HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia
Abstract
1. Introduction
2. Results
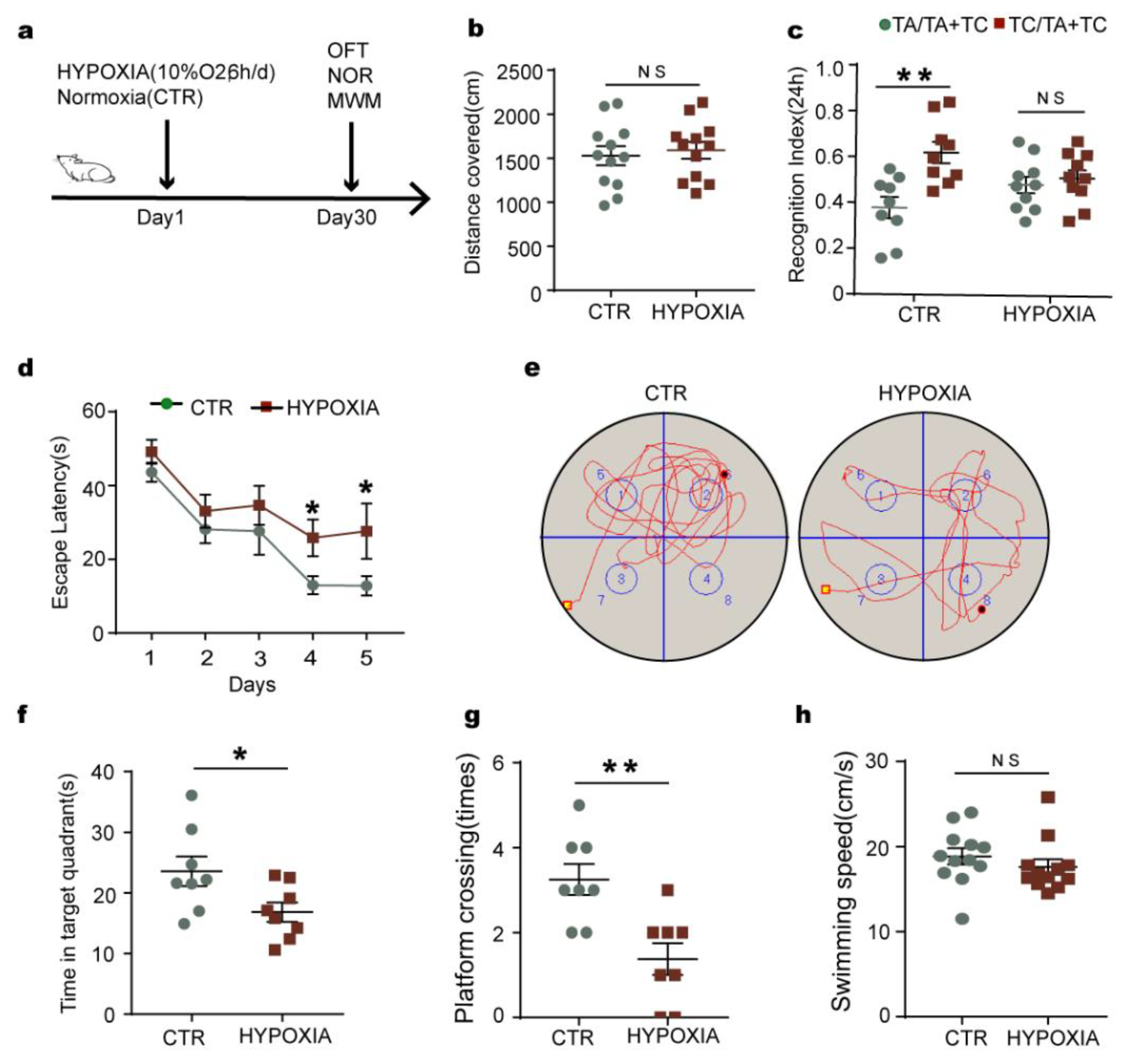
2.1. Chronic Hypoxia Impairs Cognitive Functions in Rats
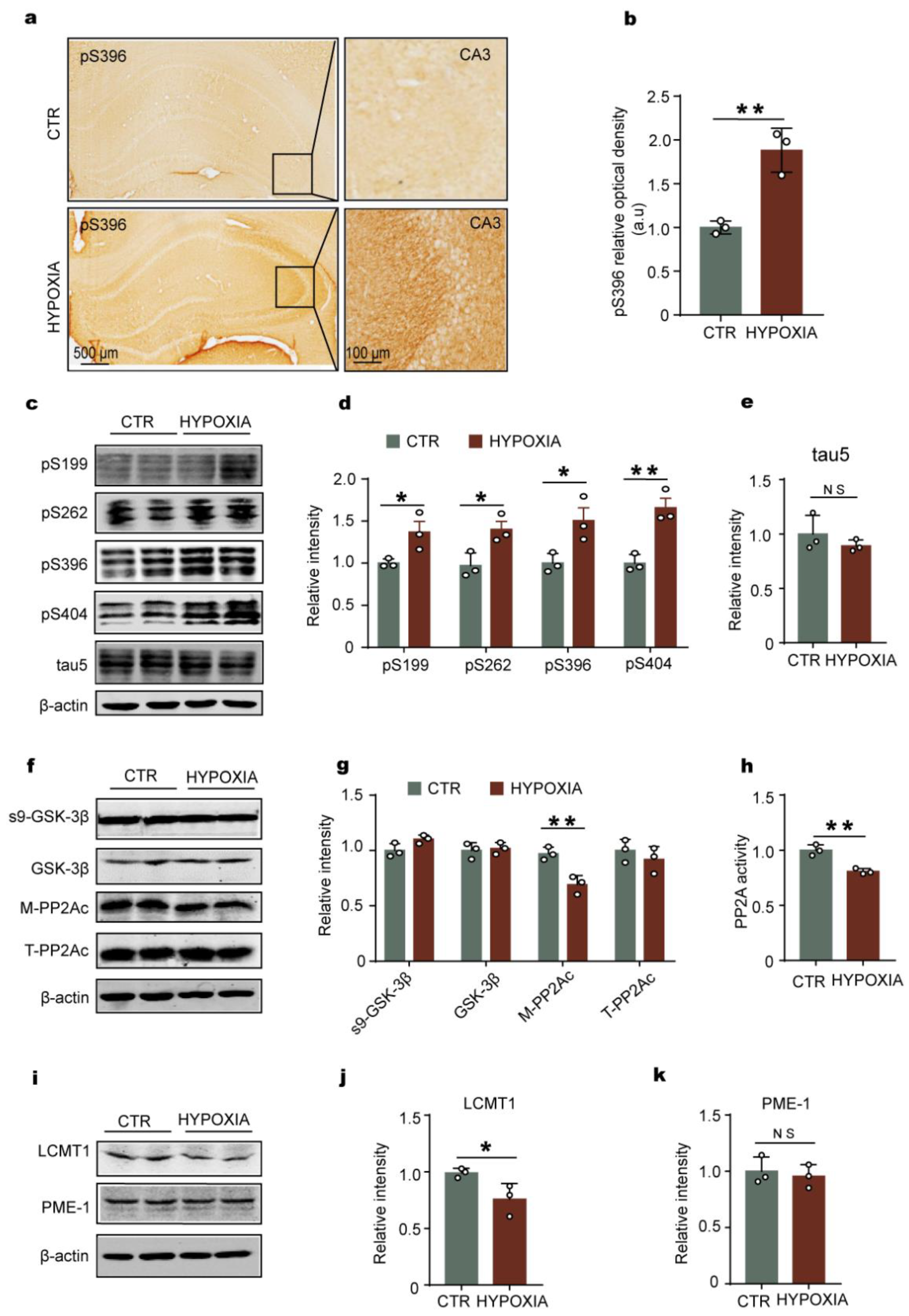
2.2. Chronic Hypoxia Leads to Tau Hyperphosphorylation Accompanied by LCMT1-Related Decrease in PP2A Activity in Rats’ Hippocampus
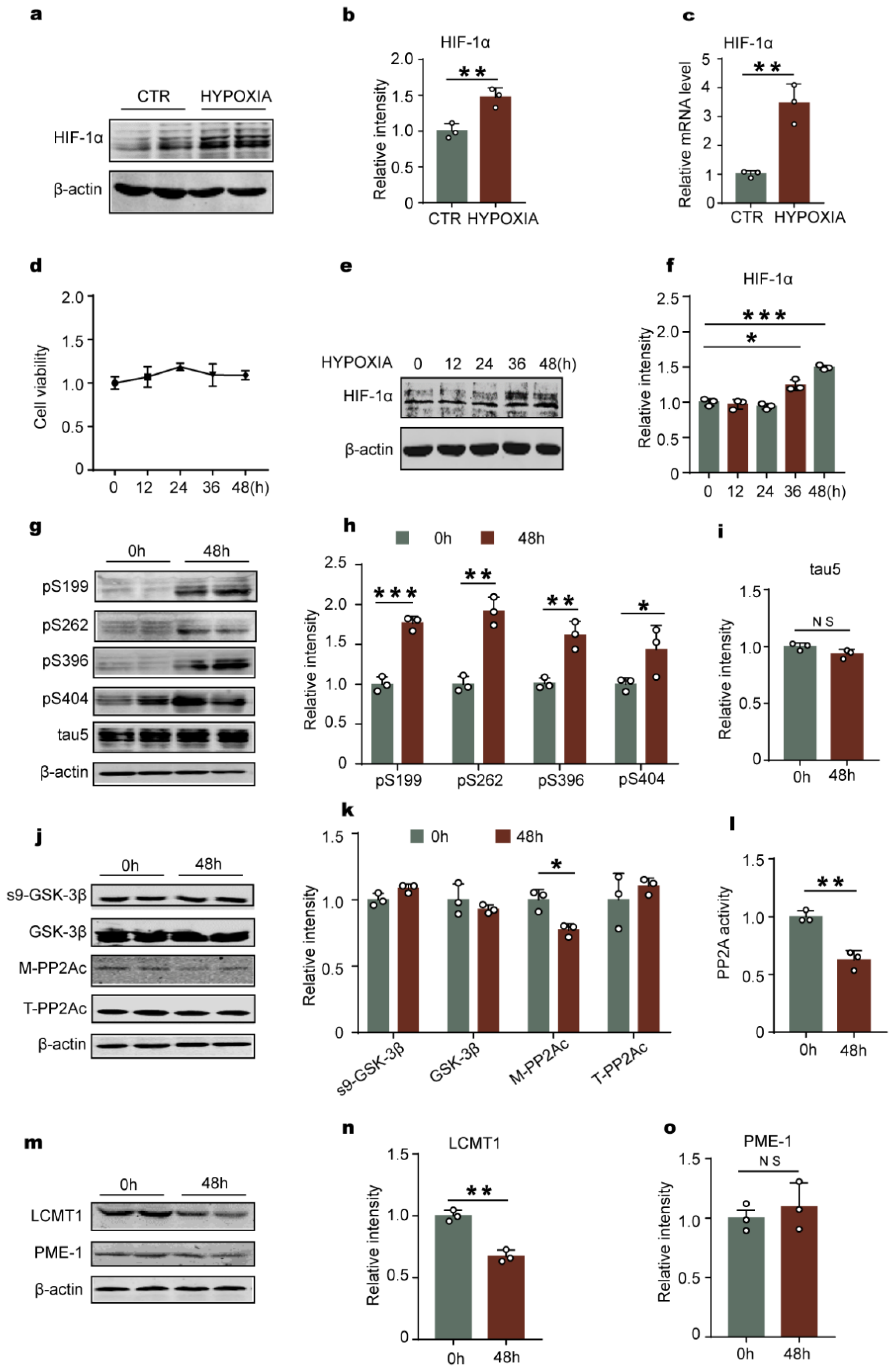
2.3. Hypoxia-Induced Tau Hyperphosphorylation Positively Correlates with HIF-1α
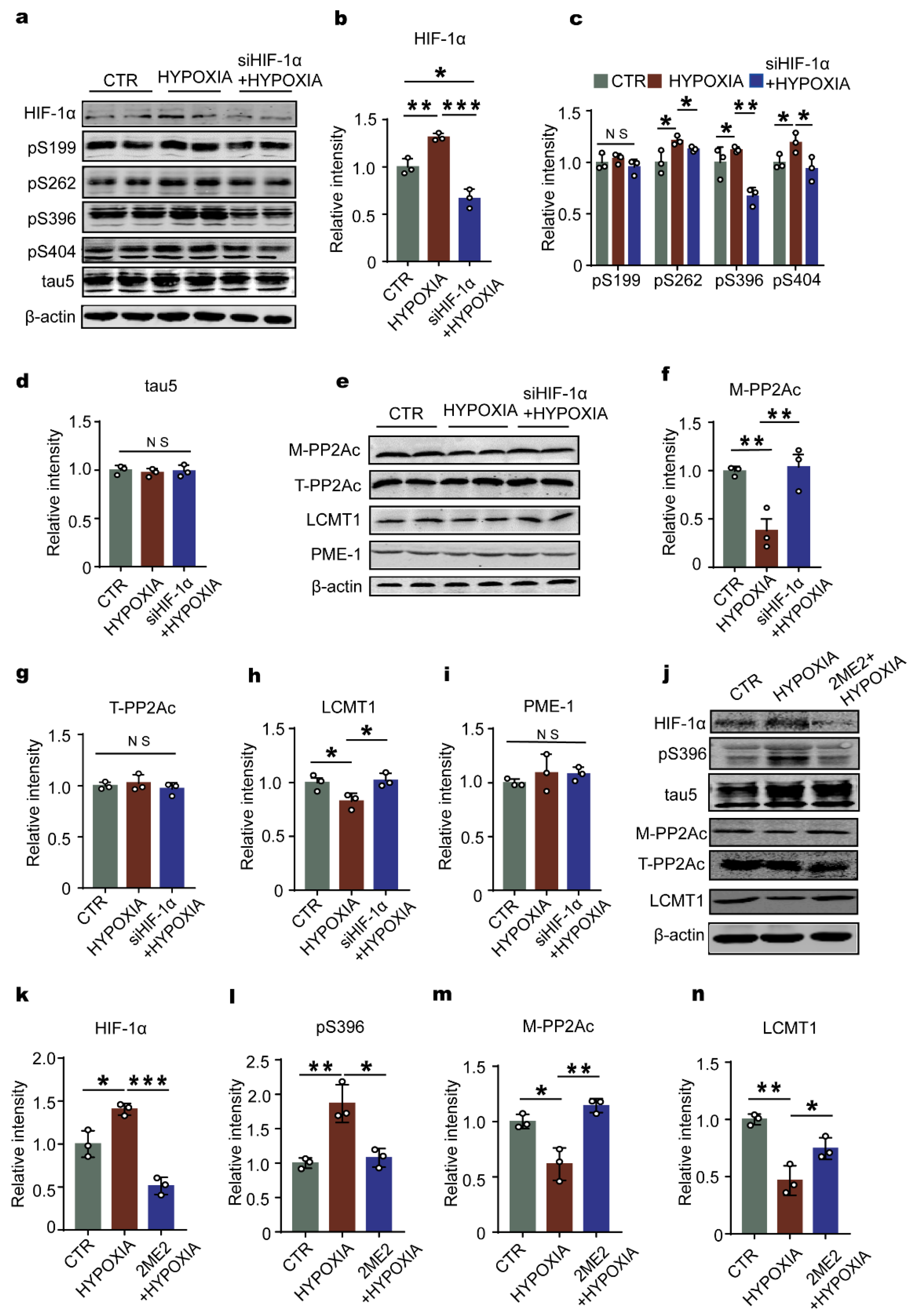
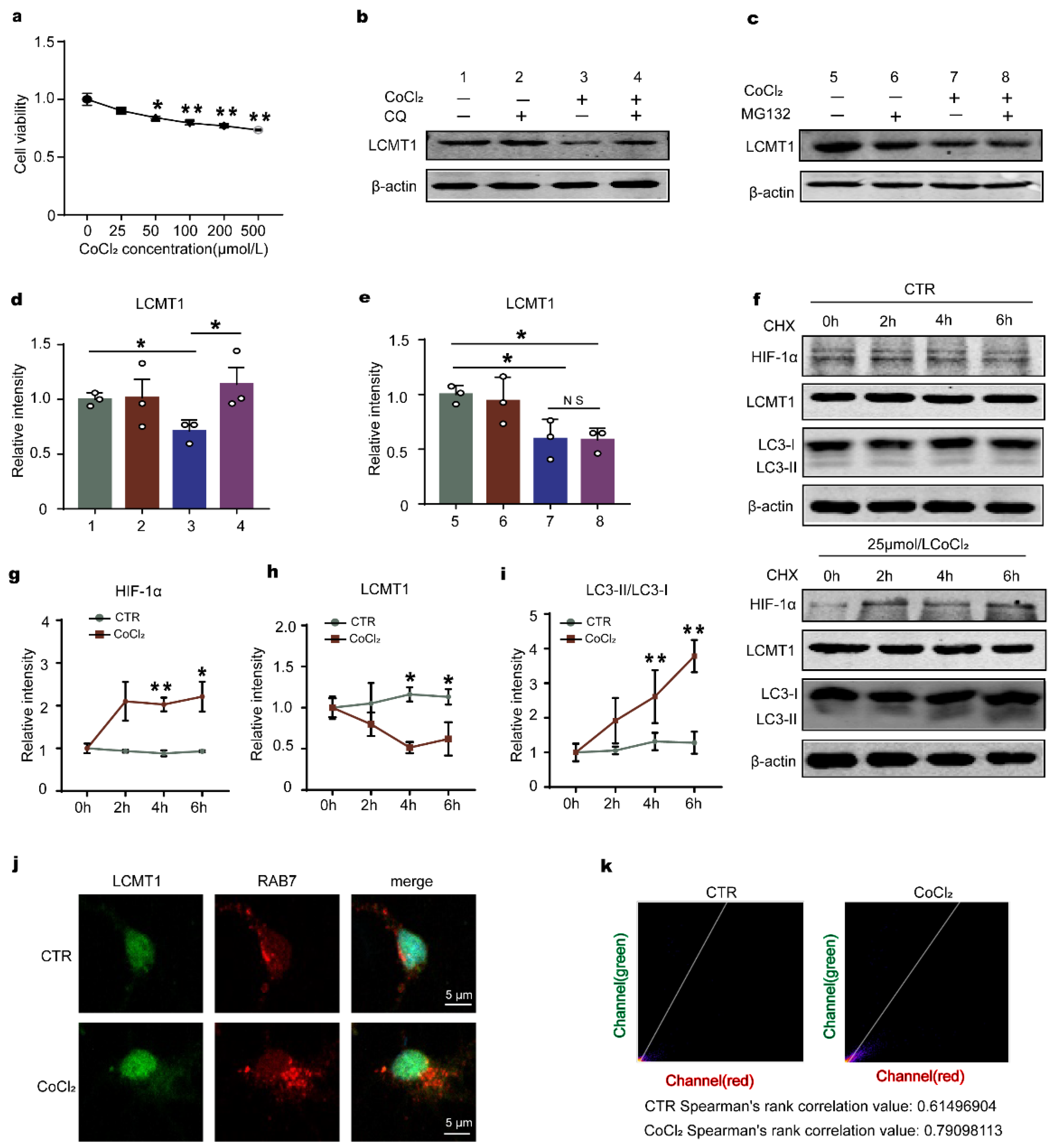
2.4. Downregulation of HIF-1 Prevents Hypoxia-Induced Alterations in the LCMT1/PP2Ac/Tau Phosphorylation Axis in Primary Hippocampal Neurons
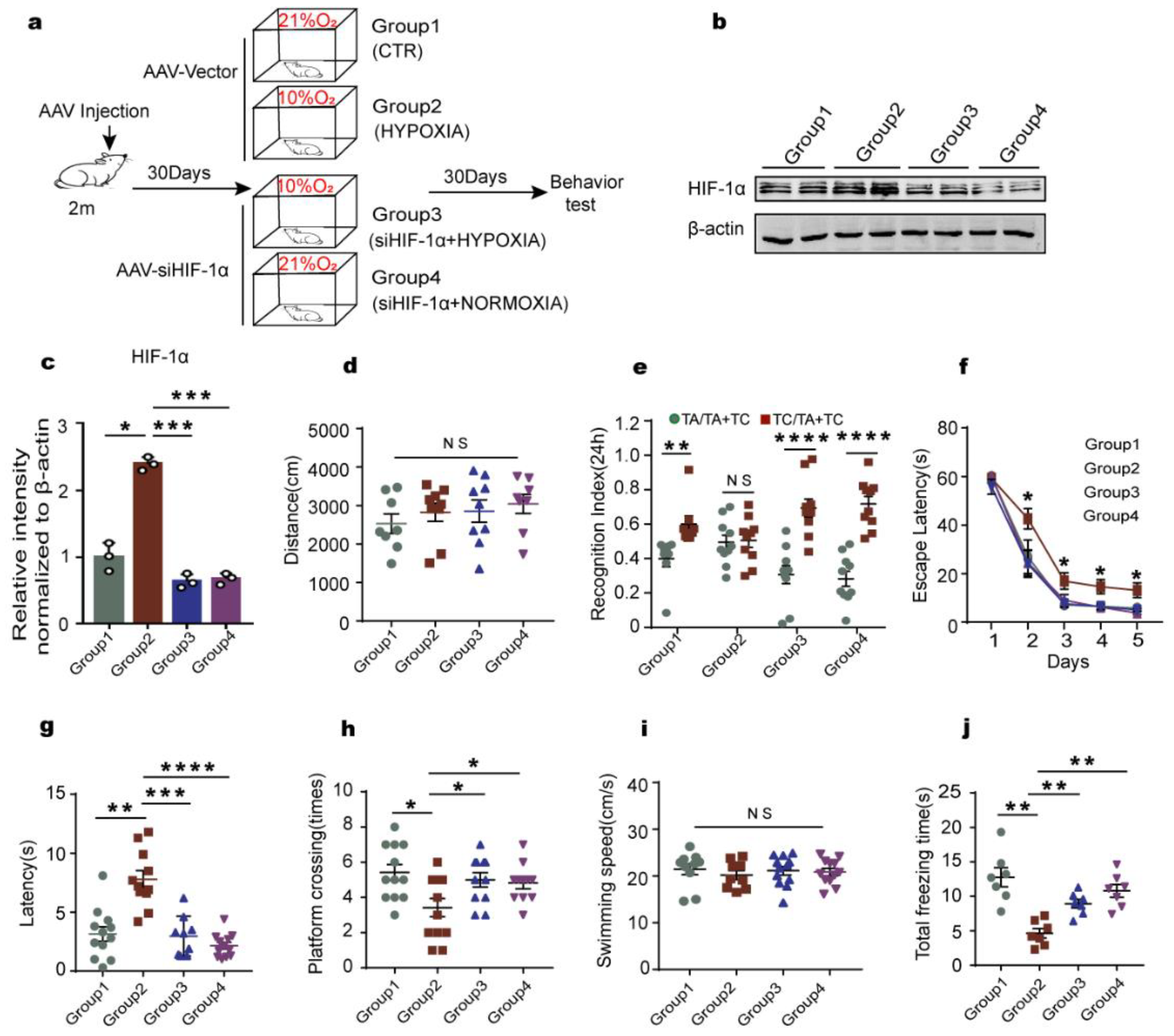
2.5. Knockdown of HIF-1α Relieves the Cognitive Dysfunction Induced by Chronic Hypoxia in Rats
2.6. Downregulation of HIF-1α Recovers LCMT1/PP2Ac/tau Axis Dysfunction in Rats’ Hippocampus
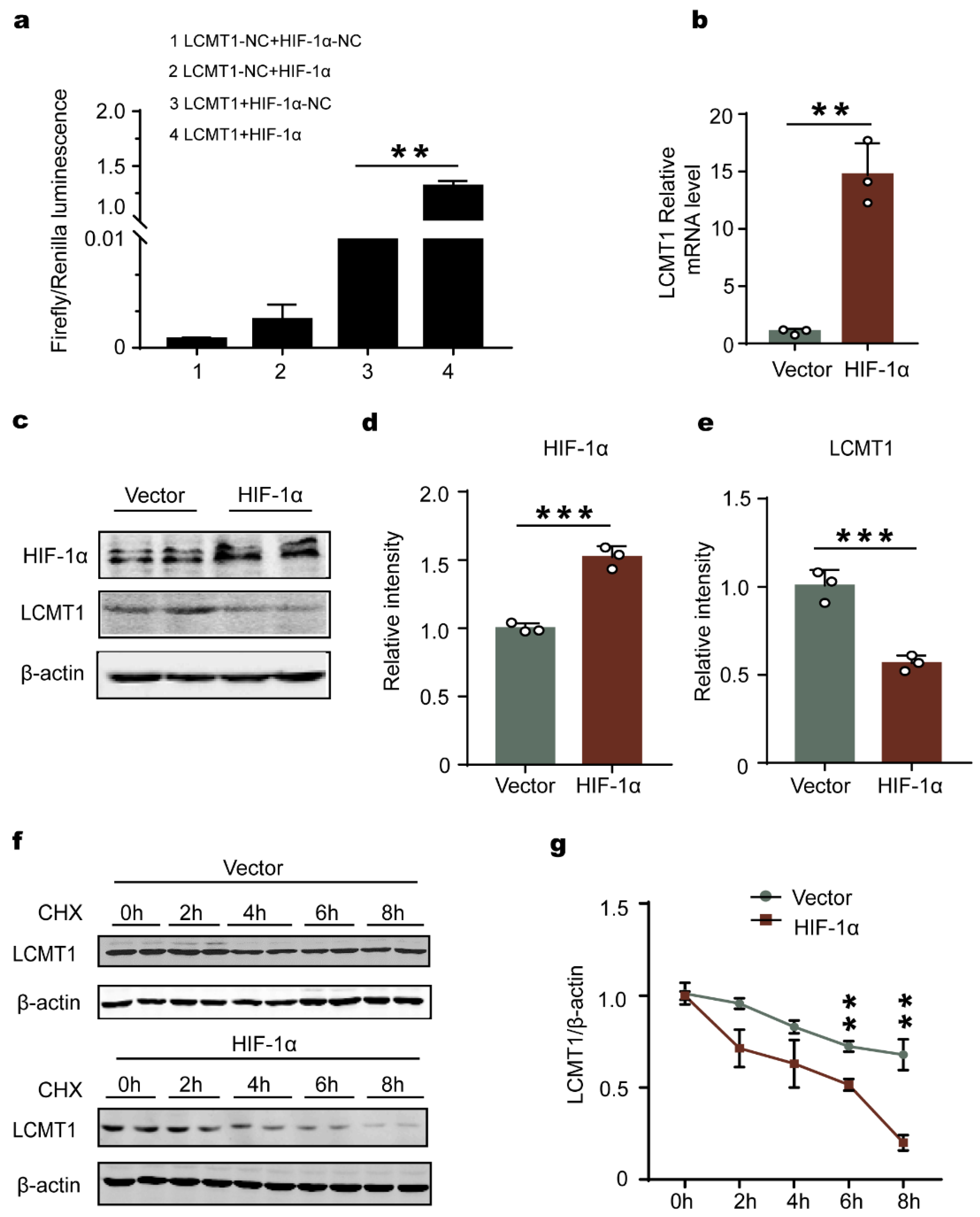
2.7. HIF-1α Accelerates LCMT1 Degradation, Counteracting its Transcriptional Increase
3. Discussion
4. Materials and Methods
4.1. Plasmids, Viruses, Chemicals, and Antibodies
4.2. Animals
4.3. Chronic Hypoxia in Animals
4.4. Lateral Ventricle Stereotactic Injection
4.5. Animal Behavior Tests
4.5.1. Open Field Test
4.5.2. Novel Object Recognition
4.5.3. Morris Water Maze Test
4.5.4. Fear Conditioning Test
4.5.5. Primary Neuron Culture and Treatment
4.5.6. C6 Cell Culture, Plasmid Transfection, and Treatment
4.5.7. Cell Counting Kit 8 Assay
4.6. Western Blotting
4.7. RNA Extraction and Real-Time PCR
4.8. PP2A Activity Assay
4.9. Immunohistochemistry
4.10. Immunofluorescence
4.11. Targeting Relationship Verification by Dual-Luciferase Reporter Gene Experiment
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-beta and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef]
