Time-Series Transcriptomic Analysis of Contrasting Rice Materials under Heat Stress Reveals a Faster Response in the Tolerant Cultivar
Abstract
1. Introduction
2. Results
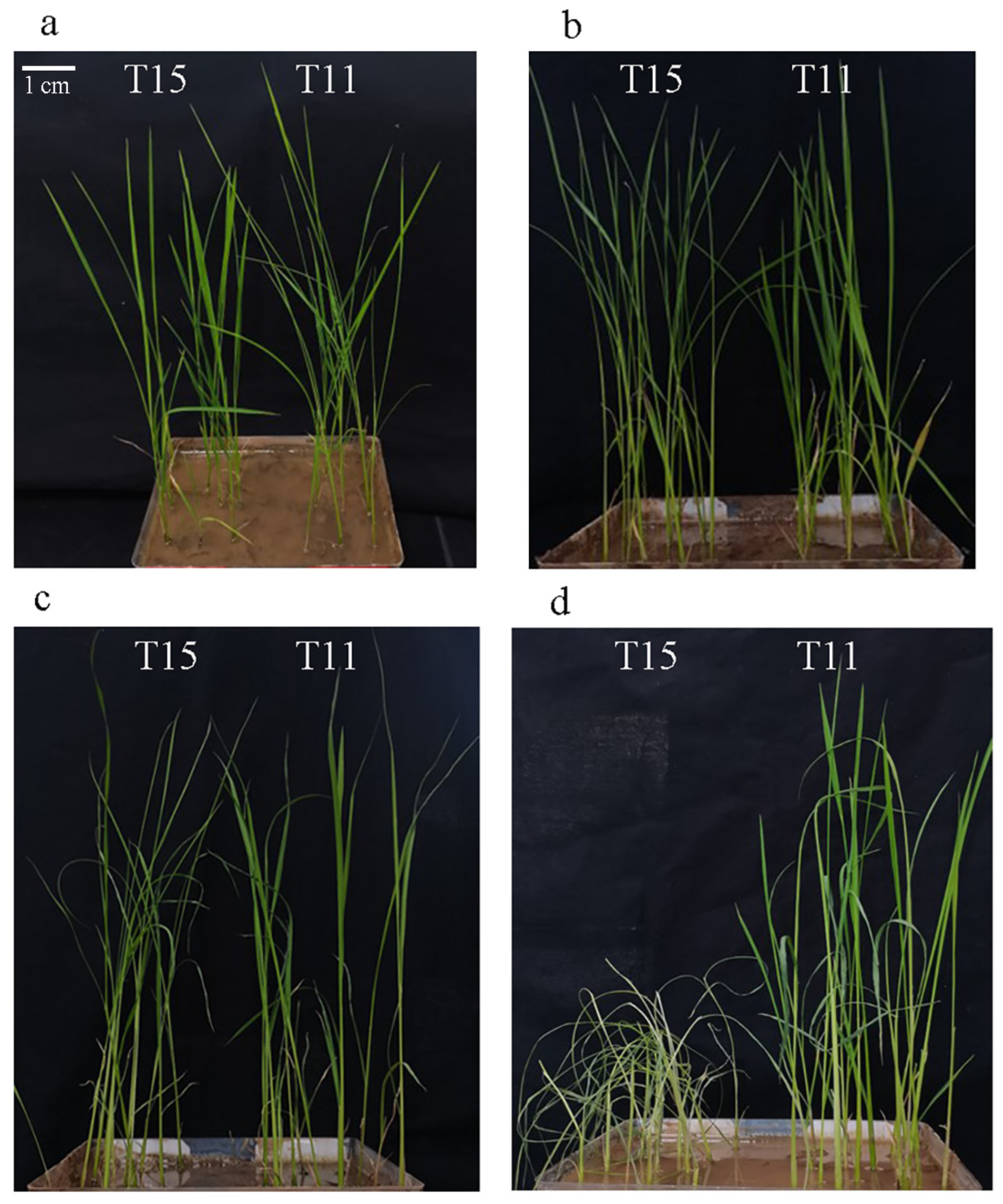
2.1. Phenotype Characteristics of T11 and T15 under Heat Stress
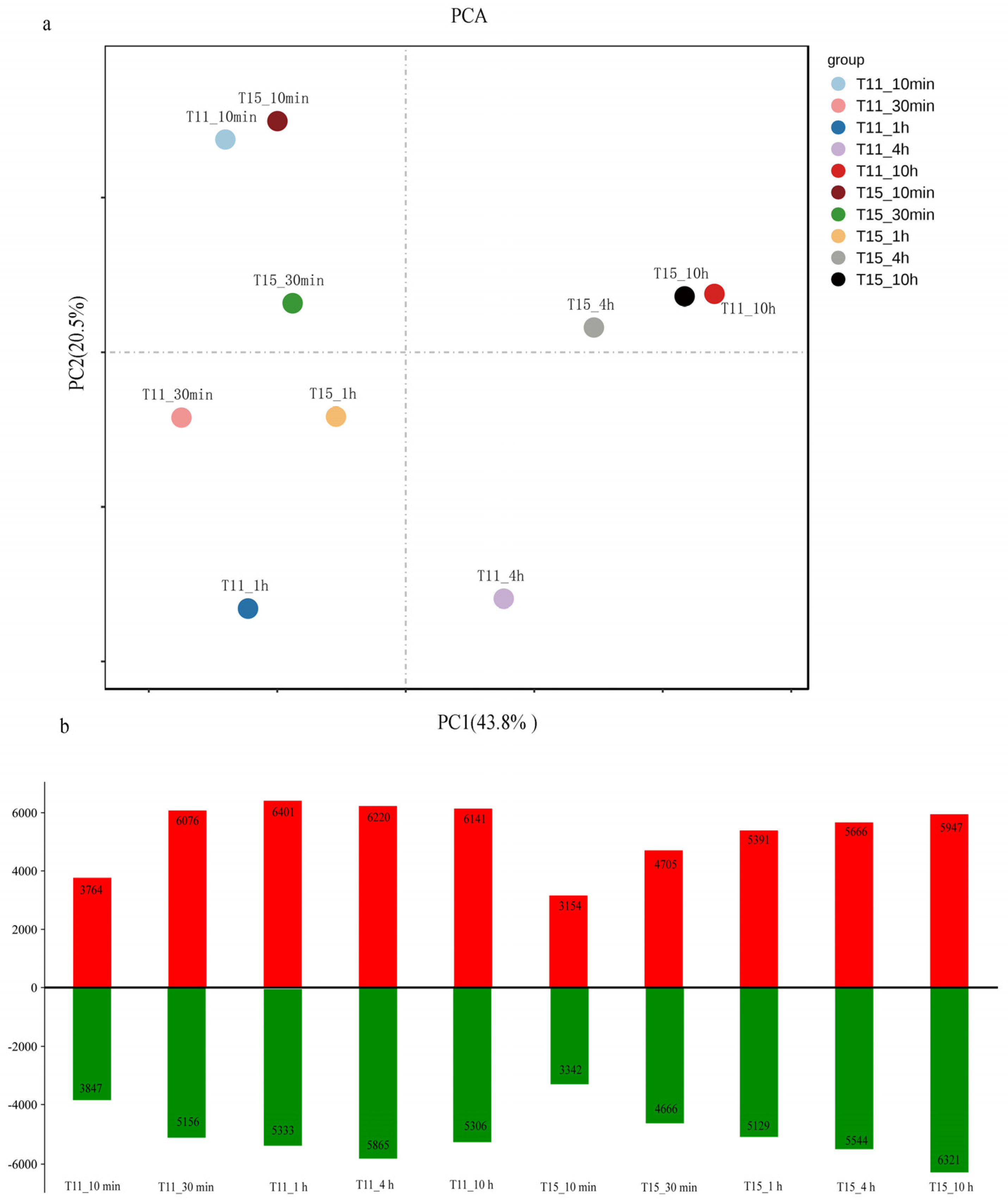
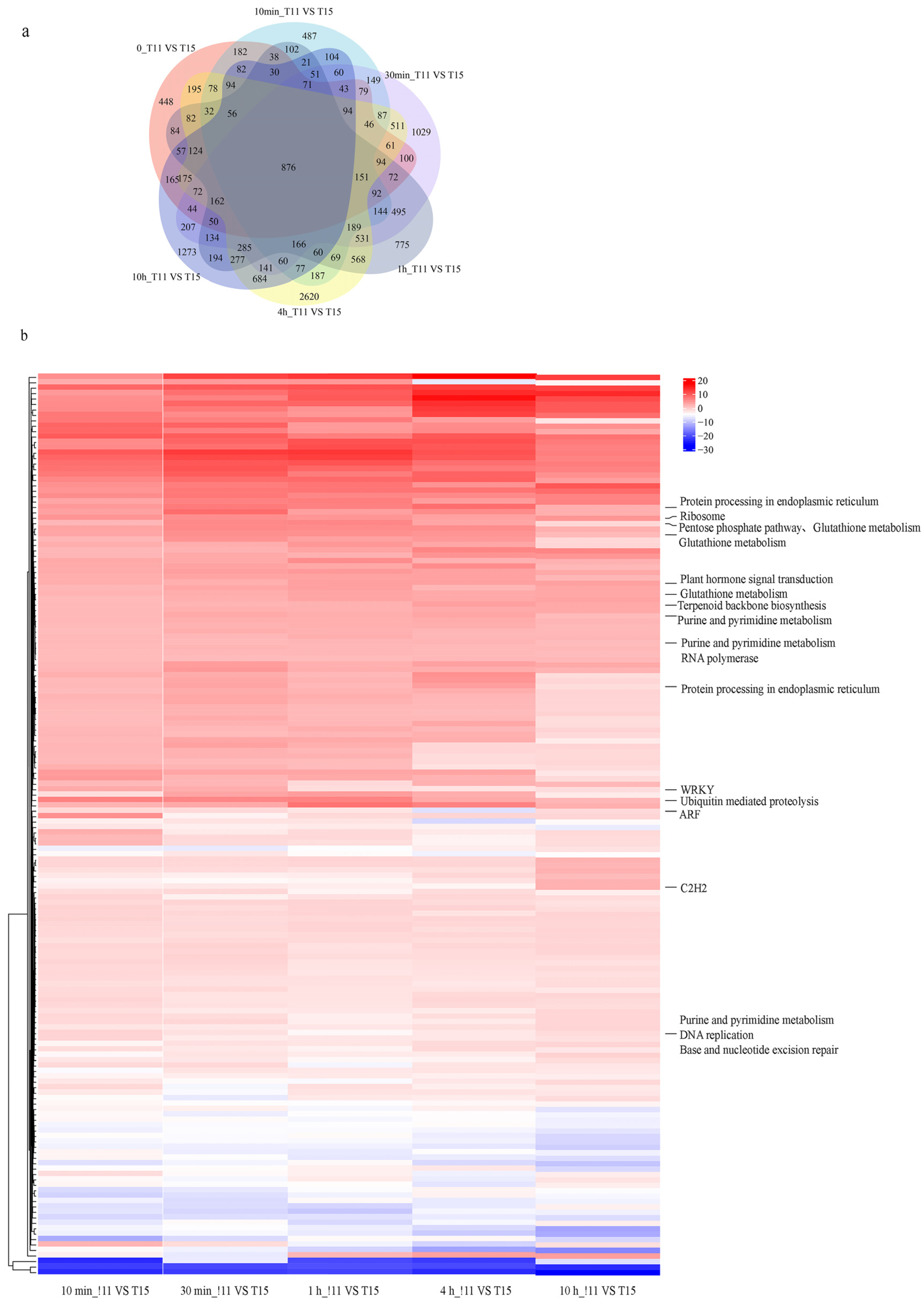
2.2. RNA–Seq Data Processing and Identification of Differentially Expressed Genes in Response to Heat Stress
