A Novel Mutation in the ADAMTS10 Associated with Weil–Marchesani Syndrome with a Unique Presentation of Developed Membranes Causing Severe Stenosis of the Supra Pulmonic, Supramitral, and Subaortic Areas in the Heart
Abstract
1. Introduction
2. Results
2.1. Clinical Presentation
2.1.1. Patient 1
2.1.2. Patient 2
2.1.3. Patient 3
2.1.4. Patient 4
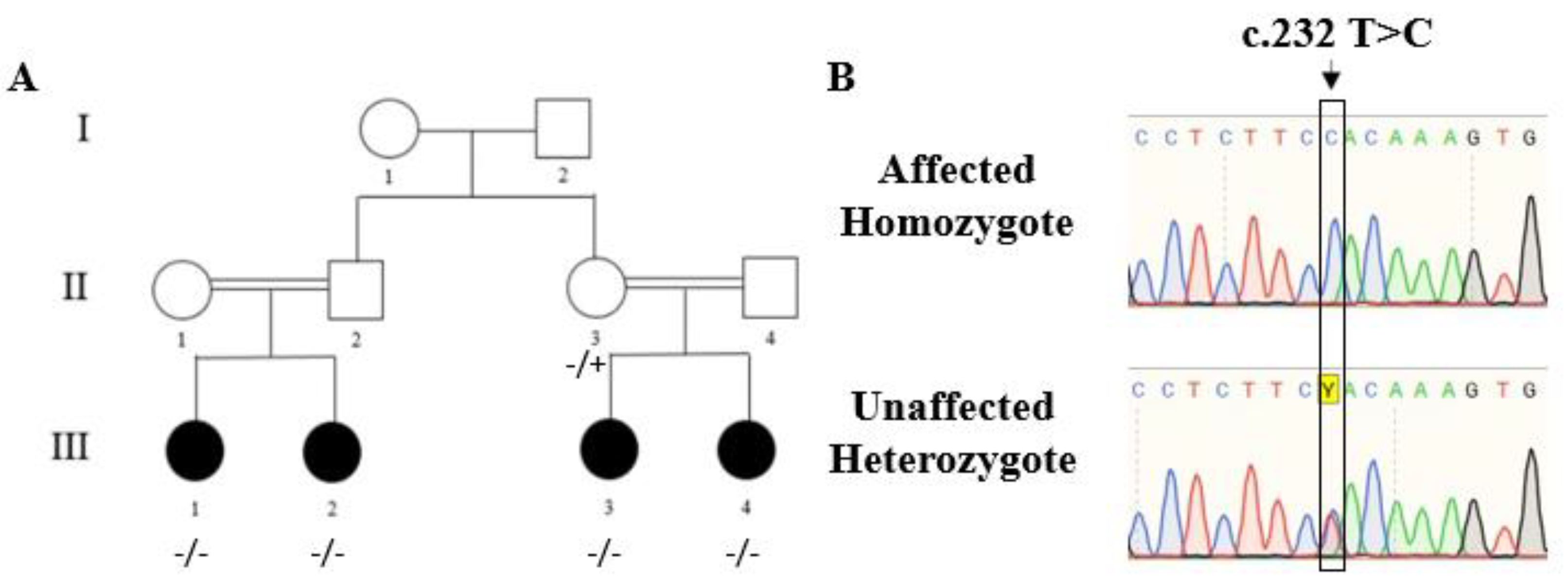
2.2. Molecular Studies
2.2.1. Identification of the ADAMTS10 Mutation
2.2.2. Verification of the Variation in the ADAMTS10 Gene
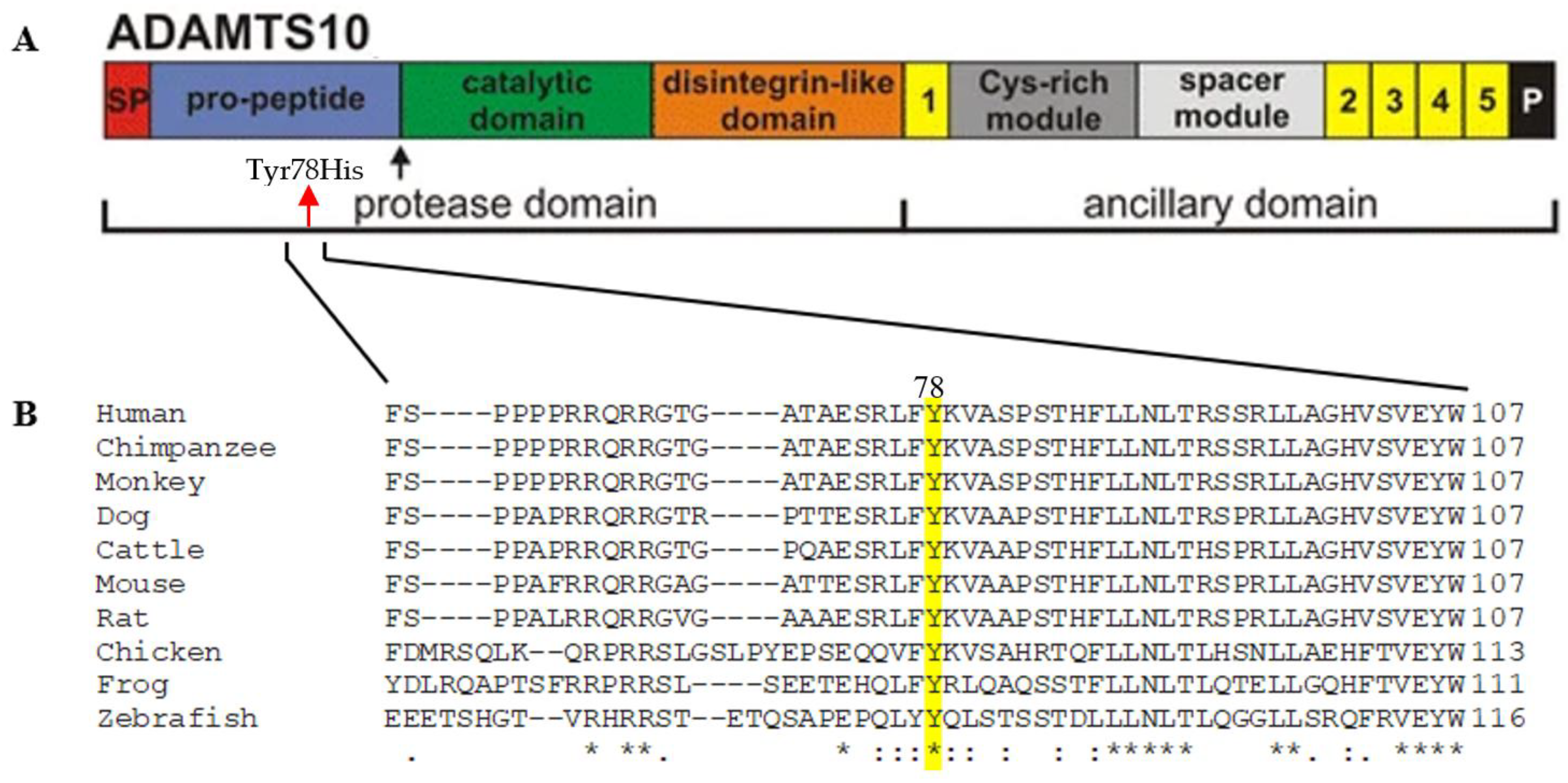
2.2.3. The Effect of the Pathogenic Variation on the ADAMTS10 Protein
3. Discussion
4. Materials and Methods
4.1. Clinic
4.2. Molecular
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karoulias, S.Z.; Beyens, A.; Balic, Z.; Symoens, S.; Vandersteen, A.; Rideout, A.L.; Dickinson, J.; Callewaert, B.; Hubmacher, D. A novel ADAMTS17 variant that causes Weill-Marchesani syndrome 4 alters fibrillin-1 and collagen type I deposition in the extracellular matrix. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 88, 1–18. [Google Scholar] [CrossRef]
- Dagoneau, N.; Benoist-Lasselin, C.; Huber, C.; Faivre, L.; Mégarbané, A.; Alswaid, A.; Dollfus, H.; Alembik, Y.; Munnich, A.; Legeai-Mallet, L.; et al. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am. J. Hum. Genet. 2004, 75, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Hubmacher, D.; Apte, S.S. Genetic and functional linkage between ADAMTS superfamily proteins and fibrillin-1: A novel mechanism influencing microfibril assembly and function. Cell. Mol. Life Sci. CMLS 2011, 68, 3137–3148. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, T.S.; Jacobi, P.C.; Krieglstein, G.K. Ciliary body is not hyperplastic in Weill-Marchesani syndrome. Acta Ophthalmol. Scand. 1998, 76, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, R.; Kato, M.; Suami, M.; Usami, Y.; Hotta, Y.; Sato, M. Chronic angle closure glaucoma secondary to frail zonular fibres and spherophakia. Acta Ophthalmol. Scand. 2003, 81, 533–535. [Google Scholar] [CrossRef]
