Pharmacological Evidence Suggests That Slo3 Channel Is the Principal K+ Channel in Boar Spermatozoa
Abstract
1. Introduction
2. Results
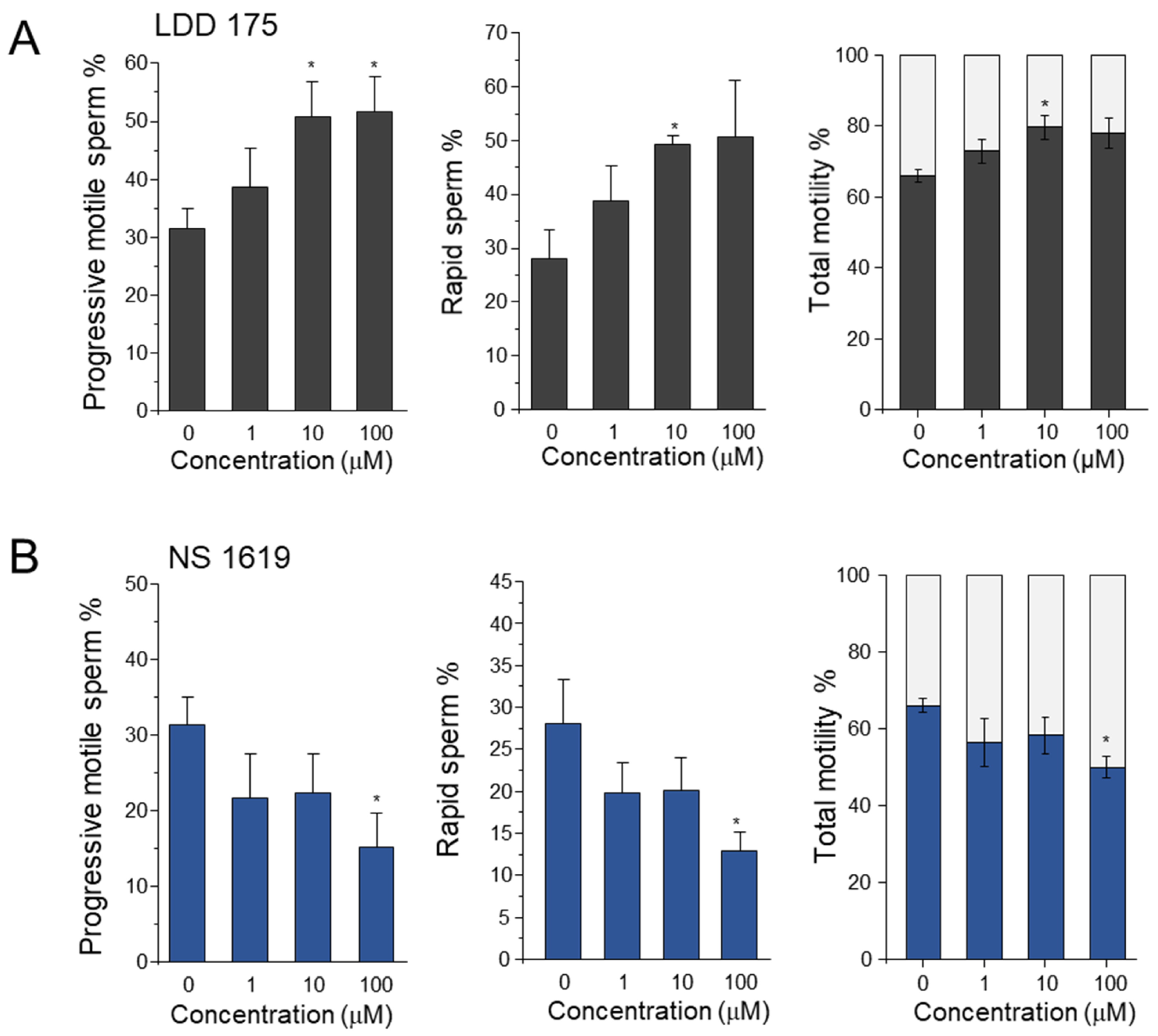
2.1. Effect of K+ Channel Modulators on Boar Sperm Motility and Kinematic Parameters
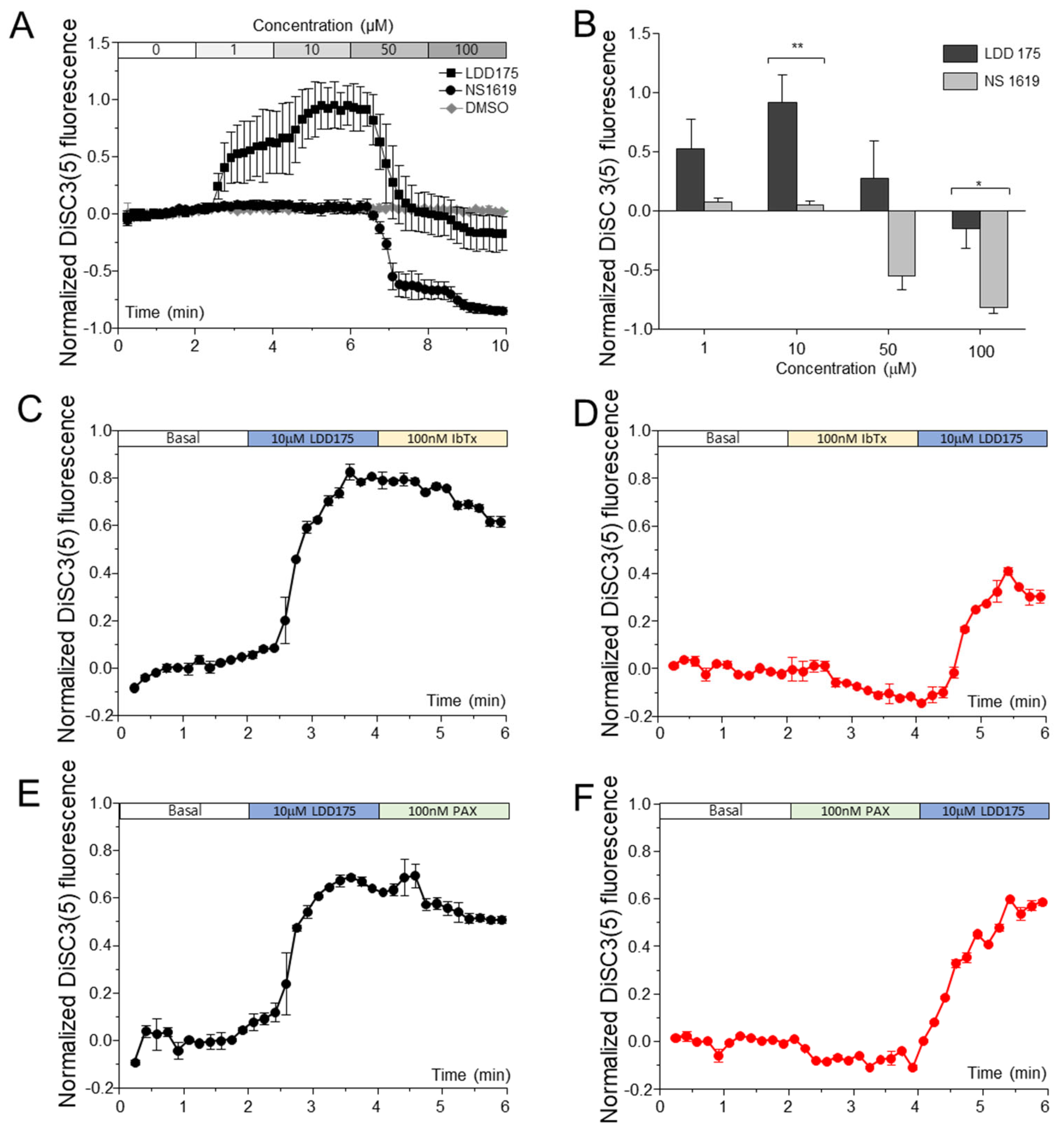
2.2. LDD175 Treatment Hyperpolarizes and NS1619 Treatment Depolarizes the Sperm Membrane, While Other Slo1 Modulators Do Not Have a Significant Effect
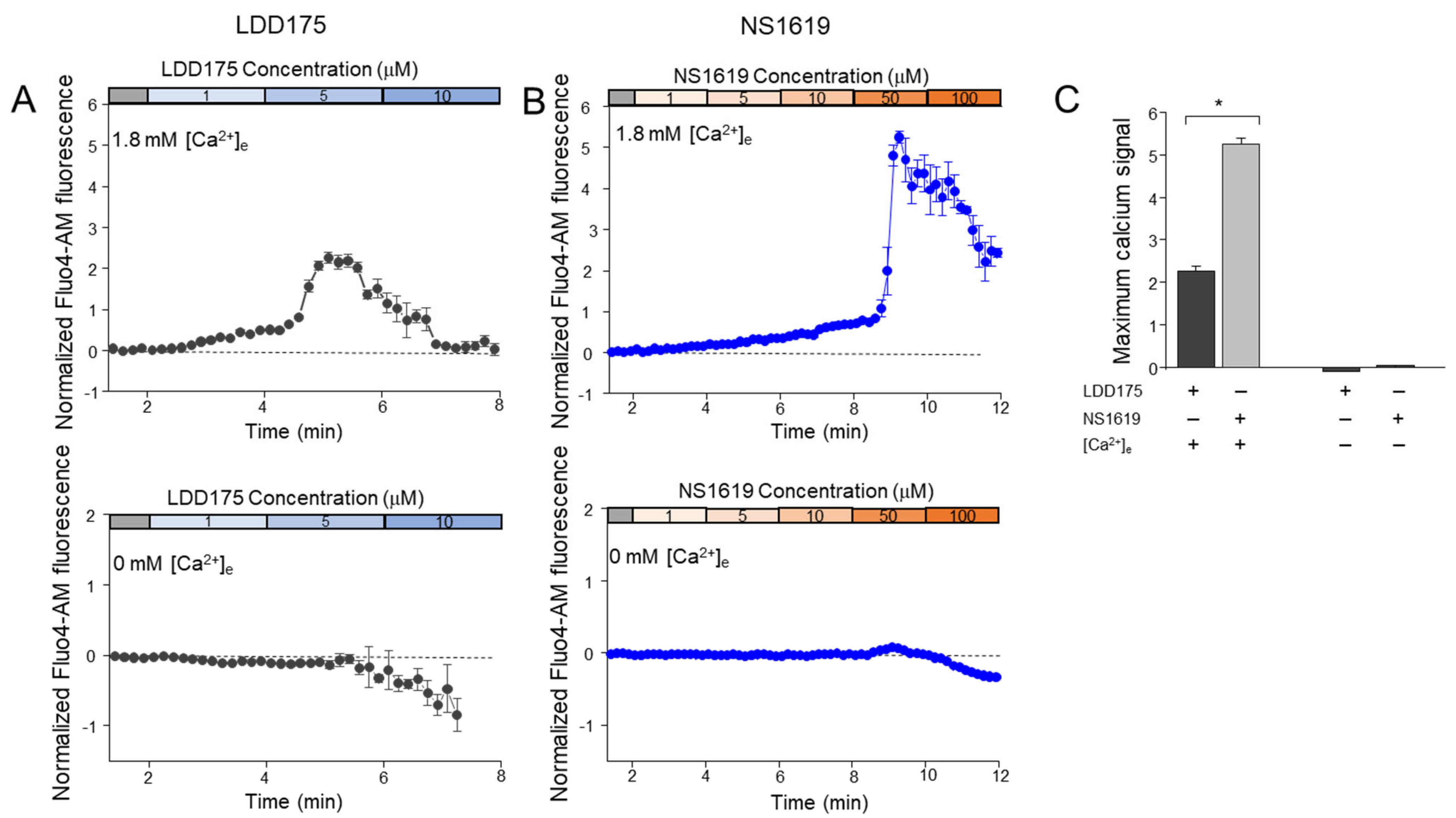
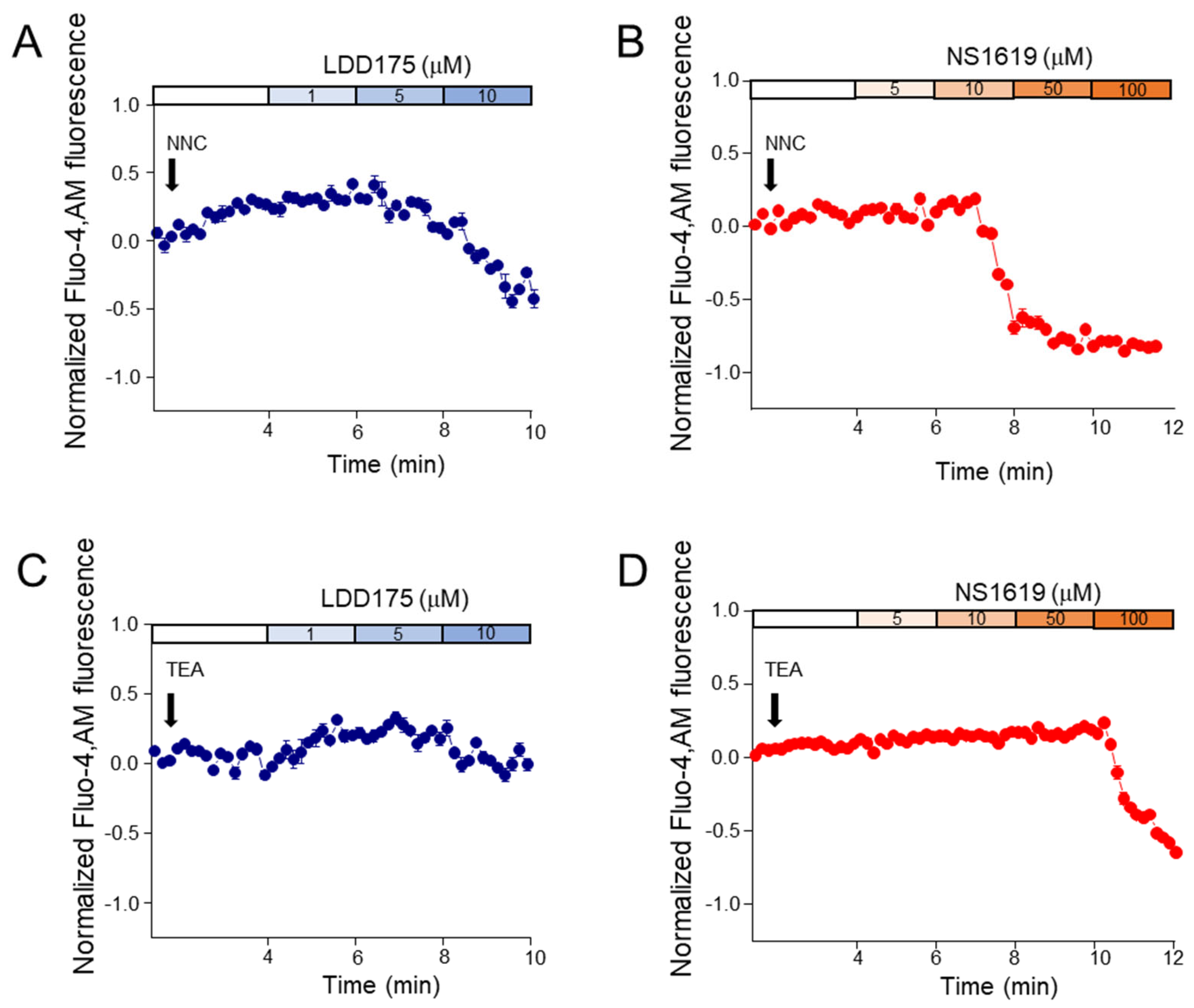
2.3. Intracellular Calcium Signals Change with K+ Channel Modulations
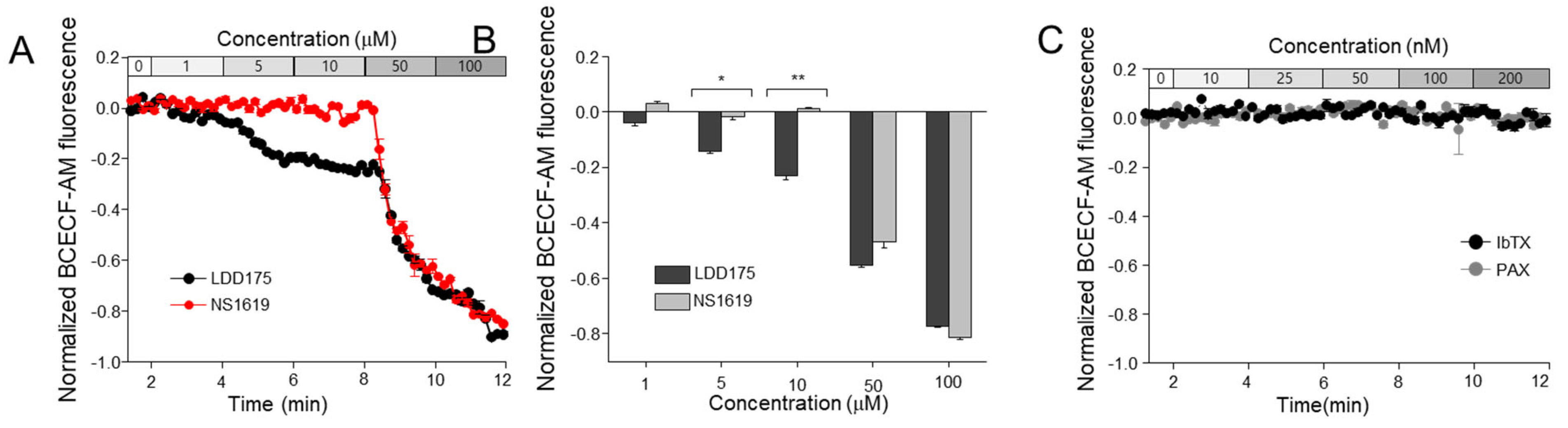
2.4. The Principal K+ Channel Affects Sperm Intracellular pH
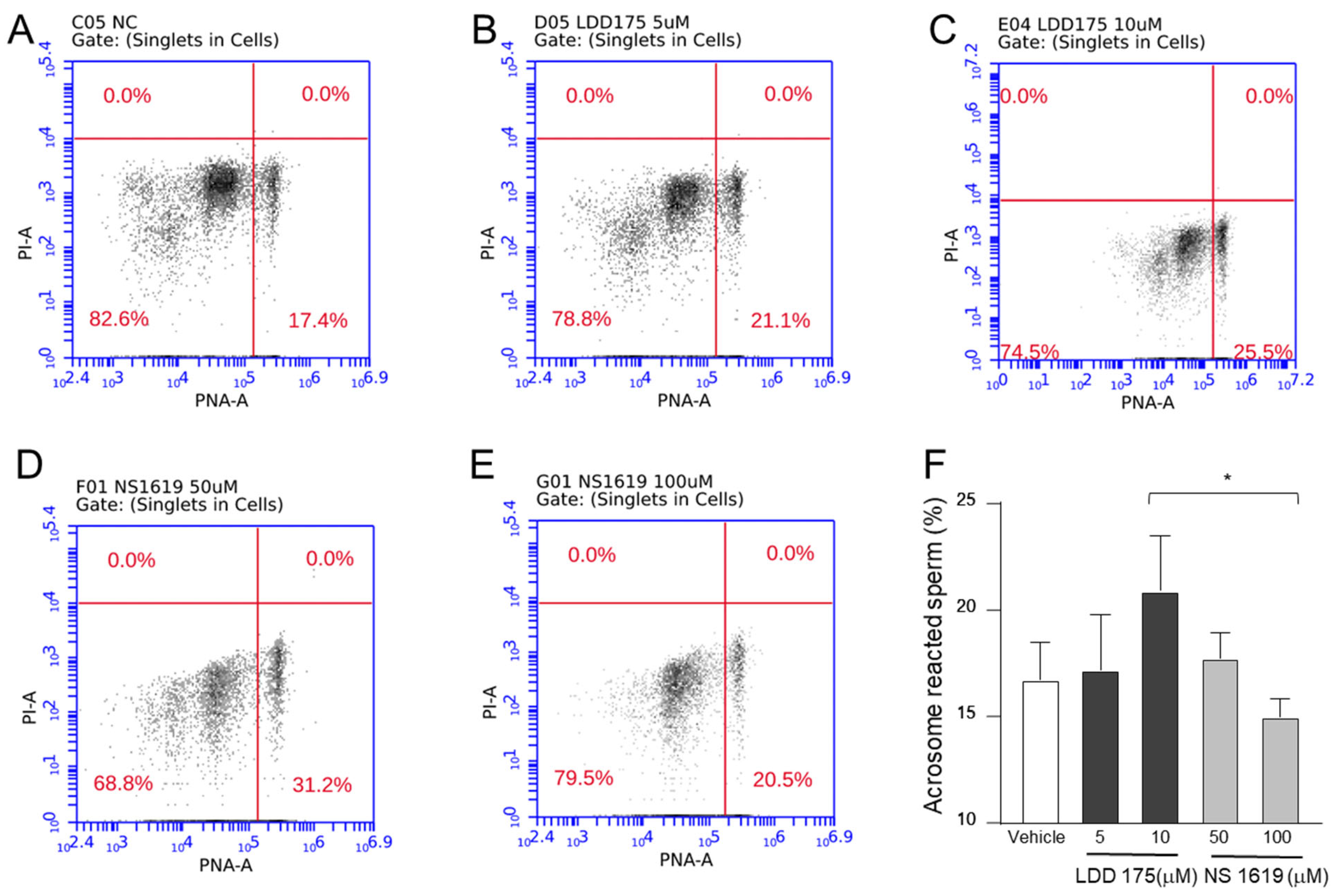
2.5. Inhibition of the Principal K+ Channel Reduces Acrosome Reaction
2.6. Slo1 and Slo3 Channels Showed Different Localizations in Boar Spermatozoa
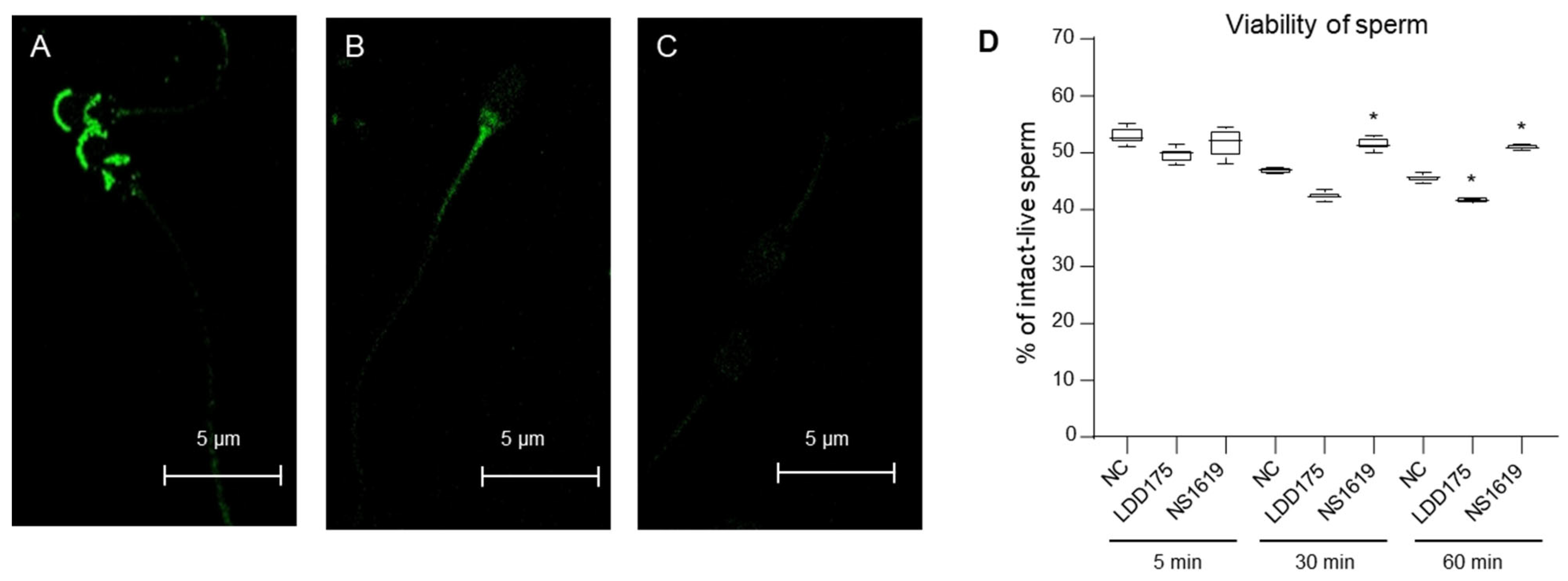
2.7. Slo3 Channel Inhibition Improves Boar Cell Viability
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Experimental Design
4.3. Sperm Medium
4.4. Sample Preparation
4.5. Computer-Assisted Sperm Analysis (CASA)
4.6. Membrane Potential Measurement
4.7. Intracellular Calcium Measurement
4.8. Intracellular pH Measurement
4.9. Acrosome Reaction
4.10. Immunocytochemistry
4.11. Apoptosis Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCECF-AM | 2′, 7′,-bis-(2-carboxyethyl)-5-(and-6-) -carboxyfluorescein acetoxymethyl ester |
| CAP | capacitating medium |
| CASA | computer-assisted sperm analysis |
| CatSper | cation channel of sperm |
| DiSC3(5) | 3,3′ dipropyl thiadicarbo cyanine iodide |
| DMSO | dimethyl sulfoxide |
| KSper | principal potassium current of sperm |
| TLFC | time-lapse flow cytometry |
| Fluo4-AM | 4-(6-Acetoxymethoxy-2,7-difluoro-3-oxo-9-xanthenyl)-4′-methyl 2,2′(ethylenedioxy)dianiline N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl) ester |
| IbTx | iberiotoxin |
| LDD175 | 4-chloro-7-trifluoromethyl-10H-benzo [4,5]furo [3,2-b]indole-1-carboxylic acid |
| NNC 55-0396 | NNC 55-0396 dihydrochloride |
| Non-CAP | non-capacitating medium |
| NS1619 | 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)-phenyl]-5-(trifluoromethyl)-2H-benzimidazole-2-one |
| PAX | paxilline |
| PNA | Arachis hypogaea lectin |
| RT | room temperature |