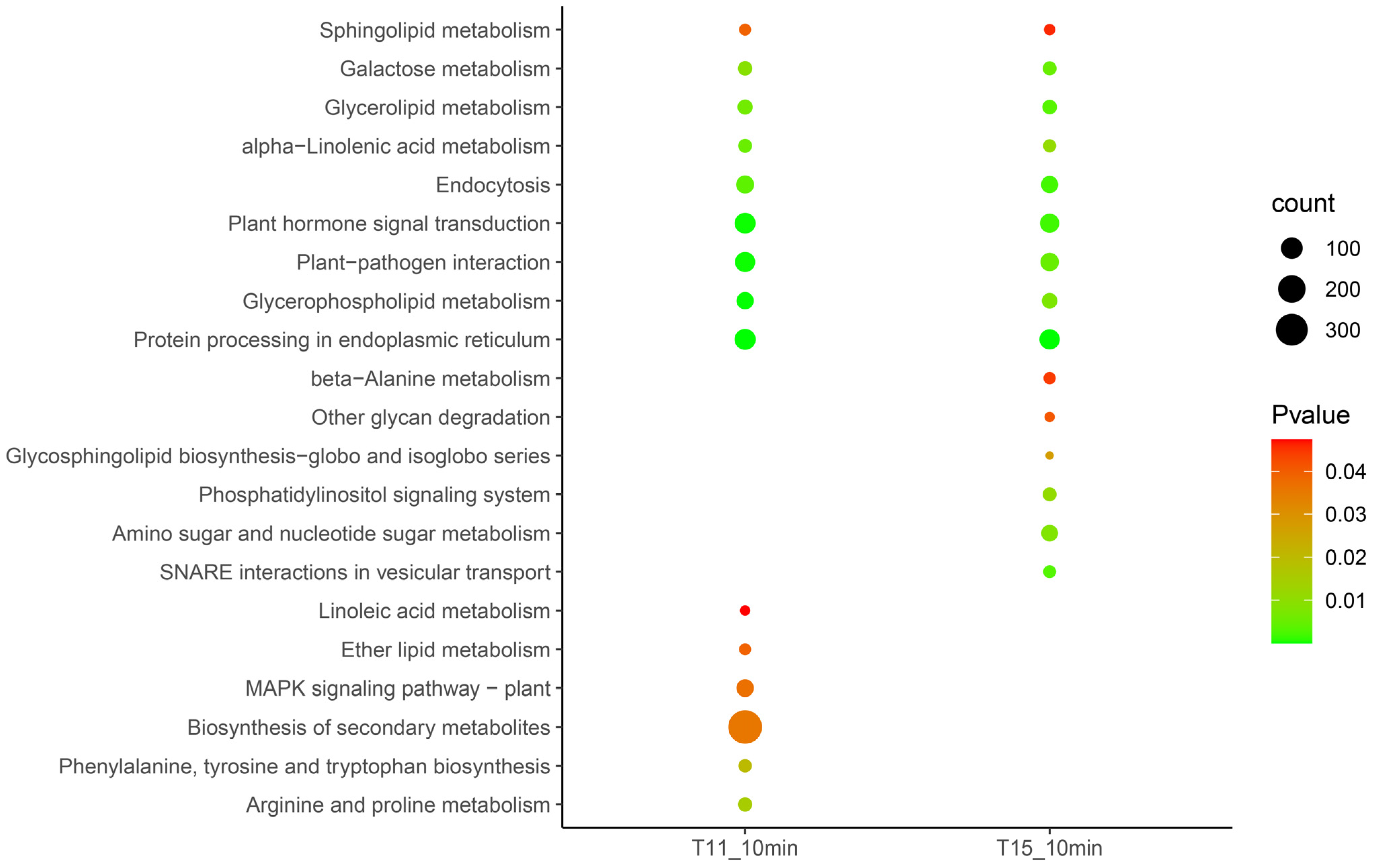
2.3. Early-Stage Response of Rice Seedlings under Heat Stress
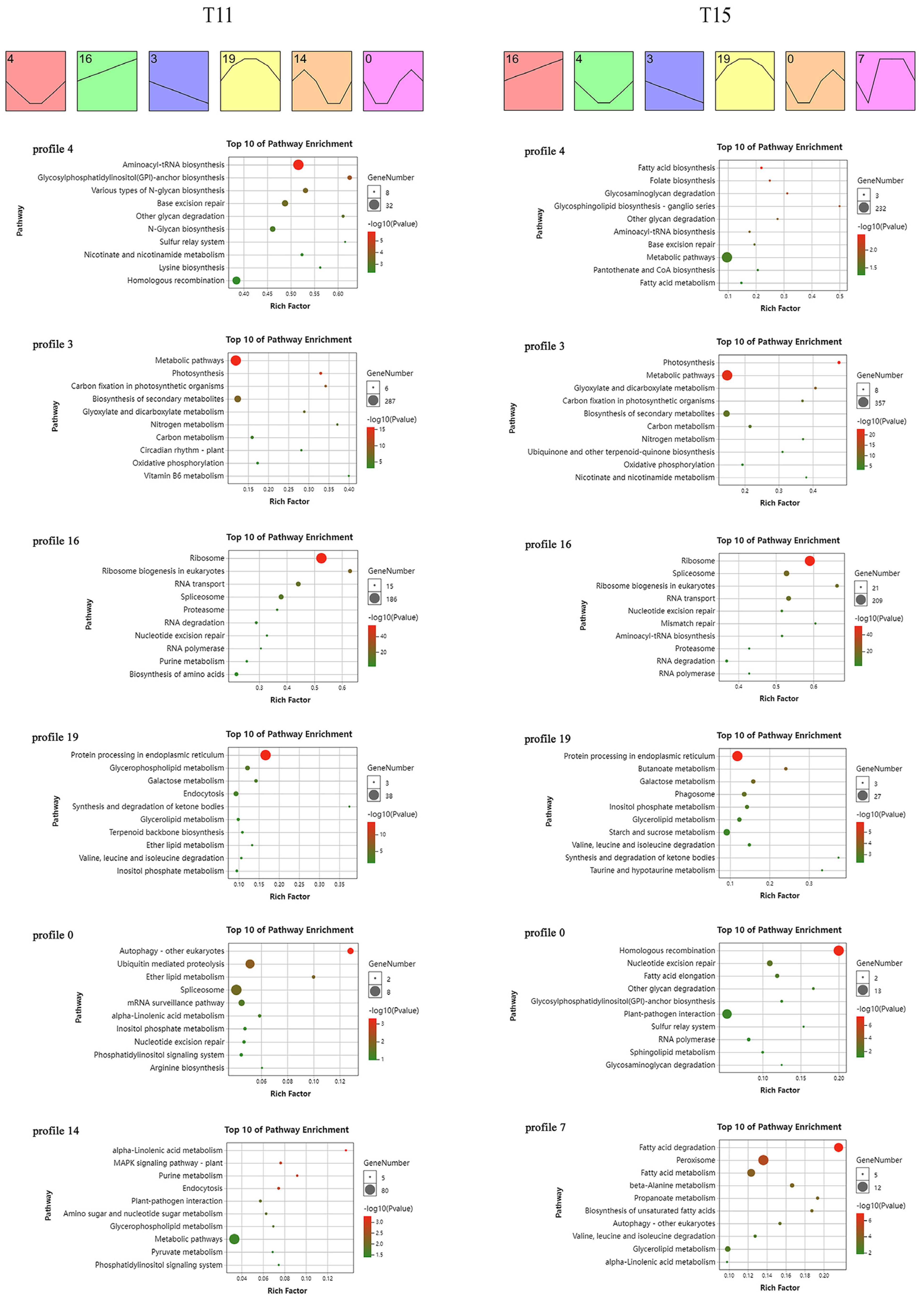
2.4. Time-Series Transcriptome Expression Trend Analysis
2.5. Identification of DEGs and Their Functional Annotations between Two Cultivars with Contrasting Heat Tolerances
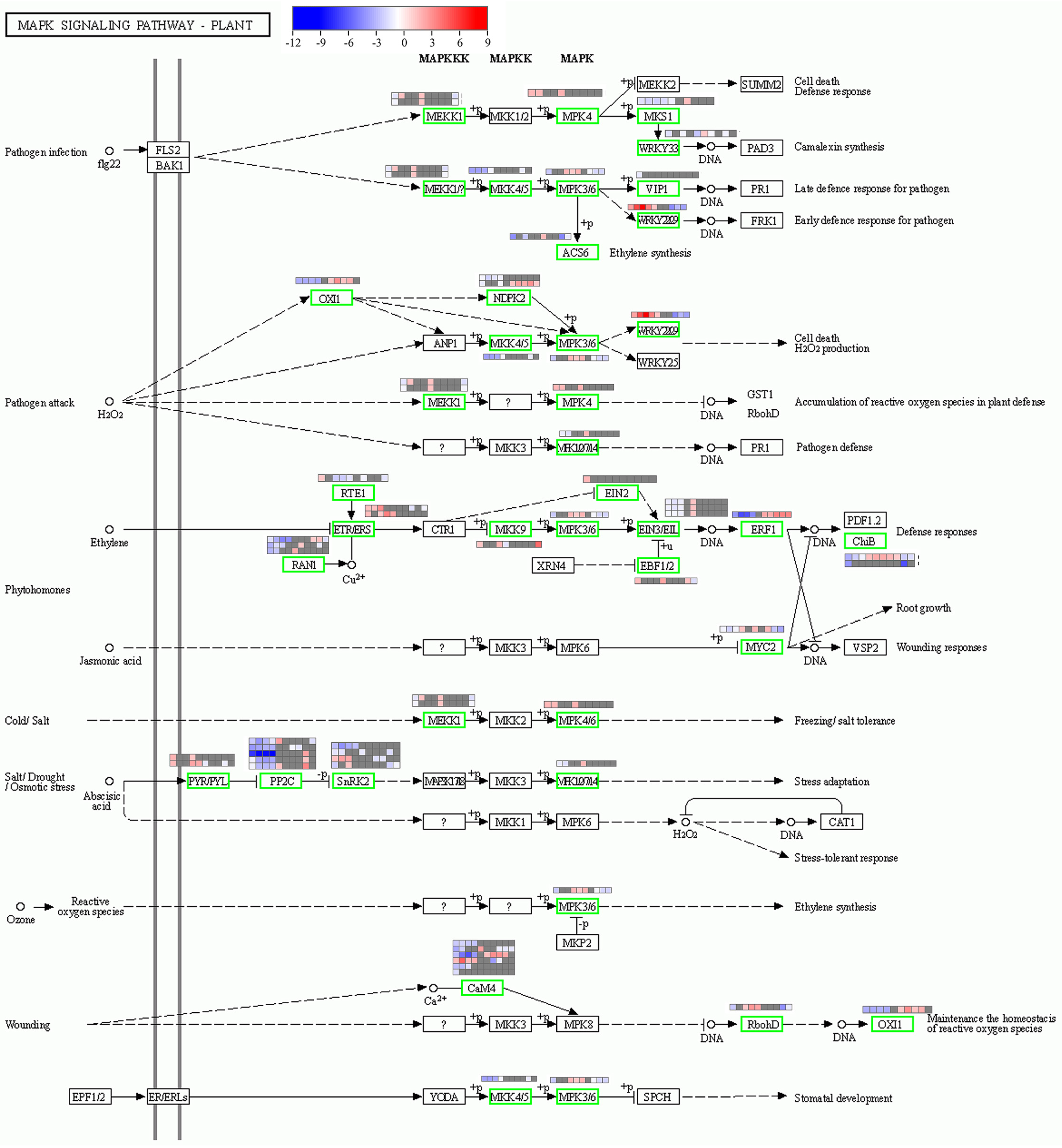
