2.1. Phosphatidic Acid (PA)
Generalized polarization (GP) measurements were carried out at 37 °C, and the reading of 0.058 suggests a fluid membrane, consistent with a reported phase transition temperature from gel to liquid crystalline at 28 °C [
20].
The addition of 50 and 100 µM Mn resulted in significant rigidification reflected by higher GP values, whereas similar additions of both 50 and 100 µM Ca or Mg only showed a limited increase for Mg (
Table 1).
Next, 1:1 mixtures combining 50 µM of each ion were analyzed in terms of ΔGP, the difference between the respective sample and the control. The increase of rigidity followed the order Ca:Mn > Mg:Mn > Ca:Mg with 2.4×, 1.9× and 1.4× over controls. A comparison to 50 µM metal values suggest that Mn plays a dominant role over Ca and Mg (
Table 1). Next, a potential impact of the order of metal additions was assessed. An initial addition of 50 µM Ca was incubated for 5 min before 50 µM Mn was added. The ΔGP readings for Mn containing systems were slightly lower than the binary counterparts, while the change for Ca:Mg was statistically lower than binary values. The order of addition played a minor role and confirmed that POPA is not a relevant binding target for the essential ions Ca and Mg
Binding constants for these ions to POPA have been published [
33,
34], and these values were used for a more detailed comparison of the binding affinities of two metals at a time.
Equations (1) and (3) show the formation of metal bound to lipid [M
1L] and [M
2L]. Equations (2) and (4) show the relationship between the binding constants K
M1 and K
M2 with the concentrations of metal [M
1] and [M
2] and lipid [L].
The total initial metal concentrations [M
1]
0 and [M
2]
0 is expressed in Equations (5) and (6) as the sum of free metal and lipid bound metal, whereas the initial lipid concentration [L]
0 is the sum of free lipid [L] and the lipid fractions that have metals bound in Equation (7).
[M
1] can be expressed from Equation (5) and inserted into Equation (2) to rearrange for K
M1. Next, [M
1L] can be derived as shown in Equation (8).
In an analogy for Equations (3) and (6), [M
2L] can be expressed (Equation (9))
The substitution of Equations (8) and (9) into Equation (7) results in a cubic equation (see the
