Hamster Sperm Possess Functional Na+/Ca2+-Exchanger 1: Its Implication in Hyperactivation
Abstract
1. Introduction
2. Results
2.1. Detection of NCX1 in Hamster Spermatozoa
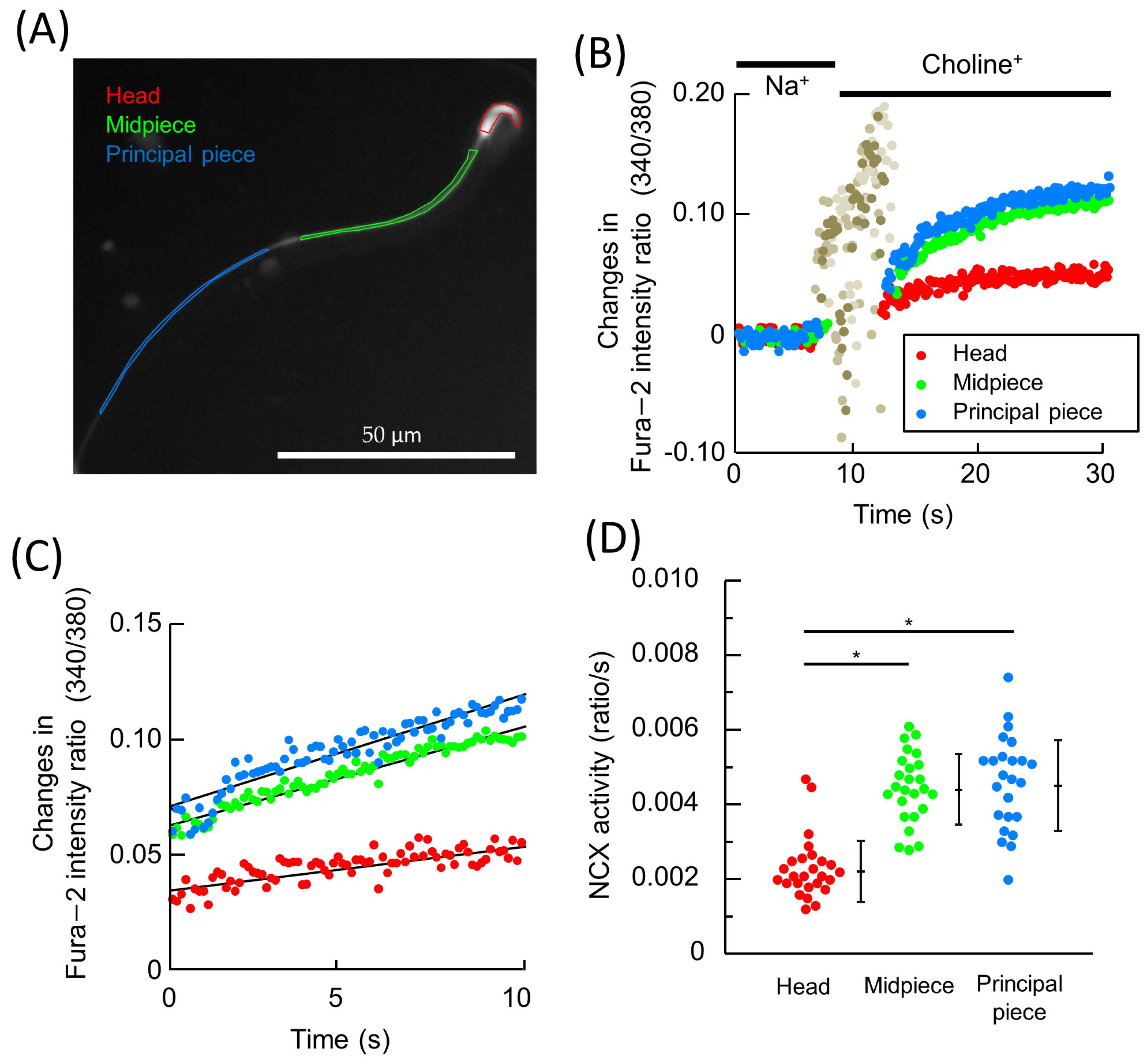
2.2. NCX1 Is Functional in Hamster Spermatozoa
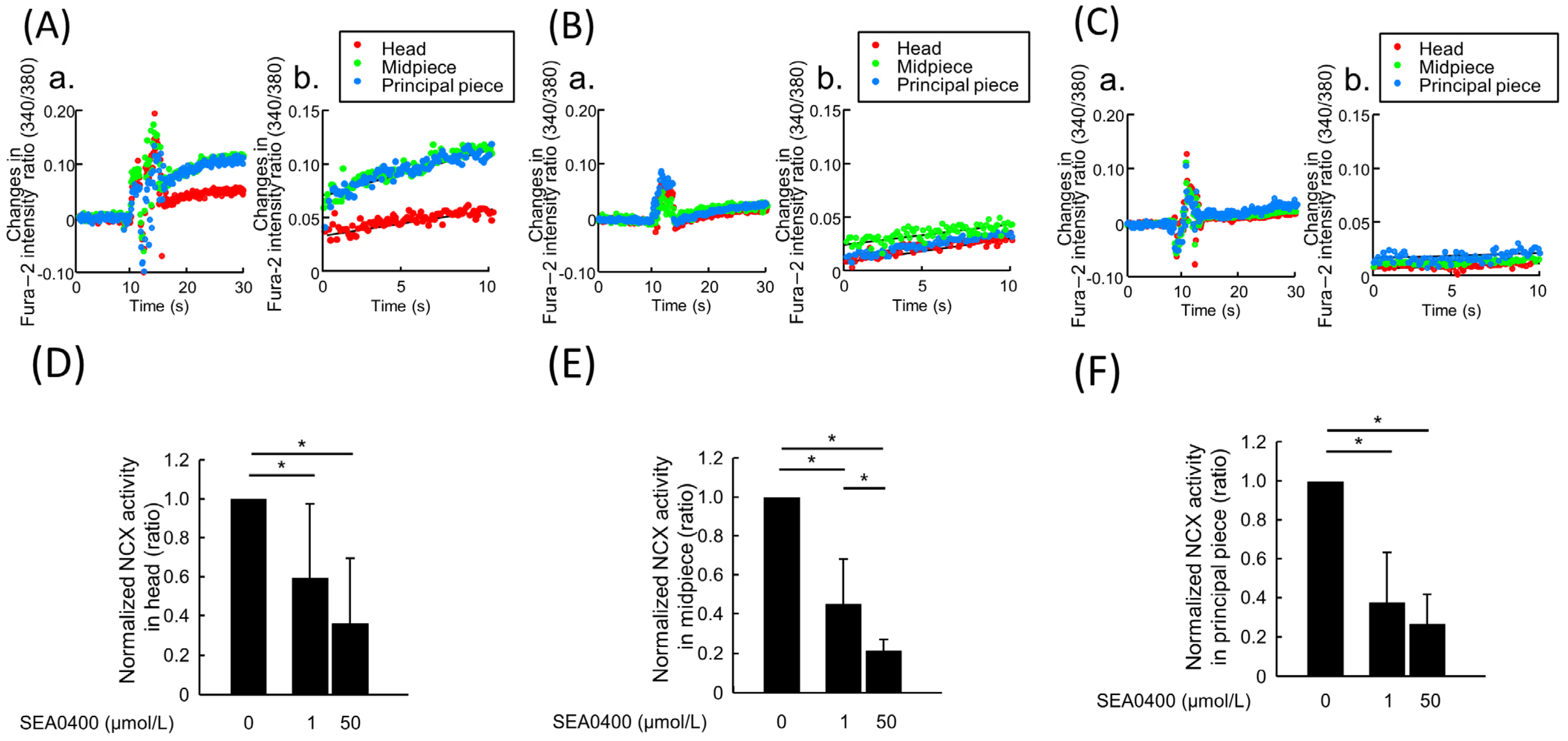
2.3. The Effect of NCX Inhibitors on NCX Activity
2.4. The NCX1 Activity Was Declined upon Capacitation
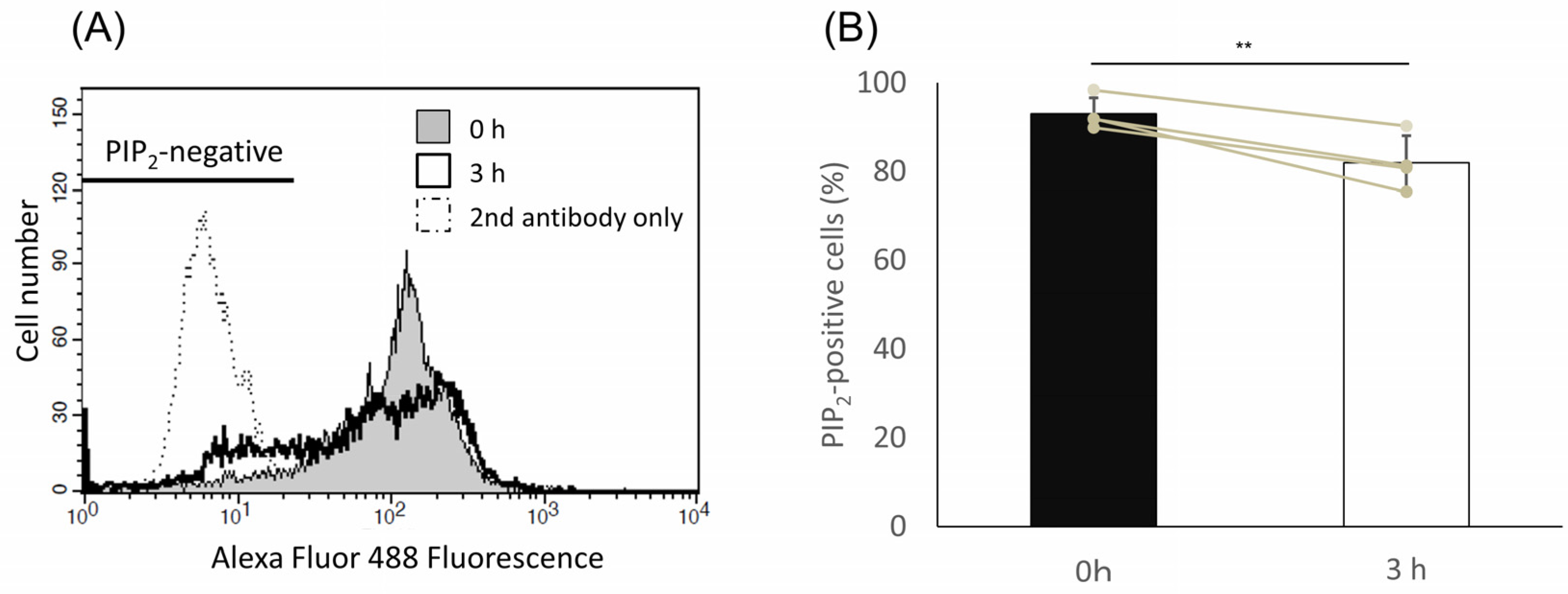
2.5. PIP2 Level Was Decreased upon Capacitation
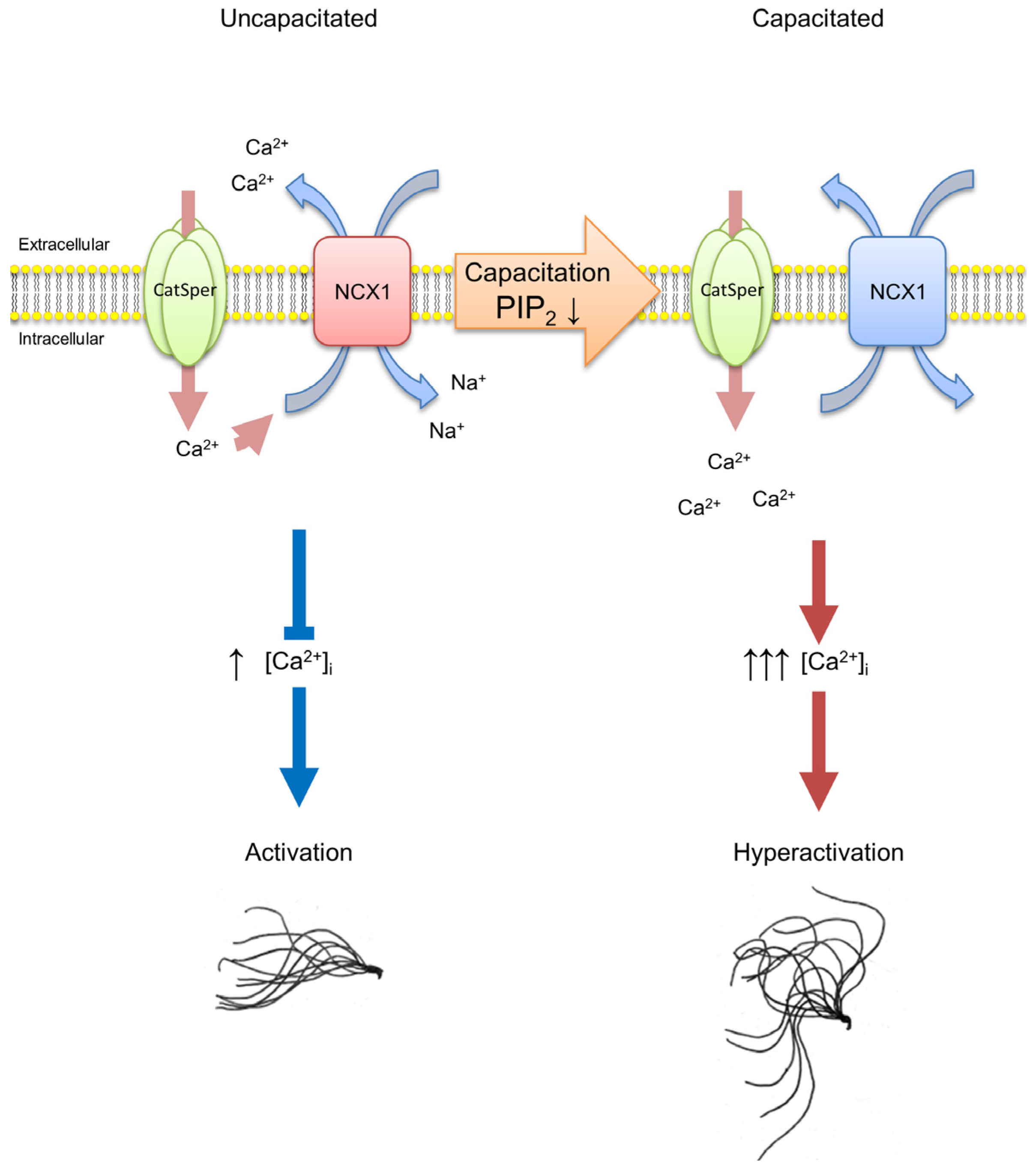
3. Discussion
4. Materials and Methods
4.1. Reagents and Solutions
4.2. Experimental Animals and Spermatozoa Collection
4.3. RNA Seq Analysis
4.4. PCR
4.5. Microsome Preparation
4.6. SDS-PAGE and Western Blotting
4.7. Measurement of NCX Activity
4.8. Analysis of PIP2 Levels by Flow Cytometry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yanagimachi, R. Mammalian fertilization. In Physiology of Reproduction, 2nd ed.; Knobil, E., Neill., J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Yanagimachi, R. The movement of golden hamster spermatozoa before and after capacitation. J. Reprod. Fert. 1970, 23, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Katz, D.F.; Owen, D.H.; Andrew, J.B.; Powell, R.L. Evidence for the function of hyperactivated motility in sperm. Biol. Reprod. 1991, 44, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Dai, X. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol. Reprod. 