Polymeric Wet-Strength Agents in the Paper Industry: An Overview of Mechanisms and Current Challenges
Abstract
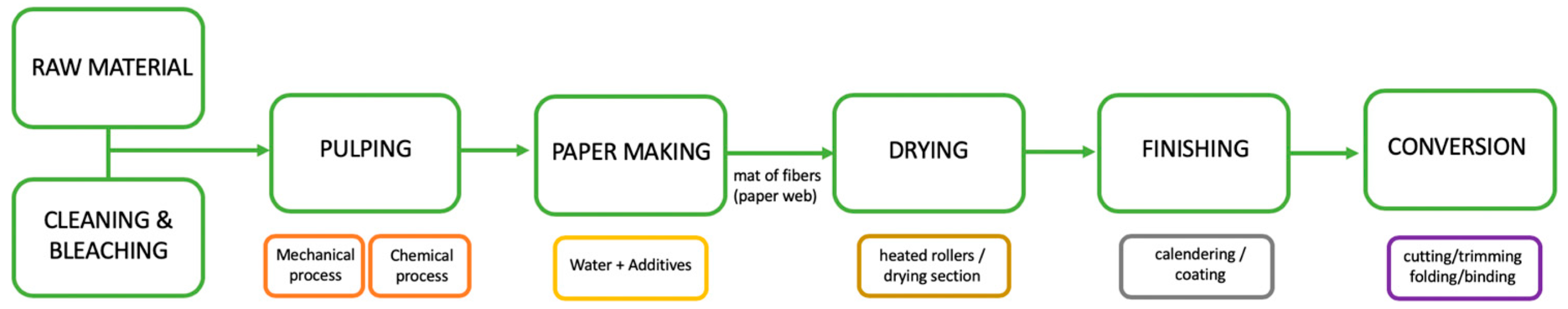
1. Introduction
2. Types of Wet-Strength Agents
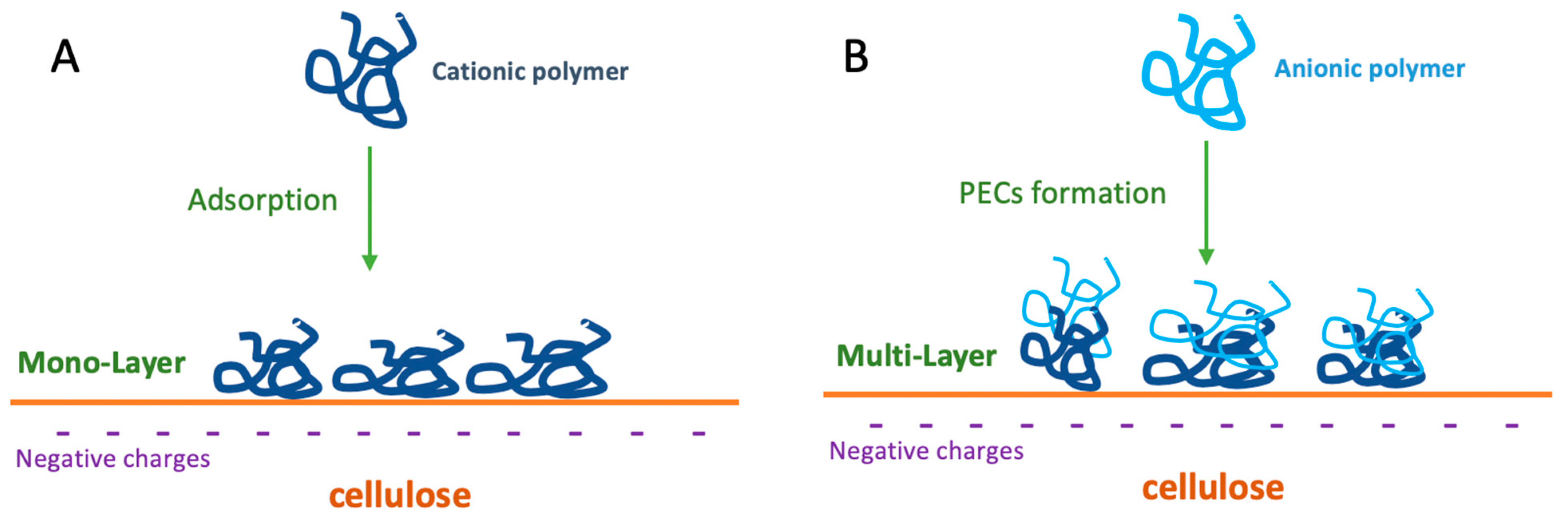
3. Mechanisms of Action of Wet-Strength Agents
4. Synthetic Wet-Strength Agents
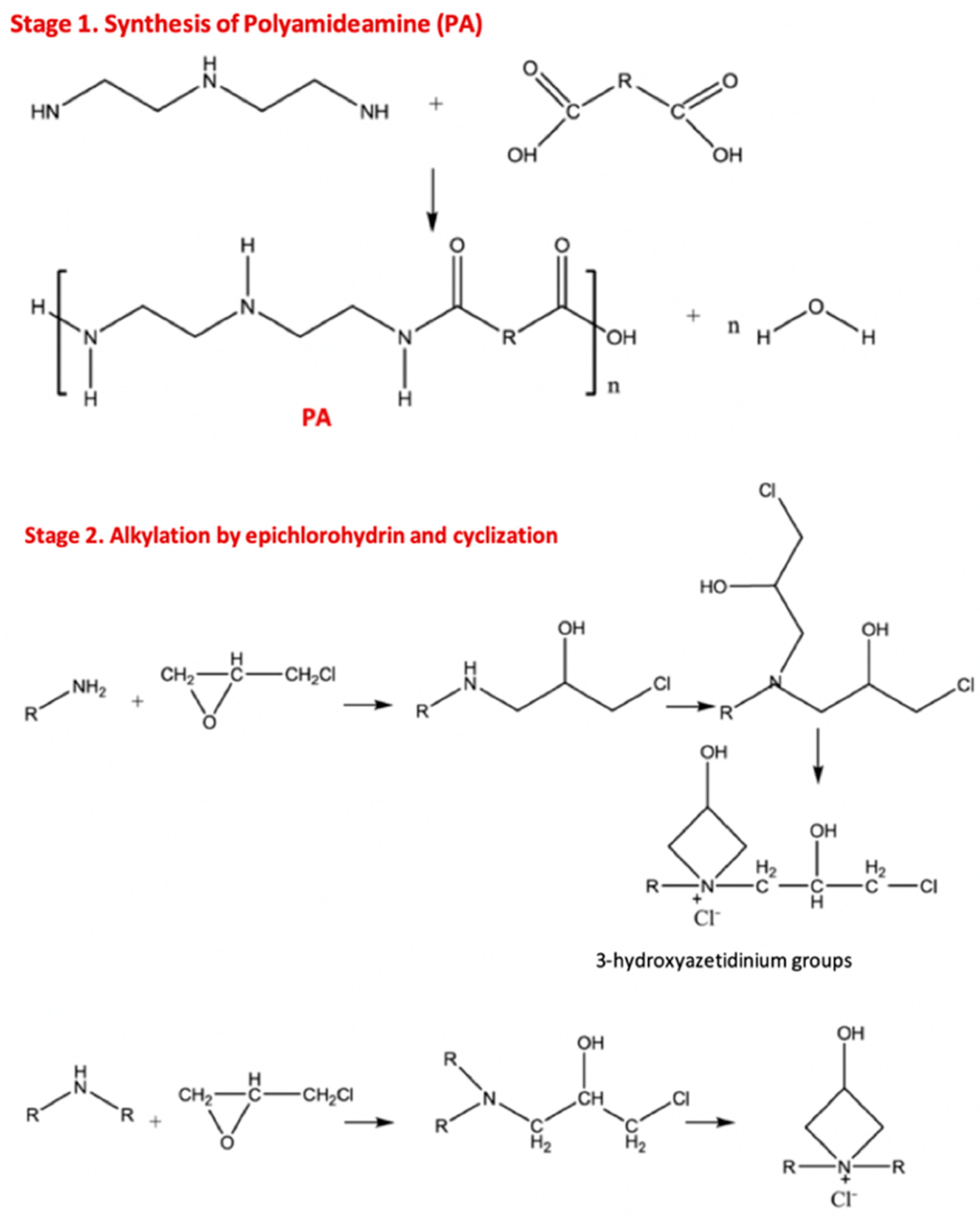
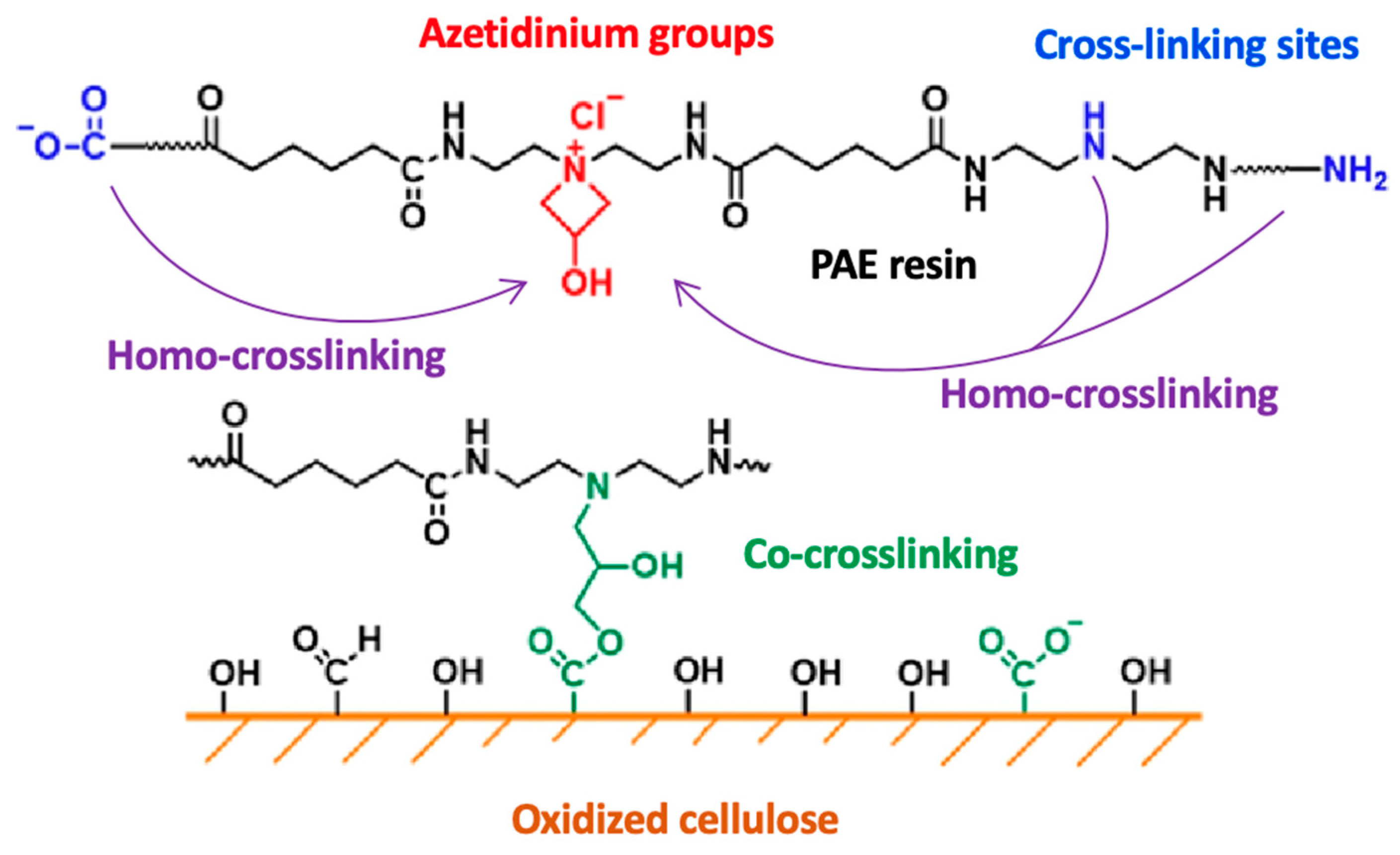
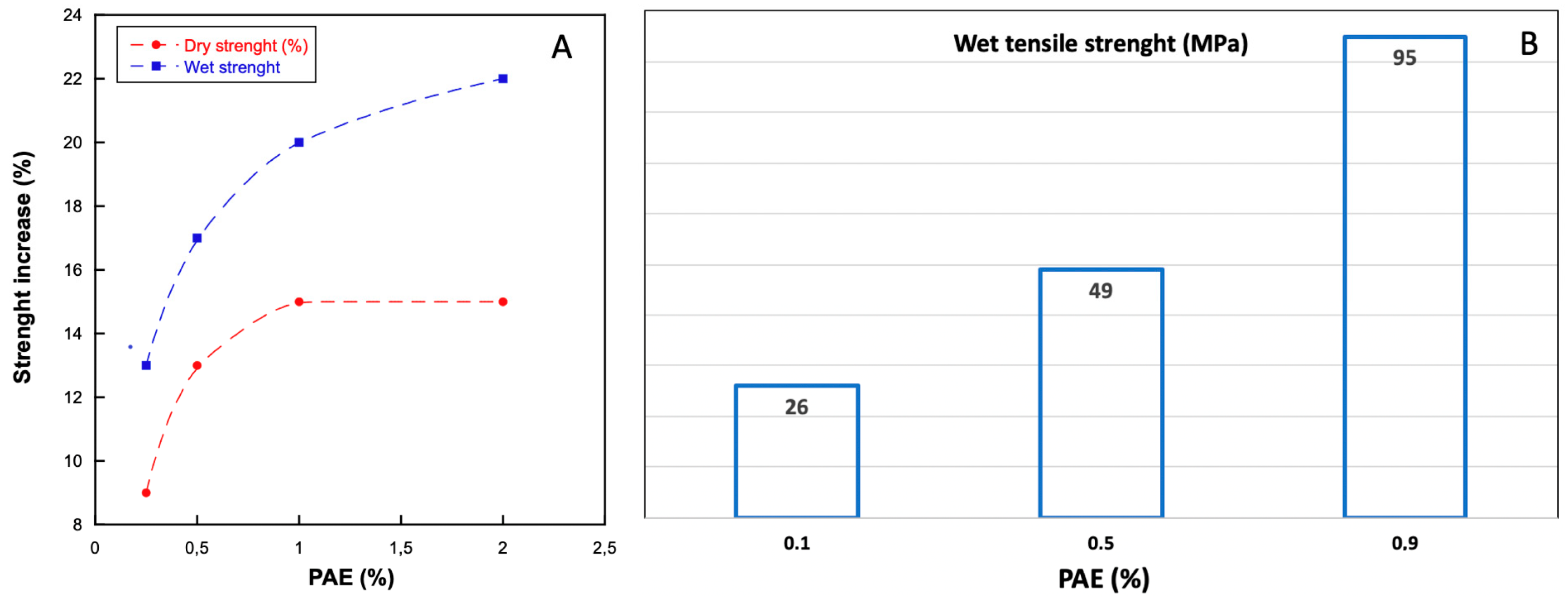
4.1. Polyamideamine-Epichlorohydrin
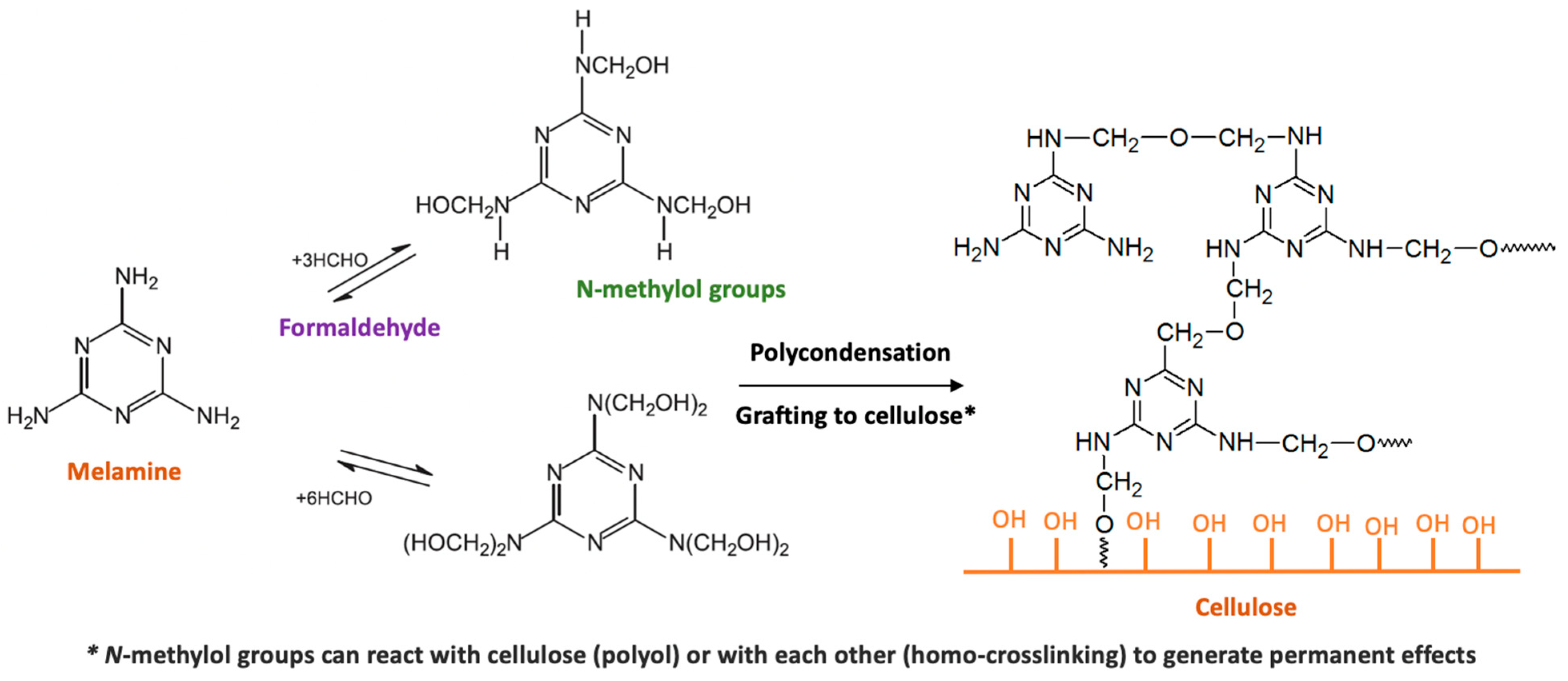
4.2. Melamine Formaldehyde Resin
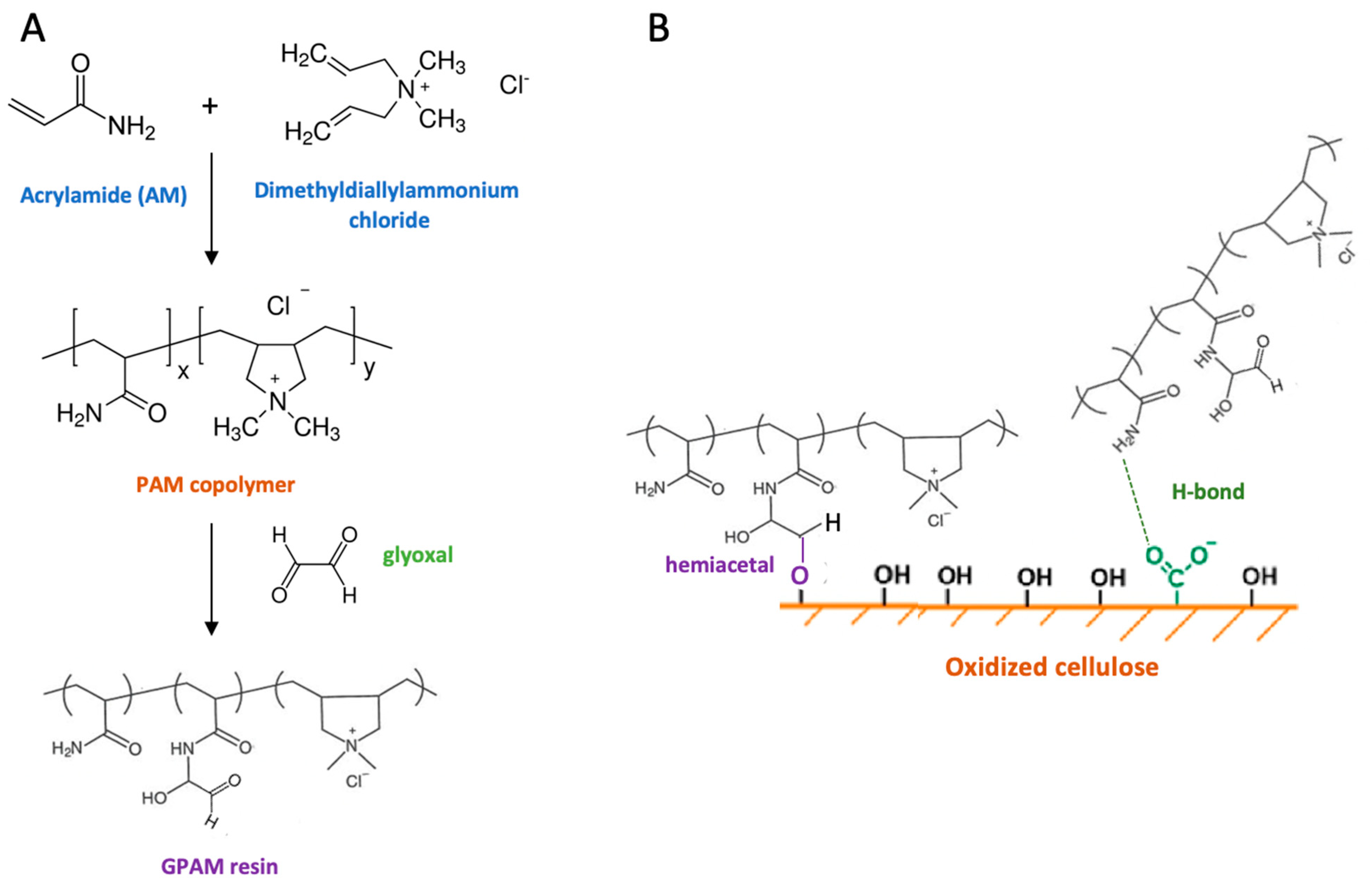
4.3. Polyacrylamide and Glyoxylated Polyacrylamide
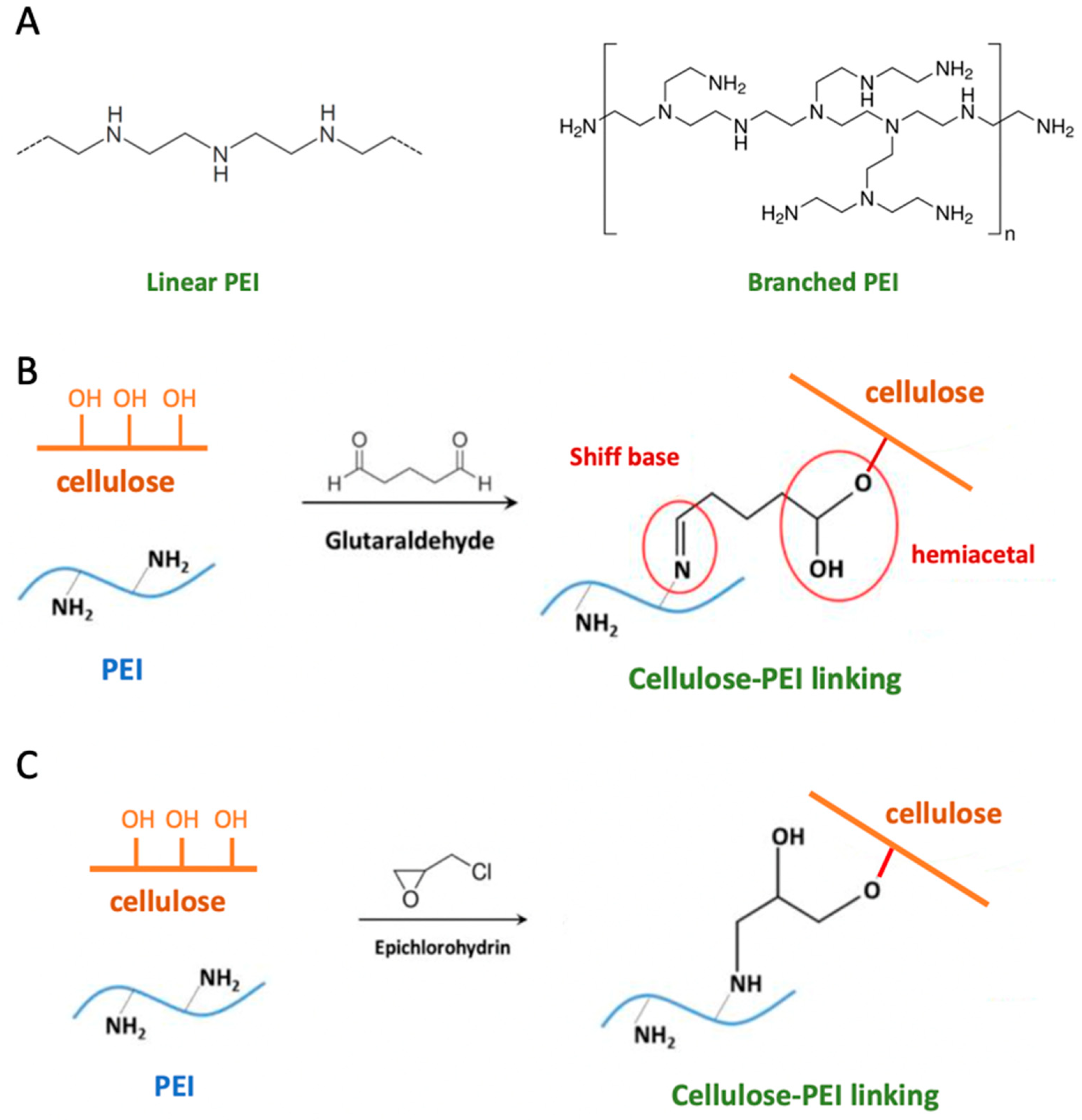
