The Importance of Subjective Cognitive Decline Recognition and the Potential of Molecular and Neurophysiological Biomarkers—A Systematic Review
Abstract
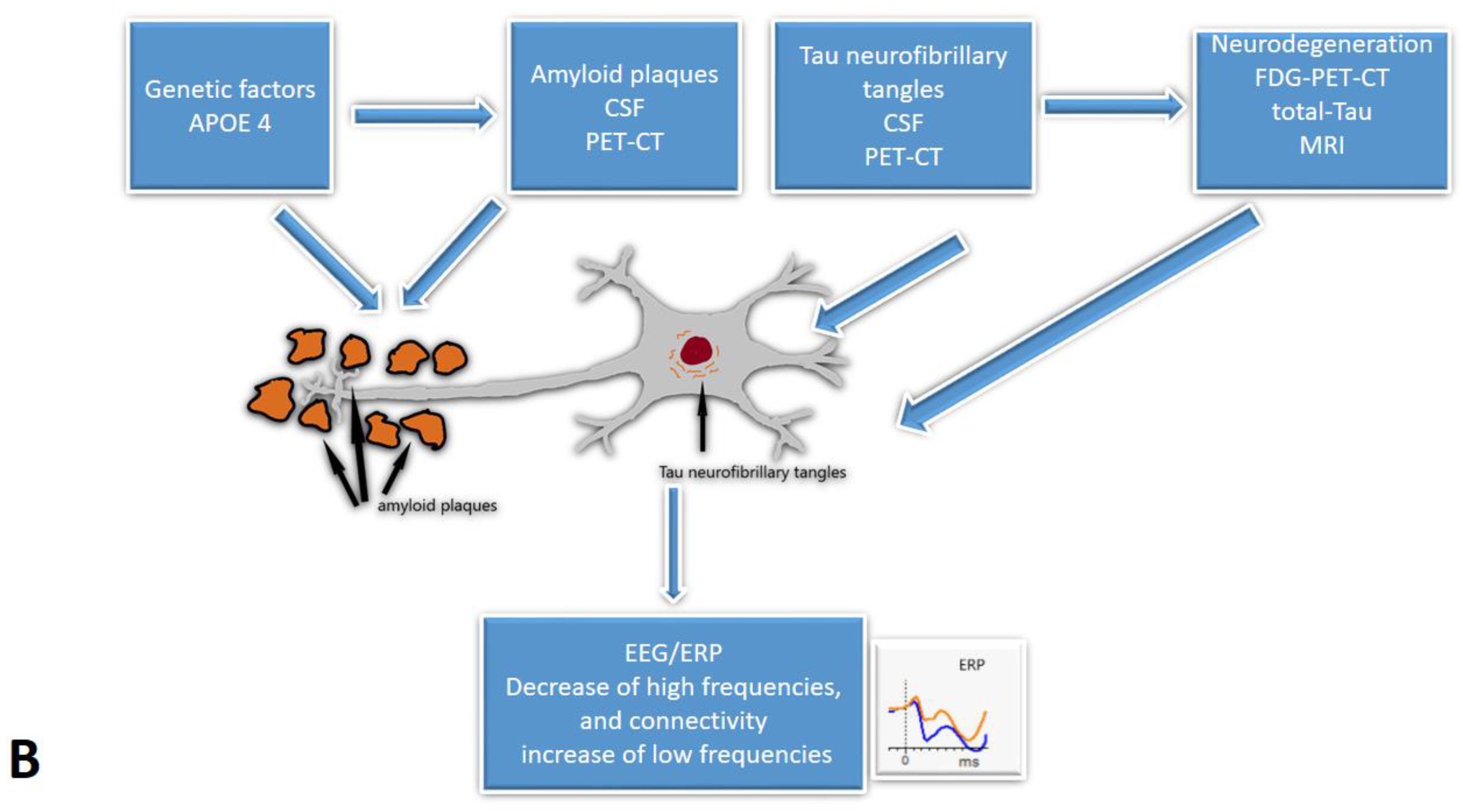
1. Introduction
2. Materials and Methods
3. Results and Discussion
| Reference | Participants | SCD Measure | Task | Observed Parameters | Main Findings | Main Limitations |
|---|---|---|---|---|---|---|
| 118: SCD (56), MCI (45), controls (17) | SCD-I criteria [3] | Resting-state | Theta, alpha, beta, and delta frequency bands | Delta frequency bands are the most discriminative for SCD and MCI | Small sample; Usage of the novel deep learning approach |
| 53: SCD (25), controls (28) | Nine-item Subjective Cognitive Decline Questionnaire [22] | Resting-state | Theta frequency band | Relative theta power: SCD > controls in left frontal region | Small sample |
| 102: SCD (22), MCI (52), AD (28) | SCD-I criteria [3] | Resting-state | Functional connectivity (EpEn; MSC; PLI; Clustering coefficient; Shortest path) | EpEn and clustering coefficient differences; Stronger long-term connectivity in SCD | Resting-state only; Small sample |
| 161: SCD (69), MCI (53), AD (39) | Cognitive complaints | Resting-state | Peak-alpha frequency; Theta/beta ratio | No significant group differences | Lack of control group |
| 73: SCD (17), MCI (23), AD (12), controls (21) | SCD-I criteria [3] | Visual attention and short-term memory task (baseline and 3 years follow-up) | Functional connectivity (Clustering coefficient; Strength; Betweenness centrality) | Controls: denser network compared to other groups; SCD converted to MCI vs. stable SCD: lower values of all functional connectivity measures at the baseline | Small sample; Lack of functional connectivity measures at the follow-up |
| 95: SCD Aβ+ (26), SCD Aβ− (69) | SCD-I criteria [3] | Resting-state | Delta, theta, alpha, beta, and gamma frequency bands | SCD Aβ+ vs. SCD Aβ−: increased relative delta power and decreased alpha activity | No control group; Lack of longitudinal data |
| 76: SCD (43), MCI (33) | Not reported | Resting-state; Game-based test | Alpha, beta, delta, and theta frequency bands | SCD < MCI in alpha, beta, and theta bands (resting and task) | Small sample; More females |
| 172: SCD Aβ− (118) SCD, Aβ+ (54) | Memory complaints | Resting-state | Alpha frequency band | SCD+ vs. SCD−: occipital alpha2 and temporal alpha3 rhythm differences | Short EEG (1 min); only 19 electrodes |
| 809: SCD (399), AD (410) | Not reported | Resting-state | Functional connectivity (PLI) | SCD vs. AD: connectivity differences | No external validation group |
| 213: SCD (47), MCI (99), controls (67) | SCD-I criteria [3] | Resting-state | Alpha, beta, theta, and delta frequency bands; SPR; DI | Baseline DI differences; 35% conversion; DI: converters > non-converters | Misdiagnosis possibility |
| 318 with SCD: Aβ+ N+ (25), Aβ+ N− (63), Aβ− N+ (51), Aβ− N− (175) | Not reported | Resting-state | Functional connectivity (PSD; MSF; SE; wSMI) | Non-linear relationship between FC measures and amyloid load | No tau protein measured |
| 318: SCD Aβ+ (88), SCD Aβ− (230) | 15-item McNair questionnaire | Resting-state; Cognitive task | Alpha:theta ratio and alpha frequency band | Decreased alpha:theta ratio; Increased prefrontal alpha (24 months) in Aβ+ group | No control group; Short follow-up |
| 169: SCD (22), MCI (58), AD (49), other pathologies (40) | SCD-I criteria [3] | Resting-state | EpEn; EEG local synchrony | EpEn: SCD > AD; Theta synchrony: SCD < AD | Small sample size |