- Kowalska, A.; Pruchnik-Wolinska, D.; Florczak, J.; Modestowicz, R.; Szczech, J.; Kozubski, W.; Rossa, G.; Wender, M. Genetic study of familial cases of Alzheimer’s disease. Acta Biochim. Pol. 2004, 51, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Grundke-Iqbal, I. Metabolic/signal transduction hypothesis of Alzheimer’s disease and other tauopathies. Acta Neuropathol. 2005, 109, 25–31. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Moroney, J.T.; Bagiella, E.; Desmond, D.W.; Paik, M.C.; Stern, Y.; Tatemichi, T.K. Cerebral hypoxia and ischemia in the pathogenesis of dementia after stroke. Ann. N. Y. Acad. Sci. 1997, 826, 433–436. [Google Scholar] [CrossRef]
- Peers, C.; Smith, I.F.; Boyle, J.P.; Pearson, H.A. Remodelling of Ca2+ homeostasis in type I cortical astrocytes by hypoxia: Evidence for association with Alzheimer’s disease. Biol. Chem. 2004, 385, 285–289. [Google Scholar] [CrossRef]
- Chavda, V.; Chaurasia, B.; Fiorindi, A.; Umana, G.E.; Lu, B.; Montemurro, N. Ischemic Stroke and SARS-CoV-2 Infection: The Bidirectional Pathology and Risk Morbidities. Neurol. Int. 2022, 14, 391–405. [Google Scholar] [CrossRef]
- Cho, J.; Park, Y.J.; Gonzales-Portillo, B.; Saft, M.; Cozene, B.; Sadanandan, N.; Borlongan, C.V. Gut dysbiosis in stroke and its implications on Alzheimer’s disease-like cognitive dysfunction. CNS Neurosci. Ther. 2021, 27, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; McEvoy, C.T.; Allen, I.E.; Yaffe, K. Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Neurol 2017, 74, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Kazim, S.F.; Sharma, A.; Saroja, S.R.; Seo, J.H.; Larson, C.S.; Ramakrishnan, A.; Wang, M.; Blitzer, R.D.; Shen, L.; Pena, C.J.; et al. Chronic Intermittent Hypoxia Enhances Pathological Tau Seeding, Propagation, and Accumulation and Exacerbates Alzheimer-like Memory and Synaptic Plasticity Deficits and Molecular Signatures. Biol. Psychiatry 2022, 91, 346–358. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Thompson, R.; Zhang, H.; Xu, H. APP processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of beta-amyloid in Alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef]
- Sun, X.; He, G.; Qing, H.; Zhou, W.; Dobie, F.; Cai, F.; Staufenbiel, M.; Huang, L.E.; Song, W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18727–18732. [Google Scholar] [CrossRef]