Supplemental Materials for details).
This equation was solved using the published binding constants [
35] and our experimental metal and lipid concentrations to get the fraction of bound metal for single metal additions at 50 and 100 µM and binary additions of 100 µM (see
Supplementary Materials for more details and
Table S2). These data were plotted against the experimental GP changes (ΔGP) (
Table S2).
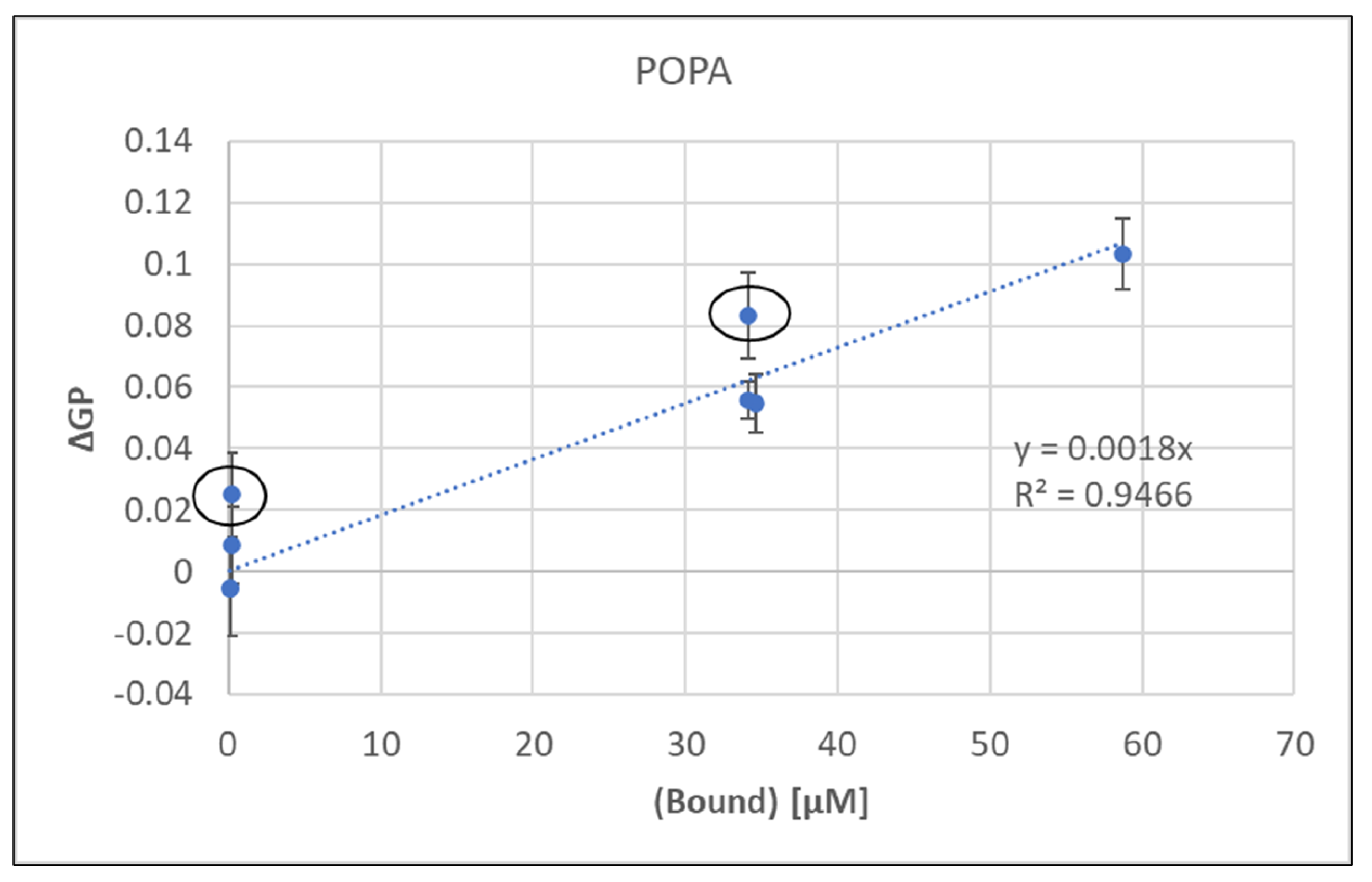
This linear regression showed a strong correlation between bound metal concentration and resulting membrane rigidity.
One additional set of experiments was the sequential addition of 50 µM of one metal with 5 min incubation, followed by 50 µM of the second metal, since we have previously observed significant GP and liposome size differences for Cd and Hg mixtures upon sequential additions [
18].
The GP values of the sequential mixtures were significantly lower for the Ca:Mg system when compared to binary results.
Since the metal concentrations were the same, the calculated values for bound metal would be identical for sequential and binary mixtures despite the differences in GP. Thus, the linear regression in
Figure 1 was used to estimate the bound concentrations for sequential additions based on the observed correlation between bound metal and induced rigidity and these values are shown in
Table 2.
While Ca:Mn and Mg:Mn were similar to the calculated binary addition (
Table S1), Ca:Mg was significantly higher (~25×) for sequential over binary addition. In fact, the points highlighted in
Figure 1 with red circles are binary additions and their ΔGP values are well above the trend line suggesting that more metal may be bound in some binary mixtures.
As previously reported for Cd, Co, Ni and Mn, the acyl side chain architecture strongly impacted metal effects, as fully saturated lipids showed enhanced metal induced membrane rigidification [
36,
37,
38].
Thus, a fully saturated PA was investigated next. Experiments with saturated DMPA were carried out at 55 °C above a reported T
m of 52 °C [
20] to assess metal binding in the physiologically relevant liquid crystalline phase. GP values for the 50 µM additions of single metals were very similar for all three ions (
Table 3).