1992, 46, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Quill, T.A.; Sugden, S.; Rossi, K.; Doolittle, L.; Hammer, R.; Garbers, D. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl. Acad. Sci. USA 2003, 100, 14869–14874. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Moran, M.M.; Navarro, B.; Chong, J.; Krapivinsky, G.; Krapivinsky, L.; Kirichok, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Wolff, C.; Suarez, S.S. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod. Fert. Dev. 2009, 21, 345–350. [Google Scholar] [CrossRef]
- Tateno, H.; Krapf, D.; Hino, T.; Sánchez-Cárdenas, C.; Darszon, A.; Yanagimachi, R.; Visconti, P.E. Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 18543–18548. [Google Scholar] [CrossRef]
- Navarrete, F.A.; Alvau, A.; Lee, H.C.; Levin, L.R.; Buck, J.; Leon, P.M.-D.; Santi, C.M.; Krapf, D.; Mager, J.; Fissore, R.A.; et al. Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Sci. Rep. 2016, 6, 33589. [Google Scholar] [CrossRef]
- Navarrete, F.A.; Aguila, L.; Martin-Hidalgo, D.; Tourzani, D.A.; Luque, G.M.; Ardestani, G.; Garcia-Vazquez, F.A.; Levin, L.R.; Buck, J.; Darszon, A.; et al. Transient Sperm Starvation Improves the Outcome of Assisted Reproductive Technologies. Front. Cell Dev. Biol. 2019, 7, 262. [Google Scholar] [CrossRef]