- Kutz, W.E.; Wang, L.W.; Bader, H.L.; Majors, A.K.; Iwata, K.; Traboulsi, E.I.; Sakai, L.Y.; Keene, D.R.; Apte, S.S. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J. Biol. Chem. 2011, 286, 17156–17167. [Google Scholar] [CrossRef]
- Marzin, P.; Cormier-Daire, V.; Tsilou, E. Weill-Marchesani Syndrome. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Haji-Seyed-Javadi, R.; Jelodari-Mamaghani, S.; Paylakhi, S.H.; Yazdani, S.; Nilforushan, N.; Fan, J.B.; Klotzle, B.; Mahmoudi, M.J.; Ebrahimian, M.J.; Chelich, N.; et al. LTBP2 mutations cause Weill-Marchesani and Weill-Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum. Mutat. 2012, 33, 1182–1187. [Google Scholar] [CrossRef]
- Gallagher, K.; Salam, T.; Sin, B.; Gupta, S.; Zambarakji, H. Retinal vascular tortuosity in a patient with weill-marchesani syndrome. Case Rep. Ophthalmol. Med. 2011, 2011, 952543. [Google Scholar] [CrossRef]
- Morales, J.; Al-Sharif, L.; Khalil, D.S.; Shinwari, J.M.; Bavi, P.; Al-Mahrouqi, R.A.; Al-Rajhi, A.; Alkuraya, F.S.; Meyer, B.F.; Al Tassan, N. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am. J. Hum. Genet. 2009, 85, 558–568. [Google Scholar] [CrossRef]
- Sengle, G.; Tsutsui, K.; Keene, D.R.; Tufa, S.F.; Carlson, E.J.; Charbonneau, N.L.; Ono, R.N.; Sasaki, T.; Wirtz, M.K.; Samples, J.R.; et al. Microenvironmental regulation by fibrillin-1. PLoS Genet. 2012, 8, e1002425. [Google Scholar] [CrossRef]
- Jones, W.; Rodriguez, J. Targeted deletion of fibrillin-1 in the mouse eye results in ectopia lentis and other ocular phenotypes associated with Marfan syndrome. Dis. Models Mech. 2019, 12, 037283. [Google Scholar] [CrossRef] [PubMed]
- Faivre, L.; Mégarbané, A.; Alswaid, A.; Zylberberg, L.; Aldohayan, N.; Campos-Xavier, B.; Bacq, D.; Legeai-Mallet, L.; Bonaventure, J.; Munnich, A.; et al. Homozygosity mapping of a Weill-Marchesani syndrome locus to chromosome 19p13.3-p13.2. Hum. Genet. 2002, 110, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Kojuri, J.; Razeghinejad, M.R.; Aslani, A. Cardiac findings in Weill-Marchesani syndrome. Am. J. Med. Genet. Part A 2007, 143, 2062–2064. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, A.; Ogawa, N.; Martinez, H.R.; Carlson, A.; Fan, Y.; Penny, D.J.; Guo, D.C.; Eisenberg, S.; Safi, H.; Estrera, A.; et al. Missense mutations in FBN1 exons 41 and 42 cause Weill-Marchesani syndrome with thoracic aortic disease and Marfan syndrome. Am. J. Med. Genet. Part A 2013, 161a, 2305–2310. [Google Scholar] [CrossRef]