| 26: SCD (8), MCI (11), controls (7) | Subjective memory complaints | Resting-state; Mental memory task | IAF peak; alpha 1, beta 1, and gamma frequency bands; FD | SCD: lower frontal IAF peak, parietal FD, beta 1 and 2, gamma; Alpha power reduction during task: frontal in controls and SCD, diffused in MCI | Small sample size |
| 637: SCD (210), MCI (230), AD (197) | SCD-I criteria [3] | Resting-state | Functional connectivity (GFP; GFS); CSF biomarkers | Differences in GFP and GFS; CSF-GFP/GFS correlations | No comparison with controls |
| 318 SCD: Aβ+ (63), Aβ− (252) | Memory complaints (6 + months) | Resting-state | Cortical amyloid load (florbetapir-PET); Functional connectivity (PLI) | No amyloid load-PLI associations | No controls or AD comparison |
| 205 amyloid positive: SCD (63), MCI (142) | SCD-I criteria [3] | Resting-state | Alpha, beta, delta, and theta frequency bands | No global EEG predictors; SCD: higher delta and theta, lower alpha power related to clinical progression | 3 different EEG systems |
| 181: SCD (64), AD (69), FTD (48) | Not reported | Resting-state | Functional connectivity (PLI; MST) | PLI and global efficiency differences: SCD, FTD, AD | No comparison with non-SCD controls |
| 755: SCD (310), MCI (285), AD (131), mixed dementia (29) | Subjective complaints | Resting-state | BA; EA; CSF markers | BA, EA frequency differences; CSF-BA/EA associations | Small sample; Mixed dementia overlap |
3.1. Resting-State EEG Spectral Analysis
3.2. Functional Connectivity Measures
3.3. Event-Related Potentials
| Reference | Participants | Measure of SCD | Task | Observed Parameters | Main Findings | Main Limitations |
|---|---|---|---|---|---|---|
| 53: SCD (25), Controls (28) | Nine-item Subjective Cognitive Decline Questionnaire (SCD-Q9) | Delayed match-to-sample task | ERP P300 amplitude and effect | Match-related P300 effect: SCD > controls in right frontal region | Small sample |
| 161: SCD (69), MCI (53), AD (39) | Cognitive complaints | Go/NoGo task | ERP P300 amplitude and latency | P300 amplitude: SCD > MCI > AD | Lack of control group |
| 136: young SCD (28), young no SCD (37), older SCD (31), older no SCD (39) | MFE-30 Questionnaire | Iowa Gambling Task | ERP P300 and FRN amplitude and latency | Older SCD worse in ambiguity phase, FRN latency differences | Highly educated participants |
| 79: SCD (41), Controls(38) | MFE-30 Questionnaire | Facial affect labelling | ERP P300, N170, LPP amplitude and latency | Mostly similar between SCD and controls; longer N170 latency in men with SCD | Highly educated participants; Stationary stimuli |
| 28 Low SCD (14), High SCD (14) | Memory complaints questionnaire | Go/NoGo auditory-visual task | ERP N200 and P300 amplitude and latency | Lower amplitudes and longer reaction times in high SCD group | Small sample; No comparison with MCI, AD, controls |
| 29 SCD | MAC-S | Source memory test and visual oddball task | ERP P300 and LPP amplitude | Higher amplitudes with correct answers and deviant stimuli | Small sample |
| 26 SCD (13), MCI (13) | Self-reported complaints | Auditory oddball paradigm | ERP N200 and P300 amplitude and latency | No significant differences in task performance or ERP | Simple task, small sample |
| 34: low SCD (18), high SCD (16) | Memory complaints questionnaire | Simon task | ERP P300 and MFN amplitude and latency | P300 and MFN differences in high SCD during incompatible conditions | Small sample; Small difference in SCD levels |