The controls values were lower than for POPA due to the higher temperature required for DMPA and thus only trends can be compared between the two lipids. In contrast to POPA, the additions of 100 µM metals resulted in statistically significant increases of GP and thus more rigid membranes (
Table 3).
The Mn readings were much higher than those of Ca and Mg while all binary metal mixtures exhibited comparable ΔGP values. The overall range of GP changes of the Mg:Mn mixture is similar to POPA, whereas the Ca:Mg is higher for DMPA.
In terms of sequential metal additions, ΔGP ranges suggest a reduced impact of Mn and stronger binding of Mg to DMPA when compared to POPA.
These differential ion interactions with POPA and DMPA were further investigated by DLS to measure the impact of metal addition on liposome size. Higher metal concentrations were used to better illustrate the changes (
Table 4).
The single addition of metal ions at 100 µM did not result in statistically relevant changes (
Table 3), while both 200 µM of either Mn or Mg increased the size. Overall, Ca had the strongest effect. This is comparable to previous results [
29,
39].
Both Mn containing mixtures increased the liposome size, whereby the high degree in standard deviation for Ca:Mn was due to increased sample turbidity, suggesting at least the onset of aggregation. In contrast, the Ca:Mg mixture had no effect as seen for their 100 µM values.
Finally, results only differed for the sequential additions in Ca:Mn systems.
The metal effects on the liposome size of DMPA membranes were much more pronounced. Single additions of 200 µM of each metal induced extensive increased between the range of 730 to 850 nm, suggesting the potential of liposome aggregation and increased turbidity of the solutions (
Table 4).
Interestingly, 1:1 mixtures with the same total metal concentrations did not induce these large increases. Ca:Mn was similar to 100 µM Ca, suggesting a stronger role. In contrast, the Mg:Mn was close to 100 µM Mn, suggest that Mn dominated the latter interaction.