- Newell, K.; Smith, W.; Ghoshhajra, B.; Isselbacher, E.; Lin, A.; Lindsay, M.E. Cervical artery dissection expands the cardiovascular phenotype in FBN1-related Weill-Marchesani syndrome. Am. J. Med. Genet. Part A 2017, 173, 2551–2556. [Google Scholar] [CrossRef]
- Alsalem, A.B.; Halees, A.S.; Anazi, S.; Alshamekh, S.; Alkuraya, F.S. Autozygome sequencing expands the horizon of human knockout research and provides novel insights into human phenotypic variation. PLoS Genet. 2013, 9, e1004030. [Google Scholar] [CrossRef]
- Hubmacher, D.; Apte, S.S. ADAMTS proteins as modulators of microfibril formation and function. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 47, 34–43. [Google Scholar] [CrossRef]
- Porter, S.; Clark, I.M.; Kevorkian, L.; Edwards, D.R. The ADAMTS metalloproteinases. Biochem. J. 2005, 386, 15–27. [Google Scholar] [CrossRef]
- Steinkellner, H.; Etzler, J.; Gogoll, L.; Neesen, J.; Stifter, E.; Brandau, O.; Laccone, F. Identification and molecular characterisation of a homozygous missense mutation in the ADAMTS10 gene in a patient with Weill-Marchesani syndrome. Eur. J. Hum. Genet. 2015, 23, 1186–1191. [Google Scholar] [CrossRef]
- Kutz, W.E.; Wang, L.W.; Dagoneau, N.; Odrcic, K.J.; Cormier-Daire, V.; Traboulsi, E.I.; Apte, S.S. Functional analysis of an ADAMTS10 signal peptide mutation in Weill-Marchesani syndrome demonstrates a long-range effect on secretion of the full-length enzyme. Hum. Mutat. 2008, 29, 1425–1434. [Google Scholar] [CrossRef]
- van de Woestijne, P.C.; Derk-Jan Ten Harkel, A.; Bogers, A.J. Two patients with Weill-Marchesani syndrome and mitral stenosis. Interact. Cardiovasc. Thorac. Surg. 2004, 3, 484–485. [Google Scholar] [CrossRef]
- Mularczyk, E.J.; Singh, M.; Godwin, A.R.F.; Galli, F.; Humphreys, N.; Adamson, A.D.; Mironov, A.; Cain, S.A.; Sengle, G.; Boot-Handford, R.P.; et al. ADAMTS10-mediated tissue disruption in Weill-Marchesani syndrome. Hum. Mol. Genet. 2018, 27, 3675–3687. [Google Scholar] [CrossRef] [PubMed]
