Sustainable Cellulose Nanofibers-Mediated Synthesis of Uniform Spinel Zn-Ferrites Nanocorals for High Performances in Supercapacitors
Abstract
1. Introduction
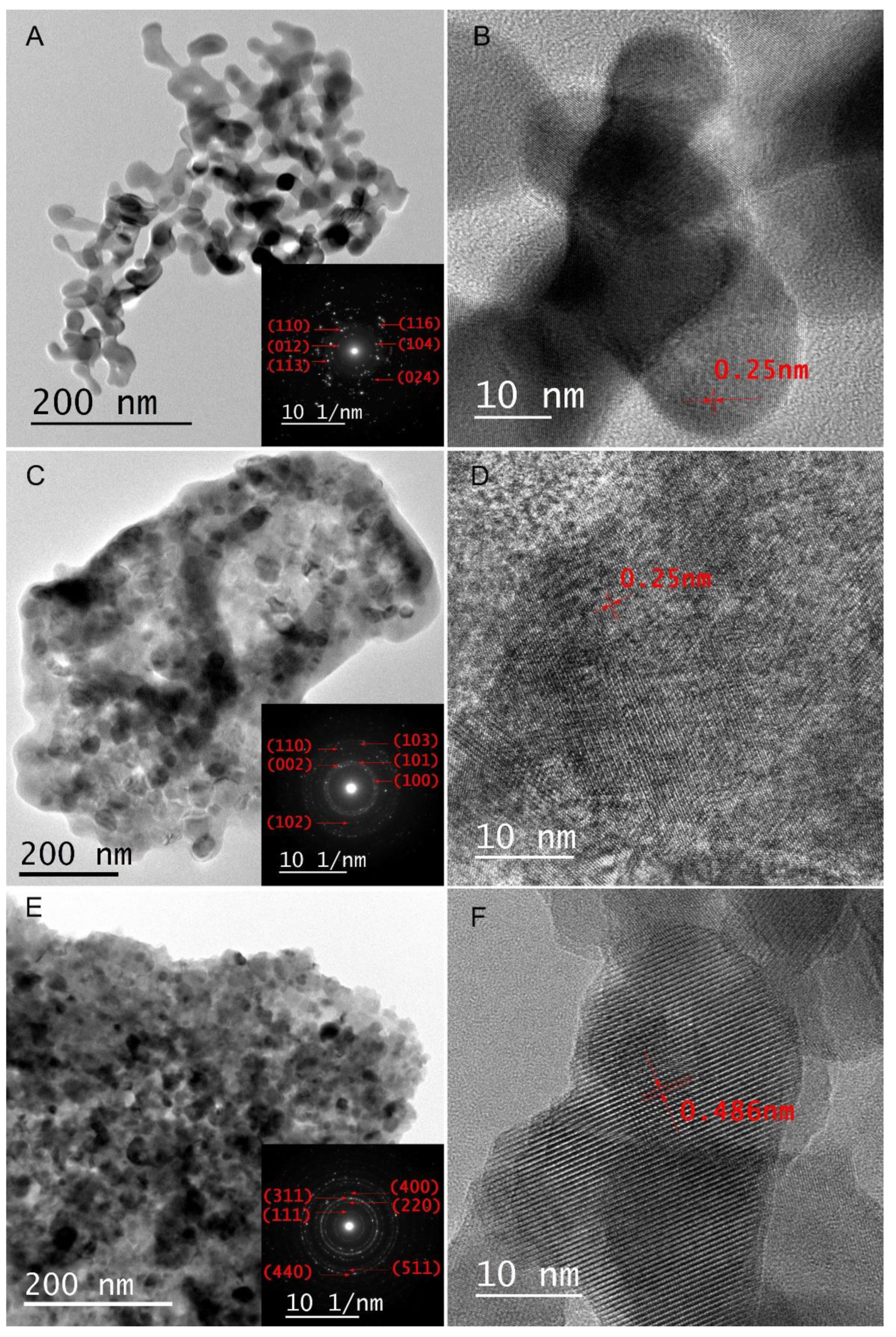
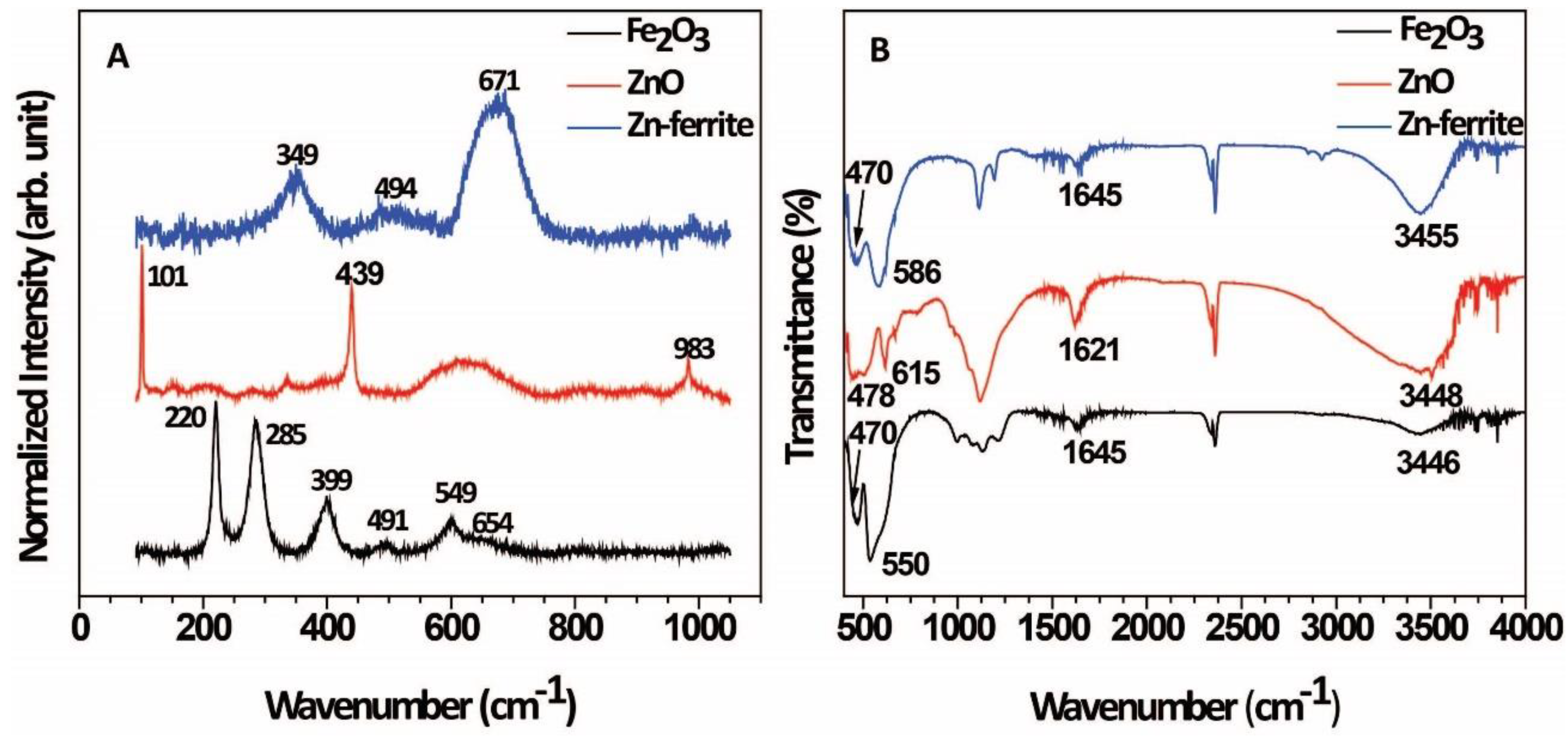
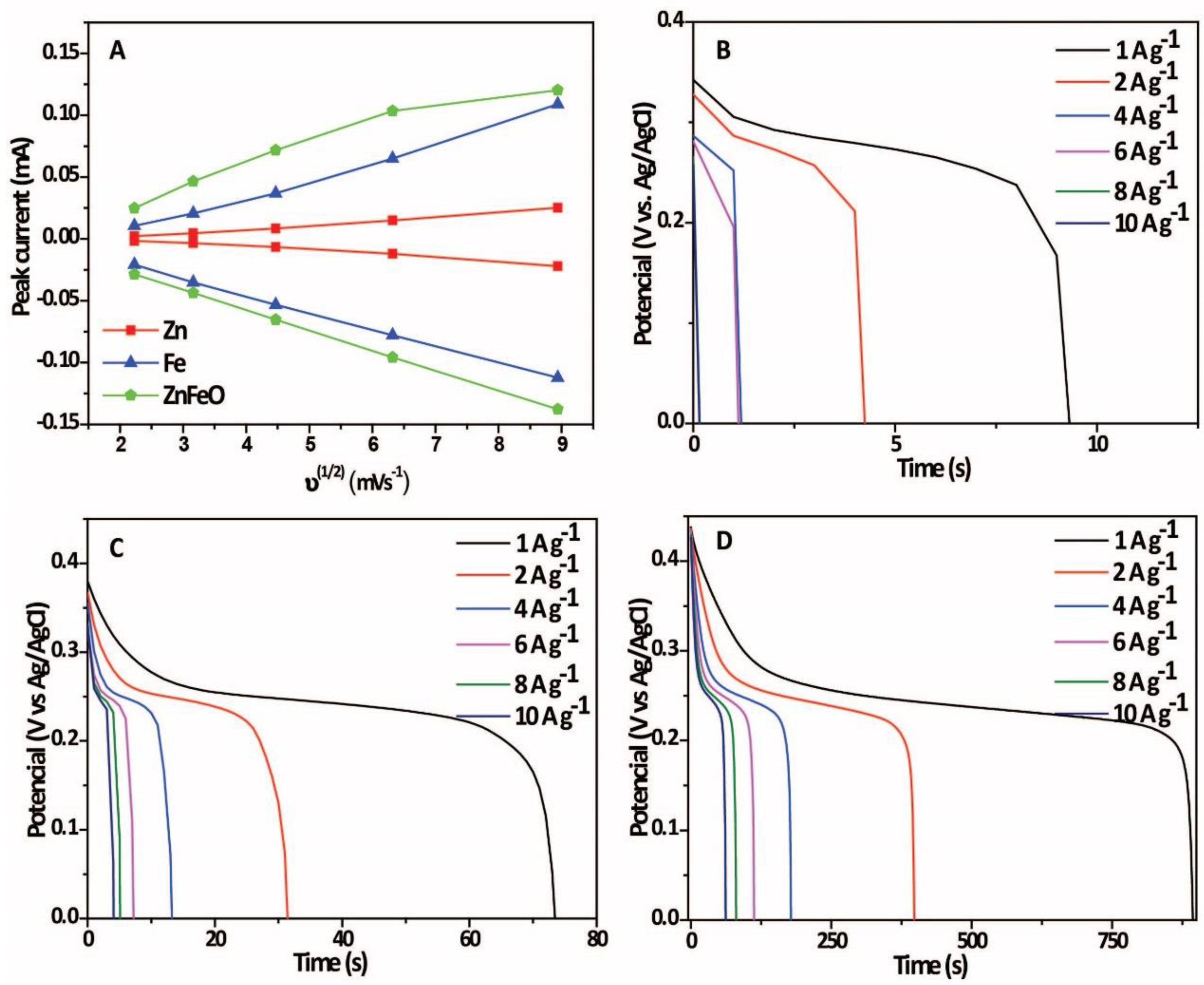
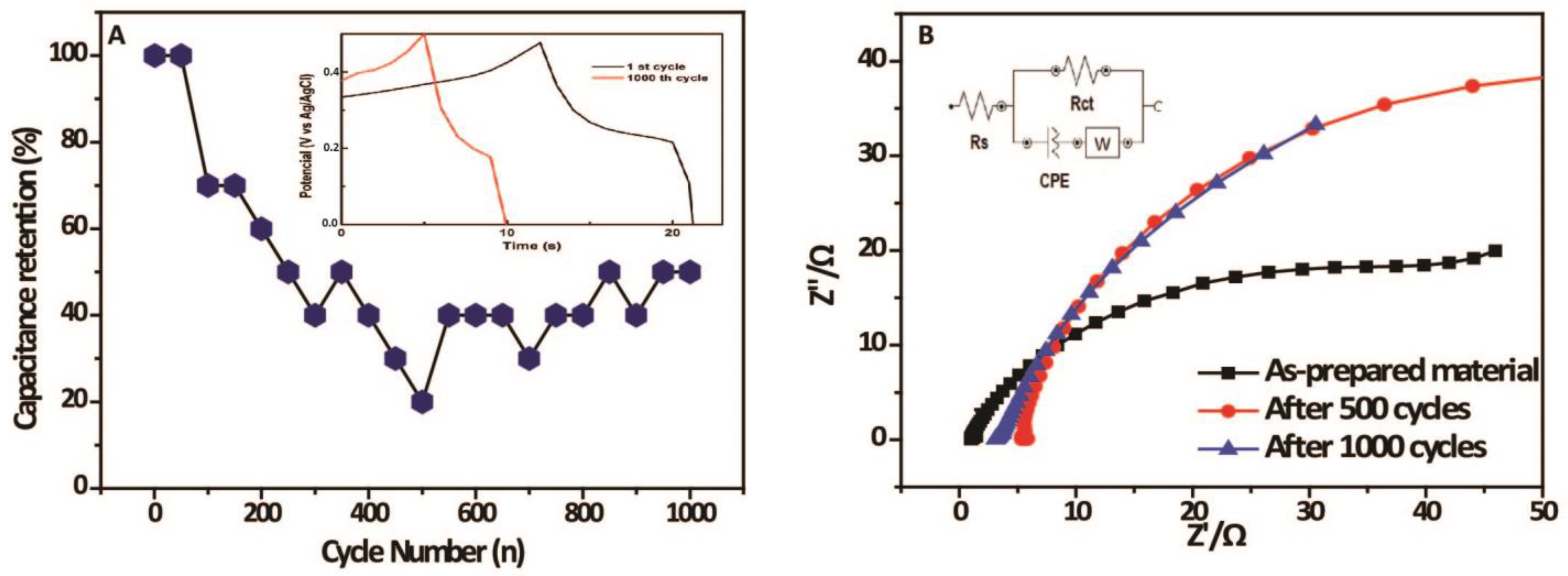
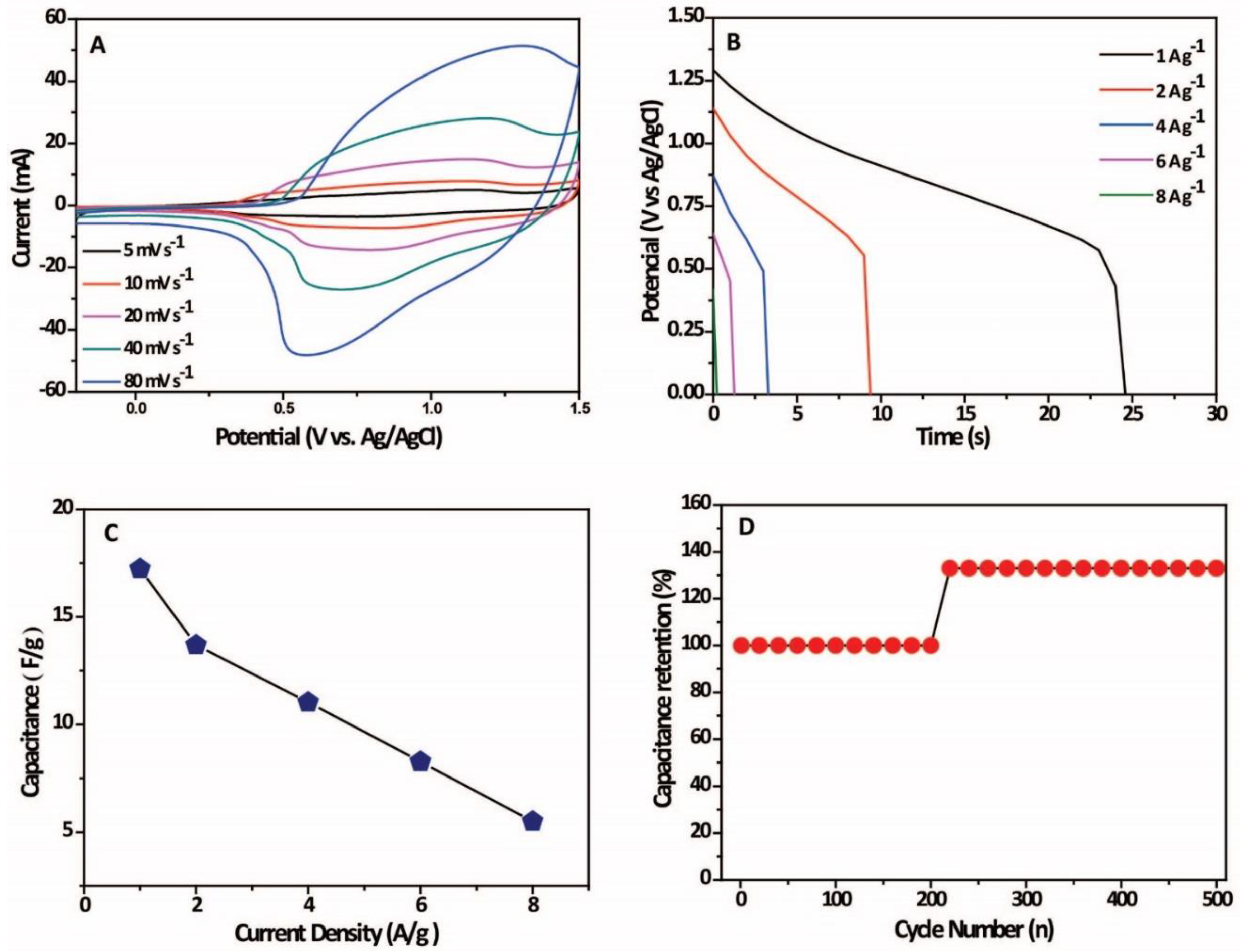
2. Results and Discussion
3. Materials and Methods
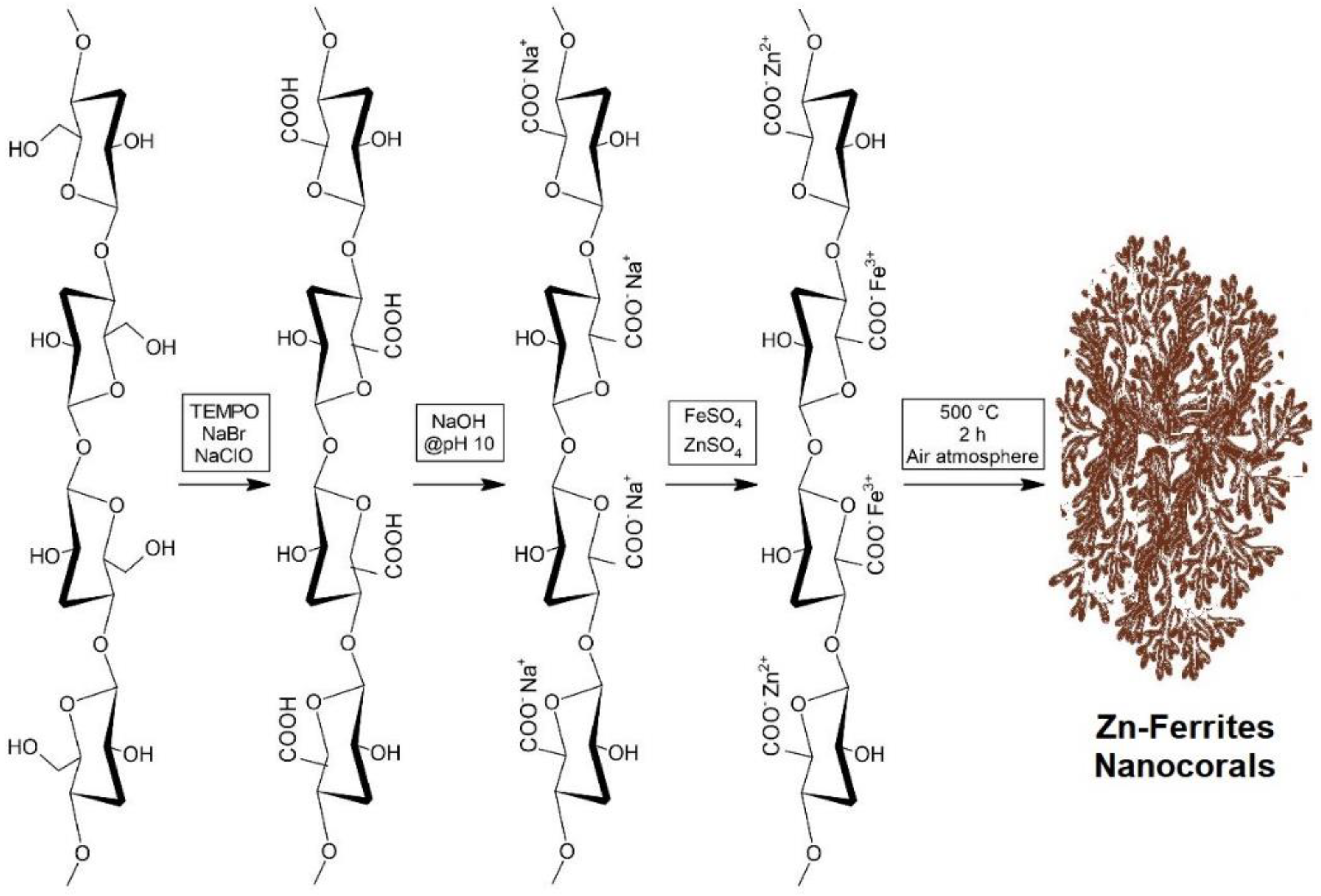
3.1. TCNF Synthesis
3.2. Synthesis of Nanocorals Mediated by TCNF
3.3. Electrode Preparation
Manufacture of Asymmetric Supercapacitor Cell
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Kumar, Y.; Yogeshwar, P.; Bajpai, S.; Jaiswal, P.; Yadav, S.; Pathak, D.P.; Sonker, M.; Tiwary, S.K. Nanomaterials: Stimulants for biofuels and renewables, yield and energy optimization. Mater. Adv. 2021, 2, 5318–5343. [Google Scholar] [CrossRef]
- Anvari, M.; Lohmann, G.; Wächter, M.; Milan, P.; Lorenz, E.; Heinemann, D.; Tabar, M.R.R.; Peinke, J. Short term fluctuations of wind and solar power systems. New J. Phys. 2016, 18, 063027. [Google Scholar] [CrossRef]
- Benavides, D.; Arévalo, P.; Tostado-Véliz, M.; Vera, D.; Escamez, A.; Aguado, J.A.; Jurado, F. An experimental study of power smoothing methods to reduce renewable sources fluctuations using supercapacitors and lithium-ion batteries. Batteries 2022, 8, 228. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent Progress in metal-organic framework-based supercapacitor electrode materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Lu, H.; Kim, K.; Kwon, Y.; Sun, X.; Ryoo, R.; Zhao, X.S. Zeolite-Templated nanoporous carbon for high-performance supercapacitors. J. Mater. Chem. A Mater. 2018, 6, 10388–10394. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Cheng, Q.; Li, J.; Liao, F.; Yang, G.; Xie, L.; Zhao, C.; Chen, L. Two-dimensional spinel structured Co-based materials for high performance supercapacitors: A critical review. Chem. Eng. J. 2020, 387, 124081. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, W.; Xu, G.; Chou, T.W. Structural supercapacitor composites: A review. Compos. Sci. Technol. 2021, 204, 108636. [Google Scholar] [CrossRef]