- Suarez, S.S.; Varosi, S.M.; Dai, X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc. Natl. Acad. Sci. USA 1993, 90, 4660–4664. [Google Scholar] [CrossRef]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujinoki, M.; Shibahara, H.; Suzuki, M. Regulation of hyperactivation by PPP2 in hamster spermatozoa. Reproduction 2010, 139, 847–856. [Google Scholar] [CrossRef]
- Alvau, A.; Battistone, M.A.; Gervasi, M.G.; Navarrete, F.A.; Xu, X.; Sánchez-Cárdenas, C.; De la Vega-Beltran, J.L.; Da Ros, V.G.; Greer, P.A.; Darszon, A.; et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 2016, 143, 2325–2333. [Google Scholar] [CrossRef]
- Takei, G.L.; Fujionki, M. Regulation of hamster sperm hyperactivation by extracellular Na+. Reproduction 2016, 151, 589–603. [Google Scholar] [CrossRef]
- Takei, G.L.; Hayashi, K. Na+/K+-ATPase α4 regulates sperm hyperactivation while Na+/K+-ATPase α1 regulates basal motility in hamster spermatozoa. Theriogenology 2020, 157, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Takei, G.L.; Kon, H. Oviductal high concentration of K+ suppresses hyperpolarization but does not prevent hyperactivation, acrosome reaction and in vitro fertilization in hamsters. Zygote 2021, 29, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Mannowetz, N. CatSper: A unique calcium channel of the sperm flagellum. Curr. Opin. Physiol. 2018, 2, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Wennemuth, G.; Babcock, D.F.; Hille, B. Calcium Clearance Mechanisms of Mouse Sperm. J. Gen. Physiol. 2003, 122, 115–128. [Google Scholar] [CrossRef]
