Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design
Abstract
1. Introduction
2. Physiology of Lesioned Nerve Regeneration: Wallerian Degeneration and Bands of Büngner
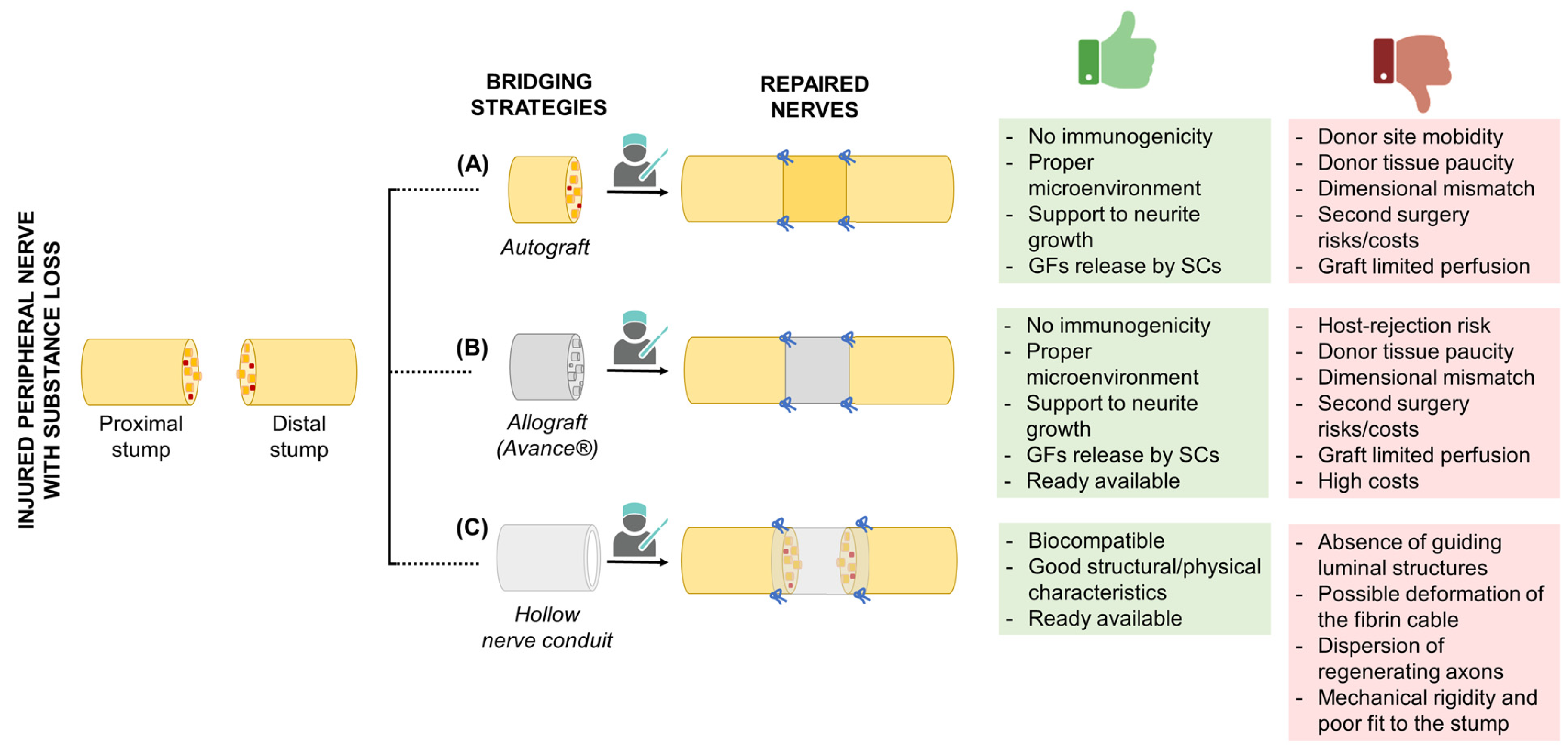
3. Current Bridging Strategies
3.1. Autografts
3.2. Allografts
3.3. Hollow Nerve Conduits
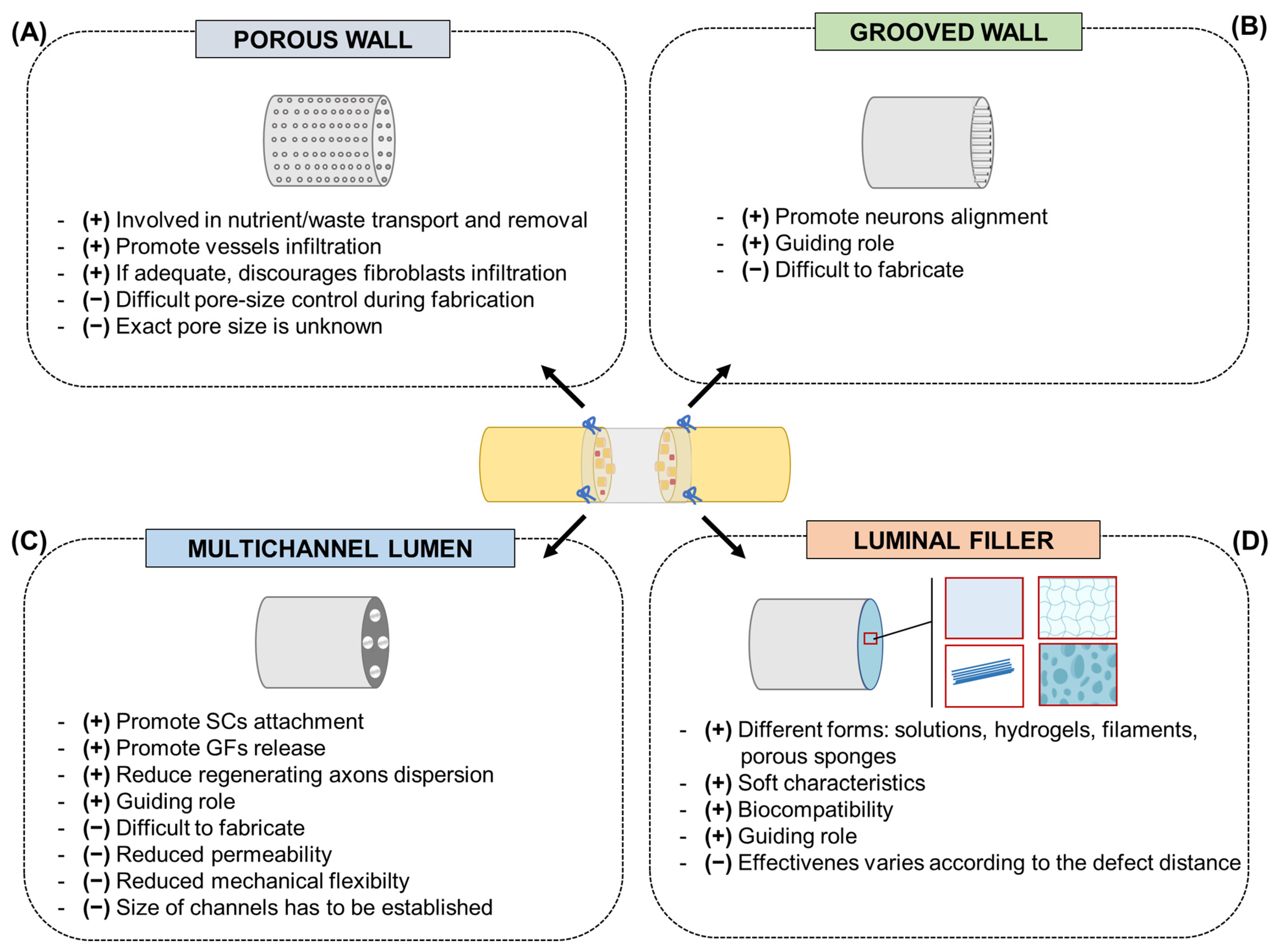
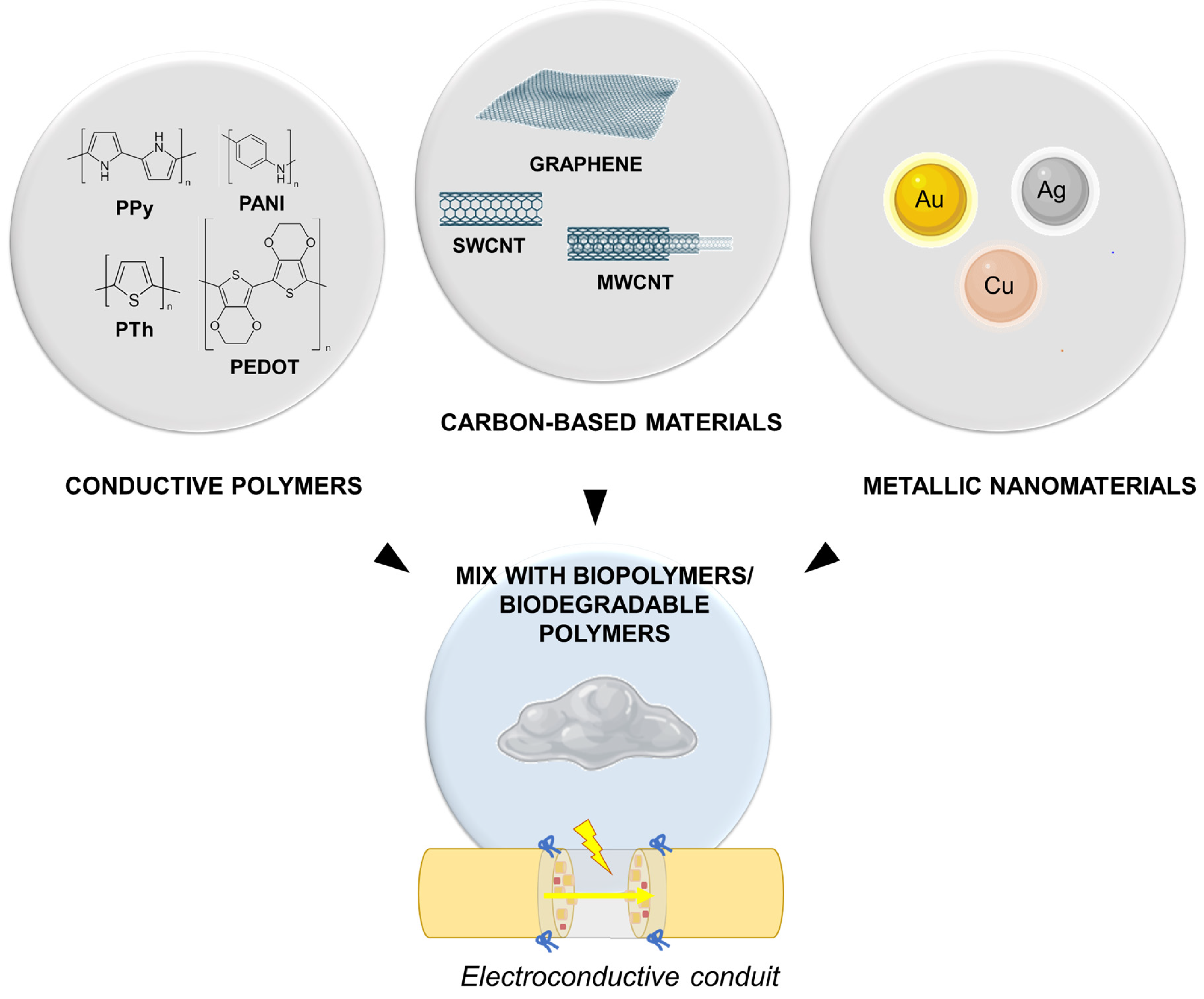
4. Future Directions in Nerve Conduits
4.1. Promising Conduits Design
4.2. Electroconductive Conduits
5. Cells
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Y.; Yin, J. Industry news: The additive manufacturing of nerve conduits for the treatment of peripheral nerve injury. Bio-Des. Manuf. 2022, 5, 6–8. [Google Scholar] [CrossRef]
- Alvites, R.; Caseiro, A.R.; Santos Pedrosa, S.; Vieira Branquinho, M.; Ronchi, G.; Geuna, S.; Varejão, A.S.P.; Maurício, A.C. Peripheral nerve injury and axonotmesis: State of the art and recent advances. Cogent Med. 2018, 5, 1466404. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Agata, V.; Trovato, B.; Roggio, F.; Castorina, A.; Vecchio, M.; Di Rosa, M.; Musumeci, G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon 2021, 7, e08281. [Google Scholar] [CrossRef]
- Kasper, M.; Deister, C.; Beck, F.; Schmidt, C.E. Bench-to-Bedside Lessons Learned: Commercialization of an Acellular Nerve Graft. Adv. Healthc. Mater. 2020, 9, e2000174. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.; Tavares, A.L.F.; Reginato, A.; Kakihata, C.M.M.; Bertolini, G.R.F.; Ribeiro, L.F.C. Low-Level Laser Therapy in Different Wavelengths on the Tibialis Anterior Muscle of Wistar Rats after Nerve Compression Injury. J. Manip. Physiol. Ther. 2020, 43, 700–707. [Google Scholar] [CrossRef]
- Ducic, I.; Fu, R.; Iorio, M.L. Innovative treatment of peripheral nerve injuries: Combined reconstructive concepts. Ann. Plast. Surg. 2012, 68, 180–187. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Macchi, V.; Tiengo, C.; Petrelli, L.; Rambaldo, A.; Borean, A.; Capelli, S.; Filippi, A.; Romanato, F.; et al. New bioresorbable wraps based on oxidized polyvinyl alcohol and leukocyte-fibrin-platelet membrane to support peripheral nerve neurorrhaphy: Preclinical comparison versus NeuraWrap. Sci. Rep. 2019, 9, 17193. [Google Scholar] [CrossRef]
- Beirowski, B.; Adalbert, R.; Wagner, D.; Grumme, D.S.; Addicks, K.; Ribchester, R.R.; Coleman, M.P. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BC Neurosci. 2005, 6, 6. [Google Scholar]
- Liu, X.; Duan, X. Mechanisms and Treatments of Peripheral Nerve Injury. Ann. Plast. Surg. 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Panzer, K.V.; Burrell, J.C.; Helm, K.V.T.; Purvis, E.M.; Zhang, Q.; Le, A.D.; O’Donnell, J.C.; Cullen, D.K. Tissue Engineered Bands of Büngner for Accelerated Motor and Sensory Axonal Outgrowth. Front. Bioeng. Biotechnol. 2020, 8, 580654. [Google Scholar] [CrossRef]
- Ribeiro-Resende, V.T.; Koenig, B.; Nichterwitz, S.; Oberhoffner, S.; Schlosshauer, B. Strategies for inducing the formation of bands of Büngner in peripheral nerve regeneration. Biomaterials 2009, 30, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Ducic, I.; Yoon, J.; Buncke, G. Chronic postoperative complications and donor site morbidity after sural nerve autograft harvest or biopsy. Microsurgery 2020, 40, 710–716. [Google Scholar] [CrossRef]