4.4. Polyethyleneimine
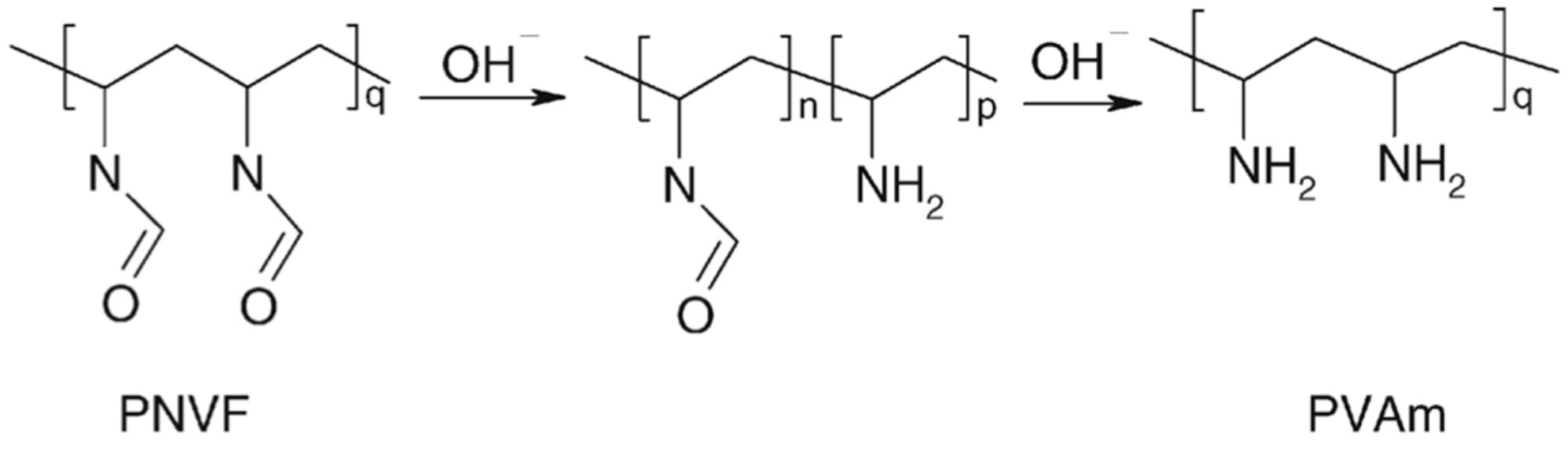
4.5. Polyvinylamine
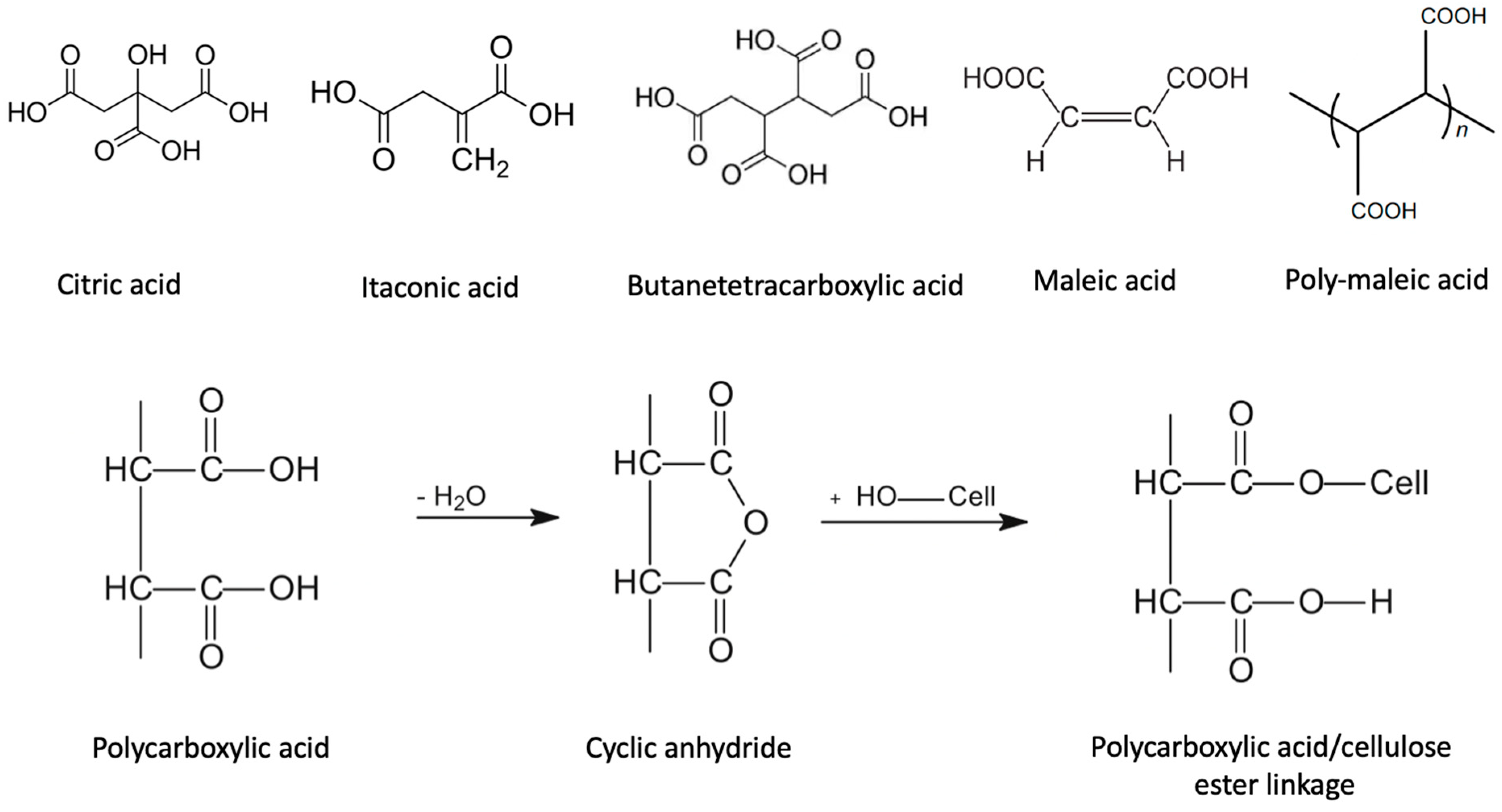
4.6. Polycarboxylic Acids
5. Natural Wet-Strength Agents
5.1. Starch
5.2. Chitosan
5.3. Cellulose Nanofibrils
5.4. Soy Protein
6. Techniques to Investigate the Effect of Wet-Strength Agents on the Physico-Chemical Properties of Paper Products
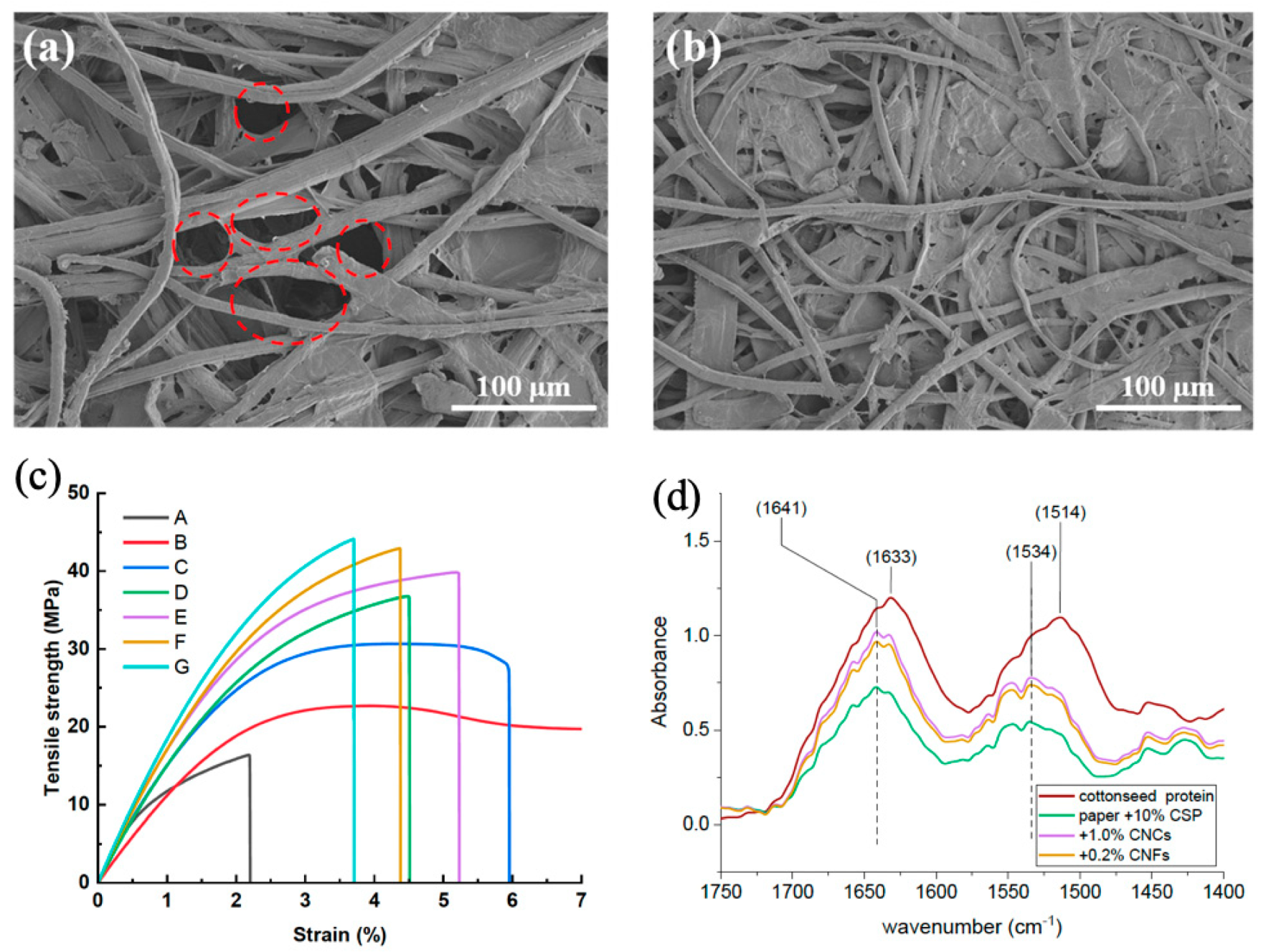
6.1. Fourier Transform Infrared Spectroscopy
6.2. X-ray Photoelectron Spectroscopy
6.3. Scanning Electron Microscopy
6.4. Tensile-Strength Testing and Wet Peeling
6.5. Dynamic Mechanical Analysis
6.6. Thermogravimetric Analysis
7. Challenges and Recent Advances
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulp and Paper Industry. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/related-industries/forest-based-industries/pulp-and-paper-industry_en (accessed on 8 May 2023).
- The Global Pulp and Paper Market Is Projected to Grow from $354.39 Billion in 2022 to $372.70 Billion by 2029, at a CAGR of 0.72% in Forecast Period 2022–2029. Available online: https://www.fortunebusinessinsights.com/pulp-and-paper-market-103447 (accessed on 8 May 2023).
- L’immagine Forte di un Settore Essenziale. Available online: https://www.industriadellacarta.it/limmagine-forte-di-un-settore-essenziale/ (accessed on 8 May 2023).