- Okunade, G.W.; Miller, M.L.; Pyne, G.J.; Sutliff, R.L.; O’Connor, K.T.; Neumann, J.C.; Andringa, A.; Miller, D.A.; Prasad, V.; Doetschman, T.; et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 2004, 279, 33742–33750. [Google Scholar] [CrossRef]
- Schuh, K.; Cartwright, E.J.; Jankevics, E.; Bundschu, K.; Liebermann, J.; Williams, J.C.; Armesilla, A.L.; Emerson, M.; Oceandy, D.; Knobeloch, K.P.; et al. Plasma Membrane Ca2+ ATPase 4 Is Required for Sperm Motility and Male Fertility. J. Biol. Chem. 2004, 279, 28220–28226. [Google Scholar] [CrossRef]
- Quednau, B.D.; Nicoll, D.A.; Philipson, K.D. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. 1997, 272, C1250–C1261. [Google Scholar] [CrossRef]
- Reddy, P.R.; Patni, A.; Sharma, A.; Gupta, S.; Tiwary, A.K. Effect of 2’,4’-dichlorobenzamil hydrochloride, a Na+-Ca2+ exchange inhibitor, on human spermatozoa. Eur. J. Pharmacol. 2001, 418, 153–155. [Google Scholar] [CrossRef]
- Krasznai, Z.; Krasznai, Z.T.; Morisawa, M.; Bazsane, Z.K.; Hernadi, Z.; Fazekas, Z.; Tron, L.; Goda, K.; Marian, T. Role of the Na+/Ca2+ exchanger in calcium homeostasis and human sperm motility regulation. Cell Motil. Cytoskelet. 2006, 63, 66–76. [Google Scholar] [CrossRef]
- Kofuji, P.; Lederer, W.J.; Schulze, D.H. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na/Ca exchanger. J. Biol. Chem. 1994, 269, 5149–5154. [Google Scholar] [CrossRef]
- Lee, S.L.; Yu, A.S.L.; Lytton, J. Tissue-specific expression of Na+-Ca2+ exchanger isoforms. J. Biol. Chem. 1994, 269, 14849–14852. [Google Scholar] [CrossRef] [PubMed]