- Hall, S.M. Regeneration in cellular and acellulax autografts in the peripheral nervous system. Neuropathol. Appl. Neurobiol. 1986, 12, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.L.; Kong, D.; Qu, B.; Su, X.J.; Li, H.; Pi, H.Y. Nerve autografts and tissue-engineered materials for the repair of peripheral nerve injuries: A 5-year bibliometric analysis. Neural Regen. Res. 2015, 10, 1003–1008. [Google Scholar]
- Stang, F.; Stollwerck, P.; Prommersberger, K.J.; van Schoonhoven, J. Posterior interosseus nerve vs. medial cutaneous nerve of the forearm: Differences in digital nerve reconstruction. Arch. Orthop. Trauma Surg. 2013, 133, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Selim, O.A.; Lakhani, S.; Midha, S.; Mosahebi, A.; Kalaskar, D.M. Three-Dimensional Engineered Peripheral Nerve: Toward a New Era of Patient-Specific Nerve Repair Solutions. Tissue Eng. Part B Rev. 2022, 28, 295–335. [Google Scholar] [CrossRef]
- Hoben, G.M.; Ee, X.; Schellhardt, L.; Yan, Y.; Hunter, D.A.; Moore, A.M.; Snyder-Warwick, A.K.; Stewart, S.; Mackinnon, S.E.; Wood, M.D. Increasing Nerve Autograft Length Increases Senescence and Reduces Regeneration. Plast. Reconstr. Surg. 2018, 142, 952–961. [Google Scholar] [CrossRef]
- Lackington, W.A.; Ryan, A.J.; O’Brien, F.J. Advances in Nerve Guidance Conduit-Based Therapeutics for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2017, 3, 1221–1235. [Google Scholar] [CrossRef]
- Smith, P.J. Nerve injuries and their repair—A critical appraisal. J. Hand Surg. 1992, 17, 120–121. [Google Scholar] [CrossRef]
- Evans, R.J.; Midha, R.; Mackinnon, S.E. The peripheral nerve allograft: A comprehensive review of regeneration and neuroimmunology. Prog. Neurobiol. 1994, 43, 187–233. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.E.; Hudson, A.R.; Falk, R.E.; Hunter, D.A. The nerve allograft response—An experimental model in the rat. Ann. Plast. Surg. 1985, 14, 334–339. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Doolabh, V.B.; Novak, C.B.; Trulock, E.P. Clinical outcome following nerve allograft transplantation. Plast. Reconstr. Surg. 2001, 107, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Suhar, R.A.; Marquardt, L.M.; Song, S.; Buabbas, H.; Doulames, V.M.; Johansson, P.K.; Klett, K.C.; Dewi, R.E.; Enejder, A.M.K.; Plant, G.W.; et al. Elastin-like Proteins to Support Peripheral Nerve Regeneration in Guidance Conduits. ACS Biomater. Sci. Eng. 2021, 7, 4209–4220. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, T.; Borger, A.; Radtke, C. Reconstruction of Critical Nerve Defects Using Allogenic Nerve Tissue: A Review of Current Approaches. Int. J. Mol. Sci. 2021, 22, 3515. [Google Scholar] [CrossRef]
- Chrząszcz, P.; Derbisz, K.; Suszyński, K.; Miodoński, J.; Trybulski, R.; Lewin-Kowalik, J.; Marcol, W. Application of peripheral nerve conduits in clinical practice: A literature review. Neurol. Neurochir. Pol. 2018, 52, 427–435. [Google Scholar] [CrossRef]
- Buncke, G. Peripheral nerve allograft: How innovation has changed surgical practice. Plast. Aesthetic Res. 2022, 9, 38. [Google Scholar] [CrossRef]
- Hudson, T.W.; Zawko, S.; Deister, C.; Lundy, S.; Hu, C.Y.; Lee, K.; Schmidt, C.E. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004, 10, 1641–1651. [Google Scholar] [CrossRef]
- Krekoski, C.A.; Neubauer, D.; Zuo, J.; Muir, D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J. Neurosci. 2001, 21, 6206–6213. [Google Scholar] [CrossRef]
- Leckenby, J.I.; Furrer, C.; Haug, L.; Juon Personeni, B.; Vögelin, E. A Retrospective Case Series Reporting the Outcomes of Avance Nerve Allografts in the Treatment of Peripheral Nerve Injuries. Plast. Reconstr. Surg. 2020, 145, 368e–381e. [Google Scholar] [CrossRef]