- Pätäri, S.; Tuppura, A.; Toppinen, A.; Korhonen, J. Global sustainability megaforces in shaping the future of the European pulp and paper industry towards a bioeconomy. For. Policy Econ. 2016, 66, 38–46. [Google Scholar] [CrossRef]
- Lindh, H.; Olsson, A.; Williams, H. Consumer perceptions of food packaging: Contributing to or counteracting environmentally sustainable development? Packag. Technol. Sci. 2016, 29, 3–23. [Google Scholar] [CrossRef]
- Raheem, D. Application of plastics and paper as food packaging materials—An overview. Emir. J. Food Agric. 2013, 25, 177–188. [Google Scholar] [CrossRef]
- Hagiopol, C.; Johnston, J.W. Chemistry of Modern Papermaking; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Messner, K.; Srebotnik, E. Biopulping: An overview of developments in an environmentally safe paper-making technology. FEMS Microbiol. Rev. 1994, 13, 351–364. [Google Scholar] [CrossRef]
- Andersson, C. New ways to enhance the functionality of paperboard by surface treatment—A review. Packag. Technol. Sci. 2008, 21, 339–373. [Google Scholar] [CrossRef]
- Lindström, T.; Wågberg, L.; Larsson, T. On the nature of joint strength in paper—A review of dry and wet strength resins used in paper manufacturing. In Proceedings of the 13th Fundamental Research Symposium, Cambridge, UK, 11–16 September 2005; Available online: https://www.researchgate.net/publication/267385974_On_the_nature_of_joint_strength_in_paper_-_A_review_of_dry_and_wet_strength_resins_in_paper_manufacturing (accessed on 8 May 2023).
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-enabled membranes for water purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Espy, H.H. The mechanism of wet-strength development in paper: A review. Tappi J. 1995, 78, 90–99. [Google Scholar]
- Laine, J.; Lindstrom, T.; Nordmark, G.G.; Risinger, G. Studies on topochemical modification of cellulosic fibres Part 3. The effect of carboxymethyl cellulose attachment on wet-strength development by alkaline-curing polyamide-amine epichlorohydrin resin. Nord. Pulp Pap. Res. J. 2002, 17, 57–60. [Google Scholar] [CrossRef]
- Aarne, N.; Vesterinen, A.-H.; Kontturi, E.; Seppala, J.; Laine, J. A systematic study of noncross-linking wet strength agents. Ind. Eng. Chem. Res. 2013, 52, 12010–12017. [Google Scholar] [CrossRef]
- Yang, D.; Sotra, A.; Pelton, R.H. Switching off PAE wet strength. Nord. Pulp Pap. Res. J. 2019, 34, 88–95. [Google Scholar] [CrossRef]
- Yang, W.; Bian, H.; Jiao, L.; Wu, W.; Deng, Y.; Dai, H. High wet-strength, thermally stable and transparent TEMPO-oxidized cellulose nanofibril film via cross-linking with poly-amide epichlorohydrin resin. RSC Adv. 2017, 7, 31567–31573. [Google Scholar] [CrossRef]
- Onur, A.; Ng, A.; Garnier, G.; Batchelor, W. The use of cellulose nanofibres to reduce the wet strength polymer quantity for development of cleaner filters. J. Clean. Prod. 2019, 215, 226–231. [Google Scholar] [CrossRef]
- Xu, G.G.; Yang, C.Q.; Deng, Y. Applications of bifunctional aldehydes to improve paper wet strength. J. Appl. Polym. Sci. 2002, 83, 2539–2547. [Google Scholar] [CrossRef]
- Solt, P.; Konnerth, J.; Gindl-Altmutter, W.; Kantner, W.; Moser, J.; Mitter, R.; van Herwijnen, H.W. Technological performance of formaldehyde-free adhesive alternatives for particleboard industry. Int. J. Adhes. Adhes. 2019, 94, 99–131. [Google Scholar] [CrossRef]
- Chen, X.; Afreen, S.; Yu, X.; Dong, C.; Kong, Q. Modified melamine-formaldehyde resins improve tensile strength along with antifouling and flame retardancy in impregnation of cellulose paper. RSC Adv. 2019, 9, 36788–36795. [Google Scholar] [CrossRef]
- Xu, W.; Yu, C.; Zhao, X.; Xu, J.; Jiang, M. Melamine formaldehyde/polyvinyl alcohol composite fiber: Structures and properties controlled by reaction-induced phase separation. J. Appl. Polym. Sci. 2016, 133, 42918. [Google Scholar] [CrossRef]
- Xu, G.G.; Yang, C.Q.; Deng, Y. Combination of bifunctional aldehydes and poly (vinyl alcohol) as the crosslinking systems to improve paper wet strength. J. Appl. Polym. Sci. 2004, 93, 1673–1680. [Google Scholar] [CrossRef]
- Yadollahi, R.; Hamzeh, Y.; Mahdavi, H.; Pourmousa, S. Synthesis and evaluation of glyoxalated polyacrylamide (GPAM) as a wet and dry-strengthening agent of paper. Sci. Technol. 2014, 27, 121–129. [Google Scholar]
- Bi, K.; Zhang, Y. Kinetic study of the polymerization of dimethyldiallylammonium chloride and acrylamide. J. Appl. Polym. Sci. 2012, 125, 1636–1641. [Google Scholar] [CrossRef]
- Grade Specific Optimisation: Permanent (PAE) versus Temporary (GPAM) Wet Strength Utilisation. Available online: https://www.tissueworldmagazine.com/technical-theme/grade-specific-optimisation-permanent-pae-versus-temporary-gpam-wet-strength-utilisation/ (accessed on 8 May 2023).
