Characterization of Early and Late Damage in a Mouse Model of Pelvic Radiation Disease
Abstract
1. Introduction
2. Results
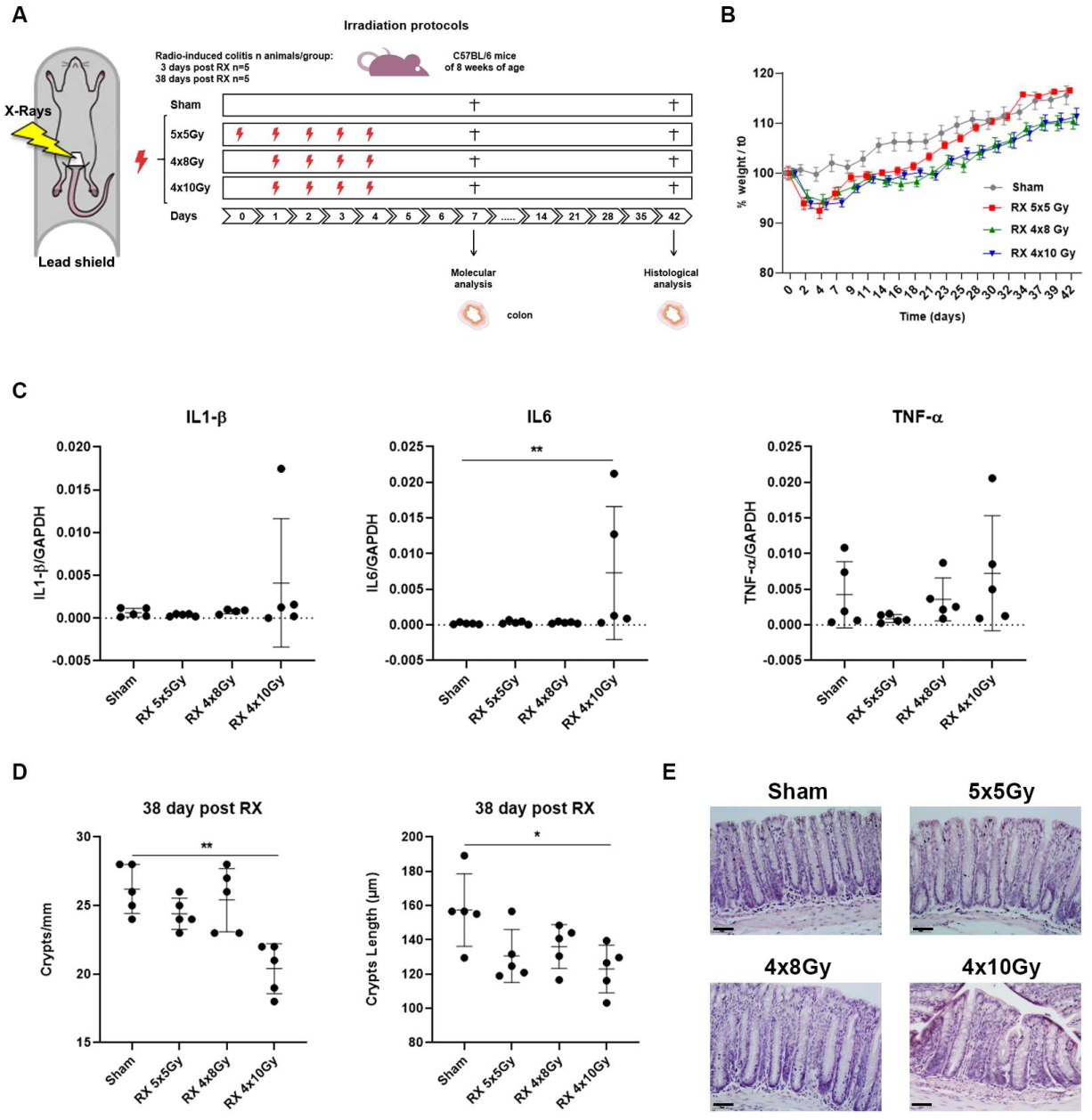
2.1. Evaluation of the Effects Induced by Different Irradiation Protocols
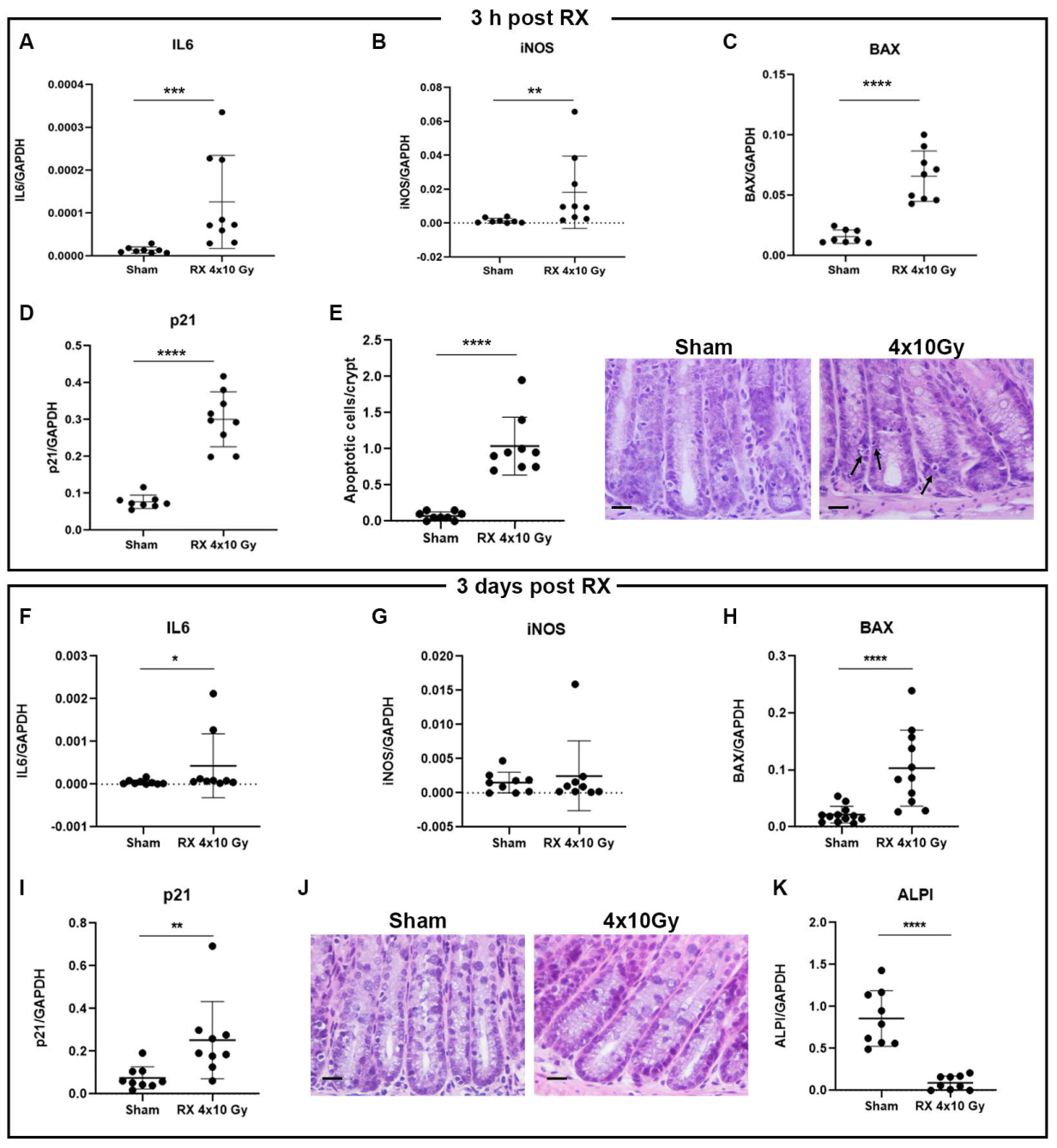
2.2. Characterization of X-ray Effect in the Colon Tissue at Early Time
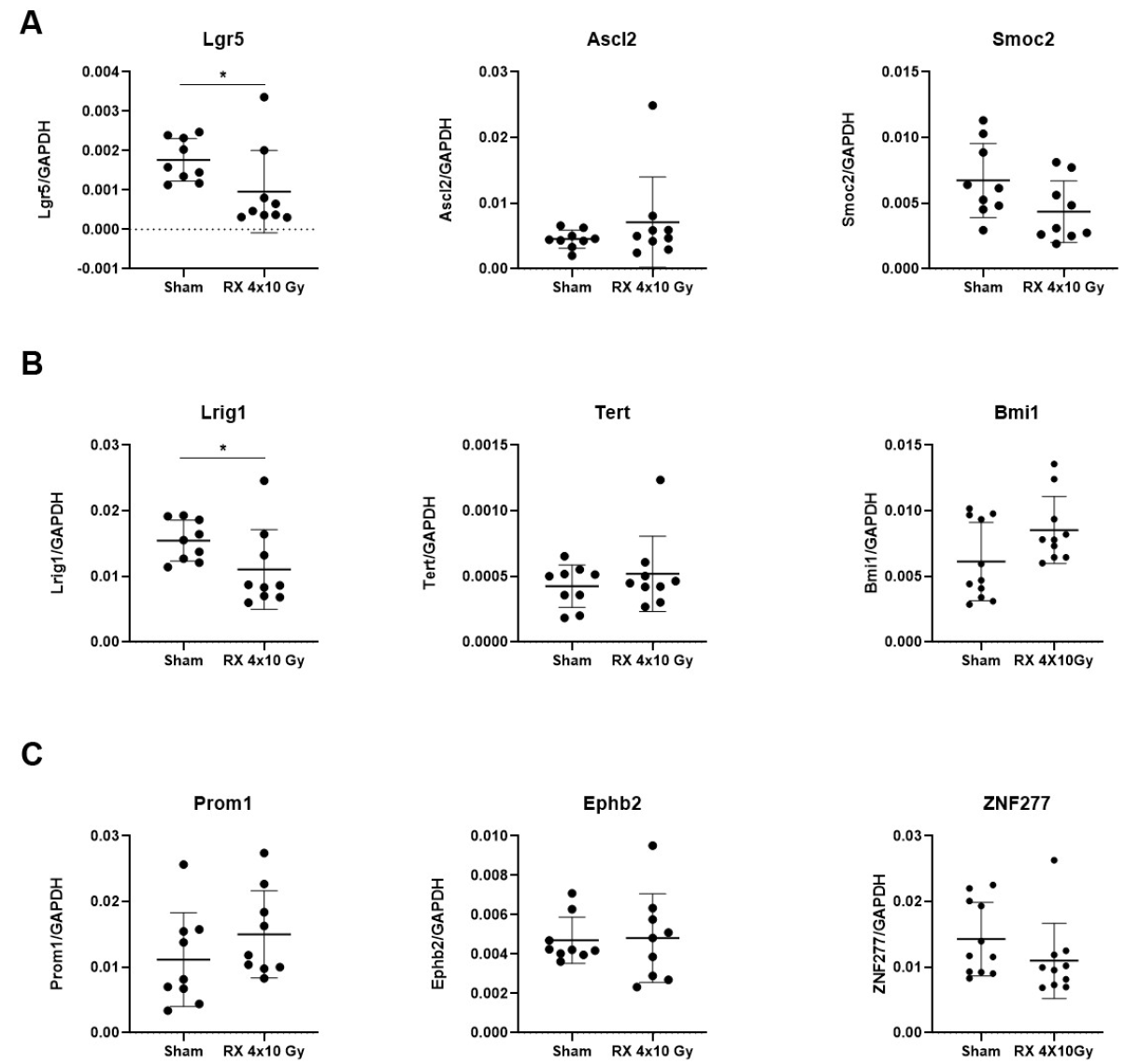
2.3. Gene Expression Analysis of Intestinal Stemness Markers following Irradiation
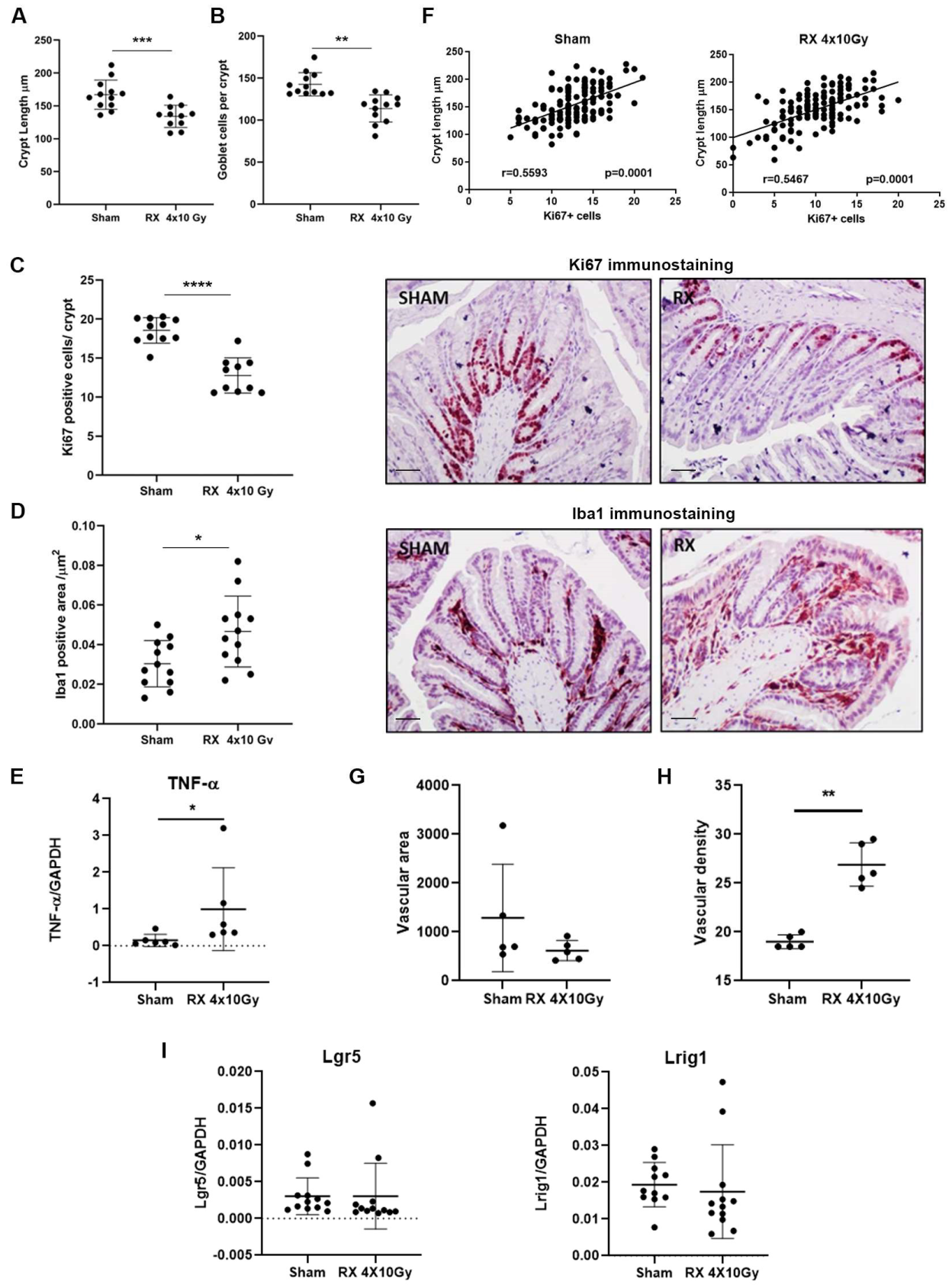
2.4. Evaluation of the Effects Induced by Irradiation on Colonic Mucosa at Long Time
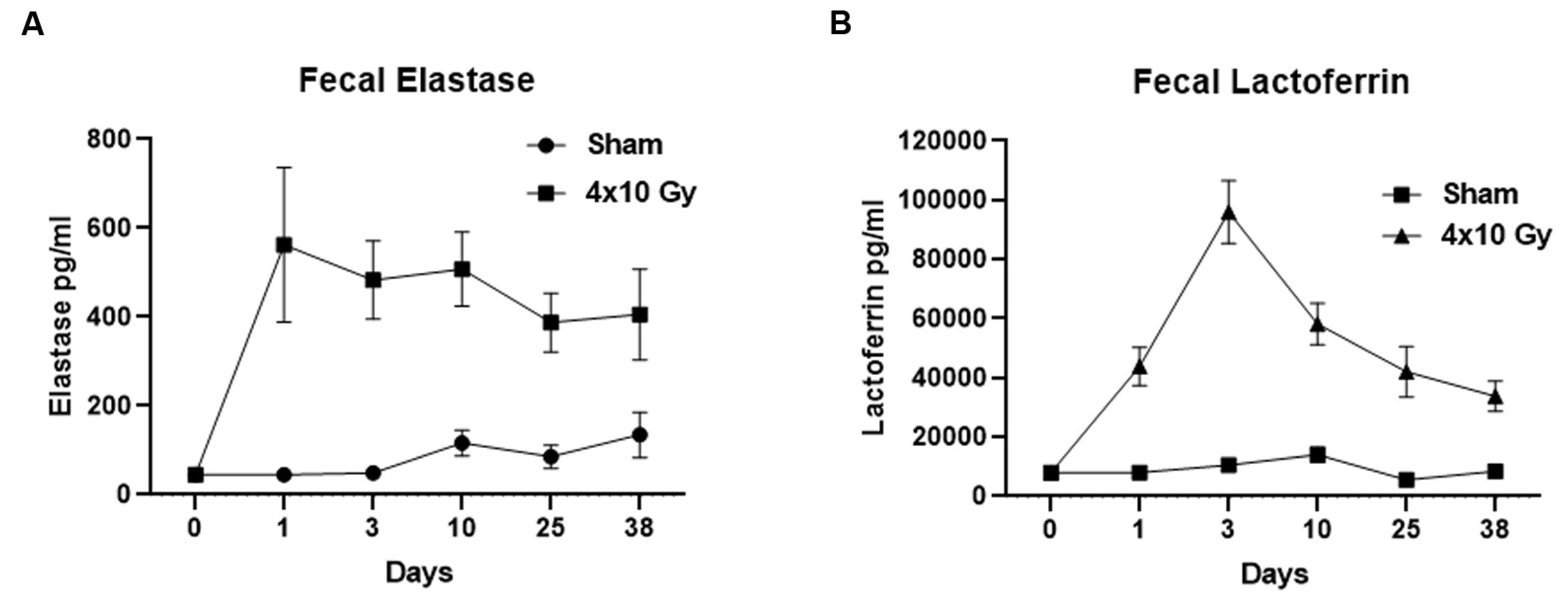
2.5. Time-Course Analysis of the Fecal Elastase and Lactoferrin after 4 × 10 Gy Irradiation
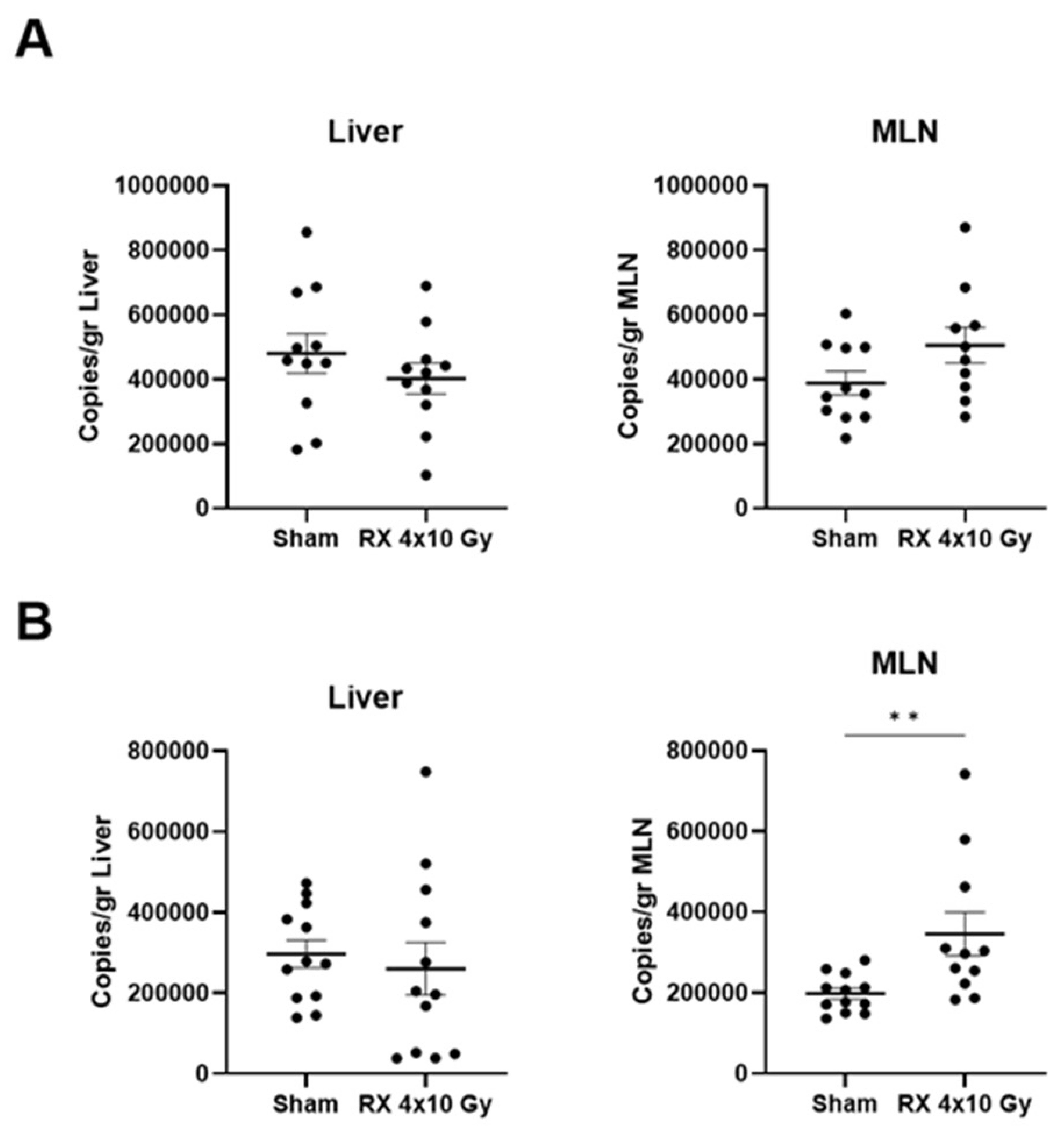
2.6. Evaluation of Bacterial Translocation
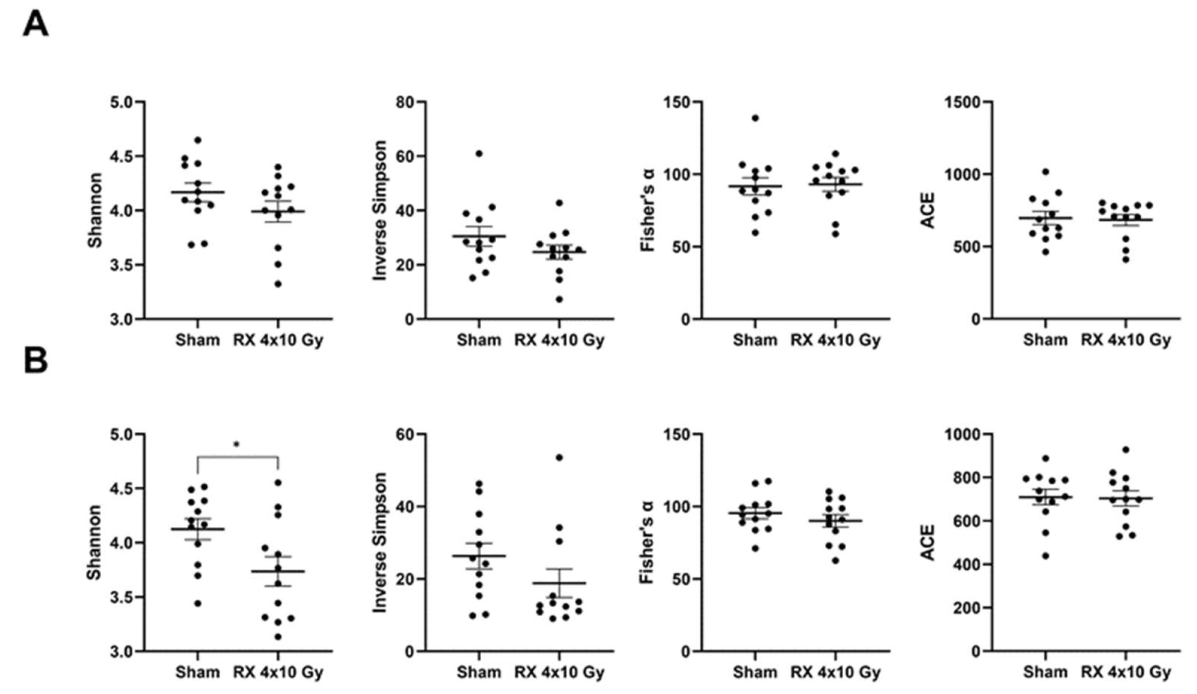
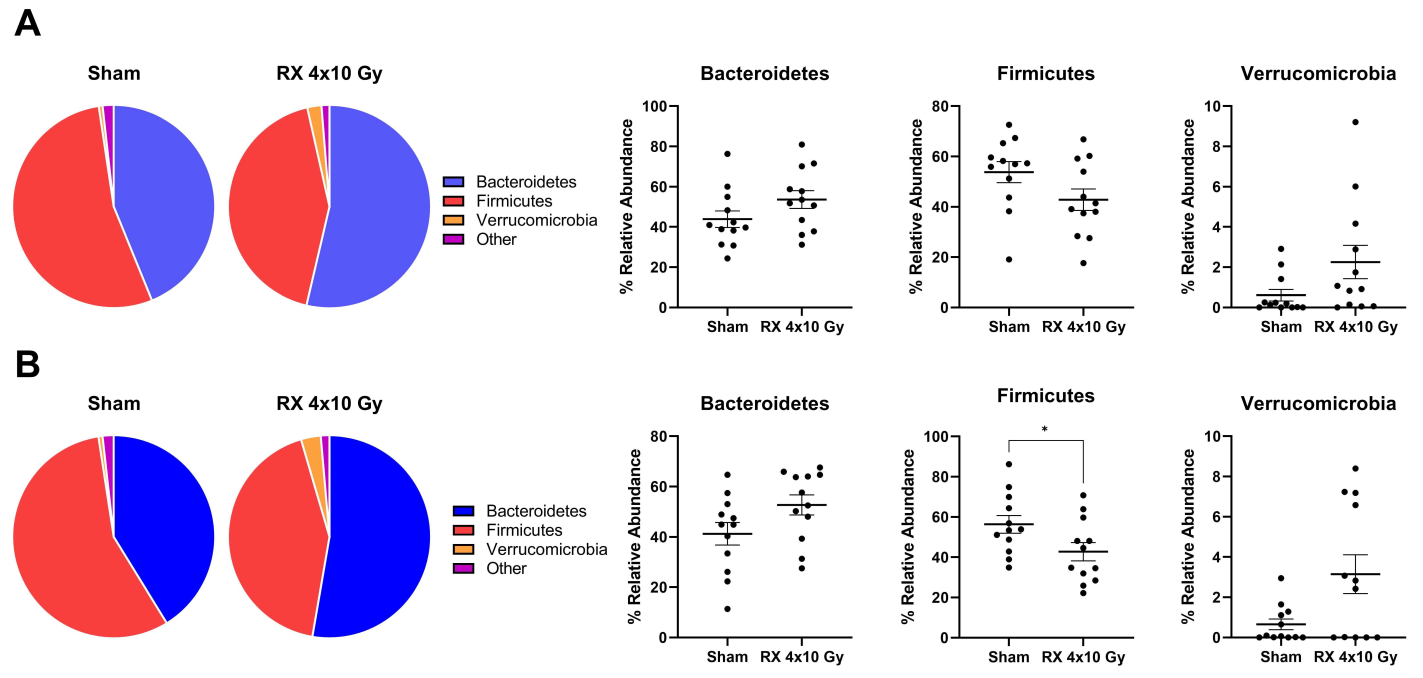
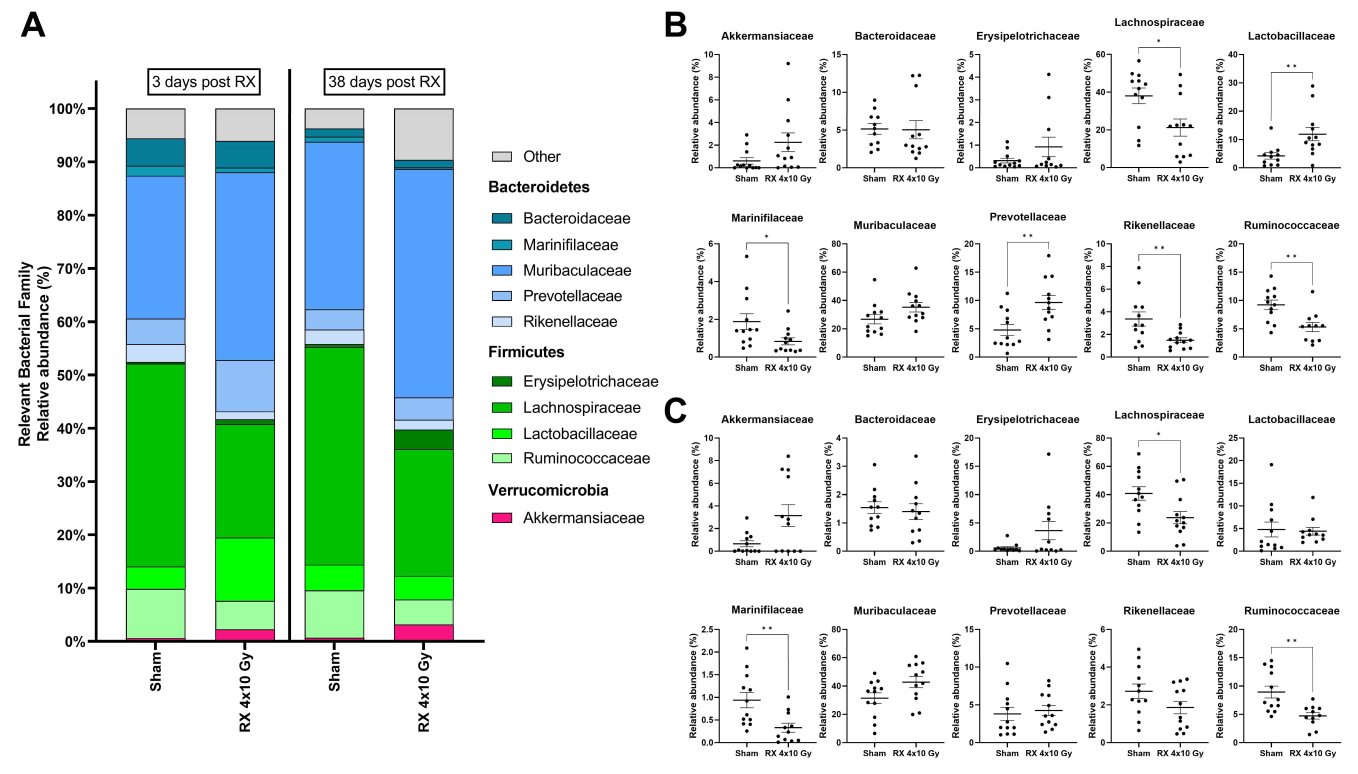
2.7. Evaluation of Microbiota Fluctuations
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Irradiation Procedures
4.3. Morphometric Analyses of Colon-Rectum
4.3.1. Morphometric Measurements
4.3.2. Assessment of Vascular Function
4.3.3. Assessment of Goblet Cell Number
4.3.4. Quantification of Apoptosis
4.4. Immunohistochemistry
4.5. RNA Isolation and Real-Time PCR Analysis
4.6. Fecal Extraction
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Quantification of Bacterial Translocation and Microbiota Profiling
4.9. Sequence Data Preparation and Statistics
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhutta, B.S.; Fatima, R.; Aziz, M. Radiation Enteritis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J. Radiation enteropathy—Pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Nanda, N.; Bhatia, A.; Akhtar, R.; Mahmood, S. Effect of antioxidant supplementation on digestive enzymes in radiation induced intestinal damage in rats. Int. J. Radiat. Biol. 2013, 89, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, J. Gastrointestinal symptoms after pelvic radiotherapy: A new understanding to improve management of symptomatic patients. Lancet Oncol. 