- Parker, B.J.; Rhodes, D.I.; O’Brien, C.M.; Rodda, A.E.; Cameron, N.R. Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: A commercial perspective. Acta Biomater. 2021, 135, 64–86. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, G.C.; Malessy, M.J.; Yaszemski, M.J.; Windebank, A.J.; Spinner, R.J. Designing ideal conduits for peripheral nerve repair. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Gander, B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J. Control. Release 2012, 161, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral nerve conduits: Technology update. Med. Devices 2014, 7, 405–424. [Google Scholar]
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to Peripheral Nerve Repair: Generations of Biomaterial Conduits Yielding to Replacing Autologous Nerve Grafts in Craniomaxillofacial Surgery. BioMed Res. Int. 2016, 2016, 3856262. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Lora, L.; Grandi, F.; Sartore, L.; Tiengo, C.; Petrelli, L.; Dalzoppo, D.; Parnigotto, P.P.; Macchi, V.; et al. Partially oxidized polyvinyl alcohol conduit for peripheral nerve regeneration. Sci. Rep. 2018, 8, 604. [Google Scholar] [CrossRef]
- Rochkind, S.; Almog, M.; Meilin, S.; Nevo, Z. Reviving Matrix for Nerve Reconstruction in Rabbit Model of Chronic Peripheral Nerve Injury with Massive Loss Defect. Front. Surg. 2021, 7, 609638. [Google Scholar] [CrossRef]
- Magaz, A.; Faroni, A.; Gough, J.E.; Reid, A.J.; Li, X.; Blaker, J.J. Bioactive Silk-Based Nerve Guidance Conduits for Augmenting Peripheral Nerve Repair. Adv. Healthc. Mater. 2018, 7, e1800308. [Google Scholar] [CrossRef]
- Gao, S.; Chen, X.; Lu, B.; Meng, K.; Zhang, K.Q.; Zhao, H. Recent advances on nerve guide conduits based on textile methods. Smart Mater. Med. 2023, 4, 368–383. [Google Scholar] [CrossRef]
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Sakiyama-Elbert, S.E. Matrices, scaffolds & carriers for cell delivery in nerve regeneration. Exp. Neurol. 2019, 319, 112837. [Google Scholar]
- Chen, M.B.; Zhang, F.; Lineaweaver, W.C. Luminal fillers in nerve conduits for peripheral nerve repair. Ann. Plast. Surg. 2006, 57, 462–471. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.; Lu, C.; Chai, Y.; Cao, Z.; Lu, J.; Zhang, Z.; Zhao, H.; Huang, Y.Y.; Yao, S.; et al. Aligned fibrin/functionalized self-assembling peptide interpenetrating nanofiber hydrogel presenting multi-cues promotes peripheral nerve functional recovery. Bioact. Mater. 2021, 8, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Lackington, W.A.; Hibbitts, A.J.; Matheson, A.; Alekseeva, T.; Stejskalova, A.; Roche, P.; O’Brien, F.J. A Physicochemically Optimized and Neuroconductive Biphasic Nerve Guidance Conduit for Peripheral Nerve Repair. Adv. Healthc. Mater. 2017, 6, 1700954. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.R.; Wrobel, S.; Meyer, C.; Brandenberger, C.; Cengiz, I.F.; López-Cebral, R.; Silva-Correia, J.; Ronchi, G.; Reis, R.L.; Grothe, C.; et al. Gellan Gum-based luminal fillers for peripheral nerve regeneration: An in vivo study in the rat sciatic nerve repair model. Biomater. Sci. 2018, 6, 1059–1075. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomater. Sci. 2018, 6, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Kim, D.; Mitchel, J.A.; Bellamkonda, R.V. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 2010, 12, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, A.; Faroni, A.; Minogue, B.M.; Downes, S.; Terenghi, G.; Reid, A.J. Polymer scaffolds with preferential parallel grooves enhance nerve regeneration. Tissue Eng. Part A 2015, 21, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, Y.Y. Biomaterials and strategies for nerve regeneration. Artif. Organs 2006, 30, 514–522. [Google Scholar] [CrossRef]