- Philipson, K.D.; Longoni, S.; Ward, R. Purification of the cardiac Na+-Ca2+ exchange protein. Biochim. Biophys. Acta 1988, 945, 298–306. [Google Scholar] [CrossRef]
- Ignotz, G.G.; Suarez, S.S. Calcium/Calmodulin and Calmodulin Kinase II Stimulate Hyperactivation in Demembranated Bovine Sperm. Biol. Reprod. 2005, 73, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Suarez, S.S. Bovine Sperm Hyperactivation Is Promoted by Alkaline-Stimulated Ca2+ Influx. Biol. Reprod. 2007, 76, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, R.R.; Jones, W.D.; Rizk, B.M. Evidence for Nitric Oxide Regulation of Hamster Sperm Hyperactivation. J. Androl. 1998, 19, 58–64. [Google Scholar] [PubMed]
- Griveau, J.E.; Renard, P.; Le Lannou, D. An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int. J. Androl. 1994, 17, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M.; Takei, G.L.; Kon, H. Non-genomic regulation and disruption of spermatozoal in vitro hyperactivation by oviductal hormones. J. Physiol. Sci. 2016, 66, 207–212. [Google Scholar] [CrossRef]
- Arai, Y.; Sakase, M.; Fukushima, M.; Harayama, H. Identification of isoforms of calyculin A-sensitive protein phosphatases which suppress full-type hyperactivation in bull ejaculated spermatozoa. Theriogenology 2019, 129, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Vacquier, V.D. A flagellar K+-dependent Na+/Ca2+ exchanger keeps Ca2+ low in sea urchin spermatozoa. Proc. Natl. Acad. Sci. USA 2002, 99, 6743–6748. [Google Scholar] [CrossRef]