- Malaie, K.; Ganjali, M.R. Spinel nano-ferrites for aqueous supercapacitors; linking abundant resources and low-cost processes for sustainable energy storage. J. Energy Storage 2021, 33, 102097. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Zhu, X.; Liu, Q. One-step preparation of one dimensional nickel ferrites/graphene composites for supercapacitor electrode with excellent cycling stability. J. Power Sources 2018, 396, 41–48. [Google Scholar] [CrossRef]
- Shinde, A.V.; Patil, S.J.; Hwang, S.K.; Rama Raju, G.S.; Huh, Y.S.; Han, Y.K.; Chodankar, N.R. Surface modified zinc ferrite as a carbon-alternative negative electrode for high-energy hybrid supercapacitor. Ceram. Int. 2021, 47, 16333–16341. [Google Scholar] [CrossRef]
- Sathiyamurthy, K.; Rajeevgandhi, C.; Guganathan, L.; Bharanidharan, S.; Savithiri, S. Enhancement of magnetic, supercapacitor applications and theoretical approach on cobalt-doped zinc ferrite nanocomposites. J. Mater. Sci. Mater. Electron. 2021, 32, 11593–11606. [Google Scholar] [CrossRef]
- Ismail, F.M.; Ramadan, M.; Abdellah, A.M.; Ismail, I.; Allam, N.K. Mesoporous spinel manganese zinc ferrite for high-performance supercapacitors. J. Electroanal. Chem. 2018, 817, 111–117. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Kolekar, S.S.; Chang, J.Y.; Ye, Z.; Ghule, A.V. Anchoring ultrafine ZnFe2O4/C nanoparticles on 3D ZnFe2O4 nanoflakes for boosting cycle stability and energy density of flexible asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 26016–26028. [Google Scholar] [CrossRef]
- Thakur, P.; Chahar, D.; Taneja, S.; Bhalla, N.; Thakur, A. A review on MnZn ferrites: Synthesis, characterization and applications. Ceram. Int. 2020, 46, 15740–15763. [Google Scholar] [CrossRef] [PubMed]
- Rana, G.; Dhiman, P.; Kumar, A.; Vo, D.V.N.; Sharma, G.; Sharma, S.; Naushad, M. Recent advances on nickel nano-ferrite: A review on processing techniques, properties and diverse applications. Chem. Eng. Res. Des. 2021, 175, 182–208. [Google Scholar] [CrossRef]