| Slo1 | calcium-activated potassium channel slo-1 |
| Slo3 | calcium-activated potassium channel subunit alpha-3 |
| TEA | tetraethylammonium chloride hydrate |
References
- Wang, H.; McGoldrick, L.L.; Chung, J.J. Sperm ion channels and transporters in male fertility and infertility. Nat. Rev. Urol. 2021, 18, 46–66. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Szymczak-Cendlak, M. Structure and function of ion channels regulating sperm motility—An overview. Int. J. Mol. Sci. 2021, 22, 3259. [Google Scholar] [CrossRef]
- López-González, I.; Torres-Rodríguez, P.; Sánchez-Carranza, O.; Solís-López, A.; Santi, C.M.; Darszon, A.; Treviño, C.L. Membrane hyperpolarization during human sperm capacitation. Mol. Hum. Reprod. 2014, 20, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, P.A.; Sanchez-Cardenas, C.; Luque, G.M.; Baro Graf, C.; Sierra, J.M.; Hernández-Cruz, A.; Visconti, P.E.; Krapf, D.; Darszon, A.; Buffone, M.G. Membrane hyperpolarization abolishes calcium oscillations that prevent induced acrosomal exocytosis in human sperm. FASEB J. 2021, 35, e21478. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Clark, E.N.; Florman, H.M. Sperm membrane potential: Hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev. Biol. 1995, 171, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.M.; Martínez-López, P.; de la Vega-Beltrán, J.L.; Butler, A.; Alisio, A.; Darszon, A.; Salkoff, L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010, 584, 1041–1046. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Mansell, S.A.; Lishko, P.V.; Williams, H.L.; Ramalingam, M.; Wilson, S.M.; Barratt, C.L.R.; Sutton, K.A.; Da Silva, S.M. Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod. 2016, 31, 1147–1157. [Google Scholar] [CrossRef]
- Zeng, X.H.; Yang, C.; Kim, S.T.; Lingle, C.J.; Xia, X.M. Deletion of the Slo3 gene abolishes alkalizationactivated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. USA 2011, 108, 5879–5884. [Google Scholar] [CrossRef]
- Vicens, A.; Vinuesa, P.; Arenas, M.; Treviño, C.L. Analyzing the functional divergence of Slo1 and Slo3 channel subfamilies. Mol. Phylogenet. Evol. 2019, 133, 33–41. [Google Scholar] [CrossRef]
- Chávez, J.C.; Vicens, A.; Wrighton, D.C.; Andrade, K.; Carmen, L.; Gutiérrez, R.M.; Lippiat, J.D.; Treviño, C.L. SHORT COMMUNICATION A cytoplasmic Slo3 isoform is expressed in somatic tissues. Mol. Biol. Rep. 2019, 46, 5561–5567. [Google Scholar] [CrossRef]