- Yuan, Z.; Hu, H. Preparation and characterization of crosslinked glyoxalated polyacrylamide paper-strengthening agent. J. Appl. Polym. Sci. 2012, 126 (Suppl. S1), E459–E469. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Punta, C. Synthesis and Application of Cellulose-Polyethyleneimine Composites and Nanocomposites: A Concise Review. Materials 2021, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Weisgerber, C.A. Paper of High Wet Strength and Processes Therefor. U.S. Patent 2,721,140, 18 October 1955. [Google Scholar]
- Pfohl, S.; Kroener, M.; Hartmann, H.; Denzinger, W. Water-Soluble Copolymers Containing Vinylamine Units as Wet Strength and Dry Strength Agent for Paper. U.S. Patent 4,978,427, 18 December 1990. [Google Scholar]
- Wang, F.; Tanaka, H. Aminated Poly-N-Vinylformamide as a Modern Retention Aid of Alkaline Paper Sizing with Acid Rosin Sizes. J. Appl. Poly. Sci. 2000, 78, 1805–1810. [Google Scholar] [CrossRef]
- Pelton, R.; Hong, J. Some properties of newsprint impregnated with poly-vinylamine. Tappi J. 2002, 1, 21–26. [Google Scholar]
- DiFlavio, J.-L.; Bertoia, R.; Pelton, R.; Leduc, M. The mechanism of polyvinylamine wet-strengthening. In Advances in Paper Science and Technology, Proceedings of the 13th Fundamental Research Symposium, Cambridge, UK, 11–16 September 2005; FRC: Manchester, UK, 2018. [Google Scholar]
- Rowland, S.P.; Welch, C.M.; Brannan, A.F.; Gallagher, D.M. Introduction of ester cross-links into cotton cellulose by a rapid curing process. Text. Res. J. 1967, 37, 933–941. [Google Scholar] [CrossRef]
- Dehabadi, V.A.; Buschmann, H.J.; Gutmann, J.S. Durable press finishing of cotton fabrics: An overview. Text. Res. J. 2013, 83, 1974–1995. [Google Scholar] [CrossRef]
- Horie, D.; Biermann, C.J. Applications of I-Press Treatment to Bleached Softwood Kraft Han. Tappi J. 1994, 77, 135–140. [Google Scholar]
- Caulifield, D.F. Ester Crosslinking to Improve Wet Performance of Paper Using Multifunctional Carboxylic Acids, Butanetetracarboxylic Acid and Citric Acid. Tappi J. 1994, 77, 205–212. [Google Scholar]
- Yang, C.Q.; Lu, Y. In-situ polymerization of maleic acid and itaconic acid and crosslinking of cotton fabric. Text. Res. J. 1999, 69, 782–789. [Google Scholar] [CrossRef]
- Yang, C.Q. FT-IR Spectroscopy Study of the Ester Crosslinking Mechanism of Cotton Cellulose. Text. Res. J. 1991, 61, 433–440. [Google Scholar] [CrossRef]
- Yang, C.Q.; Wang, X.; Kang, I.S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Text. Res. J. 1997, 67, 334–342. [Google Scholar] [CrossRef]
- Xu, G.G.; Yang, C.Q.X. Comparison of the kraft paper crosslinked by polymeric carboxylic acids of large and small molecular sizes: Dry and wet performance. J. Appl. Polym. Sci. 1999, 74, 907–912. [Google Scholar] [CrossRef]
- Tedeschi, A.M.; Di Caprio, F.; Piozzi, A.; Pagnanelli, F.; Francolini, I. Sustainable bioactive packaging based on thermoplastic starch and microalgae. Int. J. Mol. Sci. 2021, 23, 178. [Google Scholar] [CrossRef]
- Fornué, E.D.; Allan, G.G.; Quiñones, H.J.C.; González, G.T.; Saucedo, J.T. Fundamental aspects of adhesion between cellulosic surfaces in contact—A review. O Papel 2011, 72, 85–90. [Google Scholar]
- Wang, Y.; Xie, W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohydr. Polym. 2010, 80, 1172–1177. [Google Scholar] [CrossRef]
- Kuo, W.Y.; Lai, H.M. Changes of property and morphology of cationic corn starches. Carbohydr. Polym. 2007, 69, 544–553. [Google Scholar] [CrossRef]
- Nachtergaele, W. The benefits of cationic starches for the paper industry. Starch-Stärke 1989, 41, 27–31. [Google Scholar] [CrossRef]
- Shirazi, M.; Van Den Ven, T.G.M.; Garnier, G. Adsorption of modified starches on pulp fibers. Langmuir 2003, 19, 10835–10842. [Google Scholar] [CrossRef]
- Lundström-Hämälä, L.; Lindgren, J.; Svensson-Rundlöf, E.; Sennerfors, T.; Wågberg, L. The adsorption of polyelectrolyte multilayers (PEM) of starch on mechanical pulps for improved mechanical paper properties. Nord. Pulp Pap. Res. J. 2009, 24, 459–468. [Google Scholar] [CrossRef]
- van de Ven, T.G.M. Kinetic aspects of polymer and polyelectrolyte adsorption on surfaces. Adv. Colloid Interface Sci. 1994, 48, 121–140. [Google Scholar] [CrossRef]
- Eriksson, M.; Pettersson, G.; Wågberg, L. Application of polymeric multilayers of starch onto wood fibres to enhance strength properties of paper. Nord. Pulp Pap. Res. J. 2005, 20, 270–276. [Google Scholar] [CrossRef]
- Zhao, M.; Robertsén, L.; Wågberg, L.; Pettersson, T. Adsorption of paper strength additives to hardwood fibres with different surface charges and their effect on paper strength. Cellulose 2022, 29, 2617–2632. [Google Scholar] [CrossRef]
- Qin, C.; Li, J.; Wang, W.; Li, W. Improving Mechanical Strength and Water Barrier Properties of Pulp Molded Product by Wet-End Added Polyamide Epichlorohydrin/Cationic Starch. ACS Omega 2022, 7, 22173–22180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.D.; Zhang, Y.R.; Wang, X.L.; Wang, Y.Z. High carbonyl content oxidized starch prepared by hydrogen peroxide and its thermoplastic application. Starch-Stärke 2009, 61, 646–655. [Google Scholar] [CrossRef]