- Shinde, P.V.; Shinde, N.M.; Mane, R.S.; Kim, K.H. Ferrites for electrochemical supercapacitors. In Spinel Ferrite Nanostructures for Energy Storage Devices; Elsevier: Amsterdam, The Netherlands, 2020; pp. 83–122. [Google Scholar]
- Elkholy, A.E.; Heakal, F.E.; Allam, N.K. Nanostructured Spinel manganese cobalt ferrite for high-performance supercapacitors. RSC Adv. 2017, 7, 51888–51895. [Google Scholar] [CrossRef]
- Ranga, R.; Kumar, A.; Kumari, P.; Singh, P.; Madaan, V.; Kumar, K. Ferrite application as an electrochemical sensor: A review. Mater. Charact. 2021, 178, 111269. [Google Scholar] [CrossRef]
- Vadivel, M.; Babu, R.R.; Arivanandhan, M.; Ramamurthi, K.; Hayakawa, Y. Role of SDS surfactant concentrations on the structural, morphological, dielectric and magnetic properties of CoFe2O4 nanoparticles. RSC Adv. 2015, 5, 27060–27068. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Karolak, F.; García-Granda, S.; Dkhil, B.; Ammar, S.; Gadri, A. Synthesis, structural, optical, morphological and magnetic characterization of copper substituted nickel ferrite (CuxNi1−xFe2O4) through Co-precipitation method. J. Mater. Sci. Mater. Electron. 2017, 28, 18480–18488. [Google Scholar] [CrossRef]
- Gupta, N.; Jain, P.; Rana, R.; Shrivastava, S. Current development in synthesis and characterization of nickel ferrite nanoparticle. Mater. Today Proc. 2017, 4, 342–349. [Google Scholar] [CrossRef]
- Palacio Gómez, C.A.; Barrero Meneses, C.A.; Jaén, J.A. Raman, Infrared and mössbauer spectroscopic studies of solid-state synthesized Ni-Zn ferrites. J. Magn. Magn. Mater. 2020, 505, 166710. [Google Scholar] [CrossRef]
- Yadav, A.; Varshney, D. Structural and dielectric properties of copper-substituted Mg–Zn spinel ferrites. J. Supercond. Nov. Magn. 2017, 30, 1297–1302. [Google Scholar] [CrossRef]
- Sharifi, I.; Zamanian, A.; Behnamghader, A. Synthesis and characterization of Fe0.6Zn0.4Fe2O4 ferrite magnetic nanoclusters using simple thermal decomposition method. J. Magn. Magn. Mater. 2016, 412, 107–113. [Google Scholar] [CrossRef]