2007, 8, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizi, M.R.; Warshowsky, K.E.; Zobel, C.L.; Hallahan, D.E.; Sharma, G.G. Molecular and epigenetic regulatory mechanisms of normal stem cell radiosensitivity. Cell Death Discov. 2018, 4, 117. [Google Scholar] [CrossRef]
- Quan, Y.; Sun, M.; Tan, Z.; Eijkel, J.C.T.; van den Berg, A.; van der Meer, A.; Xie, Y. Organ-on-a-chip: The next generation platform for risk assessment of radiobiology. RSC Adv. 2020, 10, 39521–39530. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.M.S.; Levy, O.; et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 2018, 9, 223. [Google Scholar] [CrossRef]
- Kim, Y.H.; Han, S.H.; Kim, H.; Lee, S.J.; Joo, H.W.; Kim, M.J.; Shim, S.; Kim, K.; Lee, J.; Jang, W.S.; et al. Evaluation of the radiation response and regenerative effects of mesenchymal stem cell-conditioned medium in an intestinal organoid system. Biotechnol. Bioeng. 2020, 117, 3639–3650. [Google Scholar] [CrossRef]
- Martin, M.L.; Adileh, M.; Hsu, K.S.; Hua, G.; Lee, S.G.; Li, C.; Fuller, J.D.; Rotolo, J.A.; Bodo, S.; Klingler, S.; et al. Organoids Reveal That Inherent Radiosensitivity of Small and Large Intestinal Stem Cells Determines Organ Sensitivity. Cancer Res. 2020, 80, 1219–1227. [Google Scholar] [CrossRef]
- Yang, C.; Chen, H.X.; Zhou, Y.; Liu, M.X.; Wang, Y.; Wang, J.X.; Ren, S.P.; Han, Y.; Wu, B.Y. Manganese superoxide dismutase gene therapy protects against irradiation- induced intestinal injury. Curr. Gene Ther. 2013, 13, 305–314. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.; Park, S.; Myung, H.; Kang, J.; Kim, K.; Kim, H.; Jang, W.S.; Lee, S.J.; Shim, S.; et al. Pravastatin Attenuates Acute Radiation-Induced Enteropathy and Improves Epithelial Cell Function. Front. Pharmacol. 2018, 9, 1215. [Google Scholar] [CrossRef]