- Ezra, M.; Bushman, J.; Shreiber, D.; Schachner, M.; Kohn, J. Porous and Nonporous Nerve Conduits: The Effects of a Hydrogel Luminal Filler with and without a Neurite-Promoting Moiety. Tissue Eng. Part A 2016, 22, 818–826. [Google Scholar] [CrossRef]
- Jiang, X.; Lim, S.H.; Mao, H.Q.; Chew, S.Y. Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, H.; Wang, H.; Cao, X. Engineering topography: Effects on nerve cell behaviors and applications in peripheral nerve repair. J. Mater. Chem. B 2021, 9, 6310–6325. [Google Scholar] [CrossRef]
- Sun, M.; McGowan, M.; Kingham, P.J.; Terenghi, G.; Downes, S. Novel thin-walled nerve conduit with microgrooved surface patterns for enhanced peripheral nerve repair. J. Mater. Sci. Mater. Med. 2010, 21, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Jeftinija, S.; Mallapragada, S. Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 2002, 8, 367–378. [Google Scholar] [CrossRef]
- Clark, P.; Connolly, P.; Curtis, A.S.; Dow, J.A.; Wilkinson, C.D. Topographical control of cell behaviour: II. Multiple grooved substrata. Development 1990, 108, 635–644. [Google Scholar] [CrossRef]
- Hsu, S.; Chen, C.; Lu, P.S.; Lai, C.; Chen, C. Oriented Schwann cell growth on microgrooved surfaces. Biotechnol. Bioeng. 2005, 92, 579–588. [Google Scholar] [CrossRef]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.T.; Waggoner, P.J.; Romero, A.A.; Nelson, K.D.; Eberhart, R.C.; Smith, G.M. Poly(L-Lactide) microfilaments enhance peripheral nerve regeneration across extended nerve lesions. J. Neurosci. Res. 2003, 72, 227–238. [Google Scholar] [CrossRef]
- Yao, L.; de Ruiter, G.C.; Wang, H.; Knight, A.M.; Spinner, R.J.; Yaszemski, M.J.; Windebank, A.J.; Pandit, A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 2010, 31, 5789–5797. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, Y.; Wang, Y.; Wang, S.; Chang, J.; Liu, W.; Han, B. Multichannel nerve conduit based on chitosan derivates for peripheral nerve regeneration and Schwann cell survival. Carbohydr. Polym. 2023, 301, 120327. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Wang, H.; Wang, Y.; Zhang, K.; Fan, C.; Wang, H.; Mo, X. Biomimetic and hierarchical nerve conduits from multifunctional nanofibers for guided peripheral nerve regeneration. Acta Biomater. 2020, 117, 180–191. [Google Scholar] [CrossRef]
- Chiono, V.; Tonda-Turo, C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol. 2015, 131, 87–104. [Google Scholar] [CrossRef]
- Sarker, M.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration. Biotechnol. J. 2018, 13, e1700635. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, L. Polymers for Fabricating Nerve Conduits. Int. J. Polym. Sci. 2010, 2010, 138686. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W.; Wu, X.; Zhang, N.; Zhang, Y.; Ouyang, S.; Song, X.; Fang, X.; Seeram, R.; Xue, W.; et al. Functional Self-Assembling Peptide Nanofiber Hydrogels Designed for Nerve Degeneration. ACS Appl. Mater. Interfaces 2016, 8, 2348–2359. [Google Scholar] [CrossRef]