- Kraev, A.; Quednau, B.D.; Leach, S.; Li, X.F.; Dong, H.; Winkfein, R.; Perizzolo, M.; Cai, X.; Yang, R.; Philipson, K.D.; et al. Molecular cloning of a third member of the potassium-dependent sodium-calcium exchanger gene family, NCKX3. J. Biol. Chem. 2001, 276, 23161–23172. [Google Scholar] [CrossRef]
- Iwamoto, T.; Kita, S.; Uehara, A.; Imanaga, I.; Matsuda, T.; Baba, A.; Katsuragi, T. Molecular Determinants of Na+/Ca2+ Exchange (NCX1) Inhibition by SEA0400. J. Biol. Chem. 2004, 279, 7544–7553. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Ball, R. Regulation of Cardiac Na+, Ca2+ Exchange and KATP Potassium Channels by PIP2. Science 1996, 273, 956–959. [Google Scholar] [CrossRef]
- Iwamoto, T.; Pan, Y.; Wakabayashi, S.; Imagawa, T.; Yamanaka, H.I.; Shigekawa, M. Phosphorylation-dependent regulation of cardiac Na+-Ca2+ exchanger via protein kinase C. J. Biol. Chem. 1996, 271, 13609–13615. [Google Scholar] [CrossRef]
- He, L.P.; Cleemann, L.; Soldatov, N.M.; Morad, M. Molecular determinants of cAMP-mediated regulation of the Na+–Ca2+ exchanger expressed in human cell lines. J. Physiol. 2003, 548, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Morad, M.; Cleemann, L.; Menick, D.R. NCX1 phosphorylation dilemma: A little closer to resolution. Focus on “Full-length cardiac Na+/Ca2+ exchanger 1 protein is not phosphorylated by protein kinase A”. Am. J. Physiol. Cell Physiol. 2011, 300, C970–C973. [Google Scholar] [CrossRef] [PubMed]