| 57 SCD (14), MCI (43) | Self-report question | Resting and silent video with auditory stimuli | Auditory ERP MMN and ΔMMN amplitude and latency | MMN: SCD ≈ MCI, ΔMMN associated with episodic memory | Small sample; No comparison with unimpaired or AD patients |
| 57: SCD (14), MCI (17), AD (14), Controls (12) | SCD-I diagnostic criteria | Negative facial stimuli recognition | ERP N170 amplitude and latency; sLORETA | Reduced N170 amplitude in SCD group, similar to MCI and AD | Small sample; Negative emotions only |
| 103: SCD (27), MCI (44), AD (32) | Subjective complaints | Tone pulses task | Cortical auditory ERP MMN amplitude | MMN amplitude: MCI > SCD ≈ AD | Small sample |
| 358 SCD (54), Controls (304) | Self-report question | Vagus nerve stimulation | VSEP P100, P200, N200 amplitude and latency | Longer P200 latency in SCD | Small sample; Young and well-educated participants |
| 40 SCD (17), Controls (23) | Self-report question | Go/NoGo attention task | ERP P300 amplitude and latency | Reduced P300 amplitude in SCD group | Small sample; Well-educated participants |
3.4. Fluid Biomarkers and Their Relation to EEG and ERP Biomarkers
3.5. Neuroimaging Biomarkers and Their Relation to EEG and ERP Biomarkers
3.5.1. PET-CT Biomarkers
3.5.2. MRI Biomarkers
3.6. Genetic Biomarkers and Their Relation to EEG and ERP Biomarkers
3.7. Pharmacological Intervention
3.8. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P. Preclinical Alzheimer’s Disease: Definition, Natural History, and Diagnostic Criteria. Alzheimer Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Van Boxtel, M.; Breteler, M.; Dubois, B.; Dufouil, C.; Ellis, K.A.; Van Der Flier, W.M.; Glodzik, L.; Van Harten, A.C.; et al. A Conceptual Framework for Research on Subjective Cognitive Decline in Preclinical Alzheimer’ s Disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Parfenov, V.A.; Zakharov, V.V.; Kabaeva, A.R.; Vakhnina, N.V. Subjective Cognitive Decline as a Predictor of Future Cognitive Decline a Systematic Review. Dement. E Neuropsychol. 2020, 14, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. New Approaches to Symptomatic Treatments for Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 2. [Google Scholar] [CrossRef]
- Sheikh-Bahaei, N.; Sajjadi, S.A.; Manavaki, R.; Gillard, J.H. Imaging Biomarkers in Alzheimer’s Disease: A Practical Guide for Clinicians. J. Alzheimer’s Dis. Rep. 2017, 1, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.R.; Emek-Savaş, D.D.; Rueda-Delgado, L.; Boyle, R.; Kiiski, H.; Yener, G.; Whelan, R. A Comparison of Resting State EEG and Structural MRI for Classifying Alzheimer’s Disease and Mild Cognitive Impairment. Neuroimage 2020, 215, 116795. [Google Scholar] [CrossRef]
- Kirschstein, T.; Köhling, R. What Is the Source of the EEG? Clin. EEG Neurosci. 2009, 40, 146–149. [Google Scholar] [CrossRef]
- Horvath, A.; Szucs, A.; Csukly, G.; Sakovics, A.; Stefanics, G.; Kamondi, A. EEG and ERP Biomarkers of Alzheimer’s Disease: A Critical Review. Front. Biosci. Landmark 2018, 23, 183–220. [Google Scholar] [CrossRef]
- Pascaul-Marqui, R. Standardized Low Resolution Brain Electromagnetic Tomography (SLORETA): Technical Details. Methods Find. Exp. Clin. Pharmacol. 2002, 24 (Suppl. D), 5–12. [Google Scholar]