Sequential additions of Ca and Mn showed no significant size differences compared to binary, whereas Ca and Mg mixtures induced smaller changes (
Table 4).
As seen for GP, metal mixtures also behaved very differently compared to single ion additions in terms of liposome size changes.
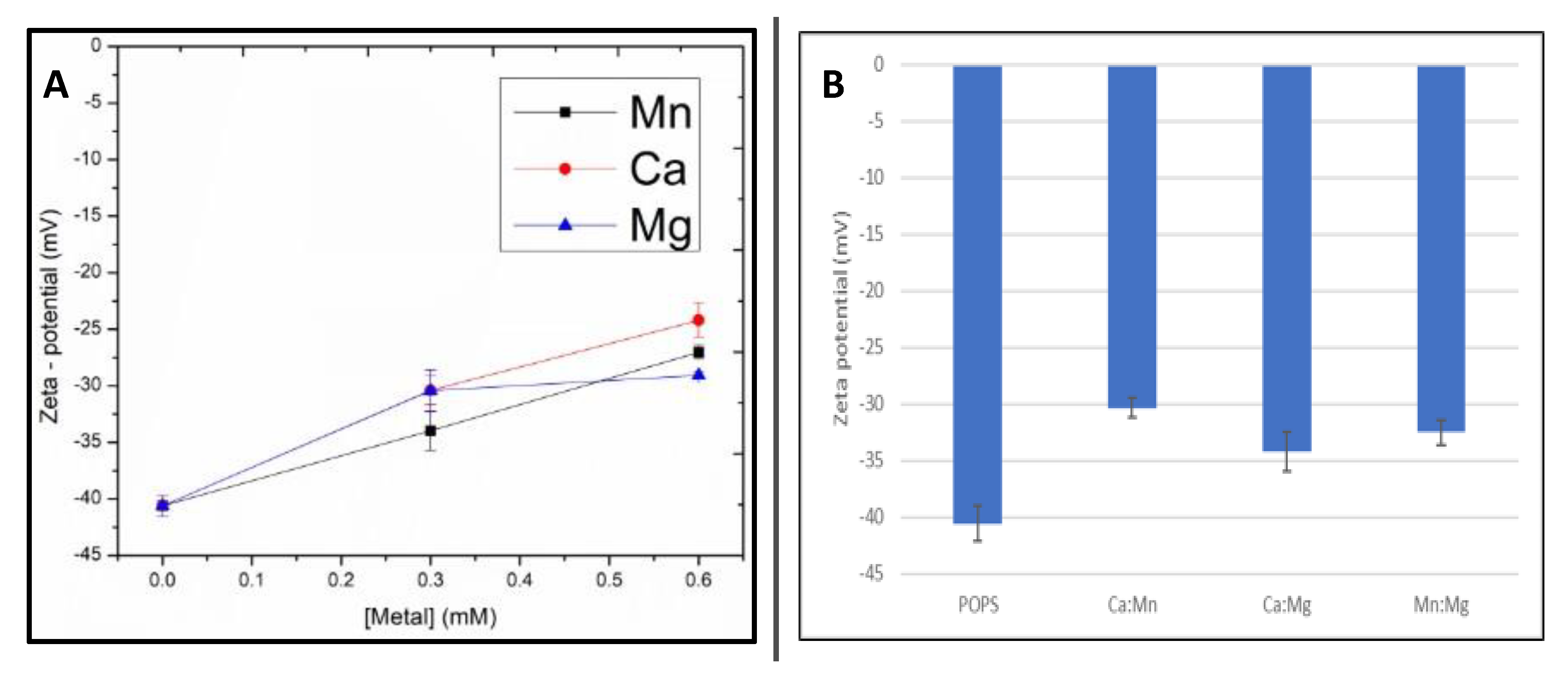
Next, changes in zeta–potential upon metal additions were determined. The observed negative zeta potential of pure POPA is expected for negatively charged liposomes (
Figure 2). The addition of each metal increased the zeta potential due to screening of negative surface charges. (
Figure 2A). At increasing metal concentration (2:1 metal-lipid), the effect of Mn was stronger than Ca and Mg. All metal mixtures induced statistically relevant increases in the zeta potential (
Figure 2B). These trends are comparable to GP results in
Table 3. The observed changes in zeta potential confirm metal binding to the liposomes, without showing statistically significant differences between the metal mixtures.
2.2. Phosphatidylserine (PS)
PS lipids provided two potential ion binding sites, the phosphate and carboxyl groups. Structural studies using NMR demonstrated that the carboxyl group was readily accessible for cation binding due to the head group tilt of PS lipids providing a cavity for metal ions to bind [
36,
37].
Measurements were carried out at 37 °C in the liquid crystalline phase, reflected in the low GP values of the control. This temperature is well above the phase transition temperature of 14 °C [
38].
POPS was significantly rigidified by Mn and Ca (3.4- and 1.5-fold increase), while smaller changes for Mg suggested less binding (
Table 5).
The 1:1 mixtures combining 50 µM of each ion exhibited significant GP increases in the order Ca:Mn > Mg:Mn > Ca:Mg with 3.8X, 2.9X and 2X. The impact of Mg was much higher compared to POPA results. In terms of sequential addition; only the ΔGP readings Ca:Mg were statistically lower than binary values. Overall, the order of addition played a minor role. POPS is a stronger binding target for Ca and Mg compared to POPA.