- Budelli, G.; Geng, Y.; Butler, A.; Magleby, K.L.; Salkoff, L. Properties of Slo1 K+ channels with and without the gating ring. Proc. Natl. Acad. Sci. USA 2013, 110, 16657–16662. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Naidoo, N.M.; Choo, S.-A.S.; Smith, J.F.; Lishko, P.V. Slo1 is the principal potassium channel of human spermatozoa. Elife 2013, 2, e01009. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Wei, A.; Yuan, A.; Gaut, J.; Saito, M.; Salkoff, L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J. Biol. Chem. 1998, 273, 3509–3516. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, M.D.; Yuan, P.; Hsiung, Y.; MacKinnon, R. Functional and structural analysis of the human SLO3 pH- and voltage-gated K+ channel. Proc. Natl. Acad. Sci. USA 2012, 109, 19274–19279. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, X.; Xia, X.M.; Lingle, C.J. pH-regulated Slo3 K+ channels: Properties of unitary currents. J. Gen. Physiol. 2006, 128, 301–315. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, X.; Lingle, C.J. Slo3 K+ channels: Voltage and pH dependence of macroscopic currents. J. Gen. Physiol. 2006, 128, 317–336. [Google Scholar] [CrossRef]
- Brenker, C.; Zhou, Y.; Müller, A.; Echeverry, F.A.; Trötschel, C.; Poetsch, A.; Xia, X.M.; Bönigk, W.; Lingle, C.J.; Kaupp, U.B.; et al. The Ca2+-activated K+ current of human sperm is mediated by Slo3. eLife 2014, 2014, e01438. [Google Scholar] [CrossRef]
- Wijerathne, T.D.; Kim, J.H.; Kim, M.J.; Kim, C.Y.; Chae, M.R.; Lee, S.W.; Lee, K.P. Onion peel extract and its constituent, quercetin inhibits human Slo3 in a pH and calcium dependent manner. Korean J. Physiol. Pharmacol. 2019, 23, 381–392. [Google Scholar] [CrossRef]
- Geng, Y.; Ferreira, J.J.; Dzikunu, V.; Butler, A.; Lybaert, P.; Yuan, P.; Magleby, K.L.; Salkoff, L.; Santi, C.M. A genetic variant of the sperm-specific SLO3 K+ channel has altered pH and Ca2+ sensitivities. J. Biol. Chem. 2017, 776013, 8978–8987. [Google Scholar] [CrossRef]
- Lv, M.; Liu, C.; Ma, C.; Yu, H.; Shao, Z.; Gao, Y.; Liu, Y.; Wu, H.; Tang, D.; Tan, Q.; et al. Homozygous mutation in SLO3 leads to severe asthenoteratozoospermia due to acrosome hypoplasia and mitochondrial sheath malformations. Reprod. Biol. Endocrinol. 2022, 20, 5. [Google Scholar] [CrossRef]