- Wu, P.; Tong, Z.; Luo, L.; Zhao, Y.; Chen, F.; Li, Y.; Huselstein, C.; Ye, Q.; Ye, Q.; Chen, Y. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact. Mater. 2021, 6, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, L.; An, H.; Zhang, P.; Liu, P. Repair of Peripheral Nerve Injury Using Hydrogels Based on Self-Assembled Peptides. Gels 2021, 7, 152. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Lamanna, A.; De Rose, E.; Zamuner, A.; Sandrin, D.; Marsotto, M.; Auditore, A.; Messina, G.M.L.; Licciardello, A.; et al. Bioactivated Oxidized Polyvinyl Alcohol towards Next-Generation Nerve Conduits Development. Polymers 2021, 13, 3372. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Zeni, E.; Cassari, L.; Zamuner, A.; Gloria, A.; Russo, T.; Boscolo-Berto, R.; Sfriso, M.M.; Macchi, V.; et al. Development of Two-Layer Hybrid Scaffolds Based on Oxidized Polyvinyl Alcohol and Bioactivated Chitosan Sponges for Tissue Engineering Purposes. Int. J. Mol. Sci. 2022, 23, 12059. [Google Scholar] [CrossRef]
- Anderson, M.; Shelke, N.B.; Manoukian, O.S.; Yu, X.; McCullough, L.D.; Kumbar, S.G. Peripheral Nerve Regeneration Strategies: Electrically Stimulating Polymer Based Nerve Growth Conduits. Crit. Rev. Biomed. Eng. 2015, 43, 131–159. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.; Li, X.; Yin, J.; Wei, P.; Qian, J.; Sun, J. Effect of Electrical and Electromechanical Stimulation on PC12 Cell Proliferation and Axon Outgrowth. Front. Bioeng. Biotechnol. 2021, 9, 757906. [Google Scholar] [CrossRef] [PubMed]
- Gisbert Roca, F.; Serrano Requena, S.; Monleón Pradas, M.; Martínez-Ramos, C. Electrical Stimulation Increases Axonal Growth from Dorsal Root Ganglia Co-Cultured with Schwann Cells in Highly Aligned PLA-PPy-Au Microfiber Substrates. Int. J. Mol. Sci. 2022, 23, 6362. [Google Scholar] [CrossRef]
- Willand, M.P.; Nguyen, M.A.; Borschel, G.H.; Gordon, T. Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabil. Neural Repair 2016, 30, 490–496. [Google Scholar] [CrossRef]
- Park, J.; Jeon, J.; Kim, B.; Lee, M.S.; Park, S.; Lim, J.; Yi, J.; Lee, H.; Yang, H.S.; Lee, J.Y. Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2003759. [Google Scholar] [CrossRef]
- Paramshetti, S.; Angolkar, M.; Al Fatease, A.; Alshahrani, S.M.; Hani, U.; Garg, A.; Ravi, G.; Osmani, R.A.M. Revolutionizing Drug Delivery and Therapeutics: The Biomedical Applications of Conductive Polymers and Composites-Based Systems. Pharmaceutics 2023, 15, 1204. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, C.A.; Kung, T.A.; Brown, D.L.; Cederna, P.S.; Kemp, S.W.P. State-of-the-Art Techniques in Treating Peripheral Nerve Injury. Plast. Reconstr. Surg. 2018, 141, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Holzwarth, J.M.; Yan, Y.; Xu, P.; Zheng, H.; Yin, Y.; Li, S.; Ma, P.X. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials 2014, 35, 225–235. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, W.; Li, D.; Shi, K.; Li, R.; Han, Y.; Jin, E.; Ding, J.; Chen, X. Functional Polymer-Based Nerve Guide Conduits to Promote Peripheral Nerve Regeneration. Adv. Mater. Interfaces 2020, 7, 2000225. [Google Scholar] [CrossRef]
- Pina, C.D.; Falletta, E. Advances in Polyaniline for Biomedical Applications. Curr. Med. Chem. 2022, 29, 329–357. [Google Scholar] [CrossRef]
- Huang, L.; Yang, X.; Deng, L.; Ying, D.; Lu, A.; Zhang, L.; Yu, A.; Duan, B. Biocompatible Chitin Hydrogel Incorporated with PEDOT Nanoparticles for Peripheral Nerve Repair. ACS Appl. Mater. Interfaces 2021, 13, 16106–16117. [Google Scholar] [CrossRef]