- Monfared, A.H.; Zamanian, A.; Beygzadeh, M.; Sharifi, I.; Mozafari, M. A rapid and efficient thermal decomposition approach for the synthesis of manganese-zinc/oleylamine core/shell ferrite Nanoparticles. J. Alloys Compd. 2017, 693, 1090–1095. [Google Scholar] [CrossRef]
- Rahmayeni, R.; Oktavia, Y.; Stiadi, Y.; Arief, S.; Zulhadjri, Z. Spinel ferrite of MnFe2O4 Synthesized in piper betle linn extract media and its application as photocatalysts and antibacterial. J. Dispers. Sci. Technol. 2021, 42, 465–474. [Google Scholar] [CrossRef]
- Das, S.; Manoharan, C.; Venkateshwarlu, M.; Dhamodharan, P. Structural, optical, morphological and magnetic properties of nickel doped cobalt ferrite nanoparticles synthesized by hydrothermal method. J. Mater. Sci. Mater. Electron. 2019, 30, 19880–19893. [Google Scholar] [CrossRef]
- Ulusoy, U. A review of particle shape effects on material properties for various engineering applications: From macro to nanoscale. Minerals 2023, 13, 91. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Wang, J.; Wang, Y.; Xiong, Y.; Liu, Q.; Li, N.; Zhou, J.; Fu, G.; Sun, D.; et al. 1-Naphthol induced Pt3Ag nanocorals as bifunctional cathode and anode catalysts of direct formic acid fuel cells. Nano Res. 2019, 12, 323–329. [Google Scholar] [CrossRef]
- Lacerda, J.N.; Franceschini, D.F.; Ponzio, E.A.; Esteves, L.M.; Guimarães, R.B.; Xing, Y.T. Manganese oxide nanofoam prepared by pulsed laser deposition for high performance supercapacitor electrodes. Mater. Chem. Phys. 2020, 242, 122459. [Google Scholar] [CrossRef]
- Xu, T.; Jiao, D.; Liu, M.; Zhang, L.; Fan, X.; Zheng, L.; Zheng, W.; Cui, X. Ni center coordination reconstructed nanocorals for efficient water splitting. Adv. Sci. 2022, 10, 202205605. [Google Scholar] [CrossRef]
- Mahyoub, S.A.; Qaraah, F.A.; Yan, S.; Hezam, A.; Zhong, J.; Cheng, Z. rational design of low loading Pd-alloyed Ag nanocorals for high current density CO2-to-CO electroreduction at elevated pressure. Mater. Today Energy 2022, 24, 100923. [Google Scholar] [CrossRef]