- Lyon, M.; Li, P.; Ferreira, J.J.; Alvarez, A.B.; Molina, L.C.P.; Lybaert, P.; Khambekar, S.; Liu, A.; Santi, C.M. A selective inhibitor of the sperm-specific potassium channel SLO3 impairs human sperm function. Proc. Natl. Acad. Sci. USA 2023, 120, e2212338120. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, Z.; Xia, X.; Lingle, C.J. Block of mouse Slo1 and Slo3 K+ channels by CTX, IbTX, TEA, 4-AP and quinidine. Channels 2010, 4, 22–41. [Google Scholar] [CrossRef]
- Noto, F.; Recuero, S.; Valencia, J.; Saporito, B.; Robbe, D.; Bonet, S.; Carluccio, A.; Yeste, M. Inhibition of potassium channels affects the ability of pig spermatozoa to elicit capacitation and trigger the acrosome exocytosis induced by progesterone. Int. J. Mol. Sci. 2021, 22, 1992. [Google Scholar] [CrossRef]
- Yeste, M.; Llavanera, M.; Pérez, G.; Scornik, F.; Puig-Parri, J.; Brugada, R.; Bonet, S.; Pinart, E. Elucidating the role of K+ channels during in vitro capacitation of boar spermatozoa: Do SLO1 channels play a crucial role? Int. J. Mol. Sci. 2019, 20, 6330. [Google Scholar] [CrossRef]
- Wijerathne, T.D.; Kim, J.; Yang, D.; Lee, K.P. Intracellular calcium-dependent regulation of the sperm-specific calcium-activated potassium channel, hSlo3, by the BKCa activator LDD175. Korean J. Physiol. Pharmacol. 2017, 21, 241–249. [Google Scholar] [CrossRef]
- Sánchez-Carranza, O.; Torres-Rodríguez, P.; Darszon, A.; Treviño, C.L.; López-González, I. Pharmacology of hSlo3 channels and their contribution in the capacitation-associated hyperpolarization of human sperm. Biochem. Biophys. Res. Commun. 2015, 466, 554–559. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, Q.Y.; Xia, O.M.; Lingle, C.J. Glycine, a determinant of paxilline block in BK channels: A novel bend in the BK S6 helix. J. Gen. Physiol. 2010, 135, 481–494. [Google Scholar] [CrossRef]
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Fritz, K.; Johnson, L. Application of preserved boar semen for arti fi cial insemination: Past, present and future challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef]
- Cruz, O.J.D.; Vassilev, A.; Uckun, F.M. Evaluation of Boar Sperm as a Model System to Study the Mechanism of Spermicidal Activity of Vanadocenes. Biochem. Biophys. Res. Commun. 2000, 830, 826–830. [Google Scholar] [CrossRef]