2.6. Plant MAPK Signaling Pathway in Response to Heat Stress
2.7. Candidate Genes in Response to Heat Stress at the Rice Seedling Stage
2.8. RNA-Seq Validation by Quantitative Real-Time PCR (RT-qPCR)
3. Discussion
3.1. MAPK Signaling Is Transient and Occurs at an Early Stage of Heat Stress
3.2. Gene Expression Dynamics of Rice in Response to Continuous Heat Stress
3.3. Candidate Genes of Rice Heat Tolerance
4. Materials and Methods
4.1. Plant Material, Heat Treatment, and Sample Preparation
4.2. Total RNA Extraction, Library Construction, Sequencing, and Bioinformatic Analysis
4.3. RT–qPCR for RNA-Seq Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehrabi, Z. Food system collapse. Nat. Clim. Change 2019, 10, 16–17. [Google Scholar] [CrossRef]
- Allen, M.; Babiker, M.; Chen, Y.; de Coninck, H.; Connors, S.; van Diemen, R.; Dube, O.; Ebi, K.; Engelbrecht, F.; Ferrat, M. Summary for Policymakers Global Warming of 1.5 °C, an IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-industrial Levels and Related Global Greenhouse Gas Emissions Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; World Meteorological Organization: Geneva, Switzerland, 2018; pp. 1–24. Available online: https://www.ipcc.ch (accessed on 7 July 2022).