- Wang, H.; Poya, Y.; Chen, X.; Jia, T.; Wang, X.; Shi, J. Hydrogen peroxide as an oxidant in starch oxidation using molybdovanadophosphate for producing a high carboxylic content. RSC Adv. 2015, 5, 45725–45730. [Google Scholar] [CrossRef]
- Silvestro, I.; Sergi, R.; D’Abusco, A.S.; Mariano, A.; Martinelli, A.; Piozzi, A.; Francolini, I. Chitosan scaffolds with enhanced mechanical strength and elastic response by combination of freeze gelation, photo-crosslinking and freeze-drying. Carbohydr. Polym. 2021, 267, 118156. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, I.; Francolini, I.; Di Lisio, V.; Martinelli, A.; Pietrelli, L.; Scotto d’Abusco, A.; Scoppio, A.; Piozzi, A. Preparation and characterization of TPP-chitosan crosslinked scaffolds for tissue engineering. Materials 2020, 13, 3577. [Google Scholar] [CrossRef]
- Crucitti, V.C.; Migneco, L.M.; Piozzi, A.; Taresco, V.; Garnett, M.; Argent, R.H.; Francolini, I. Intermolecular interaction and solid state characterization of abietic acid/chitosan solid dispersions possessing antimicrobial and antioxidant properties. Eur. J. Pharm. Biopharm. 2018, 125, 114–123. [Google Scholar] [CrossRef]
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2018, 179, 273–281. [Google Scholar] [CrossRef]
- Silvestro, I.; Ciarlantini, C.; Francolini, I.; Tomai, P.; Gentili, A.; Dal Bosco, C.; Piozzi, A. Chitosan–graphene oxide composite membranes for solid-phase extraction of pesticides. Int. J. Mol. Sci. 2021, 22, 8374. [Google Scholar] [CrossRef]
- Apriceno, A.; Silvestro, I.; Girelli, A.; Francolini, I.; Pietrelli, L.; Piozzi, A. Preparation and characterization of chitosan-coated manganese-ferrite nanoparticles conjugated with laccase for environmental bioremediation. Polymers 2021, 13, 1453. [Google Scholar] [CrossRef]
- Pietrelli, L.; Francolini, I.; Piozzi, A.; Sighicelli, M.; Silvestro, I.; Vocciante, M. Chromium (III) removal from wastewater by chitosan flakes. Appl. Sci. 2020, 10, 1925. [Google Scholar] [CrossRef]
- Laleg, M.; Pikulik, I.I. Wet-web strength increase by chitosan. Nord. Pulp Pap. Res. J. 1991, 6, 99–103. [Google Scholar] [CrossRef]
- Reis, A.B.; Yoshida, C.M.; Reis, A.P.C.; Franco, T.T. Application of chitosan emulsion as a coating on Kraft paper. Polym. Int. 2011, 60, 963–969. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Song, Z.; Qian, X. Preparation and Application of Maleic Anhydride-Acylated Chitosan for Wet Strength Improvement of Paper. BioResources 2013, 8, 3901–3911. [Google Scholar] [CrossRef]
- Einchhorn, S.J.; Dufresne, A.; Aranguren, M.M.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Veigel, S. Review: Current international research into cellulose nanofibres and composites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123. [Google Scholar] [CrossRef]
- Sehaqui, H.; Berglund, L.A.; Zhou, Q. BIOREFINERY: Nanofibrillated cellulose for enhancement of strength in high-density paper structures. Nord. Pulp Pap. Res. J. 2013, 28, 182–189. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, Y.; Kuga, S.; Wu, M.; Huang, Y. A versatile method for producing functionalized cellulose nanofibers and their application. Nanoscale 2016, 8, 3753–3759. [Google Scholar] [CrossRef]
- Ahola, S.; Österberg, M.; Laine, J. Cellulose nanofibrils—Adsorption with poly (amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive. Cellulose 2008, 15, 303–314. [Google Scholar] [CrossRef]
- Wang, A.; Wang, L.; Jiang, J.; Yao, X.; Zhao, W. Reinforcing paper strength by dual treatment of a cationic water-soluble polymer and cellulose nanofibril. Pap. Biomater. 2019, 4, 31–36. [Google Scholar]
- Charani, P.R.; Moradian, M.H. Utilization of cellulose nanofibers and cationic polymers to improve breaking length of paper. Cellul. Chem. Technol. 2019, 53, 767. [Google Scholar] [CrossRef]
- Gardlund, L.; Wagberg, L.; Gernandt, R. Polyelectrolyte complexes for surface modification of wood fibres II. Influence of complexes on wet and dry strength of paper. Colloids Surf. A 2003, 218, 137–149. [Google Scholar] [CrossRef]
- Tajik, M.; Torshizi, H.J.; Resalati, H.; Hamzeh, Y. Effects of cellulose nanofibrils and starch compared with polyacrylamide on fundamental properties of pulp and paper. Int. J. Biol. Macromol. 2021, 192, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, A.H.; Hubbe, M.A.; Tayeb, P.; Pal, L.; Rojas, O.J. Soy proteins as a sustainable solution to strengthen recycled paper and reduce deposition of hydrophobic contaminants in papermaking: A bench and pilot-plant study. ACS Sustain. Chem. Eng. 2017, 5, 7211–7219. [Google Scholar] [CrossRef]
- Jin, H.; Lucia, L.A.; Rojas, O.J.; Hubbe, M.A.; Pawlak, J.J. Survey of soy protein flour as a novel dry strength agent for papermaking furnishes. J. Agric. Food Chem. 2012, 60, 9828–9833. [Google Scholar] [CrossRef]