- Chiminelli, I.; Spicer, L.J.; Maylem, E.R.S.; Caloni, F. Emerging mycotoxins and reproductive effects in animals: A short review. Appl. Toxicol. 2022, 42, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Ritagliati, C.; Baro Graf, C.; Stival, C.; Krapf, D. Regulation mechanisms and implications of sperm membrane hyperpolarization. Mech. Dev. 2018, 154, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Cooper, T.G. Effects of the ion-channel blocker quinine on human sperm volume, kinematics and mucus penetration, and the involvement of potassium channels. Mol. Hum. Reprod. 2001, 7, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Barfield, J.P.; Yeung, C.H.; Cooper, T.G. The effects of putative K+ channel blockers on volume regulation of murine spermatozoa. Biol. Reprod. 2005, 72, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Cooper, T.G. Potassium Channels Involved in Human Sperm Volume Regulation—Quantitative Studies at the Protein and mRNA Levels. Mol. Reprod. Dev. 2008, 668, 659–668. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, D.; Singh, V.; Anand, M.; Choudhury, S.; Yadav, S.; Saxena, A.; Kumar, S. Theriogenology Molecular characterization of voltage-gated potassium channel ( Kv ) and its importance in functional dynamics in bull spermatozoa. Theriogenology 2018, 114, 229–236. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Harrison, R.A.P.; Hebel, M.; Weitze, K.F. Role of quinine-sensitive ion channels in volume regulation in boar and bull spermatozoa. Reproduction 2001, 122, 327–336. [Google Scholar] [CrossRef]
- Kirichok, Y.; Lishko, P.V. Rediscovering sperm ion channels with the patch-clamp technique. Mol. Hum. Reprod. 2011, 17, 478–499. [Google Scholar] [CrossRef]
- Bang, S.; Tanga, B.M.; Fang, X.; Seong, G.; Saadeldin, I.M.; Qamar, A.Y.; Lee, S.; Kim, K.; Park, Y.; Hamad, A.; et al. Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender. Life 2022, 12, 1155. [Google Scholar] [CrossRef]
- Martínez-López, P.; Santi, C.M.; Treviño, C.L.; Ocampo-Gutiérrez, A.Y.; Acevedo, J.J.; Alisio, A.; Salkoff, L.B.; Darszon, A. Mouse sperm K+ currents stimulated by pH and cAMP possibly coded by Slo3 channels. Biochem. Biophys. Res. Commun. 2009, 381, 204–209. [Google Scholar] [CrossRef]