- Shiota, S.; Takekawa, H.; Matsumoto, S.E.; Takeda, K.; Nurwidya, F.; Yoshioka, Y.; Takahashi, F.; Hattori, N.; Tabira, T.; Mochizuki, H.; et al. Chronic intermittent hypoxia/reoxygenation facilitate amyloid-beta generation in mice. J. Alzheimers Dis. 2013, 37, 325–333. [Google Scholar] [CrossRef]
- Ryou, M.G.; Mallet, R.T.; Metzger, D.B.; Jung, M.E. Intermittent hypoxia training blunts cerebrocortical presenilin 1 overexpression and amyloid-beta accumulation in ethanol-withdrawn rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R10–R18. [Google Scholar] [CrossRef]
- Xie, J.C.; Ma, X.Y.; Liu, X.H.; Yu, J.; Zhao, Y.C.; Tan, Y.; Liu, X.Y.; Zhao, Y.X. Hypoxia increases amyloid-beta level in exosomes by enhancing the interaction between CD147 and Hook1. Am. J. Transl. Res. 2018, 10, 150–163. [Google Scholar] [PubMed]
- Li, L.; Zhang, X.; Yang, D.; Luo, G.; Chen, S.; Le, W. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol. Aging 2009, 30, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Kerridge, C.; Kozlova, D.I.; Nalivaeva, N.N.; Turner, A.J. Hypoxia Affects Neprilysin Expression Through Caspase Activation and an APP Intracellular Domain-dependent Mechanism. Front. Neurosci. 2015, 9, 426. [Google Scholar] [CrossRef]
- Wang, J.Z.; Xia, Y.Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal hyperphosphorylation of tau: Sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimers Dis. 2013, 33 (Suppl. S1), S123–S139. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sui, D.; Dexheimer, T.; Hovde, S.; Deng, X.; Wang, K.-W.; Lin, H.L.; Chien, H.-T.; Kweon, H.K.; Kuo, N.S.; et al. Hyperphosphorylation Renders Tau Prone to Aggregate and to Cause Cell Death. Mol. Neurobiol. 2020, 57, 4704–4719. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Yardin, C.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef]
- Wang, J.Z.; Grundke-Iqbal, I.; Iqbal, K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 2007, 25, 59–68. [Google Scholar] [CrossRef]
- Maxwell, P.; Salnikow, K. HIF-1: An oxygen and metal responsive transcription factor. Cancer Biol. Ther. 2004, 3, 29–35. [Google Scholar] [CrossRef]