- Massé, D.D.; Shar, J.A.; Brown, K.N.; Keswani, S.G.; Grande-Allen, K.J.; Sucosky, P. Discrete Subaortic Stenosis: Perspective Roadmap to a Complex Disease. Front. Cardiovasc. Med. 2018, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Caballero, A.; Mao, W.; Barrett, B.; Kamioka, N.; Lerakis, S.; Sun, W. The role of stress concentration in calcified bicuspid aortic valve. J. R. Soc. Interface 2020, 17, 20190893. [Google Scholar] [CrossRef]
- Poupart, S.; Navarro-Castellanos, I.; Raboisson, M.J.; Lapierre, C.; Dery, J.; Miró, J.; Dahdah, N. Supravalvular and Valvular Pulmonary Stenosis: Predictive Features and Responsiveness to Percutaneous Dilation. Pediatr. Cardiol. 2021, 42, 814–820. [Google Scholar] [CrossRef]
- Kozel, B.A.; Barak, B.; Kim, C.A. Williams syndrome. Nat. Rev. Dis. Primers 2021, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.; Lincoln, J. The Endocardium and Heart Valves. Cold Spring Harb. Perspect. Biol. 2020, 12, a036723. [Google Scholar] [CrossRef] [PubMed]
- del Nido, P.J.; Baird, C. Congenital mitral valve stenosis: Anatomic variants and surgical reconstruction. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2012, 15, 69–74. [Google Scholar] [CrossRef]
- Karoulias, S.Z.; Taye, N.; Stanley, S.; Hubmacher, D. The ADAMTS/Fibrillin Connection: Insights into the Biological Functions of ADAMTS10 and ADAMTS17 and Their Respective Sister Proteases. Biomolecules 2020, 10, 596. [Google Scholar] [CrossRef]
- Wride, M.A.; Geatrell, J.; Guggenheim, J.A. Proteases in eye development and disease. Birth Defects Res. Part C Embryo Today Rev. 2006, 78, 90–105. [Google Scholar] [CrossRef]
- Cain, S.A.; Mularczyk, E.J.; Singh, M.; Massam-Wu, T.; Kielty, C.M. ADAMTS-10 and -6 differentially regulate cell-cell junctions and focal adhesions. Sci. Rep. 2016, 6, 35956. [Google Scholar] [CrossRef] [PubMed]
- Prins, B.P.; Mead, T.J.; Brody, J.A.; Sveinbjornsson, G.; Ntalla, I.; Bihlmeyer, N.A.; van den Berg, M.; Bork-Jensen, J.; Cappellani, S.; Van Duijvenboden, S.; et al. Exome-chip meta-analysis identifies novel loci associated with cardiac conduction, including ADAMTS6. Genome Biol. 2018, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Wünnemann, F.; Ta-Shma, A. Loss of ADAMTS19 causes progressive non-syndromic heart valve disease. Nat. Genet. 2020, 52, 40–47. [Google Scholar] [CrossRef]