- Cabrini, G.; Verkman, A.S. Potential-Sensitive Response Mechanism of DiS-C3-(5) in Biological Membranes. Membr. Biol. 1986, 92, 171–182. [Google Scholar] [CrossRef]
- Escoffier, J.; Navarrete, F.; Haddad, D.; Santi, C.M.; Darszon, A.; Visconti, P.E.; Gene, D. De Flow Cytometry Analysis Reveals That Only a Subpopulation of Mouse Sperm Undergoes Hyperpolarization During Capacitation 1. Biol. Reprod. 2015, 92, 1–11. [Google Scholar] [CrossRef]
- De La Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Krapf, D.; Hernandez-González, E.O.; Wertheimer, E.; Treviño, C.L.; Visconti, P.E.; Darszon, A. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J. Biol. Chem. 2012, 287, 44384–44393. [Google Scholar] [CrossRef]
- Gao, T.; Kun, L.; Liang, F.; Yu, J.; Liu, A.; Ni, Y.; Sun, P. KCNQ1 Potassium Channel Expressed in Human Sperm Is Involved in Sperm Motility, Acrosome Reaction, Protein Tyrosine Phosphorylation, and Ion Homeostasis During Capacitation. Front. Physiol. 2021, 12, 761910. [Google Scholar] [CrossRef]
- Dubé, C.; Tardif, S.; Leclerc, P.; Bailey, J.L. The Importance of Calcium in the Appearance of p32, a Boar Sperm Tyrosine Phosphoprotein, during In Vitro Capacitation. J. Androl. 2003, 24, 727–733. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Li, Y.; Zhao, N.; Zhen, L.; Fu, J.; Yang, Q. Calcium regulates motility and protein phosphorylation by changing cAMP and ATP concentrations in boar sperm in vitro. Anim. Reprod. Sci. 2016, 172, 39–51. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium Channels in the Development, Maturation, and Function of Spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef]
- Vicente-Carrillo, A.; Álvarez-Rodríguez, M.; Rodríguez-Martínez, H. The CatSper channel modulates boar sperm motility during capacitation. Reprod. Biol. 2017, 17, 69–78. [Google Scholar] [CrossRef]
- Yeste, M.; Fernández-Novell, J.M.; Ramió-Lluch, L.; Estrada, E.; Rocha, L.G.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Concha, I.I.; Ramírez, A.; Rodríguez-Gil, J.E. Intracellular calcium movements of boar spermatozoa during “in vitro” capacitation and subsequent acrosome exocytosis follow a multiple-storage place, extracellular calcium-dependent model. Andrology 2015, 3, 729–747. [Google Scholar] [CrossRef]
