Chelator PBT2 Forms a Ternary Cu2+ Complex with β-Amyloid That Has High Stability but Low Specificity
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. EPR Spectroscopy
4.3. Determination of the Ternary Formation Constants for Cu/PBT2/Aβ1–40 Mixtures
- (1)
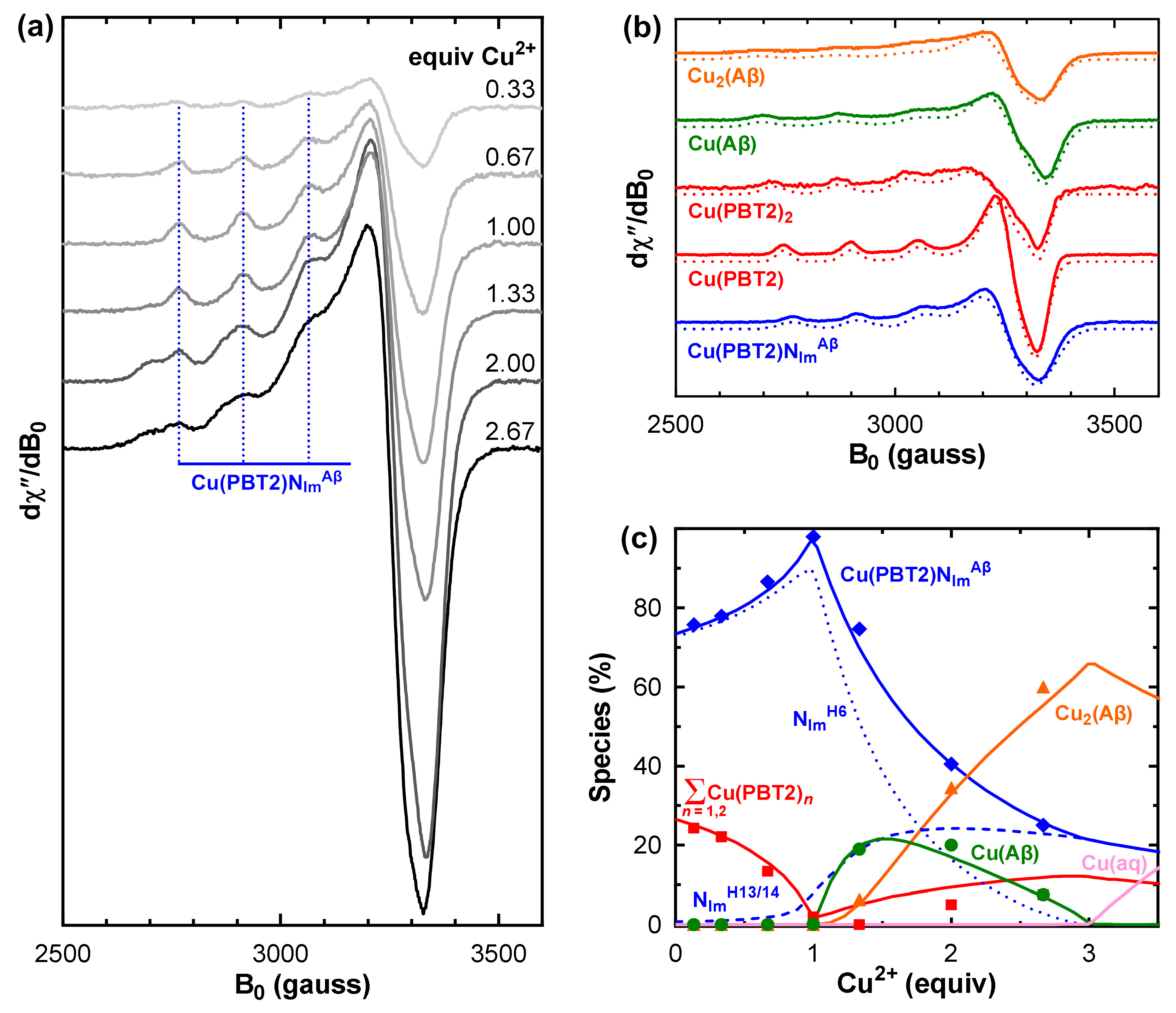
- For each value of and , the theoretical distributions of CuL, CuL2, CuA, Cu2A, CuLA, and CuLB were calculated for the condition Cu/PBT2/Aβ1–40 1:1:1 ≡ Cu/L/A/B 1:1:1:1 under which spectral features attributable to the ternary species were maximal (Figure 2c).