- Muhammad, E.; Levitas, A.; Singh, S.R.; Braiman, A.; Ofir, R.; Etzion, S.; Sheffield, V.C.; Etzion, Y.; Carrier, L.; Parvari, R. PLEKHM2 mutation leads to abnormal localization of lysosomes, impaired autophagy flux and associates with recessive dilated cardiomyopathy and left ventricular noncompaction. Hum. Mol. Genet. 2015, 24, 7227–7240. [Google Scholar] [CrossRef] [PubMed]
| Patient | Cardiac Involvement | Ophthalmic Involvement |
|---|---|---|
| 1 | 1. Severe Supra-pulmonic valve stenosis RPA + LPA stenosis 2. Discrete sub-aortic membrane: Severe sub-aortic stenosis | Microspherophakia High myopia Astigmatism Ectopia lentis |
| Refraction: Rt eye: −11.00–5.25 × 177, Lt eye: −10.25–2.50 × 37 | ||
| Stereopsis: 140 s of arc | ||
| Worth 4 Dot test: fusion for near and distance. Pupillary dilatation revealed the lenses to be clear and small. The lower and temporal equator of the lenses could be seen in the dilated pupils. Anterior segments and fundi were within normal limits. | ||
| 2 | 1. Severe supra-pulmonic valve stenosis RPA + LPA stenosis 2. Discrete sub-aortic membrane: moderate sub-aortic stenosis | Visual acuity: in both eyes 20/30 with glasses |
| Refraction: Rt eye: −1.00–5.00 × 180, Lt eye: −2.00–4.50 × 20 | ||
| Stereopsis: 140 s of arc | ||
| Worth 4 Dot test: fusion for near and distance. Pupillary dilatation revealed the lenses to be clear and small. The temporal equator of the Rt lens and the lower equator of the Lt lens could be seen in the dilated pupils. Anterior segments and fundi were within normal limits. | ||
| High Astigmatism | ||
| 3 | Supramitral valvular ring, mitral stenosis: moderate stenosis and severe mitral regurgitation | Visual acuity: Rt eye 20/25, Lt eye 20/50 with glasses |
| Refraction: Rt eye: +1.50–5.25 × 140, Lt eye: +2.50–5.75 × 25 | ||
| Stereopsis: 140 s of arc | ||
| Worth 4 Dot test: fusion for near and left eye suppression for distance. The patient had microstrabismus of Lt eye 4PD for distance. Anterior segments, lenses, and fundi were within normal limits. | ||