- Olichney, J.M.; Yang, J.C.; Taylor, J.; Kutas, M. Cognitive Event-Related Potentials: Biomarkers of Synaptic Dysfunction across the Stages of Alzheimers Disease. J. Alzheimer’s Dis. 2011, 26, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Katada, E.; Sato, K.; Ojika, K.; Ueda, R. Cognitive Event-Related Potentials: Useful Clinical Information in Alzheimer’s Disease. Curr. Alzheimer Res. 2004, 1, 63–69. [Google Scholar] [CrossRef]
- Babiloni, C.; Blinowska, K.; Bonanni, L.; Cichocki, A.; De Haan, W.; Del Percio, C.; Dubois, B.; Escudero, J.; Fernández, A.; Frisoni, G.; et al. What Electrophysiology Tells Us about Alzheimer’s Disease: A Window into the Synchronization and Connectivity of Brain Neurons. Neurobiol. Aging 2020, 85, 58–73. [Google Scholar] [CrossRef]
- Paitel, E.R.; Samii, M.R.; Nielson, K.A. A Systematic Review of Cognitive Event-Related Potentials in Mild Cognitive Impairment and Alzheimer’s Disease. Behav. Brain Res. 2021, 396, 112904. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Kazui, H.; Tanaka, T.; Ishii, R.; Canuet, L.; Pascual-Marqui, R.D.; Aoki, Y.; Ikeda, S.; Kanemoto, H.; Yoshiyama, K.; et al. Functional Connectivity Assessed by Resting State EEG Correlates with Cognitive Decline of Alzheimer’s Disease—An ELORETA Study. Clin. Neurophysiol. 2016, 127, 1269–1278. [Google Scholar] [CrossRef]
- Stuckenschneider, T.; Askew, C.D.; Weber, J.; Abeln, V.; Rüdiger, S.; Summers, M.J.; Schneider, S. Auditory Event-Related Potentials in Individuals with Subjective and Mild Cognitive Impairment. Behav. Brain Res. 2020, 391, 112700. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The Characterisation of Subjective Cognitive Decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 105906. [Google Scholar] [CrossRef]
- Sibilano, E.; Brunetti, A.; Buongiorno, D.; Lassi, M.; Grippo, A.; Bessi, V.; Micera, S.; Mazzoni, A.; Bevilacqua, V. An Attention-Based Deep Learning Approach for the Classification of Subjective Cognitive Decline and Mild Cognitive Impairment Using Resting-State EEG. J. Neural Eng. 2023, 20, 016048. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, X.; Cui, X.; Liu, X.; Zhu, X.; Jiang, Y.; Li, J. Subtle Pathophysiological Changes in Working Memory-Related Potentials and Intrinsic Theta Power in Community-Dwelling Older Adults With Subjective Cognitive Decline. Innov. Aging 2023, 7, igad004. [Google Scholar] [CrossRef] [PubMed]
- Gifford, K.A.; Liu, D.; Romano, R.R.; Jones, R.N.; Jefferson, A.L. Development of a Subjective Cognitive Decline Questionnaire Using Item Response Theory: A Pilot Study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2015, 1, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Abazid, M.; Houmani, N.; Dorizzi, B.; Boudy, J.; Mariani, J.; Kinugawa, K. Weighted Brain Network Analysis on Different Stages of Clinical Cognitive Decline. Bioengineering 2022, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Ganapathi, A.S.; Glatt, R.M.; Bookheimer, T.H.; Popa, E.S.; Ingemanson, M.L.; Richards, C.J.; Hodes, J.F.; Pierce, K.P.; Slyapich, C.B.; Iqbal, F.; et al. Differentiation of Subjective Cognitive Decline, Mild Cognitive Impairment, and Dementia Using QEEG/ERP-Based Cognitive Testing and Volumetric MRI in an Outpatient Specialty Memory Clinic. J. Alzheimer’s Dis. 2022, 90, 1761–1769. [Google Scholar] [CrossRef]
- Lazarou, I.; Georgiadis, K.; Nikolopoulos, S.; Oikonomou, V.P.; Stavropoulos, T.G.; Tsolaki, A.; Kompatsiaris, I.; Tsolaki, M.; Consortium, R.-A. Exploring Network Properties Across Preclinical Stages of Alzheimer’s Disease Using a Visual Short-Term Memory and Attention Task with High-Density Electroencephalography: A Brain-Connectome Neurophysiological Study. J. Alzheimers Dis. 2022, 87, 643–664. [Google Scholar] [CrossRef]