- Sung, J.; Sodhi, C.P.; Voltaggio, L.; Hou, X.; Jia, H.; Zhou, Q.; Čiháková, D.; Hackam, D.J. The recruitment of extra-intestinal cells to the in-jured mucosa promotes healing in radiation enteritis and chemical colitis in a mouse parabiosis model. Mucosal Immunol. 2019, 12, 503–517. [Google Scholar] [CrossRef]
- Zhang, X.M.; Hu, X.; Ou, J.Y.; Chen, S.S.; Nie, L.H.; Gao, L.; Zhu, L.L. Glycyrrhizin Ameliorates Radiation Enteritis in Mice Accompanied by the Regulation of the HMGB1/TLR4 Pathway. Evid. Based Complement. Altern. Med. 2020, 2020, 8653783. [Google Scholar] [CrossRef]
- Yuan, Q.; Peng, R.; Yu, H.; Wang, S.; Chen, Z.; Dong, S.; Li, W.; Cheng, B.; Jiang, Q.; Cong, Y.; et al. Disulfiram Protects Against Radiation-Induced Intestinal Injury in Mice. Front. Pharmacol. 2022, 13, 852669. [Google Scholar] [CrossRef]
- Gu, J.; Chen, Y.Z.; Zhang, Z.X.; Yang, Z.X.; Duan, G.X.; Qin, L.Q.; Zhao, L.; Xu, J.Y. At What Dose Can Total Body and Whole Abdominal Irradiation Cause Lethal Intestinal Injury Among C57BL/6J Mice? Dose-Response 2020, 18, 1559325820956783. [Google Scholar] [CrossRef]
- Bull, C.; Malipatlolla, D.; Kalm, M.; Sjöberg, F.; Alevronta, E.; Grandér, R.; Sultanian, P.; Persson, L.; Boström, M.; Eriksson, Y.; et al. A novel mouse model of radiation-induced cancer survivorship diseases of the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G456–G466. [Google Scholar] [CrossRef]
- Malipatlolla, D.K.; Patel, P.; Sjöberg, F.; Devarakonda, S.; Kalm, M.; Angenete, E.; Lindskog, E.B.; Grandér, R.; Persson, L.; Stringer, A.; et al. Long-term mucosal injury and repair in a murine model of pelvic radiotherapy. Sci. Rep. 2019, 9, 13803. [Google Scholar] [CrossRef]
- Singh, S.B.; Lin, H.C. Role of Intestinal Alkaline Phosphatase in Innate Immunity. Biomolecules 2021, 11, 1784. [Google Scholar] [CrossRef]