- Mali, S.S.; Betty, C.A.; Bhosale, P.N.; Patil, P.S.; Hong, C.K. From nanocorals to nanorods to nanoflowers nanoarchitecture for efficient dye-sensitized solar cells at relatively low film thickness: All hydrothermal process. Sci. Rep. 2014, 4, 5451. [Google Scholar] [CrossRef] [PubMed]
- Zainelabdin, A.; Amin, G.; Zaman, S.; Nur, O.; Lu, J.; Hultman, L.; Willander, M. CuO/ZnO nanocorals synthesis via hydrothermal technique: Growth mechanism and their application as humidity sensor. J. Mater. Chem. 2012, 22, 11583–11590. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Teixeira, L.T.; Braz, W.F.; Correia de Siqueira, R.N.; Pandoli, O.G.; Geraldes, M.C. Sulfated and carboxylated nanocellulose for Co+2 adsorption. J. Mater. Res. Technol. 2021, 15, 434–447. [Google Scholar] [CrossRef]
- Khawal, H.A.; Gawai, U.P.; Asokan, K.; Dole, B.N. Modified structural, surface morphological and optical studies of Li3+ swift heavy ion irradiation on zinc oxide nanoparticles. RSC Adv. 2016, 6, 49068–49075. [Google Scholar] [CrossRef]
- Sunny, A.; Thirumurugan, A.; Balasubramanian, K. Laser induced fano scattering, electron-phonon coupling, bond length and phonon lifetime changes in α-Fe2O3 nanostructures. Phys. Chem. Chem. Phys. 2020, 22, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Sanpo, N.; Berndt, C.C.; Wang, J. Microstructural and antibacterial properties of zinc-substituted cobalt ferrite nanopowders synthesized by sol-gel methods. J. Appl. Phys. 2012, 112, 084333. [Google Scholar] [CrossRef]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel green route of synthesis of ZnO Nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Barik, R.; Mohapatra, M. Solvent mediated surface engineering of α-Fe2O3 nanomaterials: Facet sensitive energy storage materials. CrystEngComm 2015, 17, 9203–9215. [Google Scholar] [CrossRef]
- Cao, J.; Chen, C.; Chen, K.; Lu, Q.; Wang, Q.; Zhou, P.; Liu, D.; Song, L.; Niu, Z.; Chen, J. High-strength graphene composite films by molecular level couplings for flexible supercapacitors with high volumetric capacitance. J. Mater. Chem. A Mater. 2017, 5, 15008–15016. [Google Scholar] [CrossRef]