- Shim, Y.; Yang, D.W.; Ho, S.; Hong, Y.J.; Jeong, J.H.; Park, K.H.; Kim, S.; Wang, M.J.; Choi, S.H.; Kang, S.W. Electroencephalography for Early Detection of Alzheimer’s Disease in Subjective Cognitive Decline. Dement. Neurocognitive Disord. 2022, 21, 126. [Google Scholar] [CrossRef]
- Iliadou, P.; Paliokas, I.; Zygouris, S.; Lazarou, E.; Votis, K.; Tzovaras, D.; Tsolaki, M. A Comparison of Traditional and Serious Game-Based Digital Markers of Cognition in Older Adults with Mild Cognitive Impairment and Healthy Controls. J. Alzheimers Dis. 2021, 79, 1747–1759. [Google Scholar] [CrossRef]
- Babiloni, C.; Lopez, S.; Del Percio, C.; Noce, G.; Pascarelli, M.T.; Lizio, R.; Teipel, S.J.; González-Escamilla, G.; Bakardjian, H.; George, N.; et al. Resting-State Posterior Alpha Rhythms Are Abnormal in Subjective Memory Complaint Seniors with Preclinical Alzheimer’s Neuropathology and High Education Level: The INSIGHT-PreAD Study. Neurobiol. Aging 2020, 90, 43–59. [Google Scholar] [CrossRef]
- Briels, C.T.; Schoonhoven, D.N.; Stam, C.J.; de Waal, H.; Scheltens, P.; Gouw, A.A. Reproducibility of EEG Functional Connectivity in Alzheimer’s Disease. Alzheimers. Res. Ther. 2020, 12, 68. [Google Scholar] [CrossRef]
- Engedal, K.; Barca, M.L.; Hogh, P.; Bo Andersen, B.; Winther Dombernowsky, N.; Naik, M.; Gudmundsson, T.E.; Oksengaard, A.R.; Wahlund, L.O.; Snaedal, J. The Power of EEG to Predict Conversion from Mild Cognitive Impairment and Subjective Cognitive Decline to Dementia. Dement. Geriatr. Cogn. Disord. 2020, 49, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, S.; Raimondo, F.; Houot, M.; Corsi, M.C.; Naccache, L.; Diego Sitt, J.; Hermann, B.; Oudiette, D.; Gagliardi, G.; Habert, M.O.; et al. EEG Evidence of Compensatory Mechanisms in Preclinical Alzheimer’s Disease. Brain 2019, 142, 2096–2112. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Epelbaum, S.; Nyasse, F.; Bakardjian, H.; Gagliardi, G.; Uspenskaya, O.; Houot, M.; Lista, S.; Cacciamani, F.; Potier, M.C.; et al. Cognitive and Neuroimaging Features and Brain β-Amyloidosis in Individuals at Risk of Alzheimer’s Disease (INSIGHT-PreAD): A Longitudinal Observational Study. Lancet Neurol. 2018, 17, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Houmani, N.; Vialatte, F.; Gallego-Jutglà, E.; Dreyfus, G.; Nguyen-Michel, V.H.; Mariani, J.; Kinugawa, K. Diagnosis of Alzheimer’s Disease with Electroencephalography in a Differential Framework. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, G.; De Dea, F.; Cattaruzza, T.; Manganotti, P.; Monti, F.; Accardo, A. Memorization Test and Resting State EEG Components in Mild and Subjective Cognitive Impairment. Curr. Alzheimer Res. 2018, 15, 809–819. [Google Scholar] [CrossRef]
- Smailovic, U.; Koenig, T.; Kåreholt, I.; Andersson, T.; Kramberger, M.G.; Winblad, B.; Jelic, V. Quantitative EEG Power and Synchronization Correlate with Alzheimer’s Disease CSF Biomarkers. Neurobiol. Aging 2018, 63, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Teipel, S.; Bakardjian, H.; Gonzalez-Escamilla, G.; Cavedo, E.; Weschke, S.; Dyrba, M.; Grothe, M.J.; Potier, M.C.; Habert, M.O.; Dubois, B.; et al. No Association of Cortical Amyloid Load and EEG Connectivity in Older People with Subjective Memory Complaints. Neuroimage Clin. 2018, 17, 435–443. [Google Scholar] [CrossRef]