- Cockman, M.E.; Masson, N.; Mole, D.R.; Jaakkola, P.; Chang, G.-W.; Clifford, S.C.; Maher, E.R.; Pugh, C.W.; Ratcliffe, P.J.; Maxwell, P.H. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 2000, 275, 25733–25741. [Google Scholar] [CrossRef]
- Raz, L.; Bhaskar, K.; Weaver, J.; Marini, S.; Zhang, Q.; Thompson, J.F.; Espinoza, C.; Iqbal, S.; Maphis, N.M.; Weston, L.; et al. Hypoxia promotes tau hyperphosphorylation with associated neuropathology in vascular dysfunction. Neurobiol. Dis. 2019, 126, 124–136. [Google Scholar] [CrossRef]
- Kandimalla, R.; Manczak, M.; Yin, X.; Wang, R.; Reddy, P.H. Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2018, 27, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Avila, J. GSK-3 and Tau: A Key Duet in Alzheimer’s Disease. Cells 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005, 22, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xia, Y.; Yu, G.; Shu, X.; Ge, H.; Zeng, K.; Wang, J.; Wang, X. Cleavage of GSK-3beta by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3beta activity induced by H(2)O(2). J. Neurochem. 2013, 126, 234–242. [Google Scholar] [CrossRef]
- Staniszewski, A.; Zhang, H.; Asam, K.; Pitstick, R.; Kavanaugh, M.P.; Arancio, O.; Nicholls, R.E. Reduced Expression of the PP2A Methylesterase, PME-1, or the PP2A Methyltransferase, LCMT-1, Alters Sensitivity to Beta-Amyloid-Induced Cognitive and Electrophysiological Impairments in Mice. J. Neurosci. 2020, 40, 4596–4608. [Google Scholar] [CrossRef]
- Yuan, Y.; Hilliard, G.; Ferguson, T.; Millhorn, D.E. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef]
- Samelko, L.; Caicedo, M.S.; Lim, S.J.; Della-Valle, C.; Jacobs, J.; Hallab, N.J. Cobalt-alloy implant debris induce HIF-1alpha hypoxia associated responses: A mechanism for metal-specific orthopedic implant failure. PLoS ONE 2013, 8, e67127. [Google Scholar] [CrossRef]
- Munoz-Sanchez, J.; Chanez-Cardenas, M.E. The use of cobalt chloride as a chemical hypoxia model. J. Appl. Toxicol. 2019, 39, 556–570. [Google Scholar] [CrossRef]
- Mabjeesh, N.J.; Escuin, D.; LaVallee, T.M.; Pribluda, V.S.; Swartz, G.M.; Johnson, M.S.; Willard, M.T.; Zhong, H.; Simons, J.W.; Giannakakou, P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 2003, 3, 363–375. [Google Scholar] [CrossRef]