- Kojima, A.; Matsushita, Y.; Ogura, Y.; Ishikawa, S.; Noda, T.; Murase, T.; Harayama, H. Roles of extracellular Ca2+ in the occurrence of full-type hyperactivation in boar ejaculated spermatozoa pre-incubated to induce the cAMP-triggered events. Andrology 2015, 3, 321–331. [Google Scholar] [CrossRef]
- Bhoumik, A.; Saha, S.; Majumder, G.C.; Dungdung, S.R. Optimum calcium concentration: A crucial factor in regulating sperm motility in vitro. Cell Biochem. Biophys. 2014, 70, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, L.; Marchiani, S.; Minetti, F.; Forti, G.; Muratori, M.; Baldi, E. The CatSper calcium channel in human sperm: Relation with motility and involvement in progesterone-induced acrosome reaction. Hum. Reprod. 2014, 29, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Miller, M.R.; Lishko, P.V. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl. Acad. Sci. USA 2017, 114, 5743–5748. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.C.; De la Vega-Beltrán, J.L.; José, O.; Torres, P.; Nishigaki, T.; Treviño, C.L.; Darszon, A. Acrosomal alkalization triggers Ca2+ release and acrosome reaction in mammalian spermatozoa. J. Cell. Physiol. 2018, 233, 4735–4747. [Google Scholar] [CrossRef]
- Yeste, M.; Llavanera, M.; Mateo-Otero, Y.; Catalán, J.; Bonet, S.; Pinart, E. Hvcn1 channels are relevant for the maintenance of sperm motility during in vitro capacitation of pig spermatozoa. Int. J. Mol. Sci. 2020, 21, 3255. [Google Scholar] [CrossRef]
- Aldana, A.; Carneiro, J.; Martínez-mekler, G.; Darszon, A. Discrete Dynamic Model of the Mammalian Sperm Acrosome Reaction: The Influence of Acrosomal pH and Physiological Heterogeneity. Front. Physiol. 2021, 12, 682790. [Google Scholar] [CrossRef]
- Branham, T.; Mayorga, L.S.; Tomes, C.N. Calcium-induced Acrosomal Exocytosis Requires cAMP Acting through a Protein Kinase A-independent, Epac-mediated Pathway. J. Biol. Chem. 2006, 281, 8656–8666. [Google Scholar] [CrossRef]
- Graf, C.B.; Ritagliati, C.; Torres-Monserrat, V.; Stival, C.; Carizza, C.; Buffone, M.G.; Krapf, D. Membrane Potential Assessment by Fluorimetry as a Predictor Tool of Human Sperm Fertilizing Capacity. Front. Cell Dev. Biol. 2020, 7, 383. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Wang, L.; Liu, H.; Ling, Y.; Li, Z.; Sun, L. The Catsper channel and its roles in male fertility: A systematic review. Reprod. Biol. Endocrinol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Nahed, A.R.; Martinez, G.; Hograindleur, J.P.; Le Blevec, E.; Camugli, S.; Le Boucher, R.; Ray, P.R.; Escoffier, J.; Schmitt, E.; Arnoult, C. De Slo3 K+ channel blocker clofilium extends bull and mouse sperm-fertilizing competence. Reproduction 2018, 156, 463–476. [Google Scholar] [CrossRef]