- Mihaescu, A.; Santén, S.; Jeppsson, B.; Thorlacius, H. Rho kinase signalling mediates radiation-induced inflammation and intestinal barrier dysfunction. Br. J. Surg. 2011, 98, 124–131. [Google Scholar] [CrossRef]
- Sha, H.; Gu, Y.; Shen, W.; Zhang, L.; Qian, F.; Zhao, Y.; Li, H.; Zhang, T.; Lu, W. Rheinic acid ameliorates radiation-induced acute enteritis in rats through PPAR-γ/NF-κB. Genes Genom. 2019, 41, 909–917. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 2016, 143, 3639–3649. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Carulli, A.J.; Samuelson, L.C.; Schnell, S. Unraveling intestinal stem cell behavior with models of crypt dynamics. Integr. Biol. 2014, 6, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Snippert, H.J.; van Es, J.H.; van den Born, M.; Begthel, H.; Stange, D.E.; Barker, N.; Clevers, H. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 2009, 136, 2187–2194.e1. [Google Scholar] [CrossRef]
- Maria Cambuli, F.; Rezza, A.; Nadjar, J.; Plateroti, M. Brief report: Musashi1-eGFP mice, a new tool for differential isolation of the intestinal stem cell populations. Stem Cells 2013, 31, 2273–2278. [Google Scholar] [CrossRef]
- Jang, B.G.; Kim, H.S.; Kim, K.J.; Rhee, Y.Y.; Kim, W.H.; Kang, G.H. Distribution of intestinal stem cell markers in colorectal precancerous lesions. Histopathology 2016, 68, 567–577. [Google Scholar] [CrossRef]
- Cordero, J.B.; Sansom, O.J. Wnt signalling and its role in stem cell-driven intestinal regeneration and hyperplasia. Acta Physiol. 2012, 204, 137–143. [Google Scholar] [CrossRef]
- Sémont, A.; Demarquay, C.; Bessout, R.; Durand, C.; Benderitter, M.; Mathieu, N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS ONE 2013, 8, e70170. [Google Scholar] [CrossRef]