- Lam, S.; Petit, F.; Hérard, A.-S.; Boluda, S.; Eddarkaoui, S.; Guillermier, M.; Letournel, F.; Martin-Négrier, M.-L.; Faisant, M.; Godfraind, C.; et al. Transmission of amyloid-beta and tau pathologies is associated with cognitive impairments in a primate. Acta Neuropathol. Commun. 2021, 9, 165. [Google Scholar] [CrossRef]
- Longin, S.; Zwaenepoel, K.; Martens, E.; Louis, J.V.; Rondelez, E.; Goris, J.; Janssens, V. Spatial control of protein phosphatase 2A (de)methylation. Exp. Cell Res. 2008, 314, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Pei, J.J.; Wang, X.C.; Zhou, X.W.; Tian, Q.; Winblad, B.; Wang, J.Z. Acute anoxia induces tau dephosphorylation in rat brain slices and its possible underlying mechanisms. J. Neurochem. 2005, 94, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Tian, S.; Gao, H.; Xu, Y. Hypoxia increases Abeta-induced tau phosphorylation by calpain and promotes behavioral consequences in AD transgenic mice. J. Mol. Neurosci. 2013, 51, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Peers, C.; Pearson, H.A.; Boyle, J.P. Hypoxia and Alzheimer’s disease. Essays Biochem. 2007, 43, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Y.W.; Zhang, X.; Liu, R.; Zhang, X.; Hong, S.; Xia, K.; Xia, J.; Zhang, Z.; Xu, H. Transcriptional regulation of APH-1A and increased gamma-secretase cleavage of APP and Notch by HIF-1 and hypoxia. FASEB J. 2006, 20, 1275–1277. [Google Scholar] [CrossRef]
- Olaithe, M.; Bucks, R.S.; Hillman, D.R.; Eastwood, P.R. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med. Rev. 2018, 38, 39–49. [Google Scholar] [CrossRef]
- Vanek, J.; Prasko, J.; Genzor, S.; Ociskova, M.; Kantor, K.; Holubova, M.; Slepecky, M.; Nesnidal, V.; Kolek, A.; Sova, M. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020, 72, 50–58. [Google Scholar] [CrossRef]
- Kerner, N.A.; Roose, S.P. Obstructive Sleep Apnea is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. Am. J. Geriatr. Psychiatry 2016, 24, 496–508. [Google Scholar] [CrossRef]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef]
- Saha, P.; Sen, N. Tauopathy: A common mechanism for neurodegeneration and brain aging. Mech. Ageing Dev. 2019, 178, 72–79. [Google Scholar] [CrossRef]