- Jimenez, T.; McDermott, J.P.; Sánchez, G.; Blanco, G. Na, K-ATPase α4 isoform is essential for sperm fertility. Proc. Natl. Acad. Sci. USA 2011, 108, 644–649. [Google Scholar] [CrossRef]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Yoon, S.J.; Park, Y.J.; Pang, M.G. Discovery of predictive biomarkers for litter size in boar spermatozoa. Mol. Cell. Proteom. 2015, 14, 1230–1240. [Google Scholar] [CrossRef]
- Matamoros-Volante, A.; Castillo-Viveros, V.; Torres-Rodríguez, P.; Treviño, M.B.; Treviño, C.L. Time-lapse flow cytometry: A robust tool to assess physiological parameters related to the fertilizing capability of human sperm. Int. J. Mol. Sci. 2021, 22, 93. [Google Scholar] [CrossRef]
- Álvarez-Rodriguez, M.; Vicente-Carrillo, A.; Rodriguez-Martinez, H. Hyaluronan improves neither the long-term storage nor the cryosurvival of liquid-stored CD44-bearing AI boar spermatozoa. J. Reprod. Dev. 2018, 64, 351–360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooray, A.; Kim, J.; Nirujan, B.R.; Jayathilake, N.J.; Lee, K.P. Pharmacological Evidence Suggests That Slo3 Channel Is the Principal K+ Channel in Boar Spermatozoa. Int. J. Mol. Sci. 2023, 24, 7806. https://doi.org/10.3390/ijms24097806
Cooray A, Kim J, Nirujan BR, Jayathilake NJ, Lee KP. Pharmacological Evidence Suggests That Slo3 Channel Is the Principal K+ Channel in Boar Spermatozoa. International Journal of Molecular Sciences. 2023; 24(9):7806. https://doi.org/10.3390/ijms24097806
Chicago/Turabian StyleCooray, Akila, Jeongsook Kim, Beno Ramesh Nirujan, Nishani Jayanika Jayathilake, and Kyu Pil Lee. 2023. "Pharmacological Evidence Suggests That Slo3 Channel Is the Principal K+ Channel in Boar Spermatozoa" International Journal of Molecular Sciences 24, no. 9: 7806. https://doi.org/10.3390/ijms24097806
APA StyleCooray, A., Kim, J., Nirujan, B. R., Jayathilake, N. J., & Lee, K. P. (2023). Pharmacological Evidence Suggests That Slo3 Channel Is the Principal K+ Channel in Boar Spermatozoa. International Journal of Molecular Sciences, 24(9), 7806. https://doi.org/10.3390/ijms24097806