- Gouw, A.A.; Alsema, A.M.; Tijms, B.M.; Borta, A.; Scheltens, P.; Stam, C.J.; van der Flier, W.M. EEG Spectral Analysis as a Putative Early Prognostic Biomarker in Nondemented, Amyloid Positive Subjects. Neurobiol. Aging 2017, 57, 133–142. [Google Scholar] [CrossRef]
- Yu, M.; Gouw, A.A.; Hillebrand, A.; Tijms, B.M.; Stam, C.J.; van Straaten, E.C.W.; Pijnenburg, Y.A.L. Different Functional Connectivity and Network Topology in Behavioral Variant of Frontotemporal Dementia and Alzheimer’s Disease: An EEG Study. Neurobiol. Aging 2016, 42, 150–162. [Google Scholar] [CrossRef]
- Kramberger, M.G.; Kåreholt, I.; Andersson, T.; Winblad, B.; Eriksdotter, M.; Jelic, V. Association between EEG Abnormalities and CSF Biomarkers in a Memory Clinic Cohort. Dement. Geriatr. Cogn. Disord. 2013, 36, 319–328. [Google Scholar] [CrossRef]
- Meghdadi, A.H.; Stevanović Karić, M.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting State EEG Biomarkers of Cognitive Decline Associated with Alzheimer’s Disease and Mild Cognitive Impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef]
- Abuhassan, K.; Coyle, D.; Belatreche, A.; Maguire, L. Compensating for Synaptic Loss in Alzheimer’s Disease. J. Comput. Neurosci. 2014, 36, 19–37. [Google Scholar] [CrossRef]
- Sheng, X.; Chen, H.; Shao, P.; Qin, R.; Zhao, H.; Xu, Y.; Bai, F. Brain Structural Network Compensation Is Associated With Cognitive Impairment and Alzheimer’s Disease Pathology. Front. Neurosci. 2021, 15, 630278. [Google Scholar] [CrossRef]
- de Haan, W.; Pijnenburg, Y.A.L.; Strijers, R.L.M.; van der Made, Y.; van der Flier, W.M.; Scheltens, P.; Stam, C.J. Functional Neural Network Analysis in Frontotemporal Dementia and Alzheimer’s Disease Using EEG and Graph Theory. BMC Neurosci. 2009, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, K.H. Methods for Functional Connectivity Analysis. In Computational EEG Analysis; Im, C.-H., Ed.; Springer: Singapore, 2018; pp. 125–145. ISBN 978-981-13-0908-3. [Google Scholar]
- Núnez, P.; Poza, J.; Gómez, C.; Rodríguez-González, V.; Hillebrand, A.; Tola-Arribas, M.A.; Cano, M.; Hornero, R. Characterizing the Fluctuations of Dynamic Resting-State Electrophysiological Functional Connectivity: Reduced Neuronal Coupling Variability in Mild Cognitive Impairment and Dementia Due to Alzheimer’s Disease. J. Neural Eng. 2019, 16, 056030. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, I.; Adam, K.; Georgiadis, K.; Tsolaki, A.; Nikolopoulos, S.; Kompatsiaris, I.; Tsolaki, M. Can a Novel High-Density EEG Approach Disentangle the Differences of Visual Event Related Potential (N170), Elicited by Negative Facial Stimuli, in People with Subjective Cognitive Impairment? J. Alzheimer’s Dis. 2018, 65, 543–575. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.; Garrido-Chaves, R.; Zapater-Fajari, M.; Pulopulos, M.M.; Barbosa, F.; Hidalgo, V.; Salvador, A. Deficits in Facial Emotional Valence Processing in Older People with Subjective Memory Complaints: Behavioral and Electrophysiological Evidence. Psychophysiology 2021, 59, e13989. [Google Scholar] [CrossRef]
- Garrido-Chaves, R.; Perez, V.; Perez-Alarcón, M.; Crespo-Sanmiguel, I.; Paiva, T.O.; Hidalgo, V.; Pulopulos, M.M.; Salvador, A. Subjective Memory Complaints and Decision Making in Young and Older Adults: An Event-Related Potential Study. Front. Aging Neurosci. 2021, 13, 695275. [Google Scholar] [CrossRef]
- Smart, C.M.; Segalowitz, S.J.; Mulligan, B.P.; MacDonald, S.W. Attention Capacity and Self-Report of Subjective Cognitive Decline: A P3 ERP Study. Biol. Psychol. 2014, 103, 144–151. [Google Scholar] [CrossRef]