- Ma, R.H.; Zhang, Y.; Hong, X.Y.; Zhang, J.F.; Wang, J.Z.; Liu, G.P. Role of microtubule-associated protein tau phosphorylation in Alzheimer’s disease. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Shiratsuchi, A.; Sato, S.; Omori, A.; Arioka, M.; Kobayashi, S.; Uchida, T.; Imahori, K. Glycogen synthase kinase 3 beta is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993, 325, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Krishnankutty, A.; Kimura, T.; Saito, T.; Aoyagi, K.; Asada, A.; Takahashi, S.I.; Ando, K.; Ohara-Imaizumi, M.; Ishiguro, K.; Hisanaga, S.-I. In vivo regulation of glycogen synthase kinase 3beta activity in neurons and brains. Sci. Rep. 2017, 7, 8602. [Google Scholar] [CrossRef]
- Sutherland, C.; Leighton, I.A.; Cohen, P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: New kinase connections in insulin and growth-factor signalling. Biochem. J. 1993, 296, 15–19. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Gu, J.; Yin, X.; Jin, N.; Xie, S.; Wang, Y.; Chang, H.; Qian, W.; Shi, J.; et al. Cross talk between PI3K-AKT-GSK-3beta and PP2A pathways determines tau hyperphosphorylation. Neurobiol. Aging 2015, 36, 188–200. [Google Scholar] [CrossRef]
- Taleski, G.; Sontag, E. Protein phosphatase 2A and tau: An orchestrated ‘Pas de Deux’. FEBS Lett. 2018, 592, 1079–1095. [Google Scholar] [CrossRef]
- Zhou, X.-W.; Gustafsson, J.; Tanila, H.; Bjorkdahl, C.; Liu, R.; Winblad, B.; Pei, J.-J. Tau hyperphosphorylation correlates with reduced methylation of protein phosphatase 2A. Neurobiol. Dis. 2008, 31, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Huang, Z.-T.; Yuan, M.-H.; Jing, F.; Cai, R.-L.; Zou, Q.; Pu, Y.-S.; Wang, S.-Y.; Chen, F.; Yi, W.-M.; et al. Role of Hypoxia Inducible Factor-1alpha in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 80, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, K.; Wang, R.; Cui, J.; Lipton, S.A.; Liao, F.F.; Xu, H.; Zhang, Y.-W. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J. Biol. Chem. 2007, 282, 10873–10880. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008, 582, 359–364. [Google Scholar] [CrossRef]