- Wei, Z.; Yuan, Q.; Lin, H.; Li, X.; Zhang, C.; Gao, H.; Zhang, B.; He, H.; Liu, T.; Jie, Z. Linkage analysis, GWAS, transcriptome analysis to identify candidate genes for rice seedlings in response to high temperature stress. BMC Plant Biol. 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Van Oort, P.A.; Zwart, S.J. Impacts of climate change on rice production in Africa and causes of simulated yield changes. Glob. Chang. Biol. 2017, 24, 1029–1045. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Arif, M.S.; Ahmad, R.; Hasanuzzaman, M.; Ali, B.; Hussain, A. Approaches in enhancing thermotolerance in plants: An updated review. J. Plant Growth Regul. 2020, 39, 456–480. [Google Scholar] [CrossRef]
- Haider, S.; Iqbal, J.; Naseer, S.; Shaukat, M.; Abbasi, B.A.; Yaseen, T.; Zahra, S.A.; Mahmood, T. Unfolding molecular switches in plant heat stress resistance: A comprehensive review. Plant Cell Rep. 2021, 41, 775–798. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Oh, E. PIF4 integrates multiple environmental and hormonal signals for plant growth regulation in Arabidopsis. Mol. Cells 2016, 39, 587. [Google Scholar] [CrossRef]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, L.; Zhao, R.; Sheng, J.; Zhang, S.; Li, R.; Shen, L. Knockout of SlMAPK3 enhances tolerance to heat stress involving ROS homeostasis in tomato plants. BMC Plant Biol. 2019, 19, 354. [Google Scholar] [CrossRef]
- De Vries, J.; De Vries, S.; Curtis, B.A.; Zhou, H.; Penny, S.; Feussner, K.; Pinto, D.M.; Steinert, M.; Cohen, A.M.; von Schwartzenberg, K.; et al. Heat stress response in the closest algal relatives of land plants reveals conserved stress signaling circuits. Plant J. 2020, 103, 1025–1048. [Google Scholar] [CrossRef]
- Dadras, A.; Fuerst-Jansen, J.M.; Darienko, T.; Krone, D.; Scholz, P.; Rieseberg, T.P.; Irisarri, I.; Steinkamp, R.; Hansen, M.; Buschmann, H.; et al. Environmental gradients reveal stress hubs predating plant terrestrialization. BioRxiv 2022. [Google Scholar] [CrossRef]
- Shekhawat, K.; Almeida-Trapp, M.; García-Ramírez, G.X.; Hirt, H. Beat the heat: Plant- and microbe-mediated strategies for crop thermotolerance. Trends Plant Sci. 2022, 27, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Yue, H.; Zhao, X.; Wang, M.; Song, W.; Nie, X. Genome-wide identification and analysis of MAPK and MAPKK gene families in bread wheat (Triticum aestivum L.). Genes 2017, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Hirt, H. Nuclear signaling of plant MAPKs. Front. Plant Sci. 2018, 9, 469. [Google Scholar] [CrossRef]
- Banerjee, G.; Singh, D.; Sinha, A.K. Plant cell cycle regulators: Mitogen-activated protein kinase, a new regulating switch? Plant Sci. 2020, 301, 110660. [Google Scholar] [CrossRef]
- Wu, L.; Zu, X.; Zhang, H.; Wu, L.; Xi, Z.; Chen, Y. Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana. Plant Mol. Biol. 2015, 88, 429–443. [Google Scholar] [CrossRef]
- Ye, C.; Tenorio, F.A.; Redoña, E.D.; Morales–Cortezano, P.S.; Cabrega, G.A.; Jagadish, K.S.; Gregorio, G.B. Fine-mapping and validating qHTSF4. 1 to increase spikelet fertility under heat stress at flowering in rice. Theor. Appl. Genet. 2015, 128, 1507–1517. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, T.; Yu, T.; Zhang, S.; Mao, X.; Zhao, J.; Wang, X.; Dong, J.; Liu, B. Integrating small RNA sequencing with QTL mapping for identification of miRNAs and their target genes associated with heat tolerance at the flowering stage in rice. Front. Plant Sci. 2017, 8, 43. [Google Scholar] [CrossRef]
- Ye, C.; Ishimaru, T.; Lambio, L.; Li, L.; Long, Y.; He, Z.; Htun, T.M.; Tang, S.; Su, Z. Marker-assisted pyramiding of QTLs for heat tolerance and escape upgrades heat resilience in rice (Oryza sativa L.). Theor. Appl. Genet. 2022, 135, 1345–1354. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, H.; Cai, Y.; Zeng, B.; Zhao, J.; Tang, X.; Lu, M.; Wang, H.; Zhu, X.; Wu, X. Natural variation of HTH5 from wild rice, Oryza rufipogon Griff., is involved in conferring high-temperature tolerance at the heading stage. Plant Biotechnol. J. 2022, 20, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Chao, D.Y.; Wu, Y.; Huang, X.; Chen, K.; Cui, L.G.; Su, L.; Ye, W.W.; Chen, H.; Chen, H.C.; et al. Natural alleles of a proteasome alpha2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.-R.; Zhang, H.; Gao, J.; Shan, J.-X.; Ye, W.-W.; Lin, H.-X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 2021, 8, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Yao, Y.; Xiang, D.; Du, H.; Geng, Z.; Yang, W.; Li, X.; Xie, T.; Dong, F.; Xiong, L. A MITE variation-associated heat-inducible isoform of a heat-shock factor confers heat tolerance through regulation of JASMONATE ZIM-DOMAIN genes in rice. New Phytol. 2022, 234, 1315–1331. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.F.; Kan, Y.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Guo, T.; Xiang, Y.H.; Yang, Y.B.; Li, Y.C. A genetic module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef]
- Yan, C.; Zhan, G.; Hong, X.; Yang, D. Identification and Fine Mapping of a Major QTL, TT1-2, That Plays Significant Roles in Regulating Heat Tolerance in Rice. Plant Mol. Biol. Report. 2021, 39, 376–385. [Google Scholar] [CrossRef]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef]