- Susana, C.-F.; Mónica, L.; Fernando, D. Event-Related Brain Potential Indexes Provide Evidence for Some Decline in Healthy People with Subjective Memory Complaints during Target Evaluation and Response Inhibition Processing. Neurobiol. Learn. Mem. 2021, 182, 107450. [Google Scholar] [CrossRef]
- Laptinskaya, D.; Thurm, F.; Küster, O.C.; Fissler, P.; Schlee, W.; Kolassa, S.; von Arnim, C.A.F.; Kolassa, I.T. Auditory Memory Decay as Reflected by a New Mismatch Negativity Score Is Associated with Episodic Memory in Older Adults at Risk of Dementia. Front. Aging Neurosci. 2018, 10, 5. [Google Scholar] [CrossRef]
- Idrizbegovic, E.; Hederstierna, C.; Rosenhall, U. Mismatch Negativity and Ear Laterality in Alzheimer’s Disease and in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 53, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.; Ehlis, A.C.; Haeussinger, F.B.; Beeretz, S.; Kromer, G.V.; Heinzel, S.; Maetzler, W.; Eschweiler, G.W.; Berg, D.; Fallgatter, A.J.; et al. The Relation of SMI and the VSEP in a Risk Sample for Neurodegenerative Disorders. J. Neural Transm. 2015, 122, 1167–1174. [Google Scholar] [CrossRef]
- Tarantini, L.; Bader, R.; Mecklinger, A. The ERP Correlate of Episodic Recollection Is a Neurocognitive Determinant of Subjective Memory Complaints: Implications on Their Predictive Validity. Neuropsychology 2021, 35, 742. [Google Scholar] [CrossRef] [PubMed]
- Cespón, J.; Galdo-Álvarez, S.; Díaz, F. Event-Related Potentials Reveal Altered Executive Control Activity in Healthy Elderly With Subjective Memory Complaints. Front. Hum. Neurosci. 2018, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Lavitrano, M.; Salvatore, E.; Combi, R. Molecular and Imaging Biomarkers in Alzheimer’s Disease: A Focus on Recent Insights. J. Pers. Med. 2020, 10, 61. [Google Scholar] [CrossRef]
- Visser, P.J.; Verhey, F.; Knol, D.L.; Scheltens, P.; Wahlund, L.-O.; Freund-Levi, Y.; Tsolaki, M.; Minthon, L.; Wallin, Å.K.; Hampel, H.; et al. Prevalence and Prognostic Value of CSF Markers of Alzheimer’s Disease Pathology in Patients with Subjective Cognitive Impairment or Mild Cognitive Impairment in the DESCRIPA Study: A Prospective Cohort Study. Lancet Neurol. 2009, 8, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shan, P.; Jiang, W.; Sheng, C.; Ma, L. Subjective Cognitive Decline: Preclinical Manifestation of Alzheimer’s Disease. Neurol. Sci. 2019, 40, 41–49. [Google Scholar] [CrossRef]
- Scarth, M.; Rissanen, I.; Scholten, R.J.P.M.; Geerlings, M.I. Biomarkers of Alzheimer’s Disease and Cerebrovascular Lesions and Clinical Progression in Patients with Subjective Cognitive Decline: A Systematic Review. J. Alzheimers. Dis. 2021, 83, 1089–1111. [Google Scholar] [CrossRef]
- Yu, X.; Shao, K.; Wan, K.; Li, T.; Li, Y.; Zhu, X.; Han, Y. Progress in Blood Biomarkers of Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Chin. Med. J. (Engl). 2023, 136, 505–521. [Google Scholar] [CrossRef]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-Deposition in the Human Brain and Its Relevance for the Development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Snitz, B.E.; Lopez, O.L.; McDade, E.; Becker, J.T.; Cohen, A.D.; Price, J.C.; Mathis, C.A.; Klunk, W.E. Amyloid-β Imaging in Older Adults Presenting to a Memory Clinic with Subjective Cognitive Decline: A Pilot Study. J. Alzheimer’s Dis. 2015, 48, S151–S159. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, W.; Su, L.; Xing, Y.; Jessen, F.; Sun, Y.; Shu, N.; Han, Y.; Yu, X.; Shao, K.; et al. Neuroimaging Advances Regarding Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Chin. Med. J. (Engl). 2023, 15, 1–27. [Google Scholar] [CrossRef]