- Shin, N.; Kim, H.-G.; Shin, H.J.; Kim, S.; Kwon, H.H.; Baek, H.; Yi, M.-H.; Zhang, E.; Kim, J.-J.; Hong, J.; et al. Uncoupled Endothelial Nitric Oxide Synthase Enhances p-Tau in Chronic Traumatic Encephalopathy Mouse Model. Antioxid. Redox Signal. 2019, 30, 1601–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Wang, H.H.; Liu, S.J.; Deng, Y.Q.; Zhang, Y.J.; Tian, Q.; Wang, X.-C.; Chen, X.-Q.; Yang, Y.; Zhang, J.-Y.; et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 3591–3596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-E.; Yang, X.; Li, L.; Sui, X.; Tian, Q.; Wei, W.; Wang, J.; Liu, G. Hypoxia-induced tau phosphorylation and memory deficit in rats. Neurodegener. Dis. 2014, 14, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Walker, K.R.; Tesco, G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. Aging Neurosci. 2013, 5, 29. [Google Scholar] [CrossRef]
- Liang, X.; Liu, X.; Lu, F.; Zhang, Y.; Jiang, X.; Ferriero, D.M. HIF1alpha Signaling in the Endogenous Protective Responses after Neonatal Brain Hypoxia-Ischemia. Dev. Neurosci. 2019, 1–10. [Google Scholar]
- Wang, Q.; Sun, Z.X.; Allgayer, H.; Yang, H.S. Downregulation of E-cadherin is an essential event in activating beta-catenin/Tcf-dependent transcription and expression of its target genes in Pdcd4 knockdown cells. Oncogene 2010, 29, 128–138. [Google Scholar] [CrossRef]

| Antibodies | Specific | Type | Dilution | Source |
|---|---|---|---|---|
| HIF-1α | Anti-HIF-1 alpha | pAb | 1:200 for WB | Abcam (ab179483) (Shanghai, China) |
| pS199 | Anti-Phosphorylated tau at Ser199 | pAb | 1:1000 for WB | Thermo Fisher (44–734G) (Waltham, MA, USA) |
| pS262 | Anti-Phosphorylated tau at Ser262 | pAb | 1:1000 for WB | Signalway Antibody (#21100) (Jiangsu, China) |
| pS396 | Anti-Phosphorylated tau at Ser396 | pAb | 1:1000 for WB 1:200 for IHC | Signalway Antibody (#21093) |
| pS404 | Anti-Phosphorylated tau at Ser404 | pAb | 1:1000 for WB | Signalway Antibody (#21001) |
| Tau-5 | Anti-Total tau | mAb | 1:1000 for WB | Millipore (577801) (Burlingtun, MA, USA) |