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-Based Nanocomposites for Neural Tissue Engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef]
- Kandhola, G.; Park, S.; Lim, J.W.; Chivers, C.; Song, Y.H.; Chung, J.H.; Kim, J.; Kim, J.W. Nanomaterial-Based Scaffolds for Tissue Engineering Applications: A Review on Graphene, Carbon Nanotubes and Nanocellulose. Tissue Eng. Regen. Med. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, X.; Wu, W.; Park, S.; Miller Ii, A.L.; Terzic, A.; Lu, L. Effective nerve cell modulation by electrical stimulation of carbon nanotube embedded conductive polymeric scaffolds. Biomater. Sci. 2018, 6, 2375–2385. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Farahani, M.K.; Salehi, M.; Atashi, A.; Alizadeh, M.; Kheradmandi, R.; Molzemi, S. Exploring the Physicochemical, Electroactive, and Biodelivery Properties of Metal Nanoparticles on Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng. 2023, 9, 106–138. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Zangene, E.; Manouchehri, S.; Amirabad, L.M.; Baheiraei, N.; Hadjighasem, M.R.; Farokhi, M.; Ganjali, M.R.; Walker, B.W.; Saeb, M.R.; et al. Conductive biomaterials as nerve conduits: Recent advances and future challenges. Appl. Mater. Today 2020, 20, 100784. [Google Scholar] [CrossRef]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef]
- Lin, Y.L.; Jen, J.C.; Hsu, S.H.; Chiu, M. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg. Neurol. 2008, 70, S9–S18. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Rodrigues, A.A., Jr.; Glover, L.E.; Voltarelli, J.; Borlongan, C.V. Peripheral nerve repair with cultured schwann cells: Getting closer to the clinics. Sci. World J. 2012, 2012, 413091. [Google Scholar] [CrossRef]
- Han, G.H.; Peng, J.; Liu, P.; Ding, X.; Wei, S.; Lu, S.; Wang, Y. Therapeutic strategies for peripheral nerve injury: Decellularized nerve conduits and Schwann cell transplantation. Neural Regen. Res. 2019, 14, 1343–1351. [Google Scholar] [PubMed]
- Di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1544–1552. [Google Scholar] [CrossRef]
- Rhode, S.C.; Beier, J.P.; Ruhl, T. Adipose tissue stem cells in peripheral nerve regeneration-In vitro and in vivo. J. Neurosci. Res. 2021, 99, 545–560. [Google Scholar] [CrossRef]
- Masgutov, R.; Masgutova, G.; Mullakhmetova, A.; Zhuravleva, M.; Shulman, A.; Rogozhin, A.; Syromiatnikova, V.; Andreeva, D.; Zeinalova, A.; Idrisova, K.; et al. Adipose-Derived Mesenchymal Stem Cells Applied in Fibrin Glue Stimulate Peripheral Nerve Regeneration. Front. Med. 2019, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Burks, S.S.; Diaz, A.; Haggerty, A.E.; Oliva, N.; Midha, R.; Levi, A.D. Schwann cell delivery via a novel 3D collagen matrix conduit improves outcomes in critical length nerve gap repairs. J. Neurosurg. 2021, 135, 1241–1251. [Google Scholar] [CrossRef]
- Errante, E.L.; Diaz, A.; Smartz, T.; Khan, A.; Silvera, R.; Brooks, A.E.; Lee, Y.S.; Burks, S.S.; Levi, A.D. Optimal Technique for Introducing Schwann Cells Into Peripheral Nerve Repair Sites. Front. Cell. Neurosci. 2022, 16, 929494. [Google Scholar] [CrossRef]
- Tanaka, H.; Kakinoki, R.; Kaizawa, Y.; Yurie, H.; Ikeguchi, R.; Akagi, M. Bone marrow-derived mesenchymal stem cells transplanted into a vascularized biodegradable tube containing decellularized allogenic nerve basal laminae promoted peripheral nerve regeneration; can it be an alternative of autologous nerve graft? PLoS ONE 2021, 16, e0254968. [Google Scholar] [CrossRef]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef]