- Arboleda, J.C.; Niemi, N.; Kumpunen, J.; Lucia, L.A.; Rojas, O.J. Soy protein-based polyelectrolyte complexes as biobased wood fiber dry strength agents. ACS Sustain. Chem. Eng. 2014, 2, 2267–2274. [Google Scholar] [CrossRef]
- Xu, H.; Ma, S.; Lv, W.; Wang, Z. Soy protein adhesives improved by SiO2 nanoparticles for plywoods. Pigm. Resin Technol. 2011, 40, 191. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, L.; Gao, Z.; Zhang, Y.; Shi, J.; Li, J. Formulation of a novel soybean protein-based wood adhesive with desired water resistance and technological applicability. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Lu, Y.; Gao, Z.; Gu, J. Water-resistant soybean adhesive for wood binder employing combinations of caustic degradation, nano-modification, and chemical crosslinking. BioResources 2013, 8, 1283. [Google Scholar] [CrossRef]
- Lamaming, S.Z.; Lamaming, J.; Rawi, N.F.M.; Hashim, R.; Kassim, M.H.M.; Hussin, M.H.; Bustami, Y.; Sulaiman, O.; Amini, M.H.M.; Hiziroglu, S. Improvements and limitation of soy protein-based adhesive: A review. Polym. Eng. Sci. 2021, 61, 2393–2405. [Google Scholar] [CrossRef]
- Li, X.; Pelton, R. Enhancing wet cellulose adhesion with proteins. Ind. Eng. Chem. Res. 2005, 44, 7398–7404. [Google Scholar] [CrossRef]
- Tian, P.; Shi, M.; Hou, J.; Fu, P. Cellulose-Graphene Bifunctional Paper Conservation Materials: For Reinforcement and UV Aging Protection. Coatings 2023, 13, 443. [Google Scholar] [CrossRef]
- Milotskyi, R.; Serizawa, R.; Yanagisawa, K.; Sharma, G.; Ito, E.R.D.; Fujie, T.; Wada, N.; Takahashi, K. Composite of Cellulose-Nanofiber-Reinforced Cellulose Acetate Butyrate: Improvement of Mechanical Strength by Cross-Linking of Hydroxyl Groups. J. Compos. Sci. 2023, 7, 130. [Google Scholar] [CrossRef]
- Jordan, J.H.; Cheng, H.N.; Easson, M.W.; Yao, W.; Condon, B.D.; Gibb, B.C. Effect of nanocellulose on the properties of cottonseed protein isolate as a paper strength agent. Materials 2021, 14, 4128. [Google Scholar] [CrossRef]
- Obokata, T.; Isogai, A. The mechanism of wet-strength development of cellulose sheets prepared with polyamideamine-epichlorohydrin (PAE) resin. Colloids Surf. 2007, 302, 525–531. [Google Scholar] [CrossRef]
- Marrani, A.G.; Motta, A.; Amato, F.; Schrebler, R.; Zanoni, R.; Dalchiele, E.A. Effect of electrolytic medium on the electrochemical reduction of graphene oxide on Si(111) as probed by XPS. Nanomaterials 2021, 12, 43. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Chen, S.; Tanaka, H. Surface analysis of paper containing polymer additives by X-ray photoelectron spectroscopy I: Application to paper containing dry strength additives. J. Wood Sci. 1998, 44, 303–309. [Google Scholar] [CrossRef]
- Pahimanolis, N.; Salminen, A.; Penttilä, P.A.; Korhonen, J.T.; Johansson, L.S.; Ruokolainen, J.; Serimaa, R.; Seppälä, J. Nanofibrillated cellulose/carboxymethyl cellulose composite with improved wet strength. Cellulose 2013, 20, 1459–1468. [Google Scholar] [CrossRef]
- Lai, X.; Song, Y.; Liu, M. Preparation and application of cationic blocked waterborne polyurethane as paper strength agent. J. Polym. Res. 2013, 20, 222. [Google Scholar] [CrossRef]
- Hua, L.; Flodin, P.; Rönnhult, T. Cellulose fiber-polyester composites with reduced water sensitivity (2)—Surface analysis. Polym. Compos. 1987, 8, 203–207. [Google Scholar] [CrossRef]
- Xue, Y.; Li, W.; Yang, G.; Lin, Z.; Qi, L.; Zhu, P.; Yu, J.; Chen, J. Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid. Polymers 2022, 14, 2030. [Google Scholar] [CrossRef] [PubMed]
- Häggkvist, M.; Solberg, D.; Wågberg, L.; Ödberg, L. The influence of two wet strength agents on pore size and swelling of pulp fibres and on tensile strength properties. Nord. Pulp Pap. Res. J. 1998, 13, 292–298. [Google Scholar] [CrossRef]
- Su, J.; Mosse, W.K.; Sharman, S.; Batchelor, W.; Garnier, G. Paper strength development and recyclability with polyamideamine-epichlorohydrin (PAE). BioResources 2012, 7, 913–924. [Google Scholar]
- Paper and Board—Determination of Tensile Strength after Immersion in Water (ISO 3781:2011). Available online: https://www.iso.org/obp/ui/#iso:std:iso:3781:ed-3:v1:en (accessed on 8 May 2023).
- Standard Test Methods for Wet Tensile Breaking Strength of Paper and Paper Products (ASTM: D 829-97). Available online: https://file.yzimgs.com/175706/2011090910281462.pdf (accessed on 8 May 2023).
- Ichiura, H.; Hirose, Y.; Masumoto, M.; Ohtani, Y. Ionic liquid treatment for increasing the wet strength of cellulose paper. Cellulose 2017, 24, 3469–3477. [Google Scholar] [CrossRef]
- Yang, D.; DiFlavio, J.L.; Gustafsson, E.; Pelton, R. Wet-peel: A tool for comparing wet-strength resins. Nord. Pulp Pap. Res. J. 2018, 33, 632–646. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Long, J.; Hu, J. Mechanical and dynamic mechanical analysis of PBO paper-based composites. Text. Res. J. 2022, 92, 1454–1465. [Google Scholar] [CrossRef]