- (2)
- The theoretical speciation in step 1 provided weighting factors that were used to algebraically subtract the normalized spectra of CuL, CuL2, and CuA (Figure 2b) from the experimental spectrum of Cu/PBT2/Aβ1–40 1:1:1 ≡ Cu/L/A/B 1:1:1:1, thus yielding a weighted summation of indistinguishable CuLA and CuLB spectra.
- (3)
- Linear combinations of the normalized CuL, CuL2, CuA, Cu2A, CuLA, CuLB basis spectra were used to reconstruct the experimental EPR spectra at all intermediate stoichiometries Cu/PBT2/Aβ1–40 n:1:1 ≡ Cu/L/A/B n:1:1:1 (0.33 ≤ n ≤ 2.67), and the weightings were iteratively varied using a generalized reduced gradient nonlinear solver (Frontline Systems Inc., Incline Village, NV, USA) to minimize the root-mean-squared deviation between the reconstructions and the experimental spectra.
- (4)
- The deviation between the fitted and experimental values of [CuL], [CuL2], [CuA], [Cu2A], and [CuLNImAβ] = [CuLA] + [CuLB] for all values of n was calculated.
- (5)
- New values of and were chosen and steps 1–4 were repeated until the root-mean-squared deviation was minimized.
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bush, A.I. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000, 4, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I. Drug development based on the metals hypothesis of Alzheimer’s disease. J. Alzheimers Dis. 2008, 15, 223–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adlard, P.A.; Bica, L.; White, A.R.; Nurjono, M.; Filiz, G.; Crouch, P.J.; Donnelly, P.S.; Cappai, R.; Finkelstein, D.I.; Bush, A.I. Metal ionophore treatment restores dendritic spine density and synaptic protein levels in a mouse model of Alzheimer’s disease. PLoS ONE 2011, 6, e17669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sampson, E.L.; Jenagaratnam, L.; McShane, R. Metal protein attenuating compounds for the treatment of Alzheimer’s dementia. Cochrane Database Syst. Rev. 2014, 2, CD005380. [Google Scholar] [CrossRef]
- Available online: www.alzforum.org/news/research-news/pbt2-takes-dive-phase-2-alzheimers-trial (accessed on 1 April 2014).
- Kenche, V.B.; Zawisza, I.; Masters, C.L.; Bal, W.; Barnham, K.J.; Drew, S.C. Mixed ligand Cu2+ complexes of a model therapeutic with Alzheimer’s amyloid-β peptide and monoamine neurotransmitters. Inorg. Chem. 2013, 52, 4303–4318. [Google Scholar] [CrossRef]
- Nguyen, M.; Vendier, L.; Stigliani, J.-L.; Meunier, B.; Robert, A. Structures of the Copper and Zinc Complexes of PBT2, a Chelating Agent Evaluated as Potential Drug for Neurodegenerative Diseases. Eur. J. Inorg. Chem. 2017, 2017, 600–608. [Google Scholar] [CrossRef]
- Sgarlata, C.; Arena, G.; Bonomo, R.P.; Giuffrida, A.; Tabbì, G. Simple and mixed complexes of copper(II) with 8-hydroxyquinoline derivatives and amino acids: Characterization in solution and potential biological implications. J. Inorg. Biochem. 2018, 180, 89–100. [Google Scholar] [CrossRef]
- Summers, K.L.; Roseman, G.P.; Sopasis, G.J.; Millhauser, G.L.; Harris, H.H.; Pickering, I.J.; George, G.N. Copper(II) Binding to PBT2 Differs from That of Other 8-Hydroxyquinoline Chelators: Implications for the Treatment of Neurodegenerative Protein Misfolding Diseases. Inorg. Chem. 2020, 59, 17519–17534. [Google Scholar] [CrossRef]
- Summers, K.L.; Roseman, G.; Schilling, K.M.; Dolgova, N.V.; Pushie, M.J.; Sokaras, D.; Kroll, T.; Harris, H.H.; Millhauser, G.L.; Pickering, I.J.; et al. Alzheimer’s Drug PBT2 Interacts with the Amyloid β 1–42 Peptide Differently than Other 8-Hydroxyquinoline Chelating Drugs. Inorg. Chem. 2022, 61, 14626–14640. [Google Scholar] [CrossRef]