- Wei, S.; Wang, L.; Zhang, Y.; Huang, D. Identification of early response genes to salt stress in roots of melon (Cucumis melo L.) seedlings. Mol. Biol. Rep. 2013, 40, 2915–2926. [Google Scholar] [CrossRef]
- Asano, T.; Hayashi, N.; Kobayashi, M.; Aoki, N.; Miyao, A.; Mitsuhara, I.; Ichikawa, H.; Komatsu, S.; Hirochika, H.; Kikuchi, S. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012, 69, 26–36. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef]
- Oh, V.K.S.; Li, R.W. Temporal dynamic methods for bulk RNA-Seq time series data. Genes 2021, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Thorne, T. Approximate inference of gene regulatory network models from RNA-Seq time series data. BMC Bioinform. 2018, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, S.; Gao, D.; Ding, H.; Yan, X. Time-series transcriptomic analysis reveals potential genes and pathways involved in the process of monospore formation in Phycocalida chauhanii. J. Appl. Phycol. 2021, 33, 1925–1937. [Google Scholar] [CrossRef]
- Yi, F.; Huo, M.; Li, J.; Yu, J. Time-series transcriptomics reveals a drought-responsive temporal network and crosstalk between drought stress and the circadian clock in foxtail millet. Plant J. 2022, 110, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Soren, K.; Tripathi, S.; Pareek, S.; Hembram, M.; Gangwar, P.; Abrol, S.; Bohra, A.; Kumar, K.; Konda, A.; Shanmugavadivel, P. Comparative Time Series RNA-seq Analysis of Pigeonpea Root Tissues in Response to Fusarium udum Infection. Front. Fungal Biol. 2021, 19, 664953. [Google Scholar] [CrossRef]
- Yan, J.; Buer, H.; Wang, Y.P.; Zhula, G.; Bai, Y.E. Transcriptomic Time-Series Analyses of Gene Expression Profile during Zygotic Embryo Development in Picea Mongolica. Front. Genet. 2021, 12, 738649. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Galli, M.; Gallavotti, A. Mechanisms of temperature-regulated growth and thermotolerance in crop species. Curr. Opin. Plant Biol. 2021, 65, 102134. [Google Scholar] [CrossRef]
- Kornhuber, K.; Coumou, D.; Vogel, E.; Lesk, C.; Donges, J.F.; Lehmann, J.; Horton, R.M.J.N.C.C. Amplified Rossby waves enhance risk of concurrent heatwaves in major breadbasket regions. Nat. Clim. Chang. 2020, 10, 48–53. [Google Scholar] [CrossRef]
- Bokszczanin, K.L.; Fragkostefanakis, S.; Bostan, H.; Bovy, A.; Chaturvedi, P.; Chiusano, M.L.; Firon, N.; Iannacone, R.; Jegadeesan, S.; Klaczynskid, K. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front. Plant Sci. 2013, 4, 315. [Google Scholar] [CrossRef]
- Li, Z.; Ye, X. Transcriptome response of maize (Zea mays L.) seedlings to heat stress. Protoplasma 2021, 259, 357–369. [Google Scholar] [CrossRef]
- Liu, Y.; He, C. A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol. 2017, 11, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Zelicourt, A.d.; Colcombet, J.; Hirt, H. The Role of MAPK Modules and ABA during Abiotic Stress Signaling. Trends Plant Sci. 2016, 21, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Rodrigues, A.; Saez, A.; Rubio, S.; Antoni, R.; Dupeux, F.; Park, S.Y.; Márquez, J.A.; Cutler, S.R.; Rodriguez, P.L. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009, 60, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, X.; Modrego, A.; Rodriݩguez, D.; Gonzaݩlez-Garciݩa, M.P.; Sanz, L.; Nicolaݩs, G.; Lorenzo, O. The nuclear interactor PYL8/RCAR3 of Fagus sylvatica FsPP2C1 is a positive regulator of abscisic acid signaling in seeds and stress. Plant Physiol. Biochem. 2010, 152, 133–150. [Google Scholar] [CrossRef]
- Wang, H.; Ngwenyama, N.; Liu, Y.; Walker, J.C.; Zhang, S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 2007, 19, 63–73. [Google Scholar] [CrossRef]
- Shin, H.; You, M.K.; Jeung, J.U.; Shin, J.S. OsMPK3 is a TEY-type rice MAPK in Group C and phosphorylates OsbHLH65, a transcription factor binding to the E-box element. Plant Cell Rep. 2014, 33, 1343–1353. [Google Scholar] [CrossRef]
- Kumar, R.R.; Dubey, K.; Arora, K.; Dalal, M.; Rai, G.K.; Mishra, D.; Chaturvedi, K.K.; Rai, A.; Kumar, S.N.; Singh, B. Characterizing the putative mitogen-activated protein kinase (MAPK) and their protective role in oxidative stress tolerance and carbon assimilation in wheat under terminal heat stress. Biotechnol. Rep. 2021, 29, e00597. [Google Scholar] [CrossRef]
- Danquah, A.; de Zélicourt, A.; Boudsocq, M.; Neubauer, J.; Frei dit Frey, N.; Leonhardt, N.; Pateyron, S.; Gwinner, F.; Tamby, J.P.; Ortiz-Masia, D. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J. 2015, 82, 232–244. [Google Scholar] [CrossRef]
- Yang, L.; Jin, Y.; Huang, W.; Sun, Q.; Liu, F.; Huang, X. Full-length transcriptome sequences of ephemeral plant Arabidopsis pumila provides insight into gene expression dynamics during continuous salt stress. BMC Genom. 2018, 19, 717. [Google Scholar] [CrossRef]
- Sun, S.; Lin, M.; Qi, X.; Chen, J.; Gu, H.; Zhong, Y.; Sun, L.; Muhammad, A.; Bai, D.; Hu, C.; et al. Full-length transcriptome profiling reveals insight into the cold response of two kiwifruit genotypes (A. arguta) with contrasting freezing tolerances. BMC Plant Biol. 2021, 21, 365. [Google Scholar] [CrossRef] [PubMed]