- Mihara, I.; Sakaemura, T.; Yamauchi, T. Mechanism of paper strength development by the addition of dry strength resin and its distribution within and around a fiber wall. Nord. Pulp Pap. Res. J. 2008, 23, 382–388. [Google Scholar] [CrossRef]
- Mihara, I.; Yamauchi, T. Dynamic mechanical properties of paper containing a polyacrylamide dry-strength resin additive and its distribution within a fiber wall: Effect of the application method. J. Appl. Polym. Sci. 2008, 110, 3836–3842. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric analysis properties of cellulosic natural fiber polymer composites: A review on influence of chemical treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Sun, B.; Zhang, R. Improving the wet strength of hemicelluloses based composite films by citric acid crosslinking. J. Wood Chem. Technol. 2021, 41, 1–9. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Jiao, M.; Hu, L. Lignin as a wood-inspired binder enabled strong, water stable, and biodegradable paper for plastic replacement. Adv. Funct. Mater. 2020, 30, 1906307. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell. Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Di Consiglio, M.; Sturabotti, E.; Brugnoli, B.; Piozzi, A.; Migneco, L.M.; Francolini, I. Synthesis of sustainable eugenol/hydroxyethylmethacrylate-based polymers with antioxidant and antimicrobial properties. Polym. Chem. 2023, 14, 432–442. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Du, G.; Belić, D.; Del Giudice, A.; Alfredsson, V.; Carnerup, A.M.; Zhu, K.; Nyström, B.; Wang, Y.; Galantini, L.; Schillén, K. Condensed supramolecular helices: The twisted sisters of DNA. Angew. Chem. 2022, 134, e202113279. [Google Scholar] [CrossRef]
- Cautela, J.; Stenqvist, B.; Schillén, K.; Belić, D.; Månsson, L.K.; Hagemans, F.; Seuss, M.; Fery, A.; Crassous, J.J.; Galantini, L. Supracolloidal Atomium. ACS Nano 2020, 14, 15748–15756. [Google Scholar] [CrossRef]
- Morais, F.P.; Carta, A.M.M.; Amaral, M.E.; Curto, J.M. Cellulose fiber enzymatic modification to improve the softness, strength, and absorption properties of tissue papers. BioResources 2021, 16, 846. [Google Scholar] [CrossRef]
- Martinelli, A.; Giannini, L.; Branduardi, P. Enzymatic modification of cellulose to unlock its exploitation in advanced materials. ChemBioChem 2021, 22, 974–981. [Google Scholar] [CrossRef]
- Witayakran, S.; Ragauskas, A.J. Modification of high-lignin softwood kraft pulp with laccase and amino acids. Enzym. Microb. Technol. 2009, 44, 176–181. [Google Scholar] [CrossRef]
- Ballinas-Casarrubias, L.; Villanueva-Solís, L.; Espinoza-Hicks, C.; Camacho-Dávila, A.; Piñón Castillo, H.A.; Pérez, S.B.; Duarte Villa, E.; De Dios Hernández, M.; González-Sánchez, G. Effect of laccase-mediated biopolymer grafting on Kraft pulp fibers for enhancing paper’s mechanical properties. Polymers 2017, 9, 570. [Google Scholar] [CrossRef] [PubMed]
| Wet Strength Agents | |||
|---|---|---|---|
| Synthetic Resin | Structure | Natural Resin | Structure |
| Polyamidemine-epichlorohydrin (PAE) | 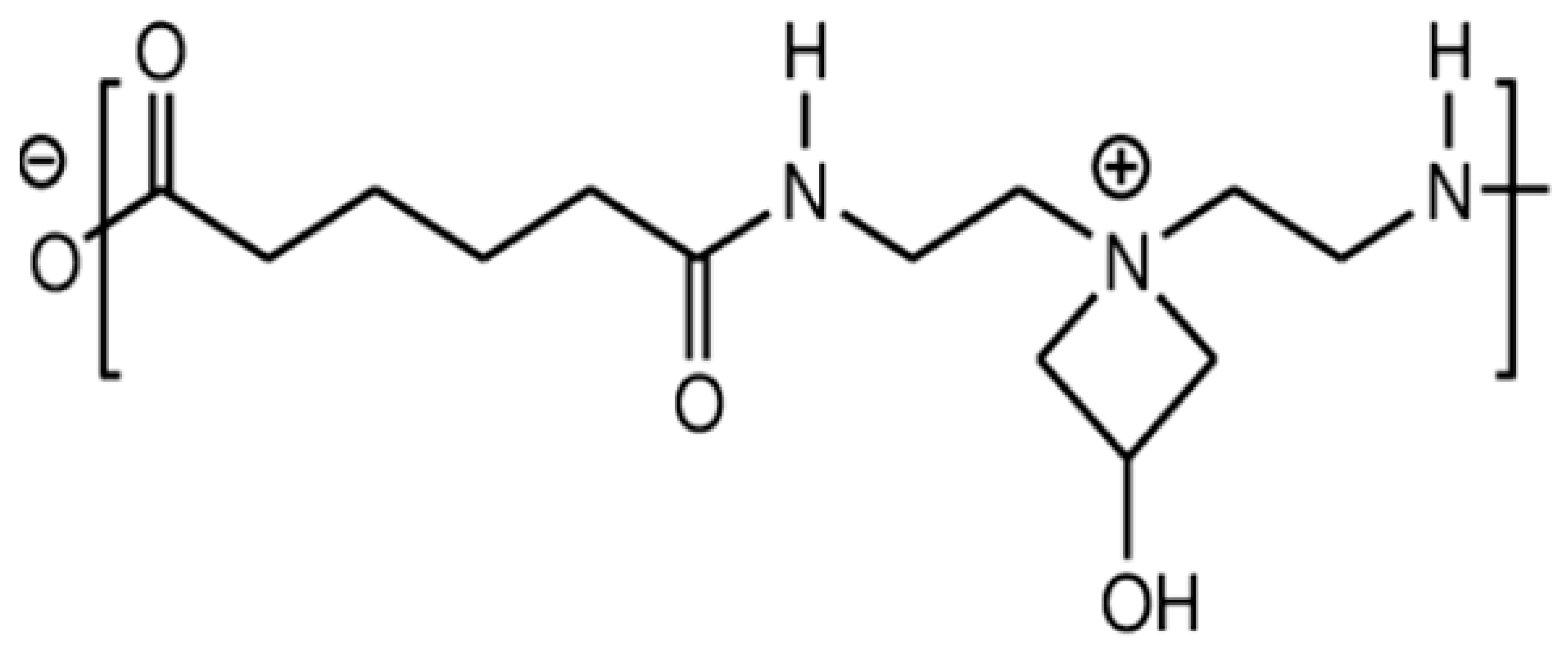 | Starch | 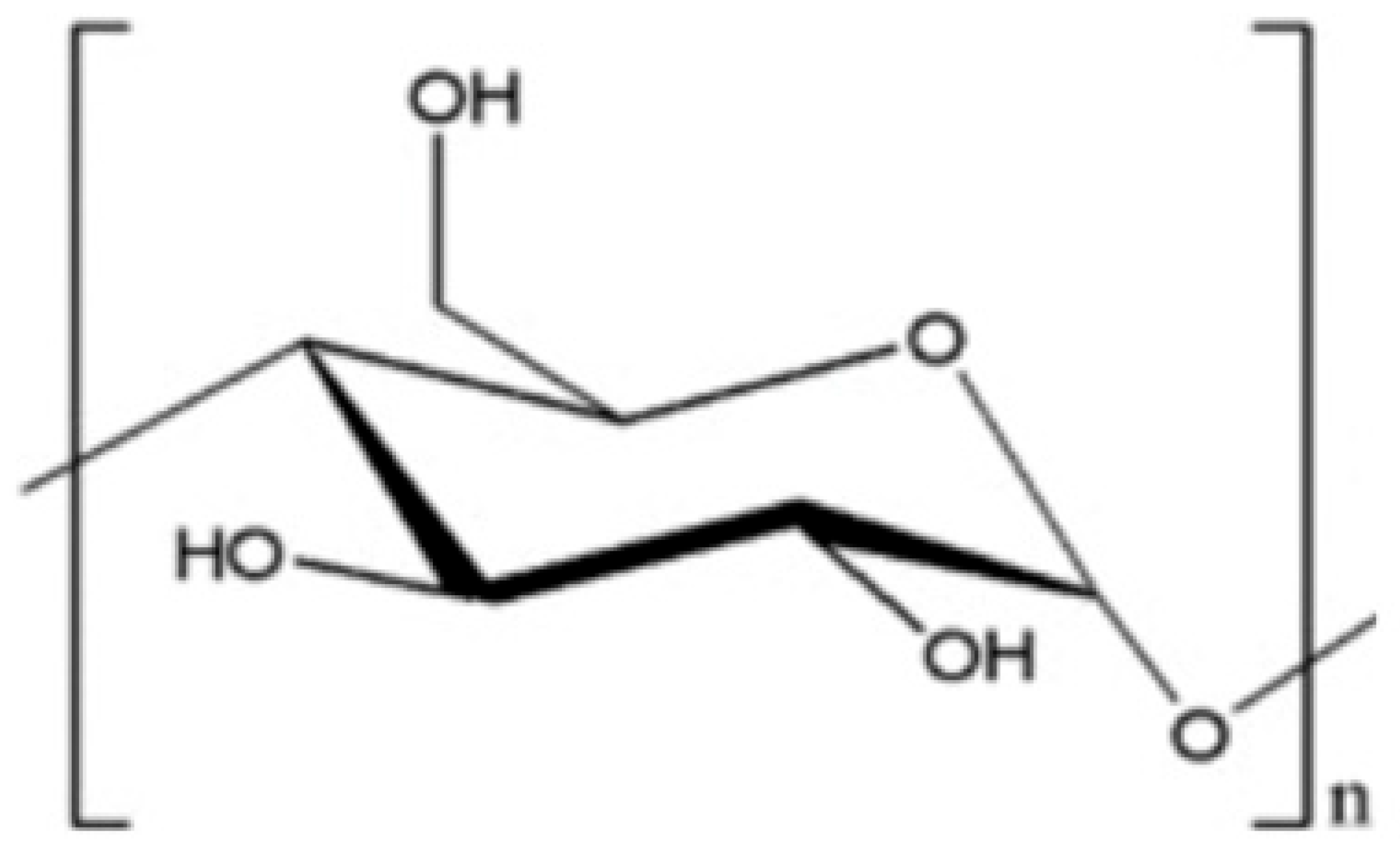 |
| Melamine formaldehyde (MF) | 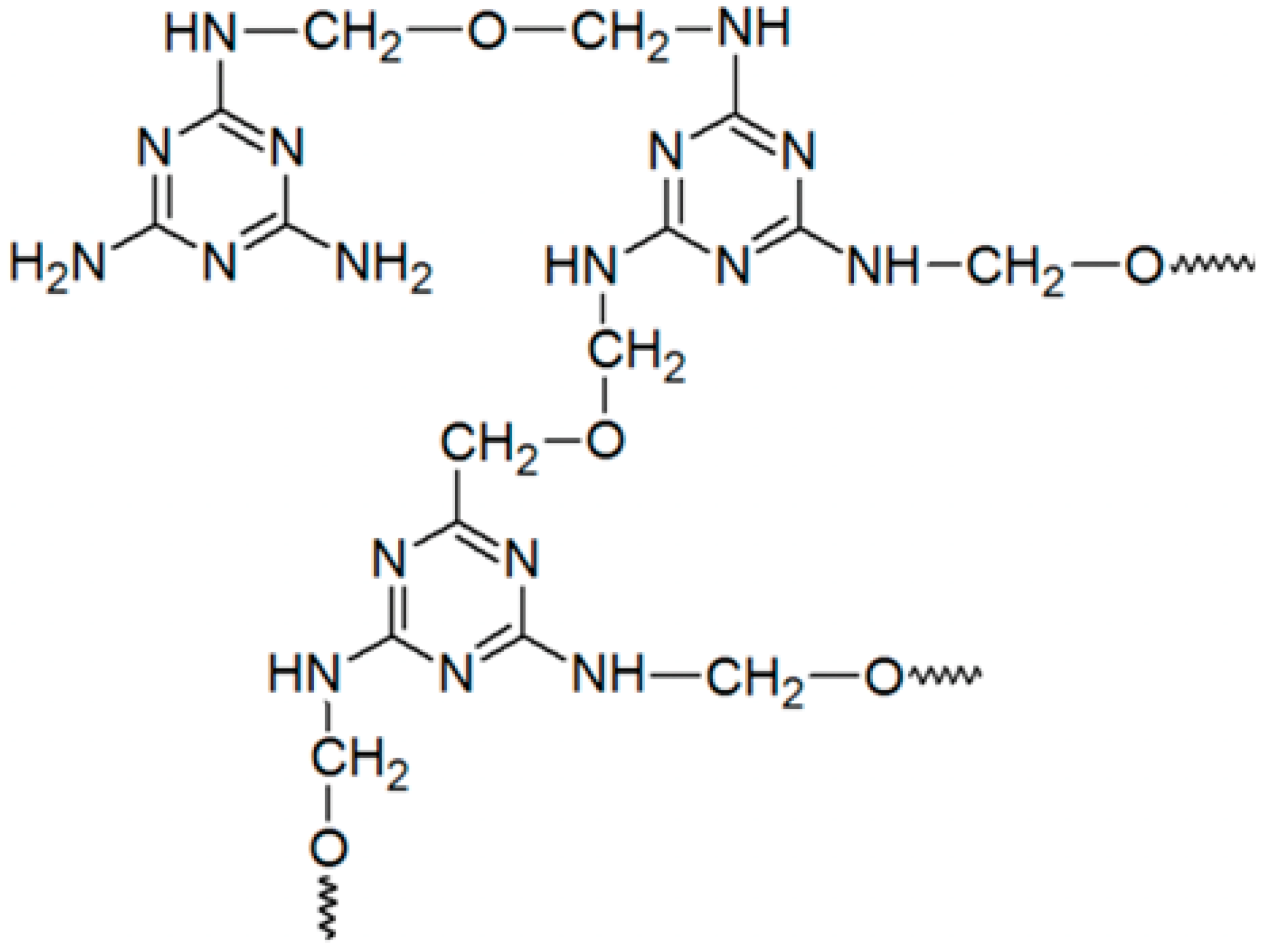 | Cationic starch | 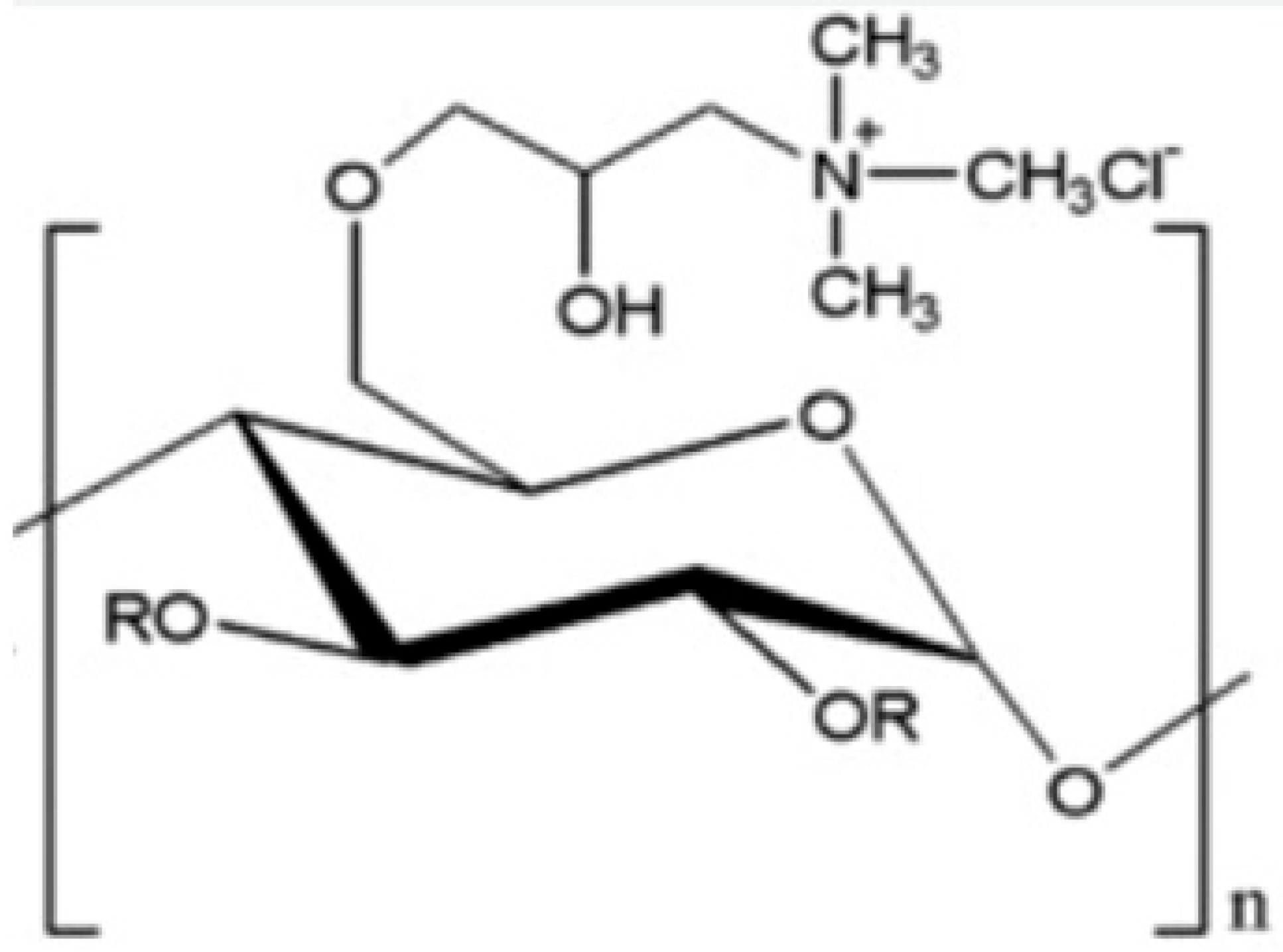 |
| Polyacrylamide (PAM) |  | Chitosan | 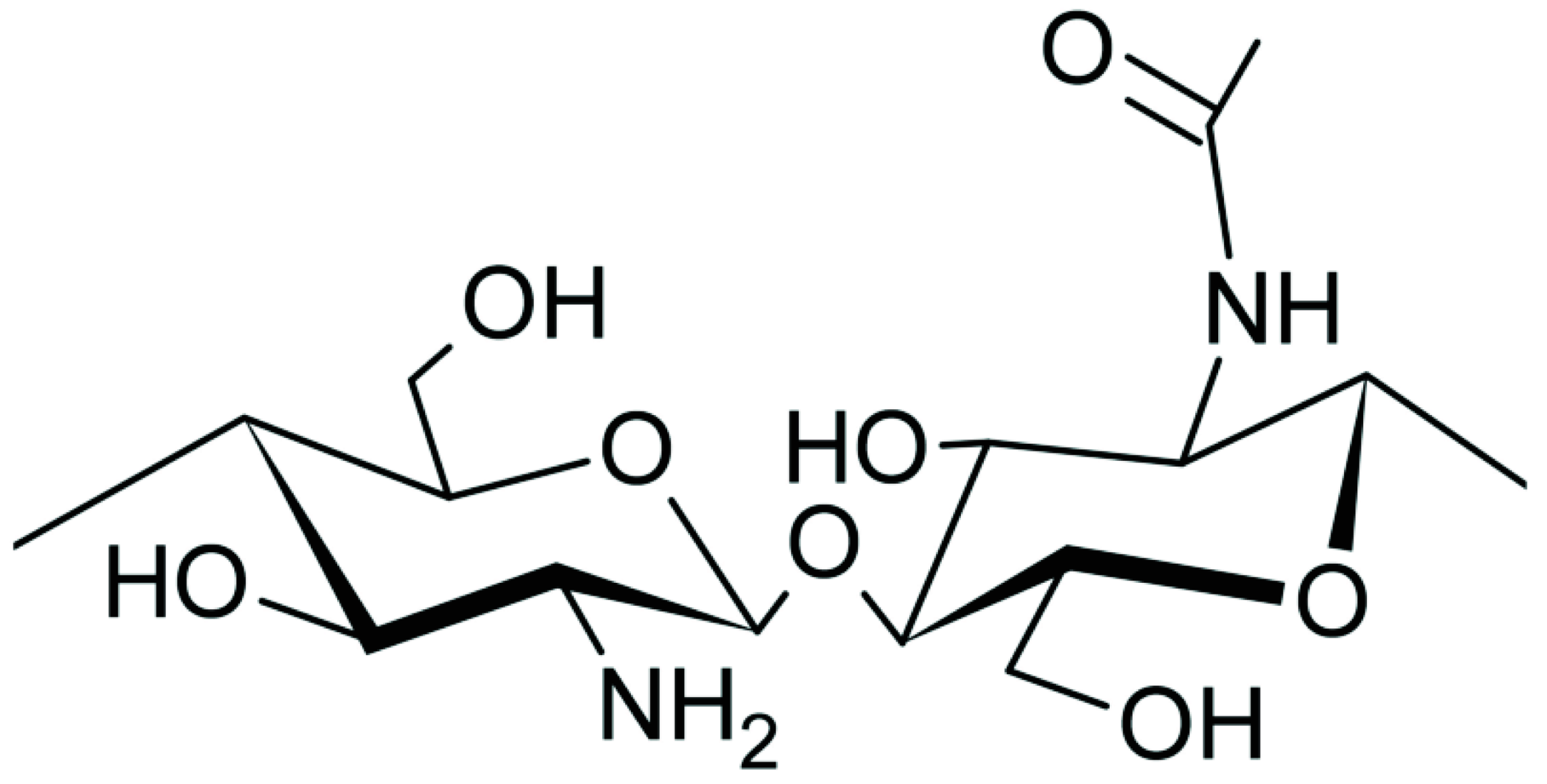 |
| Glyoxylated polyacrylamide (GPAM) | 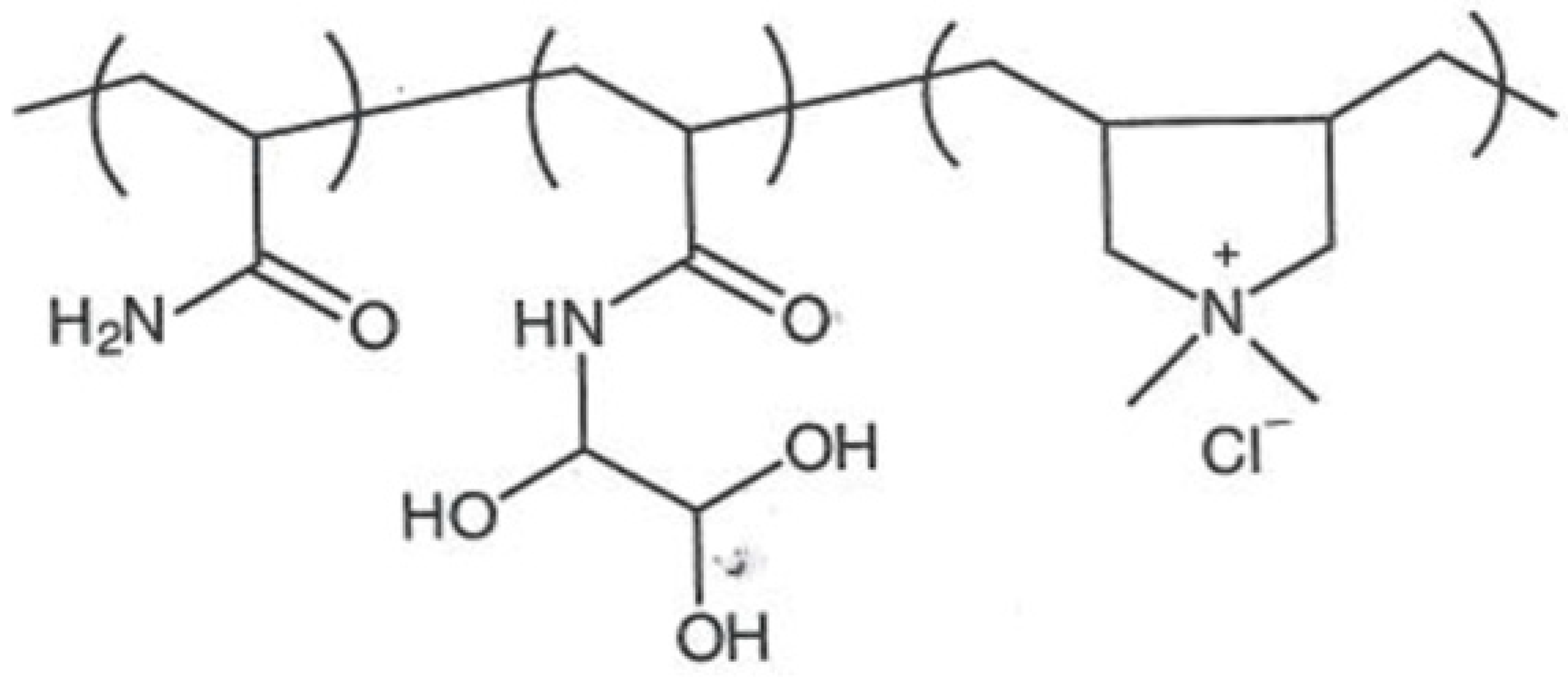 | Cellulose nanofibrils (CNF) | 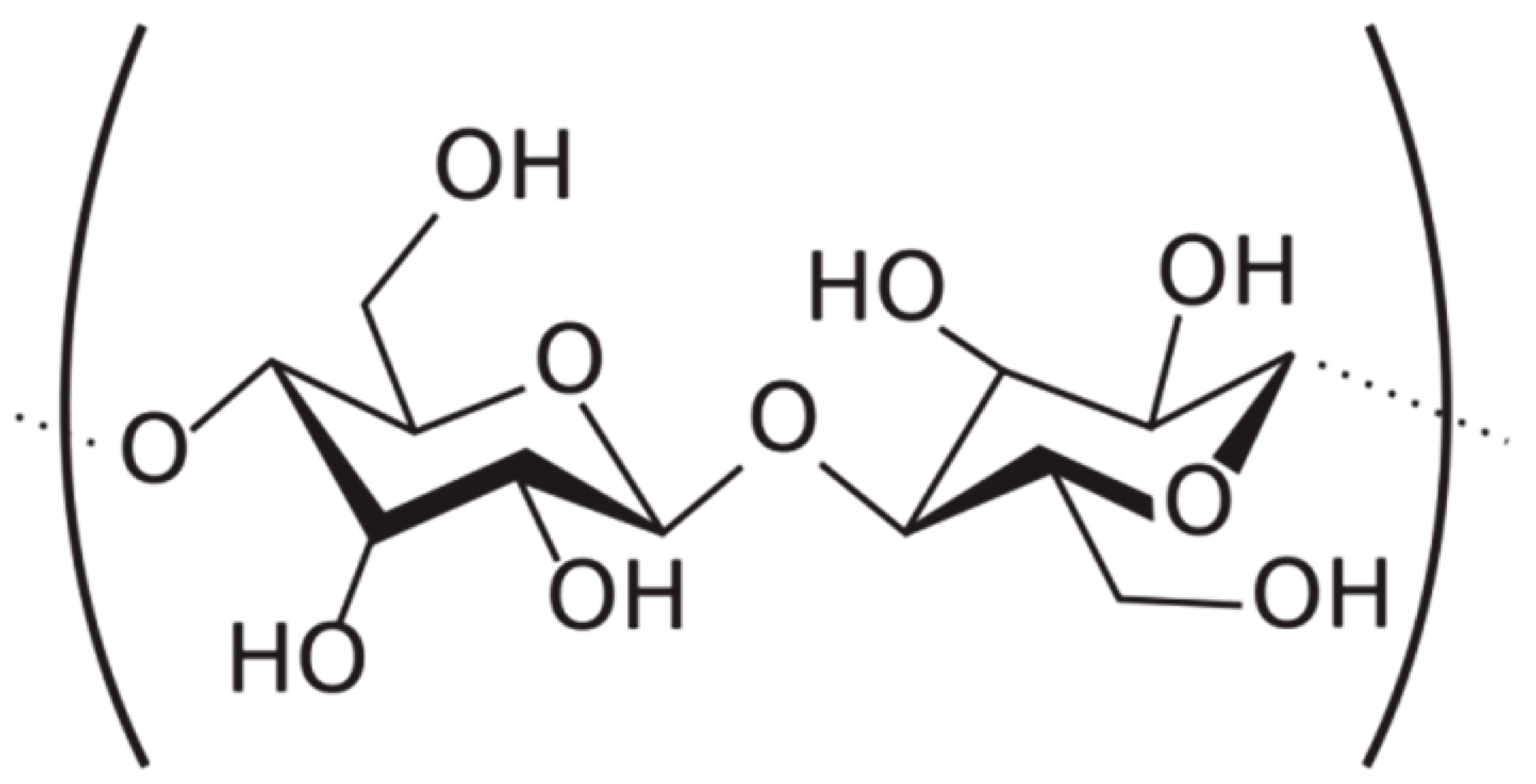 |
| Polyethyleneimine (PEI) | 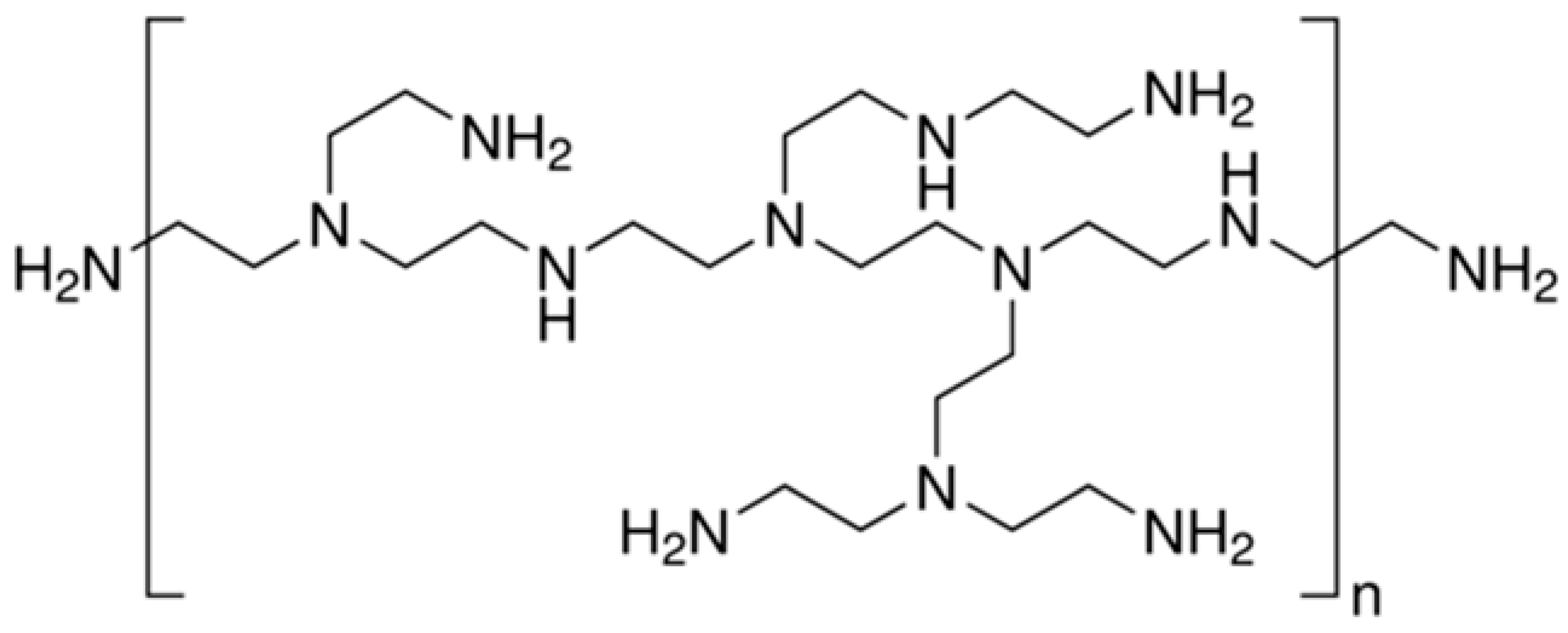 | Soy protein | - |
| Polyvinylamine (PVAm) | 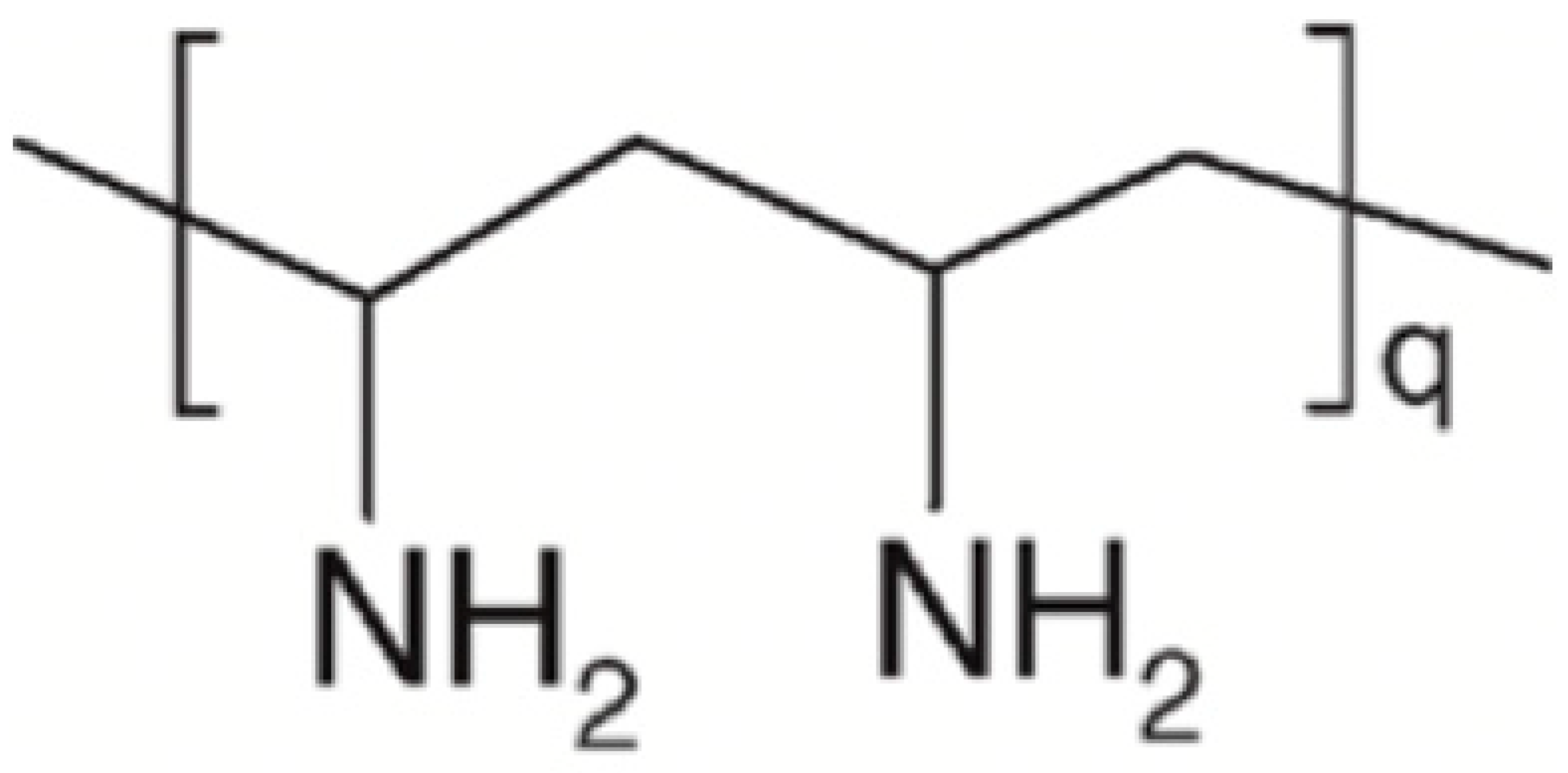 | Lignin | 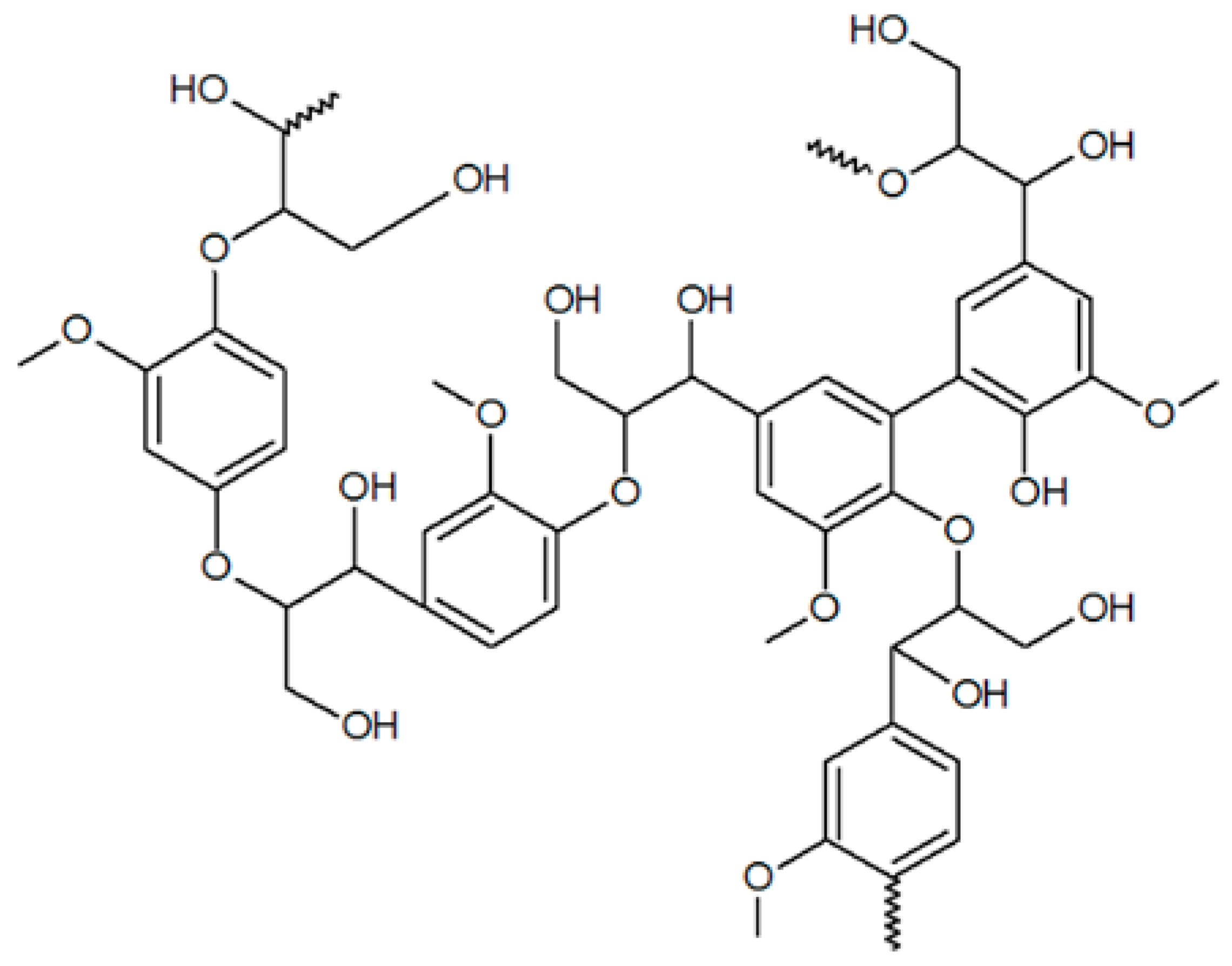 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francolini, I.; Galantini, L.; Rea, F.; Di Cosimo, C.; Di Cosimo, P. Polymeric Wet-Strength Agents in the Paper Industry: An Overview of Mechanisms and Current Challenges. Int. J. Mol. Sci. 2023, 24, 9268. https://doi.org/10.3390/ijms24119268
Francolini I, Galantini L, Rea F, Di Cosimo C, Di Cosimo P. Polymeric Wet-Strength Agents in the Paper Industry: An Overview of Mechanisms and Current Challenges. International Journal of Molecular Sciences. 2023; 24(11):9268. https://doi.org/10.3390/ijms24119268
Chicago/Turabian StyleFrancolini, Iolanda, Luciano Galantini, Fernando Rea, Cristiano Di Cosimo, and Pierpaolo Di Cosimo. 2023. "Polymeric Wet-Strength Agents in the Paper Industry: An Overview of Mechanisms and Current Challenges" International Journal of Molecular Sciences 24, no. 11: 9268. https://doi.org/10.3390/ijms24119268
APA StyleFrancolini, I., Galantini, L., Rea, F., Di Cosimo, C., & Di Cosimo, P. (2023). Polymeric Wet-Strength Agents in the Paper Industry: An Overview of Mechanisms and Current Challenges. International Journal of Molecular Sciences, 24(11), 9268. https://doi.org/10.3390/ijms24119268