As described for POPA, the degree of metal bound were calculated as outlined above using published binding constants [
34,
40] (see
Table S3). These calculated values were plotted against the experimental GP data in
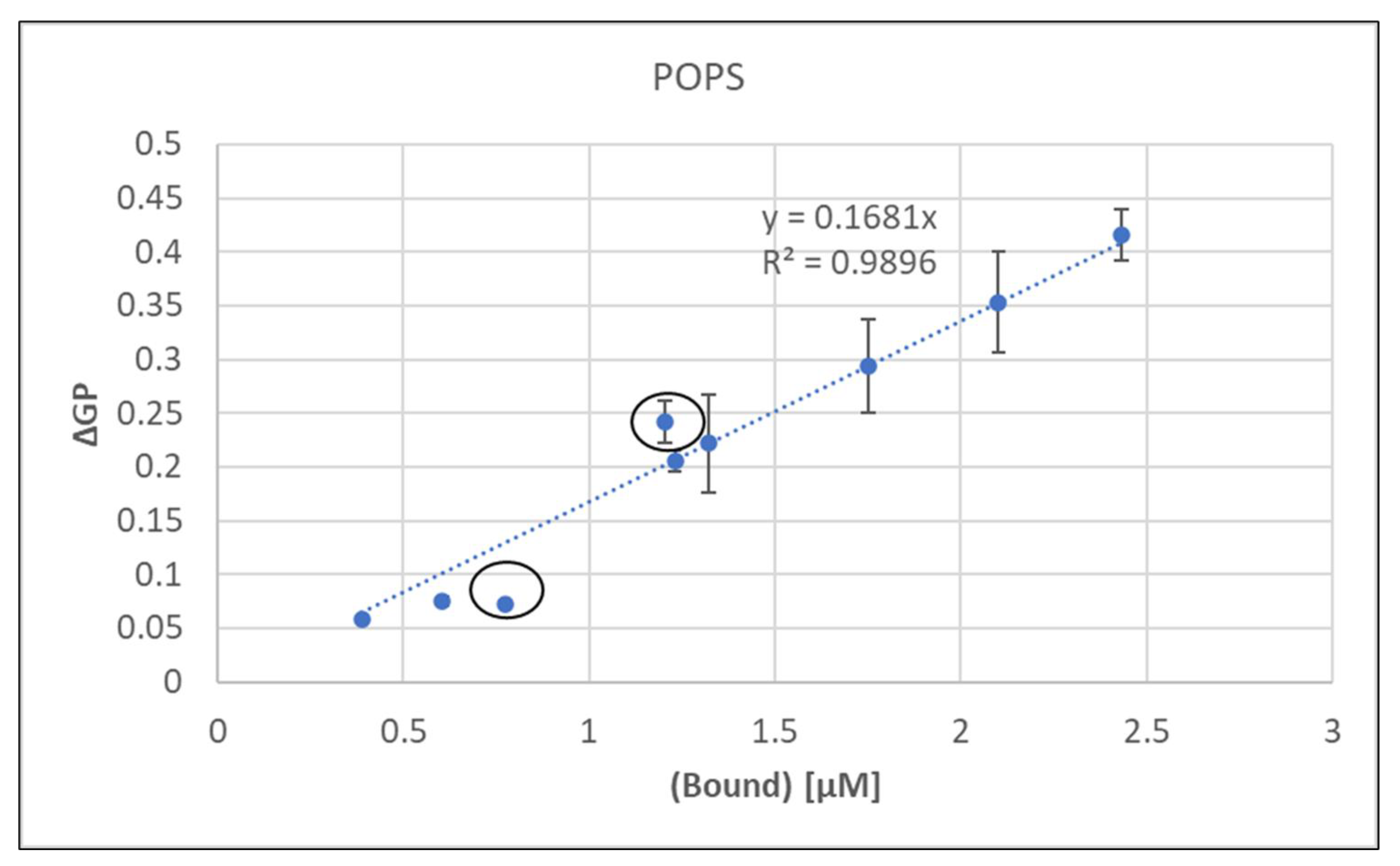
Figure 3, which showed a very good correlation between bound metal and induced membrane rigidity.
As discussed for POPA, calculated values for binary and sequential additions of metal would be identical, although their ΔGP values clearly differ (
Table 6). Thus, the equation for the linear regression in
Figure 3 was used to calculate bound metal concentrations for sequential additions (
Table 6).