- Reilly, L.; Howie, J.; Wypijewski, K.; Ashford, M.L.J.; Hilgemann, D.W.; Fuller, W. Palmitoylation of the Na/Ca exchanger cytoplasmic loop controls its inactivation and internalization during stress signaling. FASEB J. 2015, 29, 4532–4543. [Google Scholar] [CrossRef] [PubMed]
- Porzig, H.; Li, Z.; Nicoll, D.A.; Philipson, K.D. Mapping of the cardiac sodium-calcium exchanger with monoclonal antibodies. Am. J. Physiol. Cell Physiol. 1993, 265, C748–C756. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Islam, R.; Takayanagi, M.; Yasumuro, H.; Inami, W.; Kunahong, A.; Casco-Robles, R.M.; Toyama, F.; Chiba, C. A Transcriptome for the Study of Early Processes of Retinal Regeneration in the Adult Newt, Cynops pyrrhogaster. PLoS ONE 2014, 9, e109831. [Google Scholar] [CrossRef] [PubMed]
- Ujihara, Y.; Iwasaki, K.; Takatsu, S.; Hashimoto, K.; Naruse, K.; Mohri, S.; Katanosaka, Y. Induced NCX1 overexpression attenuates pressure overload-induced pathological cardiac remodelling. Cardiovasc. Res. 2016, 111, 348–361. [Google Scholar] [CrossRef]
- Ogura, Y.; Ito, H.; Sugita, S.; Nakamura, M.; Ujihara, Y. Decrease in Ca2+ concentration in quail cardiomyocytes is faster than that in rat cardiomyocytes. Processes 2022, 10, 508. [Google Scholar] [CrossRef]
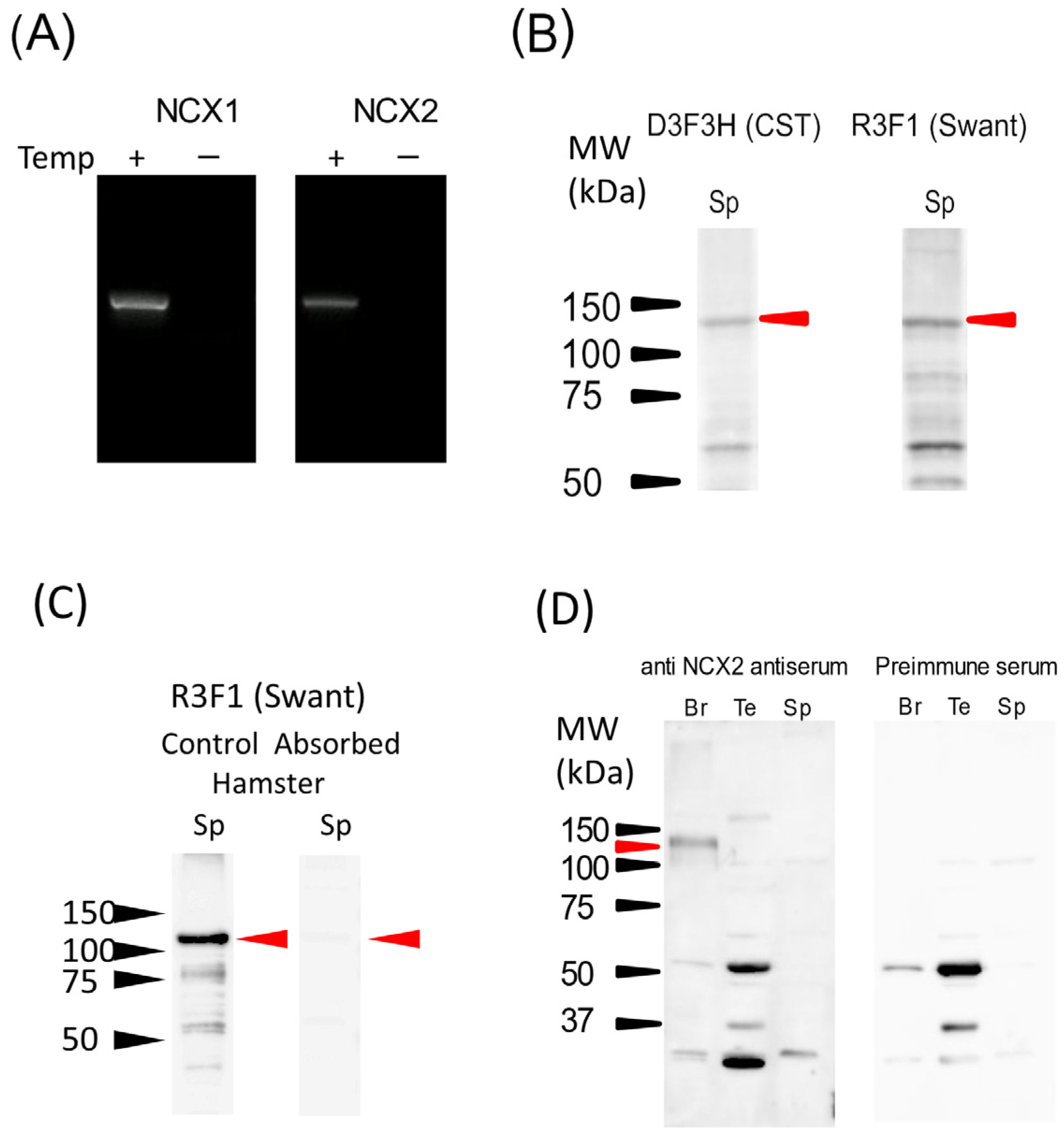

| Genes | Sequence ID | Species | Bit Score | e-Value | Identical (%) | TPM | FPKM |