- Li, L.; Bi, H.; Gai, S.; He, F.; Gao, P.; Dai, Y.; Zhang, X.; Yang, D.; Zhang, M.; Yang, P. Uniformly dispersed ZnFe2O4 nanoparticles on nitrogen-modified graphene for high-performance supercapacitor as electrode. Sci. Rep. 2017, 7, 43116. [Google Scholar] [CrossRef]
- Wang, G.; Huang, J.; Chen, S.; Gao, Y.; Cao, D. Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam. J. Power Sources 2011, 196, 5756–5760. [Google Scholar] [CrossRef]
- Barzegar, F.; Bello, A.; Momodu, D.; Madito, M.J.; Dangbegnon, J.; Manyala, N. Preparation and characterization of porous carbon from expanded graphite for high energy density supercapacitor in aqueous electrolyte. J. Power Sources 2016, 309, 245–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Yu, C.; Liu, Z.; Yu, X.; Meng, F. Synthesis, structure and supercapacitive behavior of spinel NiFe2O4 and NiO@NiFe2O4 nanoparticles. Ceram. Int. 2021, 47, 10063–10071. [Google Scholar] [CrossRef]
- Sharifi, S.; Rahimi, K.; Yazdani, A. Highly improved supercapacitance properties of MnFe2O4 nanoparticles by MoS2 nanosheets. Sci. Rep. 2021, 11, 8378. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Bhise, S.C.; Patil, S.K.; Patil, S.A.; Pawar, D.K.; Ghule, A.V.; Patil, P.S.; Kolekar, S.S. Mechanochemical growth of a porous ZnFe2O4 nano-flake thin film as an electrode for supercapacitor application. RSC Adv. 2015, 5, 45935–45942. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Kolekar, S.S.; Deshpande, N.G.; Chang, J.Y.; Kashale, A.A.; Ghule, A.V.F. Binder-free chemical synthesis of ZnFe2O4 thin films for asymmetric supercapacitor with improved performance. Ionics 2017, 23, 741–749. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Park, C.; Kim, Y.; Lee, H.S.; Yoon, S.S. Bimetallic ZnFe2O4 nanosheets prepared via electrodeposition as binder-free high-performance supercapacitor electrodes. Appl. Surf. Sci. 2021, 559, 149951. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Vattikuti, S.V.P.; Kim, K.H. Structural and electrochemical properties of mixed calcium-zinc spinel ferrites nanoparticles. Ceram. Int. 2022, 49, 4365–4371. [Google Scholar] [CrossRef]