Unlike POPS, all three metals caused significant membrane rigidification of the fully saturated DMPS, with the strongest effect for Mn, followed by Ca and Mg (
Table 7. Experiments were carried out at 50 °C, in the liquid crystalline phase and well above the T
m of 35 °C [
41], Metal concentrations had to be reduced to 200 µM to avoid liposome aggregation. Thus, stronger effects were observed at much lower metal concentrations than those used for POPS.
GP values for 200 μM Mn and Ca (
Table 8) were similar to the effect of 1000 μM on POPS (
Table 6). The most striking contrast to POPS is seen for Mg, with a ΔGP of 0.136 over 0.072 for POPS, although the latter was exposed to a 5× higher metal concentration (
Table 6 and
Table 8). Mg values were similar to Mn values.
The Mn containing binary mixtures exhibited comparable ΔGP values that were similar to single Mn or Mg additions. Finally, Ca:Mg was lower than the combined values for 100 μM of Ca and Mg. These data again demonstrated that the impact of some metal mixtures clearly deviated from single metal additions.
Sequential additions of the metals did not show a dependence on order for Ca:Mn, while Mg:Mn data were lower than the binary data. Most strikingly was the significant increase of GP for the sequential addition of Ca:Mg over the binary addition. Overall, the impact of Mg was significantly increased for DMPS compared to POPS. These results confirmed that single addition data could not be extrapolated to binary mixtures and in some cases, stepwise additions were different as well.
Next, changes in liposome size were determined by DLS (
Table 8). Control liposomes for POPS were 104.8 ± 0.7 nm with a PDI of 0.18. Again, higher metal concentrations were used to better illustrate the effects. The addition of 500 µM metal only showed minor increases, but 1000 µM Mn and Ca doubled the liposome diameter. Indeed, Ca has been shown to induce fusion in PS liposomes [
28]. A doubling of the surface area of the POPS liposomes after a fusion event would result in a calculated diameter of 156 nm. Thus, Mn and Ca data suggested membrane fusion.
Alike the Laurdan GP results, Mg only induced a minor change of the hydrodynamic diameter and its effects at 500 μM were much less than size increases for Mg and POPA at 100 and 200 μM (
Table 4).
Remarkably, for sequential additions, Mn containing mixtures induced larger size changes compared to binary (
Table 9). A more pronounced role for the order of addition was seen for Ca:Mg where Ca(1):Mg(2) induced larger liposomes in the fusion size range, whereas Mg(1):Ca(2) results were similar to the binary system.
In contrast, binary and sequential additions of Ca and Mg did not induce significant size changes in POPA (
Table 4).
The addition of the metals to DMPS liposomes resulted in extensive aggregation at concentrations above 200 µM and lower metal concentrations compared to POPS had to be used.
All three metals at 100 and 200 µM resulted in moderate and comparable size increases (
Table 8) that were much smaller than seen for DMPA at the same metal concentrations that exhibited up to ~6-fold size increases (
Table 4).
Metal mixtures induced moderate changes that were similar for all systems and well below results for DMPA with 2–2.5-fold size increases (
Table 4).
For sequential additions, Ca:Mn mixtures were higher than the binary, especially Ca(1):Mn(2) (
Table 9).
Alike POPA membranes, the zeta potential for POPS was determined and control POPS liposomes had a highly negative zeta potential, as expected for negatively charged lipids (
Figure 4).
All metal mixtures induced statistically relevant increases in the zeta potential, whereby, at a 1:1 ratio, Ca and Mg were the strongest (1.7-fold increase). Mn was slightly weaker, in contrast to being the strongest with POPA (
Figure 2). At increasing metal concentrations (2:1 metal:lipid), Ca maintained the strongest role, followed by Mn and a slightly reduced effect of Mg. The observed changes in the zeta potential confirmed metal binding to liposomes without showing statistically significant differences between the metals.
Binary additions resulted in the following order Ca:Mn > Mg:Mn and Ca:Mg, with changes within the standard deviations. These trends agreed with the GP trends in
Table 4.