- Liang, L.; Shen, L.; Fu, G.; Yao, Y.; Li, G.; Deng, Y.; Zhang, H.; Zhou, M.; Yang, W.; Hua, G.; et al. Regulation of the regeneration of intestinal stem cells after irradiation. Ann. Transl. Med. 2020, 8, 1063. [Google Scholar] [CrossRef]
- Montgomery, R.K.; Carlone, D.L.; Richmond, C.A.; Farilla, L.; Kranendonk, M.E.; Henderson, D.E.; Baffour-Awuah, N.Y.; Ambruzs, D.M.; Fogli, L.K.; Algra, S.; et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 179–184. [Google Scholar] [CrossRef]
- Powell, A.E.; Wang, Y.; Li, Y.; Poulin, E.J.; Means, A.L.; Washington, M.K.; Higginbotham, J.N.; Juchheim, A.; Prasad, N.; Levy, S.E.; et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 2012, 149, 146–158. [Google Scholar] [CrossRef]
- Sangiorgi, E.; Capecchi, M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008, 40, 915–920. [Google Scholar] [CrossRef]
- Yan, K.S.; Chia, L.A.; Li, X.; Ootani, A.; Su, J.; Lee, J.Y.; Su, N.; Luo, Y.; Heilshorn, S.C.; Amieva, M.R.; et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 2012, 109, 466–471. [Google Scholar] [CrossRef]
- Han, B.; Yan, S.; Wei, S.; Xiang, J.; Liu, K.; Chen, Z.; Bai, R.; Sheng, J.; Xu, Z.; Gao, X. YTHDF1-mediated translation amplifies Wnt-driven intestinal stemness. EMBO Rep. 2020, 21, e49229. [Google Scholar] [CrossRef]
- Suzuki, F.; Loucas, B.D.; Ito, I.; Asai, A.; Suzuki, S.; Kobayashi, M. Survival of Mice with Gastrointestinal Acute Radiation Syndrome through Control of Bacterial Translocation. J. Immunol. 2018, 201, 77–86. [Google Scholar] [CrossRef]
- Nam, Y.D.; Kim, H.J.; Seo, J.G.; Kang, S.W.; Bae, J.W. Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS ONE 2013, 8, e82659. [Google Scholar] [CrossRef]
- Wang, B.; Jin, Y.X.; Dong, J.L.; Xiao, H.W.; Zhang, S.Q.; Li, Y.; Chen, Z.Y.; Yang, X.D.; Fan, S.J.; Cui, M. Low-Intensity Exercise Modulates Gut Microbiota to Fight Against Radiation-Induced Gut Toxicity in Mouse Models. Front. Cell Dev. Biol. 2021, 9, 706–755. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.; Park, S.J. High-Throughput 16S RRNA Gene Sequencing Reveals Alterations of Mouse Intestinal Microbiota after Radiotherapy. Anaerobe 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Wang, A.; Ling, Z.; Yang, Z.; Kiela, P.R.; Wang, T.; Wang, C.; Cao, L.; Geng, F.; Shen, M.; Ran, X.; et al. Gut Microbial Dysbiosis May Predict Diarrhea and Fatigue in Patients Undergoing Pelvic Cancer Radiotherapy: A Pilot Study. PLoS ONE 2015, 10, e0126312. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Wang, X.; Zhu, L.; Chen, J.; Zhang, B.; Chen, Y.; Yuan, Z. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J. Cell. Mol. Med. 2019, 23, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, H.; Zhang, Y.; Li, Q.; Yu, L.; Li, Q.; Liu, C.; Xie, Y.; Chen, K.; Ye, F.; et al. Alterations of the Gut Microbiome Composition and Lipid Metabolic Profile in Radiation Enteritis. Front. Cell. Infect. Microbiol. 2020, 10, 541178. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhao, Y.; Yang, Y.; Wang, T.; Jin, S.; Guo, J.; Liu, Z. The Protective Role of Short-Chain Fatty Acids Acting as Signal Molecules in Chemotherapy- or Radiation-Induced Intestinal Inflammation. Am. J. Cancer Res. 2020, 10, 3508–3531. [Google Scholar] [PubMed]
- Teofani Teofani, A.; Marafini, I.; Laudisi, F.; Pietrucci, D.; Salvatori, S.; Unida, V.; Biocca, S.; Monteleone, G.; Desideri, A. Intestinal Taxa Abundance and Diversity in Inflammatory Bowel Disease Patients: An Analysis including Covariates and Confounders. Nutrients 2022, 14, 260. [Google Scholar] [CrossRef]
- Larsen, A.; Hovdenak, N.; Karlsdottir, A.; Wentzel-Larsen, T.; Dahl, O.; Fagerhol, M.K. Faecal calprotectin and lactoferrin as markers of acute radiation proctitis: A pilot study of eight stool markers. Scand. J. Gastroenterol. 2004, 39, 1113–1118. [Google Scholar] [CrossRef]