| High Astigmatism | ||
| 4 | 1. Mild supra-pulmonic valve stenosis 2. Discrete sub-aortic membrane: moderate sub-aortic stenosis | Visual acuity: Rt eye 20/40, Lt eye 20/60 with glasses |
| Refraction: Rt eye: +4.00–5.00 × 170, Lt eye: +5.00–5.75 × 10 | ||
| Stereopsis: Fly test positive | ||
| Worth 4 Dot test: fusion for near | ||
| The patient had accommodative esotropia. Anterior segments, lenses, and fundi were within normal limits. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levitas, A.; Aspit, L.; Lowenthal, N.; Shaki, D.; Krymko, H.; Slanovic, L.; Yagev, R.; Parvari, R. A Novel Mutation in the ADAMTS10 Associated with Weil–Marchesani Syndrome with a Unique Presentation of Developed Membranes Causing Severe Stenosis of the Supra Pulmonic, Supramitral, and Subaortic Areas in the Heart. Int. J. Mol. Sci. 2023, 24, 8864. https://doi.org/10.3390/ijms24108864
Levitas A, Aspit L, Lowenthal N, Shaki D, Krymko H, Slanovic L, Yagev R, Parvari R. A Novel Mutation in the ADAMTS10 Associated with Weil–Marchesani Syndrome with a Unique Presentation of Developed Membranes Causing Severe Stenosis of the Supra Pulmonic, Supramitral, and Subaortic Areas in the Heart. International Journal of Molecular Sciences. 2023; 24(10):8864. https://doi.org/10.3390/ijms24108864
Chicago/Turabian StyleLevitas, Aviva, Liam Aspit, Neta Lowenthal, David Shaki, Hanna Krymko, Leonel Slanovic, Ronit Yagev, and Ruti Parvari. 2023. "A Novel Mutation in the ADAMTS10 Associated with Weil–Marchesani Syndrome with a Unique Presentation of Developed Membranes Causing Severe Stenosis of the Supra Pulmonic, Supramitral, and Subaortic Areas in the Heart" International Journal of Molecular Sciences 24, no. 10: 8864. https://doi.org/10.3390/ijms24108864
APA StyleLevitas, A., Aspit, L., Lowenthal, N., Shaki, D., Krymko, H., Slanovic, L., Yagev, R., & Parvari, R. (2023). A Novel Mutation in the ADAMTS10 Associated with Weil–Marchesani Syndrome with a Unique Presentation of Developed Membranes Causing Severe Stenosis of the Supra Pulmonic, Supramitral, and Subaortic Areas in the Heart. International Journal of Molecular Sciences, 24(10), 8864. https://doi.org/10.3390/ijms24108864