- Arif, F.; Rahman, M.F.; Khan, C.F. Adipose derived stem cells for the peripheral nerve regeneration: Review of techniques and clinical implications. J. Pak. Med. Assoc. 2023, 73, S148–S154. [Google Scholar] [CrossRef]
- Zhang, R.; Rosen, J.M. The role of undifferentiated adipose-derived stem cells in peripheral nerve repair. Neural Regen. Res. 2018, 13, 757–763. [Google Scholar] [PubMed]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Barbon, S.; Stocco, E.; Dalzoppo, D.; Contran, M.; De Rose, E.; Parnigotto, P.P.; Macchi, V.; Grandi, C.; De Caro, R. Development of Oxidized Polyvinyl Alcohol-Based Nerve Conduits Coupled with the Ciliary Neurotrophic Factor. Materials 2019, 12, 1996. [Google Scholar] [CrossRef]
- Joung, D.; Lavoie, N.S.; Guo, S.Z.; Park, S.H.; Parr, A.M.; McAlpine, M.C. 3D Printed Neural Regeneration Devices. Adv. Funct. Mater. 2020, 30, 1906237. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Yang, Y.; Yang, Y.; Liu, W. Material advancement in tissue-engineered nerve conduit. Nanotechnol. Rev. 2021, 10, 488–503. [Google Scholar] [CrossRef]
- Kang, N.U.; Lee, S.J.; Gwak, S.J. Fabrication Techniques of Nerve Guidance Conduits for Nerve Regeneration. Yonsei Med. J. 2022, 63, 114–123. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Grandi, F.; Gamba, P.G.; Borgio, L.; Del Gaudio, C.; Dalzoppo, D.; Lora, S.; Rajendran, S.; Porzionato, A.; et al. Partially oxidized polyvinyl alcohol as a promising material for tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2060–2070. [Google Scholar] [CrossRef]
- Barbon, S.; Stocco, E.; Negro, A.; Dalzoppo, D.; Borgio, L.; Rajendran, S.; Grandi, F.; Porzionato, A.; Macchi, V.; De Caro, R.; et al. In vitro assessment of TAT—Ciliary Neurotrophic Factor therapeutic potential for peripheral nerve regeneration. Toxicol. Appl. Pharmacol. 2016, 309, 121–128. [Google Scholar] [CrossRef]
- Grandi, F.; Stocco, E.; Barbon, S.; Rambaldo, A.; Contran, M.; Fascetti Leon, F.; Gamba, P.G.; Parnigotto, P.P.; Macchi, V.; De Caro, R.; et al. Composite Scaffolds Based on Intestinal Extracellular Matrices and Oxidized Polyvinyl Alcohol: A Preliminary Study for a New Regenerative Approach in Short Bowel Syndrome. BioMed Res. Int. 2018, 2018, 7824757. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.; Stocco, E.; Dalzoppo, D.; Todros, S.; Canale, A.; Boscolo-Berto, R.; Pavan, P.; Macchi, V.; Grandi, C.; De Caro, R.; et al. Halogen-Mediated Partial Oxidation of Polyvinyl Alcohol for Tissue Engineering Purposes. Int. J. Mol. Sci. 2020, 21, 801. [Google Scholar] [CrossRef]
- Todros, S.; Barbon, S.; Stocco, E.; Favaron, M.; Macchi, V.; De Caro, R.; Porzionato, A.; Pavan, P.G. Time-dependent mechanical behavior of partially oxidized polyvinyl alcohol hydrogels for tissue engineering. J. Mech. Behav. Biomed. Mater. 2022, 125, 104966. [Google Scholar] [CrossRef]
- Todros, S.; Spadoni, S.; Barbon, S.; Stocco, E.; Confalonieri, M.; Porzionato, A.; Pavan, P.G. Compressive Mechanical Behavior of Partially Oxidized Polyvinyl Alcohol Hydrogels for Cartilage Tissue Repair. Bioengineering 2022, 9, 789. [Google Scholar] [CrossRef] [PubMed]
- Angius, D.; Wang, H.; Spinner, R.J.; Gutierrez-Cotto, Y.; Yaszemski, M.J.; Windebank, A.J. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials 2012, 33, 8034–8039. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.; Razavi, S.; Bakhtiari, A. The advances in nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays. J. Tissue Eng. Regen. Med. 2019, 13, 2077–2100. [Google Scholar] [CrossRef] [PubMed]