- Larsen, A.; Bjørge, B.; Klementsen, B.; Helgeland, L.; Wentzel-Larsen, T.; Fagerhol, M.K.; Hovdenak, N.; Dahl, O. Time patterns of changes in biomarkers, symptoms and histopathology during pelvic radiotherapy. Acta Oncol. 2007, 46, 639–650. [Google Scholar] [CrossRef]
- Hille, A.; Rave-Fränk, M.; Christiansen, H.; Herrmann, M.K.; Kertesz, T.; Hermann, R.M.; Wolff, H.A.; Schirmer, M.; Hess, C.F.; Ramadori, G. Faecal calprotectin and lactoferrin values during irradiation of prostate cancer correlate with chronic radiation proctitis: Results of a prospective study. Scand. J. Gastroenterol. 2009, 44, 939–946. [Google Scholar] [CrossRef]
- Dandin, Ö.; Akin, M.L.; Balta, A.Z.; Yücel, E.; Karakaş, D.Ö.; Demirbaş, S.; Özdemir, S.; Haholu, A. The Efficacy of Probiotic (Lactobacillus rhamnosus GG) and 5-ASA (Aminosalicylic Acid) in the Treatment of Experimental Radiation Proctitis in Rats. Indian J. Surg. 2015, 77, 563–569. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhang, G.; Ma, Y.; Zhang, Q.; Li, Z.; Ran, J.; Hou, X.; Geng, Y.; Yang, Z.; et al. The impact of pelvic radiotherapy on the gut microbiome and its role in radiation-induced diarrhoea: A systematic review. Radiat. Oncol. 2021, 16, 187. [Google Scholar] [CrossRef]
- Manichanh, C.; Varela, E.; Martinez, C.; Antolin, M.; Llopis, M.; Doré, J.; Giralt, J.; Guarner, F.; Malagelada, J.R. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am. J. Gastroenterol. 2008, 103, 1754–1761. [Google Scholar] [CrossRef]
- Nadkarni, M.A.; Martin, F.E.; Jacques, N.A.; Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002, 148, 257–266. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S RRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, e00009–e00015. [Google Scholar] [CrossRef]