- Agyemang, F.O.; Kim, H. Electrospun ZnFe2O4-based nanofiber composites with enhanced supercapacitive properties. Mater. Sci. Eng. B Solid. State Mater. Adv. Technol. 2016, 211, 141–148. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Zhou, Y.; Zhuo, C.; Huang, J.; Li, S. Facile solvothermal synthesis of porous ZnFe2O4 microspheres for capacitive pseudocapacitors. RSC Adv. 2015, 5, 39270–39277. [Google Scholar] [CrossRef]
- Ghaly, H.A.; El-Deen, A.G.; Souaya, E.R.; Allam, N.K. Asymmetric supercapacitors based on 3D graphene-wrapped V2O5 nanospheres and Fe3O4@3D graphene electrodes with high power and energy densities. Electrochim. Acta 2019, 310, 58–69. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Yin, M.; Shi, J.; Zhang, W.; Wang, X.; Wu, Y.; Ma, J.; Yuan, D.; Jia, C. Co3O4@Co3S4 core-shell neuroid network for high cycle-stability hybrid-supercapacitors. J. Power Sources 2021, 485, 229315. [Google Scholar] [CrossRef]
- Li, J.; Xiong, D.; Wang, L.; Hirbod, M.K.S.; Li, X. High-performance self-assembly MnCo2O4 nanosheets for asymmetric supercapacitors. J. Energy Chem. 2019, 37, 66–72. [Google Scholar] [CrossRef]



| Material | Structure | Synthesis | Electrolyte | Specific Capacitance (F g−1) | Current Density (A g−1) | Ref. |
|---|---|---|---|---|---|---|
| Zn-ferrite | Nanocorals | Thermal | 2.0 M KOH | 2031.8 F g−1 | 1.0 A g−1 | This study |
| NiO@NiFe2O4 | Nanoparticles | Hydrothermal | 1.0 M KOH | 248.4 | 1.0 A g−1 | [51] |
| ZnFe2O4/RNG | Nanoparticles | Solvothermal | 1.0 M KOH | 244.0 | 0.5 A g−1 | [48] |
| MnFe2O4/MoS2 | Nanosheets | Hydrothermal | 3.0 M KOH | 2093.0 | 1.0 A g−1 | [52] |
| MnZnFe2O4 | Nanoneedles | Co-precipitation method | 0.5 M H2SO4 | 550.0 | 0.8 A g−1 | [15] |
| ZnFe2O4 | Nanoflakes | Mechanochemical growth | 3.0 M KOH | 768.0 | 5.0 mA cm−2 | [53] |
| ZnFe2O4 thin films | Nanospheres | Successive ionic layer adsorption and reaction method | 6.0 M KOH | 615.0 | 3 mA cm−2 | [54] |
