuable nutraceutical with numerous potential applications in the promotion of human and animal health. Anim. Sci. Pap. Rep. 2022, 40, 119–134. [Google Scholar]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More than resveratrol: New insights into stilbene-based compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Brooks, M.S.L.; Celli, G.B. (Eds.) Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health; Royal Society of Chemistry: London, UK, 2019; p. 315. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Demerdash, A.; Horbanczuk, O.K.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; Santini, A.; et al. Apple polyphenols in human and animal health. Anim. Sci. Pap. Rep. 2021, 39, 105–118. [Google Scholar]
- Spínola, V.; Pinto, J.; Llorent-Martínez, E.J.; Tomás, H.; Castilho, P.C. Evaluation of Rubus grandifolius L. (wild blackberries) activities targeting management of type-2 diabetes and obesity using in vitro models. Food Chem. Toxicol. 2019, 123, 443–452. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz ND, R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, D.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef]
- Evaluation of Certain Food Additives: Eighty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives; (WHO Technical Report Series; No. 1020). Licence: CC BY-NC-SA 3.0 IGO; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2019.
- Davinelli, S.; Bertoglio, J.C.; Zarrelli, A.; Pina, R.; Scapagnini, G. A randomized clinical trial evaluating the efficacy of an anthocyanin–maqui berry extract (Delphinol®) on oxidative stress biomarkers. J. Am. Coll. Nutr. 2015, 34 (Suppl. S1), 28–33. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef]
- Choi, K.; Choi, S.-I.; Park, M.H.; Han, J.-S. Cyanidin-3-O-glucoside Ameliorates Postprandial Hyperglycemia in Diabetic Mice. J. Life Sci. 2017, 27, 32–37. [Google Scholar] [CrossRef]
- Ştefănuţ, M.N.; Căta, A.; Pop, R.; Tănasie, C.; Boc, D.; Ienaşcu, I.; Ordodi, V. Anti-hyperglycemic effect of bilberry, blackberry and mulberry ultrasonic extracts on diabetic rats. Plant Foods Hum Nutr. 2013, 68, 378–384. [Google Scholar] [CrossRef]
- Sun, C.D.; Zhang, B.; Zhang, J.K.; Xu, C.J.; Wu, Y.L.; Li, X.; Chen, K.S. Cyanidin-3-glucoside-rich extract from Chinese bayberry fruit protects pancreatic β cells and ameliorates hyperglycemia in streptozotocin-induced diabetic mice. J. Med. Food. 2012, 15, 288–298. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Su, L.; Hu, Q.; Li, W.; He, J.; Zhao, L. Cyanidin-3-O-glucoside ameliorates palmitic-acid-induced pancreatic beta cell dysfunction by modulating chop-mediated endoplasmic reticulum stress pathways. Nutrients 2022, 14, 1835. [Google Scholar] [CrossRef]
- Liu, M.; Weiss, M.A.; Arunagiri, A.; Yong, J.; Rege, N.; Sun, J.; Haataja, L.; Kaufman, R.J.; Arvan, P. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes. Metab. 2018, 20, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kaufman, R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012, 197, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ling, W. The update of anthocyanins on obesity and type 2 diabetes: Experimental evidence and clinical perspectives. Rev. Endocr. Metab. Disord. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Guo, H.; Guo, J.; Jiang, X.; Li, Z.; Ling, W. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 2012, 50, 3040–3047. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Galvano, F.; Masella, R. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef]
- Hiukka, A.; Maranghi, M.; Matikainen, N.; Taskinen, M.R. PPARα: An emerging therapeutic target in diabetic microvascular damage. Nat. Rev. Endocrinol. 2010, 6, 454–463. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.E.; Berger, J.P. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Int. J. Obes. 2003, 27, S17–S21. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Kim, Y.-S.; Yang, S.O.; Kim, Y.; Kim, J.-S.; Jeong, M.-Y.; Lee, J.H.; Kim, B.; Lee, S.; et al. A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun. Biol. 2020, 3, 514. [Google Scholar] [CrossRef]
- Solverson, P. Anthocyanin bioactivity in obesity and diabetes: The essential role of glucose transporters in the gut and periphery. Cells 2020, 9, 2515. [Google Scholar] [CrossRef]
- Sasaki, R.; Nishimura, N.; Hoshino, H.; Isa, Y.; Kadowaki, M.; Ichi, T.; Tanaka, A.; Nishiumi, S.; Fukuda, I.; Ashida, H.; et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. 2007, 74, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Kahn, B.B. Facilitative glucose transporters: Regulatory mechanisms and dysregulation in diabetes. J. Clin. Investig. 1992, 89, 1367–1374. [Google Scholar] [CrossRef]
- Matsukawa, T.; Inaguma, T.; Han, J.; Villareal, M.O.; Isoda, H. Cyanidin-3-glucoside derived from black soybeans ameliorate type 2 diabetes through the induction of differentiation of preadipocytes into smaller and insulin-sensitive adipocytes. J. Nutr. Biochem. 2015, 26, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef] [PubMed]
- Yogosawa, S.; Mizutani, S.; Ogawa, Y.; Izumi, T. Activin Receptor-Like Kinase 7 Suppresses Lipolysis to Accumulate Fat in Obesity Through Downregulation of Peroxisome Proliferator–Activated Receptor γ and C/EBPα. Diabetes 2013, 62, 115–123. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartel, I.; Koszarska, M.; Strzałkowska, N.; Tzvetkov, N.T.; Wang, D.; Horbańczuk, J.O.; Wierzbicka, A.; Atanasov, A.G.; Jóźwik, A. Cyanidin-3-O-glucoside as a Nutrigenomic Factor in Type 2 Diabetes and Its Prominent Impact on Health. Int. J. Mol. Sci. 2023, 24, 9765. https://doi.org/10.3390/ijms24119765
Bartel I, Koszarska M, Strzałkowska N, Tzvetkov NT, Wang D, Horbańczuk JO, Wierzbicka A, Atanasov AG, Jóźwik A. Cyanidin-3-O-glucoside as a Nutrigenomic Factor in Type 2 Diabetes and Its Prominent Impact on Health. International Journal of Molecular Sciences. 2023; 24(11):9765. https://doi.org/10.3390/ijms24119765
Chicago/Turabian StyleBartel, Iga, Magdalena Koszarska, Nina Strzałkowska, Nikolay T. Tzvetkov, Dongdong Wang, Jarosław O. Horbańczuk, Agnieszka Wierzbicka, Atanas G. Atanasov, and Artur Jóźwik. 2023. "Cyanidin-3-O-glucoside as a Nutrigenomic Factor in Type 2 Diabetes and Its Prominent Impact on Health" International Journal of Molecular Sciences 24, no. 11: 9765. https://doi.org/10.3390/ijms24119765
APA StyleBartel, I., Koszarska, M., Strzałkowska, N., Tzvetkov, N. T., Wang, D., Horbańczuk, J. O., Wierzbicka, A., Atanasov, A. G., & Jóźwik, A. (2023). Cyanidin-3-O-glucoside as a Nutrigenomic Factor in Type 2 Diabetes and Its Prominent Impact on Health. International Journal of Molecular Sciences, 24(11), 9765. https://doi.org/10.3390/ijms24119765