- Comeau, A.M.; Douglas, G.M.; Langille, M.G.I. Microbiome Helper: A Custom and Streamlined Workflow for Microbiome Research. mSystems 2017, 2, e00127-16. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmo1. Bolger AM, Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Marcon, E.; Hérault, B. Entropart: An R Package to Measure and Partition Diversity. J. Stat. Softw. 2015, 67, 1–26. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package (Version 2.5-6). Compr. R Arch. Netw. 2019, 2, 1–295. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and Microbial Differential Abundance Strategies Depend upon Data Characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitali, R.; Palone, F.; De Stefano, I.; Fiorente, C.; Novelli, F.; Pasquali, E.; Fratini, E.; Tanori, M.; Leonardi, S.; Tanno, B.; et al. Characterization of Early and Late Damage in a Mouse Model of Pelvic Radiation Disease. Int. J. Mol. Sci. 2023, 24, 8800. https://doi.org/10.3390/ijms24108800
Vitali R, Palone F, De Stefano I, Fiorente C, Novelli F, Pasquali E, Fratini E, Tanori M, Leonardi S, Tanno B, et al. Characterization of Early and Late Damage in a Mouse Model of Pelvic Radiation Disease. International Journal of Molecular Sciences. 2023; 24(10):8800. https://doi.org/10.3390/ijms24108800
Chicago/Turabian StyleVitali, Roberta, Francesca Palone, Ilaria De Stefano, Chiara Fiorente, Flavia Novelli, Emanuela Pasquali, Emiliano Fratini, Mirella Tanori, Simona Leonardi, Barbara Tanno, and et al. 2023. "Characterization of Early and Late Damage in a Mouse Model of Pelvic Radiation Disease" International Journal of Molecular Sciences 24, no. 10: 8800. https://doi.org/10.3390/ijms24108800
APA StyleVitali, R., Palone, F., De Stefano, I., Fiorente, C., Novelli, F., Pasquali, E., Fratini, E., Tanori, M., Leonardi, S., Tanno, B., Colantoni, E., Soldi, S., Galletti, S., Grimaldi, M., Morganti, A. G., Fuccio, L., Pazzaglia, S., Pioli, C., Mancuso, M., & Vesci, L. (2023). Characterization of Early and Late Damage in a Mouse Model of Pelvic Radiation Disease. International Journal of Molecular Sciences, 24(10), 8800. https://doi.org/10.3390/ijms24108800