- Mital, M.; Zawisza, I.A.; Wiloch, M.Z.; Wawrzyniak, U.E.; Kenche, V.; Wróblewski, W.; Bal, W.; Drew, S.C. Copper Exchange and Redox Activity of a Prototypical 8-Hydroxyquinoline: Implications for Therapeutic Chelation. Inorg Chem. 2016, 55, 7317–7319. [Google Scholar] [CrossRef]
- Drew, S.C.; Barnham, K.J. The Heterogeneous Nature of Cu2+ Interactions with Alzheimer’s Amyloid-β Peptide. Acc. Chem. Res. 2011, 44, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C.; La Penna, G. Metal ions and intrinsically disordered proteins and peptides: From Cu/Zn amyloid-β to general principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Drew, S.C.; Noble, C.J.; Masters, C.L.; Hanson, G.R.; Barnham, K.J. Pleomorphic copper coordination by Alzheimer’s amyloid-β peptide. J. Am. Chem. Soc. 2009, 131, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Alies, B.; Renaglia, E.; Rózga, M.; Bal, W.; Faller, P.; Hureau, C. Cu(II) affinity for the Alzheimer’s peptide: Tyrosine fluorescence studies revisited. Anal. Chem. 2013, 85, 1501–1508. [Google Scholar] [CrossRef]
- Bossak-Ahmad, K.; Bal, W.; Frączyk, T.; Drew, S.C. Ternary Cu2+ Complexes of Human Serum Albumin and Glycyl-L-histidyl-L-lysine. Inorg. Chem. 2021, 60, 16927–16931. [Google Scholar] [CrossRef]
- Bossak-Ahmad, K.; Wiśniewska, M.; Bal, W.; Drew, S.C.; Frączyk, T. Ternary Cu(II) complex with GHK peptide and cis-urocanic acid as a potential physiologically functional copper chelate. Int. J. Mol. Sci. 2020, 21, 6190. [Google Scholar] [CrossRef]
- Drew, S.C. The case for abandoning therapeutic chelation of copper ions in Alzheimer’s disease. Front. Neurosci. 2017, 11, 317. [Google Scholar] [CrossRef][Green Version]
- Bohlmann, L.; De Oliveira, D.M.P.; El-Deeb, I.M.; Brazel, E.B.; Harbison-Price, N.; Ong, C.Y.; Rivera-Hernandez, T.; Ferguson, S.A.; Cork, A.J.; Phan, M.D.; et al. Chemical Synergy between Ionophore PBT2 and Zinc Reverses Antibiotic Resistance. mBio 2018, 9, e02391-18. [Google Scholar] [CrossRef][Green Version]
- Brazel, E.B.; Tan, A.; Neville, S.L.; Iverson, A.R.; Udagedara, S.R.; Cunningham, B.A.; Sikanyika, M.; De Oliveira, D.M.P.; Keller, B.; Bohlmann, L.; et al. Dysregulation of Streptococcus pneumoniae zinc homeostasis breaks ampicillin resistance in a pneumonia infection model. Cell Rep. 2022, 38, 110202. [Google Scholar] [CrossRef]
- Harbison-Price, N.; Ferguson, S.A.; Heikal, A.; Taiaroa, G.; Hards, K.; Nakatani, Y.; Rennison, D.; Brimble, M.A.; El-Deeb, I.M.; Bohlmann, L.; et al. Multiple Bactericidal Mechanisms of the Zinc Ionophore PBT2. mSphere 2020, 5, e00157-20. [Google Scholar] [CrossRef][Green Version]
- Irving, H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Kuipers, B.J.H.; Gruppen, H. Prediction of Molar Extinction Coefficients of Proteins and Peptides Using UV Absorption of the Constituent Amino Acids at 214 nm To Enable Quantitative Reverse Phase High-Performance Liquid Chromatography-Mass Spectrometry Analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Gautier, E.C.L.; Kok, G.B.; Krippner, G. 8-Hydroxy quinoline derivatives. U.S. Patent 20080161353A1, 3 July 2008. [Google Scholar]
- Wertz, J.E.; Orton, J.W.; Auzins, P. Electron spin resonance studies of radiation effects in inorganic solids. Discuss. Faraday Soc. 1961, 31, 140–150. [Google Scholar] [CrossRef]