|---|---|---|---|---|---|---|---|
| NCX1 | XP_005077303.1 | Mesocricetus auratus | 1497.6 | 0 | 100 | 0.27 | 0.25 |
| NCX1 | XP_014420366.1 | Camelus ferus | 193.4 | 5.07 × 10−62 | 98.97 | 0.52 | 0.47 |
| NCX2 | XP_013853997.1 | Sus scrofa | 386.3 | 7.85 × 10−131 | 100 | 1.82 | 1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takei, G.L.; Ogura, Y.; Ujihara, Y.; Toyama, F.; Hayashi, K.; Fujita, T. Hamster Sperm Possess Functional Na+/Ca2+-Exchanger 1: Its Implication in Hyperactivation. Int. J. Mol. Sci. 2023, 24, 8905. https://doi.org/10.3390/ijms24108905
Takei GL, Ogura Y, Ujihara Y, Toyama F, Hayashi K, Fujita T. Hamster Sperm Possess Functional Na+/Ca2+-Exchanger 1: Its Implication in Hyperactivation. International Journal of Molecular Sciences. 2023; 24(10):8905. https://doi.org/10.3390/ijms24108905
Chicago/Turabian StyleTakei, Gen L., Yuhei Ogura, Yoshihiro Ujihara, Fubito Toyama, Keitaro Hayashi, and Tomoe Fujita. 2023. "Hamster Sperm Possess Functional Na+/Ca2+-Exchanger 1: Its Implication in Hyperactivation" International Journal of Molecular Sciences 24, no. 10: 8905. https://doi.org/10.3390/ijms24108905
APA StyleTakei, G. L., Ogura, Y., Ujihara, Y., Toyama, F., Hayashi, K., & Fujita, T. (2023). Hamster Sperm Possess Functional Na+/Ca2+-Exchanger 1: Its Implication in Hyperactivation. International Journal of Molecular Sciences, 24(10), 8905. https://doi.org/10.3390/ijms24108905