| ZnFe2O4 | Nanosheets | Electrodeposition | 6.0 M KOH | 1093.0 | 1.0 A g−1 | [55] |
| Ca0.1Zn0.9Fe2O4 | Nanoparticles | Solvothermal | 2.0 M Na2SO4 | 208.0 | 2.0 A g−1 | [56] |
| ZnFe2O4 | Nanofibers | Electrospun | 3.0 M KOH | 590.0 | 1.0 A g−1 | [57] |
| ZnFe2O4 | Microspheres | Solvothermal | 1.0 M KOH | 131.0 | 0.1 A g−1 | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, L.T.; de Lima, S.L.S.; Rosado, T.F.; Liu, L.; Vitorino, H.A.; dos Santos, C.C.; Mendonça, J.P.; Garcia, M.A.S.; Siqueira, R.N.C.; da Silva, A.G.M. Sustainable Cellulose Nanofibers-Mediated Synthesis of Uniform Spinel Zn-Ferrites Nanocorals for High Performances in Supercapacitors. Int. J. Mol. Sci. 2023, 24, 9169. https://doi.org/10.3390/ijms24119169
Teixeira LT, de Lima SLS, Rosado TF, Liu L, Vitorino HA, dos Santos CC, Mendonça JP, Garcia MAS, Siqueira RNC, da Silva AGM. Sustainable Cellulose Nanofibers-Mediated Synthesis of Uniform Spinel Zn-Ferrites Nanocorals for High Performances in Supercapacitors. International Journal of Molecular Sciences. 2023; 24(11):9169. https://doi.org/10.3390/ijms24119169
Chicago/Turabian StyleTeixeira, Lucas T., Scarllet L. S. de Lima, Taissa F. Rosado, Liying Liu, Hector A. Vitorino, Clenilton C. dos Santos, Jhonatam P. Mendonça, Marco A. S. Garcia, Rogério N. C. Siqueira, and Anderson G. M. da Silva. 2023. "Sustainable Cellulose Nanofibers-Mediated Synthesis of Uniform Spinel Zn-Ferrites Nanocorals for High Performances in Supercapacitors" International Journal of Molecular Sciences 24, no. 11: 9169. https://doi.org/10.3390/ijms24119169
APA StyleTeixeira, L. T., de Lima, S. L. S., Rosado, T. F., Liu, L., Vitorino, H. A., dos Santos, C. C., Mendonça, J. P., Garcia, M. A. S., Siqueira, R. N. C., & da Silva, A. G. M. (2023). Sustainable Cellulose Nanofibers-Mediated Synthesis of Uniform Spinel Zn-Ferrites Nanocorals for High Performances in Supercapacitors. International Journal of Molecular Sciences, 24(11), 9169. https://doi.org/10.3390/ijms24119169