- Vela, F.J.; Martínez-Chacón, G.; Ballestín, A.; Campos, J.L.; Sánchez-Margallo, F.M.; Abellán, E. Animal models used to study direct peripheral nerve repair: A systematic review. Neural Regen. Res. 2020, 15, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Meena, P.; Kakkar, A.; Kumar, M.; Khatri, N.; Nagar, R.K.; Singh, A.; Malhotra, P.; Shukla, M.; Saraswat, S.K.; Srivastava, S.; et al. Advances and clinical challenges for translating nerve conduit technology from bench to bed side for peripheral nerve repair. Cell Tissue Res. 2021, 383, 617–644. [Google Scholar] [CrossRef] [PubMed]
| Commercial Device | Manufacturer | Composition | Gap | Degradation Time | Maximum Length; Inner Diameter | Device ID, Year of FDA Approval |
|---|---|---|---|---|---|---|
| NeuroTubeTM | Synovis Micro Companies Alliance, Inc., Birmingham, AL, USA | PGA | 2.0–4.0 cm | 3 months | 30 mm; 2–8 mm | K983007, 1999 |
| SaluBridgeTM | Salumedica LLC, Atlanta, GA, USA | PVA | 4.0–6.35 cm | Not resorbable | 40 mm; -- | K002098, 2000 |
| NeuraGenTM | Integra LifeSciences, Princeton, NJ, USA | Collagen type I + glycosaminoglycan (chondroitin-6-sulfate) | 0.5–1.7 cm | 4 years | 20–30 mm; 15–30 mm | K011168, 2001 |
| NeuroMatrixTM | Collagen Matrix, Inc., Franklin Lakes, NJ, USA | Collagen type I | 2.5–3.0 cm | 4–8 months | 25 mm; 2–6 mm | K012814, 2001 |
| SaluTunnelTM | Salumedica LLC, Atlanta, GA, USA | PVA | 4.0–6.35 cm | Not resorbable | 40 mm; -- | K100382, 2010 |
| NeurolacTM | Polyganics Inc., Groningen, The Netherlands | D,L-PLCL | Up to 2.0 cm | 16 months | 25 mm; N/A | K050573, 2005 K112267, 2011 |
| NeuroMendTM | Collagen Matrix, Inc., Franklin Lakes, NJ, USA | Collagen type I | 0.9–2.5 cm | 4–8 months | 25–50 mm; 4–12 mm | K060952, 2006 |
| Avance® Nerve Graft | Axogen, Inc. Alachua, FL, USA | Decellularized cadaveric nerve | 2.5–3.0 cm | -- | 15–70 mm; 3–5 mm | 2007 |
| NeuroFlexTM | Collagen Matrix, Inc., Franklin Lakes, NJ, USA | Collagen type I | 2.5 cm | 4–8 months | 25 mm; 2–6 mm | K131541, 2014 |
| NeuraGenTM 3D Matrix | Integra LifeSciences, Princeton, NJ, USA | Collagen type I + glycosaminoglycan (chondroitin-6-sulfate) | 2.5 cm | 4–8 months | 20–30 mm; 1.5–7 mm | K011168, 2014 |
| ReaxonTM Plus | Medovent GmbH, Mainz, Germany | Chitosan | Up to 1.0 cm | Significant macroscopic signs of degradation after 74/77 weeks | 14 mm; 2.1–6.0 mm | K143711, 2015 |
| AxoGuardTM Nerve Connector | AxoGen, Inc., Alachua, FL, USA | Porcine SIS (mainly collagen types I, III, IV, and VI) | 0.5–1.0 cm | 3–4 months | 10 mm; 1.5–7 mm | K162741, 2016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. https://doi.org/10.3390/ijms24119170
Stocco E, Barbon S, Emmi A, Tiengo C, Macchi V, De Caro R, Porzionato A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. International Journal of Molecular Sciences. 2023; 24(11):9170. https://doi.org/10.3390/ijms24119170
Chicago/Turabian StyleStocco, Elena, Silvia Barbon, Aron Emmi, Cesare Tiengo, Veronica Macchi, Raffaele De Caro, and Andrea Porzionato. 2023. "Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design" International Journal of Molecular Sciences 24, no. 11: 9170. https://doi.org/10.3390/ijms24119170
APA StyleStocco, E., Barbon, S., Emmi, A., Tiengo, C., Macchi, V., De Caro, R., & Porzionato, A. (2023). Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. International Journal of Molecular Sciences, 24(11), 9170. https://doi.org/10.3390/ijms24119170