- Stoll, S.; Britt, R.D. General and efficient simulation of pulse EPR spectra. Phys. Chem. Chem. Phys. 2009, 11, 6614–6625. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Haigh, C.L.; Tumpach, C.; Collins, S.J.; Drew, S.C. A 2-substituted 8-hydroxyquinoline stimulates neural stem cell proliferation by modulating ROS signalling. Cell Biochem. Biophys. 2016, 74, 297–306. [Google Scholar] [CrossRef]
- Drew, S.C. α-synuclein and β-amyloid form a bridged copper complex. Appl. Magn. Reson. 2015, 46, 1041–1052. [Google Scholar] [CrossRef]
- Sarell, C.J.; Syme, C.D.; Rigby, S.E.; Viles, J.H. Copper(II) binding to amyloid-beta fibrils of Alzheimer’s disease reveals a picomolar affinity: Stoichiometry and coordination geometry are independent of Abeta oligomeric form. Biochemistry 2009, 48, 4388–4402. [Google Scholar] [CrossRef]
- Kowalik-Jankowska, T.; Ruta, M.; Wiśniewska, K.; Lankiewicz, L. Coordination abilities of the 1-16 and 1-28 fragments of beta-amyloid peptide towards copper(II) ions: A combined potentiometric and spectroscopic study. J. Inorg. Biochem. 2003, 95, 270–282. [Google Scholar] [CrossRef]
- Sjöberg, S. Critical evaluation of stability constants of metal-imidazole and metal-histamine systems (Technical Report). Pure Appl. Chem. 1997, 69, 1549–1570. [Google Scholar] [CrossRef]
- Ringbom, A. The analyst and the inconstant constants. J. Chem. Educ. 1958, 35, 282–288. [Google Scholar] [CrossRef]

| Complex | gz | Az (63Cu) a | Reference | ||
|---|---|---|---|---|---|
| L = PBT2 | |||||
| CuL | 2.259 ± 0.002 | 151 ± 1 | This work b | ||
| CuL2 | 2.283 ± 0.002 | 148 ± 3 | This work b | ||
| CuLNImX | |||||
| X = Aβ1–40 | 2.249 ± 0.002 | 147 ± 2 | This work b | ||
| X = imidazole | 2.248 ± 0.001 | 143 ± 1 | This work b | ||
| X = histamine | 2.248 ± 0.001 | 143 ± 1 | This work b | ||
| X = Aβ1–42 | 2.242 ± 0.002 | 142 ± 3 | 10 c | ||
| L = non-chlorinated PBT2 homologue | |||||
| CuL | 2.255 ± 0.001 | 153 ± 1 | 6 | ||
| CuL2 | 2.267 ± 0.001 | 149 ± 1 | 6 | ||
| CuLNImX | |||||
| X = imidazole | 2.245 ± 0.001 | 144 ± 1 | This work b, 6, 11 | ||
| X = histamine | 2.245 ± 0.001 | 145 ± 1 | This work b, 6, 11 | ||
| Aβ | |||||
| Cu(Aβ1–40) | 2.268 ± 0.002 | 174 ± 2 | This work d | ||
| Cu2(Aβ1–40) | |||||
| first site | 2.268 ± 0.002 | 174 ± 2 | This work e | ||
| second site | 2.309 ± 0.005 | 168 ± 5 | This work e | ||
| Complex | Formation Constant a | /(1 M−1)] at pH 7.4 | Reference |
|---|---|---|---|
| CuL | 13.61 ± 0.05 | 8 | |
| CuL2 | 5.95 ± 0.07 | 8 | |
| CuLNImAβ (His6) | 6.4 ± 0.1 | This work | |
| CuLNImAβ (His13/14) | 4.4 ± 0.1 | This work | |
| CuLNImimidazole | 4.22 ± 0.09 | This work | |
| CuLNImhistamine | 4.00 ± 0.05 | This work | |
| Cu(Aβ1–40) | 10.0 ± 0.1 | This work | |
| Cu2(Aβ1–40) | 8.0 ± 0.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drew, S.C. Chelator PBT2 Forms a Ternary Cu2+ Complex with β-Amyloid That Has High Stability but Low Specificity. Int. J. Mol. Sci. 2023, 24, 9267. https://doi.org/10.3390/ijms24119267
Drew SC. Chelator PBT2 Forms a Ternary Cu2+ Complex with β-Amyloid That Has High Stability but Low Specificity. International Journal of Molecular Sciences. 2023; 24(11):9267. https://doi.org/10.3390/ijms24119267
Chicago/Turabian StyleDrew, Simon C. 2023. "Chelator PBT2 Forms a Ternary Cu2+ Complex with β-Amyloid That Has High Stability but Low Specificity" International Journal of Molecular Sciences 24, no. 11: 9267. https://doi.org/10.3390/ijms24119267
APA StyleDrew, S. C. (2023). Chelator PBT2 Forms a Ternary Cu2+ Complex with β-Amyloid That Has High Stability but Low Specificity. International Journal of Molecular Sciences, 24(11), 9267. https://doi.org/10.3390/ijms24119267