- Molinier, J.; Ramos, C.; Fritsch, O.; Hohn, B. CENTRIN2 modulates homologous recombination and nucleotide excision repair in Arabidopsis. Plant Cell 2004, 16, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Howell, S.H. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 2010, 22, 2930–2942. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.H. Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 2013, 64, 477–499. [Google Scholar] [CrossRef]
- Liu, Y.; Burgos, J.S.; Deng, Y.; Srivastava, R.; Howell, S.H.; Bassham, D.C. Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell 2012, 24, 4635–4651. [Google Scholar] [CrossRef]
- Zwiewka, M.; Nodzyński, T.; Robert, S.; Vanneste, S.; Friml, J. Osmotic stress modulates the balance between exocytosis and clathrin-mediated endocytosis in Arabidopsis thaliana. Mol. Plant 2015, 8, 1175–1187. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Gao, C.; Zeng, Y.; Cui, Y.; Shen, W.; Jiang, L. The roles of endomembrane trafficking in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 55–69. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Xu, Q.; Wang, D.; Di, H.; Huang, J.; Yang, X.; Wang, Z.; Zhang, L.; Dong, L. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seq approaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Jugulam, M. Role of cytochrome P450 enzymes in plant stress response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Rosic, N.N.; Pernice, M.; Dunn, S.; Dove, S.; Hoegh-Guldberg, O.J.A. Differential regulation by heat stress of novel cytochrome P450 genes from the dinoflagellate symbionts of reef-building corals. Appl. Environ. Microbiol. 2010, 76, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.; Mamidi, S.; Rahman, M. Genome-wide association study of heat stress-tolerance traits in spring-type Brassica napus L. under controlled conditions. Crop J. 2018, 6, 115–125. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Xian, W.; Wang, J.; Liao, H. Identification and expression analysis of the Glycine max CYP707A gene family in response to drought and salt stresses. Ann. Bot. 2012, 110, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.; Kusolwa, P.; Rathinasabapathi, B. Heat stress tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant Sci. 2018, 9, 1578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, D.; Wang, R.; Kong, N.; Zhang, C.; Yang, C.; Wu, W.; Ma, H.; Chen, Q. Genome-wide analysis of the potato Hsp20 gene family: Identification, genomic organization and expression profiles in response to heat stress. BMC Genom. 2018, 19, 61. [Google Scholar] [CrossRef]
- Muthusamy, S.K.; Dalal, M.; Chinnusamy, V.; Bansal, K.C. Genome-wide identification and analysis of biotic and abiotic stress regulation of small heat shock protein (HSP20) family genes in bread wheat. J. Plant Physiol. 2017, 211, 100–113. [Google Scholar] [CrossRef]
- Jung, K.; An, G. Application of MapMan and RiceNet drives systematic analyses of the early heat stress transcriptome in rice seedlings. J. Plant Biol. 2012, 55, 436–449. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef]
- Wang, H.; Cai, H.; Zhu, J.; Wei, X.; Zhang, S.; Liu, G.; He, Y.; Li, B.; Xu, L.; Jiao, C.; et al. Dynamic Resistant Starch Accumulation in Contrasting Wheat Genotypes Highlights the Lipid Metabolic Pathway Related to Resistant Starch Synthesis. Agriculture 2022, 12, 308. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Li, B.; Cai, H.; Liu, K.; An, B.; Wang, R.; Yang, F.; Zeng, C.; Jiao, C.; Xu, Y. DNA Methylation Alterations and Their Association with High Temperature Tolerance in Rice Anthesis. J. Plant Growth Regul. 2022, 42, 780–794. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Wang, H.; Zhou, L.; Li, B.; Zhang, S.; He, Y.; Guo, Y.; You, A.; Jiao, C.; Xu, Y. Time-Series Transcriptomic Analysis of Contrasting Rice Materials under Heat Stress Reveals a Faster Response in the Tolerant Cultivar. Int. J. Mol. Sci. 2023, 24, 9408. https://doi.org/10.3390/ijms24119408
Cai H, Wang H, Zhou L, Li B, Zhang S, He Y, Guo Y, You A, Jiao C, Xu Y. Time-Series Transcriptomic Analysis of Contrasting Rice Materials under Heat Stress Reveals a Faster Response in the Tolerant Cultivar. International Journal of Molecular Sciences. 2023; 24(11):9408. https://doi.org/10.3390/ijms24119408
Chicago/Turabian StyleCai, Haiya, Hongpan Wang, Lei Zhou, Bo Li, Shuo Zhang, Yonggang He, Ying Guo, Aiqing You, Chunhai Jiao, and Yanhao Xu. 2023. "Time-Series Transcriptomic Analysis of Contrasting Rice Materials under Heat Stress Reveals a Faster Response in the Tolerant Cultivar" International Journal of Molecular Sciences 24, no. 11: 9408. https://doi.org/10.3390/ijms24119408
APA StyleCai, H., Wang, H., Zhou, L., Li, B., Zhang, S., He, Y., Guo, Y., You, A., Jiao, C., & Xu, Y. (2023). Time-Series Transcriptomic Analysis of Contrasting Rice Materials under Heat Stress Reveals a Faster Response in the Tolerant Cultivar. International Journal of Molecular Sciences, 24(11), 9408. https://doi.org/10.3390/ijms24119408