| M-PP2Ac | Anti-Methylated PP2Ac at Leu309 | mAb | 1:500 for WB | Millipore (041479) |
| T-PP2Ac | Anti-PP2A catalytic subunit | pAb | 1:1000 for WB | Cell Signaling (#2038) (Danvers, MA, USA) |
| S9-GSK3β | Anti-Phosphorylated GSK-β at Ser 9 | pAb | 1:1000 for WB | Upstate (AP0039) (Thermofisher) |
| GSK3β | Anti-Glycogen synthase kinase-3β | pAb | 1:1000 for WB | Signalway Antibody (40989) |
| β-actin | Anti-β-actin | pAb | 1:1000 for WB | Abcam (ab8227) |
| LCMT1 | Anti-Leucine carboxyl methyltransferase1 | pAb | 1:1000 for WB | Cell Signaling (#5691) |
| PME-1 | Anti-Protein phosphatase methylesterase1 | mAb | 1:1000 for WB | Cell Signaling (#29135) |
| LC3B | Anti-LC3B | pAb | 1:1000 for WB 1:200 for IF | Abcam(ab192890) |
| Rab7 | Anti- Rab7 | mAb | 1:200 for IF | Cell Signaling (#9367) |
| Gene Name | NCBI No. | Primer | |
|---|---|---|---|
| HIF-1α | 024359.2 | (5–3) (3–5) | ACACACAGAAATGGCCCAGTG AATCAGCACCAAGCACGTCA |
| LCMT1 | 199405.3 | (5–3) (3–5) | GTTGAATGGGTGGGAGACGG TTATCTCCTTCAAACCCAGCTC |
| GAPDH | 017008.4 | (5–3) (3–5) | GAAGGTCGGTGTGAACGGAT CCCATTTGATGTTAGCGGGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, L.; Feng, J.; Wu, G.; Wei, Z.; Wang, J.-Z.; Zhang, B.; Liu, R.; Liu, F.; Wang, X.; Li, H.-L. HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia. Int. J. Mol. Sci. 2022, 23, 16140. https://doi.org/10.3390/ijms232416140
Lei L, Feng J, Wu G, Wei Z, Wang J-Z, Zhang B, Liu R, Liu F, Wang X, Li H-L. HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia. International Journal of Molecular Sciences. 2022; 23(24):16140. https://doi.org/10.3390/ijms232416140
Chicago/Turabian StyleLei, Ling, Jun Feng, Gang Wu, Zhen Wei, Jian-Zhi Wang, Bin Zhang, Rong Liu, Fei Liu, Xiaochuan Wang, and Hong-Lian Li. 2022. "HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia" International Journal of Molecular Sciences 23, no. 24: 16140. https://doi.org/10.3390/ijms232416140
APA StyleLei, L., Feng, J., Wu, G., Wei, Z., Wang, J.-Z., Zhang, B., Liu, R., Liu, F., Wang, X., & Li, H.-L. (2022). HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia. International Journal of Molecular Sciences, 23(24), 16140. https://doi.org/10.3390/ijms232416140