- Pennanen, C.; Kivipelto, M.; Tuomainen, S.; Hartikainen, P.; Hänninen, T.; Laakso, M.P.; Hallikainen, M.; Vanhanen, M.; Nissinen, A.; Helkala, E.-L.; et al. Hippocampus and Entorhinal Cortex in Mild Cognitive Impairment and Early AD. Neurobiol. Aging 2004, 25, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D. Looking into the Functional Architecture of the Brain with Diffusion MRI. Nat. Rev. Neurosci. 2003, 4, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Li, Y.; Sun, Y.; Sheng, C.; Li, H.; Li, X.; Yu, Y.; Chen, G.; Hu, X.; et al. Abnormal Resting-State Functional Connectivity Strength in Mild Cognitive Impairment and Its Conversion to Alzheimer’s Disease. Neural Plast. 2016, 2016, 4680972. [Google Scholar] [CrossRef]
- Bhome, R.; Berry, A.J.; Huntley, J.D.; Howard, R.J. Interventions for Subjective Cognitive Decline: Systematic Review and Meta-Analysis. BMJ Open. 2018, 8, e021610. [Google Scholar] [CrossRef]
- Larson, M.J.; Carbine, K.A. Sample Size Calculations in Human Electrophysiology (EEG and ERP) Studies: A Systematic Review and Recommendations for Increased Rigor. Int. J. Psychophysiol. 2017, 111, 33–41. [Google Scholar] [CrossRef]
- Vozzi, A.; Ronca, V.; Aricò, P.; Borghini, G.; Sciaraffa, N.; Cherubino, P.; Trettel, A.; Babiloni, F.; Di Flumeri, G. The Sample Size Matters: To What Extent the Participant Reduction Affects the Outcomes of a Neuroscientific Research. a Case-Study in Neuromarketing Field. Sensors 2021, 21, 6088. [Google Scholar] [CrossRef]


| Keywords | Nr. of Results in PubMed | Nr. of Results in Web of Science | |
|---|---|---|---|
| subjective cognitive decline AND | electroencephalography OR | 476 | 70 |
| event-related potentials OR | 270 | 42 | |
| event-related synchronisation desynchronisation | 8 | 0 | |
| subjective cognitive impairment AND | electroencephalography OR | 587 | 118 |
| event-related potentials OR | 337 | 68 | |
| event-related synchronisation desynchronisation | 7 | 0 | |
| subjective memory complaint AND | electroencephalography OR | 26 | 39 |
| event-related potentials OR | 17 | 21 | |
| event-related synchronisation desynchronisation | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulbl, J.; Rakusa, M. The Importance of Subjective Cognitive Decline Recognition and the Potential of Molecular and Neurophysiological Biomarkers—A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10158. https://doi.org/10.3390/ijms241210158
Ulbl J, Rakusa M. The Importance of Subjective Cognitive Decline Recognition and the Potential of Molecular and Neurophysiological Biomarkers—A Systematic Review. International Journal of Molecular Sciences. 2023; 24(12):10158. https://doi.org/10.3390/ijms241210158
Chicago/Turabian StyleUlbl, Janina, and Martin Rakusa. 2023. "The Importance of Subjective Cognitive Decline Recognition and the Potential of Molecular and Neurophysiological Biomarkers—A Systematic Review" International Journal of Molecular Sciences 24, no. 12: 10158. https://doi.org/10.3390/ijms241210158
APA StyleUlbl, J., & Rakusa, M. (2023). The Importance of Subjective Cognitive Decline Recognition and the Potential of Molecular and Neurophysiological Biomarkers—A Systematic Review. International Journal of Molecular Sciences, 24(12), 10158. https://doi.org/10.3390/ijms241210158







